Oxidative Stress and the Use of Antioxidants in Stroke
Abstract
:1. Introduction
2. Sources of Reactive Oxygen Species (ROS)
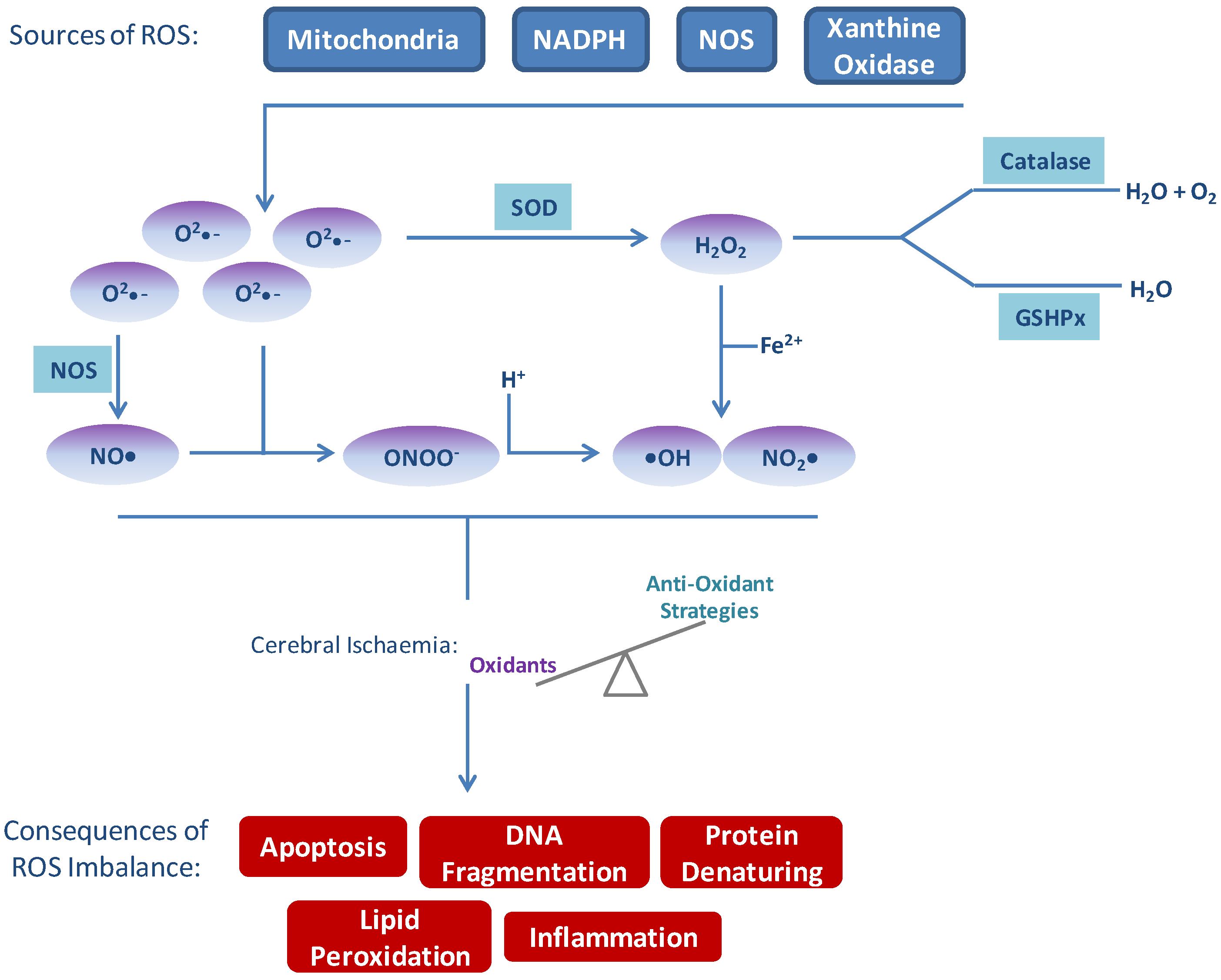
3. Consequences of ROS Imbalance
3.1. Apoptosis
3.2. Blood-Brain Barrier (BBB) Disruption
3.3. Immune Response
4. Antioxidant Strategies
4.1. Inhibition of Free Radical Production
4.2. Free Radical Scavengers
4.3. Free Radical Degradation
4.4. Mitochondrial Targeted Anti-Oxidants
4.5. Upregulation of Endogenous Antioxidants
4.6. Combination Therapy
4.7. Reasons for Failure?
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (dalys) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Townsend, N.; Wickramasinghe, K.; Bhatnagar, P.; Smolina, K.; Nichols, M.; Leal, J.; Luengo-Fernandez, R.; Rayner, M. Coronary Heart Disease Statistics; British Heart Foundation: London, UK, 2012; pp. 1–206. [Google Scholar]
- Li, B.-H.; Ding, X.; Yin, Y.-W.; Liu, Y.; Gao, C.-Y.; Zhang, L.-L.; Li, J.-C. Meta-analysis of clinical outcomes of intravenous recombinant tissue plasminogen activator for acute ischemic stroke: Within 3 h versus 3–4.5 h. Curr. Med. Res. Opin. 2013, 29, 1105–1114. [Google Scholar] [CrossRef]
- Adeoye, O.; Hornung, R.; Khatri, P.; Kleindorfer, D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the united states: A doubling of treatment rates over the course of 5 years. Stroke 2011, 42, 1952–1955. [Google Scholar] [CrossRef]
- Herculano-Houzel, S. Scaling of brain metabolism with a fixed energy budget per neuron: Implications for neuronal activity, plasticity and evolution. PLoS One 2011, 6, e17514. [Google Scholar] [CrossRef]
- Pauwels, P.J.; Opperdoes, F.R.; Trouet, A. Effects of antimycin, glucose deprivation, and serum on cultures of neurons, astrocytes, and neuroblastoma cells. J. Neurochem. 1985, 44, 143–148. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Kahles, T.; Brandes, R.P. NADPH oxidases as therapeutic targets in ischemic stroke. Cell. Mol. Life Sci. 2012, 69, 2345–2363. [Google Scholar] [CrossRef]
- Sanderson, T.H.; Reynolds, C.A.; Kumar, R.; Przyklenk, K.; Huttemann, M. Molecular mechanisms of ischemia-reperfusion injury in brain: Pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol. Neurobiol. 2013, 47, 9–23. [Google Scholar] [CrossRef]
- Vergeade, A.; Mulder, P.; Vendeville, C.; Ventura-Clapier, R.; Thuillez, C.; Monteil, C. Xanthine oxidase contributes to mitochondrial ros generation in an experimental model of cocaine-induced diastolic dysfunction. J. Cardiovasc. Pharmacol. 2012, 60, 538–543. [Google Scholar] [CrossRef]
- Rice, M.E. H2O2: A dynamic neuromodulator. Neuroscientist 2011, 17, 389–406. [Google Scholar] [CrossRef]
- Liu, S.; Shi, H.; Liu, W.; Furuichi, T.; Timmins, G.S.; Liu, K.J. Interstitial pO2 in ischemic penumbra and core are differentially affected following transient focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 2004, 24, 343–349. [Google Scholar] [PubMed]
- Ying, W.; Han, S.K.; Miller, J.W.; Swanson, R.A. Acidosis potentiates oxidative neuronal death by multiple mechanisms. J. Neurochem. 1999, 73, 1549–1556. [Google Scholar] [PubMed]
- Stanika, R.I.; Villanueva, I.; Kazanina, G.; Andrews, S.B.; Pivovarova, N.B. Comparative impact of voltage-gated calcium channels and nmda receptors on mitochondria-mediated neuronal injury. J. Neurosci. 2012, 32, 6642–6650. [Google Scholar] [CrossRef]
- Yamato, M.; Egashira, T.; Utsumi, H. Application of in vivo ESR spectroscopy to measurement of cerebrovascular ROS generation in stroke. Free Radic. Biol. Med. 2003, 35, 1619–1631. [Google Scholar] [CrossRef]
- Peters, O.; Back, T.; Lindauer, U.; Busch, C.; Megow, D.; Dreier, J.; Dirnagl, U. Increased formation of reactive oxygen species after permanent and reversible middle cerebral artery occlusion in the rat. J. Cereb. Blood Flow Metab. 1998, 18, 196–205. [Google Scholar] [PubMed]
- Chen, Q.; Moghaddas, S.; Hoppel, C.L.; Lesnefsky, E.J. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am. J. Physiol. Cell. Physiol. 2008, 294, C460–C466. [Google Scholar] [PubMed]
- Niatsetskaya, Z.V.; Sosunov, S.A.; Matsiukevich, D.; Utkina-Sosunova, I.V.; Ratner, V.I.; Starkov, A.A.; Ten, V.S. The oxygen free radicals originating from mitochondrial complex I contribute to oxidative brain injury following hypoxia-ischemia in neonatal mice. J. Neurosci. 2012, 32, 3235–3244. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Methner, C.; Nadtochiy, S.M.; Logan, A.; Pell, V.R.; Ding, S.; James, A.M.; Cocheme, H.M.; Reinhold, J.; Lilley, K.S.; et al. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat. Med. 2013, 19, 753–759. [Google Scholar] [CrossRef]
- Chen, H.; Song, Y.S.; Chan, P.H. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J. Cereb. Blood Flow Metab. 2009, 29, 1262–1272. [Google Scholar] [CrossRef]
- Bokoch, G.M.; Knaus, U.G. NADPH oxidases: Not just for leukocytes anymore! Trends Biochem. Sci. 2003, 28, 502–508. [Google Scholar] [CrossRef]
- Cheng, G.; Ritsick, D.; Lambeth, J.D. Nox3 regulation by NOXO1, p47phox, and p67phox. J. Biol. Chem. 2004, 279, 34250–34255. [Google Scholar] [CrossRef]
- Matsushima, S.; Kuroda, J.; Ago, T.; Zhai, P.; Park, J.Y.; Xie, L.H.; Tian, B.; Sadoshima, J. Increased oxidative stress in the nucleus caused by Nox4 mediates oxidation of HDAC4 and cardiac hypertrophy. Circ. Res. 2013, 112, 651–663. [Google Scholar] [CrossRef]
- Granger, D.N.; Rutili, G.; McCord, J.M. Superoxide radicals in feline intestinal ischemia. Gastroenterology 1981, 81, 22–29. [Google Scholar] [PubMed]
- Parks, D.A.; Granger, D.N. Xanthine oxidase: Biochemistry, distribution and physiology. Acta Physiol. Scand. Suppl. 1986, 548, 87–99. [Google Scholar] [PubMed]
- Crack, P.J.; Taylor, J.M. Reactive oxygen species and the modulation of stroke. Free Radic. Biol. Med. 2005, 38, 1433–1444. [Google Scholar] [CrossRef]
- Gursoy-Ozdemir, Y.; Can, A.; Dalkara, T. Reperfusion-induced oxidative/nitrative injury to neurovascular unit after focal cerebral ischemia. Stroke 2004, 35, 1449–1453. [Google Scholar] [CrossRef]
- Nelson, C.W.; Wei, E.P.; Povlishock, J.T.; Kontos, H.A.; Moskowitz, M.A. Oxygen radicals in cerebral ischemia. Am. J. Physiol. 1992, 263, H1356–H1362. [Google Scholar] [PubMed]
- McCracken, E.; Valeriani, V.; Simpson, C.; Jover, T.; McCulloch, J.; Dewar, D. The lipid peroxidation by-product 4-hydroxynonenal is toxic to axons and oligodendrocytes. J. Cereb. Blood Flow Metab. 2000, 20, 1529–1536. [Google Scholar] [PubMed]
- Matsuda, S.; Umeda, M.; Uchida, H.; Kato, H.; Araki, T. Alterations of oxidative stress markers and apoptosis markers in the striatum after transient focal cerebral ischemia in rats. J. Neural Transm. 2009, 116, 395–404. [Google Scholar] [CrossRef]
- Liu, P.K.; Hsu, C.Y.; Dizdaroglu, M.; Floyd, R.A.; Kow, Y.W.; Karakaya, A.; Rabow, L.E.; Cui, J.K. Damage, repair, and mutagenesis in nuclear genes after mouse forebrain ischemia-reperfusion. J. Neurosci. 1996, 16, 6795–6806. [Google Scholar] [PubMed]
- Chen, J.; Jin, K.; Chen, M.; Pei, W.; Kawaguchi, K.; Greenberg, D.A.; Simon, R.P. Early detection of DNA strand breaks in the brain after transient focal ischemia: Implications for the role of DNA damage in apoptosis and neuronal cell death. J. Neurochem. 1997, 69, 232–245. [Google Scholar] [PubMed]
- Nagayama, T.; Simon, R.P.; Chen, D.; Henshall, D.C.; Pei, W.; Stetler, R.A.; Chen, J. Activation of poly(adp-ribose) polymerase in the rat hippocampus may contribute to cellular recovery following sublethal transient global ischemia. J. Neurochem. 2000, 74, 1636–1645. [Google Scholar] [PubMed]
- Kawase, M.; Fujimura, M.; Morita-Fujimura, Y.; Chan, P.H. Reduction of apurinic/apyrimidinic endonuclease expression after transient global cerebral ischemia in rats: Implication of the failure of DNA repair in neuronal apoptosis. Stroke 1999, 30, 441–449. [Google Scholar] [CrossRef]
- Kirkland, R.A.; Windelborn, J.A.; Kasprzak, J.M.; Franklin, J.L. A bax-induced pro-oxidant state is critical for cytochrome c release during programmed neuronal death. J. Neurosci. 2002, 22, 6480–6490. [Google Scholar] [PubMed]
- Sugawara, T.; Lewen, A.; Gasche, Y.; Yu, F.; Chan, P.H. Overexpression of SOD1 protects vulnerable motor neurons after spinal cord injury by attenuating mitochondrial cytochrome c release. FASEB J. 2002, 16, 1997–1999. [Google Scholar] [PubMed]
- Croall, D.E.; DeMartino, G.N. Calcium-activated neutral protease (calpain) system: Structure, function, and regulation. Physiol. Rev. 1991, 71, 813–847. [Google Scholar] [PubMed]
- Scorrano, L.; Penzo, D.; Petronilli, V.; Pagano, F.; Bernardi, P. Arachidonic acid causes cell death through the mitochondrial permeability transition. Implications for tumor necrosis factor-alpha aopototic signaling. J. Biol. Chem. 2001, 276, 12035–12040. [Google Scholar] [CrossRef] [PubMed]
- Enari, M.; Sakahira, H.; Yokoyama, H.; Okawa, K.; Iwamatsu, A.; Nagata, S. A caspase-activated dnase that degrades DNA during apoptosis, and its inhibitor icad. Nature 1998, 391, 43–50. [Google Scholar] [CrossRef]
- Jänicke, R.U.; Sprengart, M.L.; Wati, M.R.; Porter, A.G. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J. Biol. Chem. 1998, 273, 9357–9360. [Google Scholar] [CrossRef]
- Hanus, J.; Kalinowska-Herok, M.; Widlak, P. The major apoptotic endonuclease DFF40/CAD is a deoxyribose-specific and double-strand-specific enzyme. Apoptosis 2008, 13, 377–382. [Google Scholar] [CrossRef]
- Widlak, P.; Li, P.; Wang, X.; Garrard, W.T. Cleavage preferences of the apoptotic endonuclease DFF40 (caspase-activated dnase or nuclease) on naked DNA and chromatin substrates. J. Biol. Chem. 2000, 275, 8226–8232. [Google Scholar] [CrossRef]
- Dalton, T.P.; Shertzer, H.G.; Puga, A. Regulation of gene expression by reactive oxygen. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 67–101. [Google Scholar] [CrossRef]
- Pautz, A.; Art, J.; Hahn, S.; Nowag, S.; Voss, C.; Kleinert, H. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide 2010, 23, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Raval, A.P.; Hirsch, N.; Perez-Pinzon, M.A. Ischemic preconditioning mediates cyclooxygenase-2 expression via nuclear factor-kappa B activation in mixed cortical neuronal cultures. Transl. Stroke Res. 2010, 1, 40–47. [Google Scholar] [CrossRef]
- Hsieh, H.L.; Wang, H.H.; Wu, W.B.; Chu, P.J.; Yang, C.M. Transforming growth factor-beta1 induces matrix metalloproteinase-9 and cell migration in astrocytes: Roles of ros-dependent ERK- and JNK-NF-kappaB pathways. J. Neuroinflamm. 2010, 7, 88. [Google Scholar] [CrossRef]
- Park, T.Y.; Baik, E.J.; Lee, S.H. Prostaglandin e(2)-induced intercellular adhesion molecule-1 expression is mediated by cAMP/Epac signalling modules in bEnd.3 brain endothelial cells. Br. J. Pharmacol. 2013, 169, 604–618. [Google Scholar] [CrossRef]
- Deng, X.; Xiao, L.; Lang, W.; Gao, F.; Ruvolo, P.; May, W.S., Jr. Novel role for JNK as a stress-activated Bcl2 kinase. J. Biol. Chem. 2001, 276, 23681–23688. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Chambers, T.C. Role of mitogen-activated protein kinases in the response of tumor cells to chemotherapy. Drug Resist. Updat 2001, 4, 253–267. [Google Scholar] [CrossRef]
- Soberanes, S.; Urich, D.; Baker, C.M.; Burgess, Z.; Chiarella, S.E.; Bell, E.L.; Ghio, A.J.; de Vizcaya-Ruiz, A.; Liu, J.; Ridge, K.M.; et al. Mitochondrial complex III-generated oxidants activate ASK1 and JNK to induce alveolar epithelial cell death following exposure to particulate matter air pollution. J. Biol. Chem. 2009, 284, 2176–2186. [Google Scholar] [CrossRef]
- De Vries, H.E.; Kuiper, J.; de Boer, A.G.; van Berkel, T.J.; Breimer, D.D. The blood-brain barrier in neuroinflammatory diseases. Pharmacol. Rev. 1997, 49, 143–155. [Google Scholar] [PubMed]
- Grieb, P.; Forster, R.E.; Strome, D.; Goodwin, C.W.; Pape, P.C. O2 exchange between blood and brain tissues studied with 18O2 indicator-dilution technique. J. Appl. Physiol. 1985, 58, 1929–1941. [Google Scholar] [PubMed]
- Neumann-Haefelin, T.; Kastrup, A.; de Crespigny, A.; Yenari, M.A.; Ringer, T.; Sun, G.H.; Moseley, M.E. Serial mri after transient focal cerebral ischemia in rats: Dynamics of tissue injury, blood-brain barrier damage, and edema formation. Stroke 2000, 31, 1965–1973. [Google Scholar] [CrossRef]
- Rosell, A.; Cuadrado, E.; Ortega-Aznar, A.; Hernández-Guillamon, M.; Lo, E.H.; Montaner, J. Mmp-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke 2008, 39, 1121–1126. [Google Scholar] [CrossRef]
- Cortez, D.M.; Feldman, M.D.; Mummidi, S.; Valente, A.J.; Steffensen, B.; Vincenti, M.; Barnes, J.L.; Chandrasekar, B. Il-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-β, NF-κB, and AP-1 activation. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3356–H3365. [Google Scholar] [CrossRef]
- Mark, K.S.; Davis, T.P. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1485–H1494. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.D.; Witt, K.A.; Hom, S.; Egleton, R.D.; Mark, K.S.; Davis, T.P. Inflammatory pain alters blood-brain barrier permeability and tight junctional protein expression. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1241–H1248. [Google Scholar] [PubMed]
- Yamagata, K.; Tagami, M.; Takenaga, F.; Yamori, Y.; Itoh, S. Hypoxia-induced changes in tight junction permeability of brain capillary endothelial cells are associated with IL-1beta and nitric oxide. Neurobiol. Dis. 2004, 17, 491–499. [Google Scholar] [CrossRef]
- Pokutta, S.; Herrenknecht, K.; Kemler, R.; Engel, J. Conformational changes of the recombinant extracellular domain of E-cadherin upon calcium binding. Eur. J. Biochem. 1994, 223, 1019–1026. [Google Scholar] [CrossRef]
- Cipolla, M.J.; Crete, R.; Vitullo, L.; Rix, R.D. Transcellular transport as a mechanism of blood-brain barrier disruption during stroke. Front. Biosci. 2004, 9, 777–785. [Google Scholar] [CrossRef]
- Xu, H.; Dawson, R.; Crane, I.J.; Liversidge, J. Leukocyte diapedesis in vivo induces transient loss of tight junction protein at the blood-retina barrier. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2487–2494. [Google Scholar] [CrossRef]
- Konsman, J.P.; Drukarch, B.; van Dam, A.M. (Peri)vascular production and action of pro-inflammatory cytokines in brain pathology. Clin. Sci. 2007, 112, 1–25. [Google Scholar] [CrossRef]
- Peerschke, E.I.; Yin, W.; Ghebrehiwet, B. Complement activation on platelets: Implications for vascular inflammation and thrombosis. Mol. Immunol. 2010, 47, 2170–2175. [Google Scholar] [CrossRef]
- Pinsky, D.J.; Naka, Y.; Liao, H.; Oz, M.C.; Wagner, D.D.; Mayadas, T.N.; Johnson, R.C.; Hynes, R.O.; Heath, M.; Lawson, C.A.; et al. Hypoxia-induced exocytosis of endothelial cell weibel-palade bodies. A mechanism for rapid neutrophil recruitment after cardiac preservation. J. Clin. Investig. 1996, 97, 493–500. [Google Scholar] [CrossRef]
- Yilmaz, G.; Granger, D.N. Leukocyte recruitment and ischemic brain injury. Neuromol. Med. 2010, 12, 193–204. [Google Scholar] [CrossRef]
- Atochin, D.N.; Wang, A.; Liu, V.W.; Critchlow, J.D.; Dantas, A.P.; Looft-Wilson, R.; Murata, T.; Salomone, S.; Shin, H.K.; Ayata, C.; et al. The phosphorylation state of enos modulates vascular reactivity and outcome of cerebral ischemia in vivo. J. Clin. Investig. 2007, 117, 1961–1967. [Google Scholar] [CrossRef]
- Del Zoppo, G.J.; Schmid-Schonbein, G.W.; Mori, E.; Copeland, B.R.; Chang, C.M. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke 1991, 22, 1276–1283. [Google Scholar] [CrossRef]
- Melani, A.; Turchi, D.; Vannucchi, M.G.; Cipriani, S.; Gianfriddo, M.; Pedata, F. ATP extracellular concentrations are increased in the rat striatum during in vivo ischemia. Neurochem. Int. 2005, 47, 442–448. [Google Scholar] [CrossRef]
- Korcok, J.; Raimundo, L.N.; Ke, H.Z.; Sims, S.M.; Dixon, S.J. Extracellular nucleotides act through P2X7 receptors to activate NF-kappaB in osteoclasts. J. Bone Miner. Res. 2004, 19, 642–651. [Google Scholar] [CrossRef]
- Lyons, A.; Downer, E.J.; Crotty, S.; Nolan, Y.M.; Mills, K.H.; Lynch, M.A. CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: A role for IL-4. J. Neurosci. 2007, 27, 8309–8313. [Google Scholar] [CrossRef]
- Denes, A.; Ferenczi, S.; Halasz, J.; Kornyei, Z.; Kovacs, K.J. Role of CX3CR1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. J. Cereb. Blood Flow Metab. 2008, 28, 1707–1721. [Google Scholar] [CrossRef]
- Chen, G.Y.; Nunez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef]
- Marsh, B.J.; Williams-Karnesky, R.L.; Stenzel-Poore, M.P. Toll-like receptor signaling in endogenous neuroprotection and stroke. Neuroscience 2009, 158, 1007–1020. [Google Scholar] [CrossRef]
- Facchinetti, F.; Dawson, V.L.; Dawson, T.M. Free radicals as mediators of neuronal injury. Cell. Mol. Neurobiol. 1998, 18, 667–682. [Google Scholar] [CrossRef]
- Margaill, I.; Plotkine, M.; Lerouet, D. Antioxidant strategies in the treatment of stroke. Free Radic. Biol. Med. 2005, 39, 429–443. [Google Scholar] [CrossRef]
- Tang, L.L.; Ye, K.; Yang, X.F.; Zheng, J.S. Apocynin attenuates cerebral infarction after transient focal ischaemia in rats. J. Int. Med. Res. 2007, 35, 517–522. [Google Scholar] [CrossRef]
- Genovese, T.; Mazzon, E.; Paterniti, I.; Esposito, E.; Bramanti, P.; Cuzzocrea, S. Modulation of NADPH oxidase activation in cerebral ischemia/reperfusion injury in rats. Brain Res. 2011, 1372, 92–102. [Google Scholar] [CrossRef]
- McCann, S.K.; Dusting, G.J.; Roulston, C.L. Early increase of NOX4 NADPH oxidase and superoxide generation following endothelin-1-induced stroke in conscious rats. J. Neurosci. Res. 2008, 86, 2524–2534. [Google Scholar] [CrossRef]
- Yoshioka, H.; Niizuma, K.; Katsu, M.; Okami, N.; Sakata, H.; Kim, G.S.; Narasimhan, P.; Chan, P.H. NADPH oxidase mediates striatal neuronal injury after transient global cerebral ischemia. J. Cereb. Blood Flow Metab. 2011, 31, 868–880. [Google Scholar] [CrossRef]
- Serrander, L.; Cartier, L.; Bedard, K.; Banfi, B.; Lardy, B.; Plastre, O.; Sienkiewicz, A.; Forro, L.; Schlegel, W.; Krause, K.H. NOX4 activity is determined by MRNA levels and reveals a unique pattern of ros generation. Biochem. J. 2007, 406, 105–114. [Google Scholar] [CrossRef]
- Chen, H.; Kim, G.S.; Okami, N.; Narasimhan, P.; Chan, P.H. NADPH oxidase is involved in post-ischemic brain inflammation. Neurobiol. Dis. 2011, 42, 341–348. [Google Scholar] [CrossRef]
- De Silva, T.M.; Brait, V.H.; Drummond, G.R.; Sobey, C.G.; Miller, A.A. NOX2 oxidase activity accounts for the oxidative stress and vasomotor dysfunction in mouse cerebral arteries following ischemic stroke. PLoS One 2011, 6, e0028393. [Google Scholar]
- Kleinschnitz, C.; Grund, H.; Wingler, K.; Armitage, M.E.; Jones, E.; Mittal, M.; Barit, D.; Schwarz, T.; Geis, C.; Kraft, P.; et al. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010, 8, e1000479. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Hattori, K.; Hamanaka, J.; Murase, T.; Egashira, Y.; Mishiro, K.; Ishiguro, M.; Tsuruma, K.; Hirose, Y.; Tanaka, H.; et al. Pharmacological inhibition of TLR4-NOX4 signal protects against neuronal death in transient focal ischemia. Sci. Rep. 2012, 2. [Google Scholar] [CrossRef]
- Jackman, K.A.; Miller, A.A.; Drummond, G.R.; Sobey, C.G. Importance of NOX1 for angiotensin II-induced cerebrovascular superoxide production and cortical infarct volume following ischemic stroke. Brain Res. 2009, 1286, 215–220. [Google Scholar] [CrossRef]
- Kahles, T.; Kohnen, A.; Heumueller, S.; Rappert, A.; Bechmann, I.; Liebner, S.; Wittko, I.M.; Neumann-Haefelin, T.; Steinmetz, H.; Schroeder, K.; et al. NADPH oxidase NOX1 contributes to ischemic injury in experimental stroke in mice. Neurobiol. Dis. 2010, 40, 185–192. [Google Scholar] [CrossRef]
- Radermacher, K.A.; Wingler, K.; Kleikers, P.; Altenhofer, S., Jr.; Hermans, J.; Kleinschnitz, C.; Hhw Schmidt, H. The 1027th target candidate in stroke: Will NADPH oxidase hold up? Exp. Transl. Stroke Med. 2012, 4, 11. [Google Scholar] [CrossRef]
- Altenhofer, S.; Radermacher, K.A.; Kleikers, P.; Wingler, K.; Schmidt, H.H. Evolution of NADPH oxidase inhibitors: Selectivity and mechanisms for target engagement. Antioxid. Redox Signal. 2014, in press. [Google Scholar]
- Khan, F.; George, J.; Wong, K.; McSwiggan, S.; Struthers, A.D.; Belch, J.J. Allopurinol treatment reduces arterial wave reflection in stroke survivors. Cardiovasc. Ther. 2008, 26, 247–252. [Google Scholar] [CrossRef]
- Muir, K.W. Glutamate-based therapeutic approaches: Clinical trials with nmda antagonists. Curr. Opin. Pharmacol. 2006, 6, 53–60. [Google Scholar] [CrossRef]
- Dawson, J.; Quinn, T.J.; Harrow, C.; Lees, K.R.; Walters, M.R. The effect of allopurinol on the cerebral vasculature of patients with subcortical stroke; a randomized trial. Br. J. Clin. Pharmacol. 2009, 68, 662–668. [Google Scholar] [CrossRef]
- Park, C.K.; Hall, E.D. Dose-response analysis of the effect of 21-aminosteroid tirilazad mesylate (U-74006F) upon neurological outcome and ischemic brain damage in permanent focal cerebral ischemia. Brain Res. 1994, 645, 157–163. [Google Scholar] [CrossRef]
- Xue, D.; Slivka, A.; Buchan, A.M. Tirilazad reduces cortical infarction after transient but not permanent focal cerebral ischemia in rats. Stroke 1992, 23, 894–899. [Google Scholar] [CrossRef]
- Sena, E.; Wheble, P.; Sandercock, P.; Macleod, M. Systematic review and meta-analysis of the efficacy of tirilazad in experimental stroke. Stroke 2007, 38, 388–394. [Google Scholar] [CrossRef]
- RANTTAS. A randomized trial of tirilazad mesylate in patients with acute stroke (ranttas). The ranttas investigators. Stroke 1996, 27, 1453–1458. [Google Scholar] [CrossRef]
- Fleishaker, J.C.; Hulst-Pearson, L.K.; Peters, G.R. Effect of gender and menopausal status on the pharmacokinetics of tirilazad mesylate in healthy subjects. Am. J. Ther. 1995, 2, 553–560. [Google Scholar] [CrossRef]
- Kuroda, S.; Tsuchidate, R.; Smith, M.L.; Maples, K.R.; Siesjo, B.K. Neuroprotective effects of a novel nitrone, NXY-059, after transient focal cerebral ischemia in the rat. J. Cereb. Blood Flow Metab. 1999, 19, 778–787. [Google Scholar] [PubMed]
- Zhao, Z.; Cheng, M.; Maples, K.R.; Ma, J.Y.; Buchan, A.M. NXY-059, a novel free radical trapping compound, reduces cortical infarction after permanent focal cerebral ischemia in the rat. Brain Res. 2001, 909, 46–50. [Google Scholar] [CrossRef]
- Sydserff, S.G.; Borelli, A.R.; Green, A.R.; Cross, A.J. Effect of NXY-059 on infarct volume after transient or permanent middle cerebral artery occlusion in the rat; studies on dose, plasma concentration and therapeutic time window. Br. J. Pharmacol. 2002, 135, 103–112. [Google Scholar] [CrossRef]
- Marshall, J.W.; Duffin, K.J.; Green, A.R.; Ridley, R.M. NXY-059, a free radical—Trapping agent, substantially lessens the functional disability resulting from cerebral ischemia in a primate species. Stroke 2001, 32, 190–198. [Google Scholar] [CrossRef]
- Marshall, J.W.; Cummings, R.M.; Bowes, L.J.; Ridley, R.M.; Green, A.R. Functional and histological evidence for the protective effect of NXY-059 in a primate model of stroke when given 4 h after occlusion. Stroke 2003, 34, 2228–2233. [Google Scholar] [CrossRef]
- Lees, K.R.; Davalos, A.; Davis, S.M.; Diener, H.C.; Grotta, J.; Lyden, P.; Shuaib, A.; Ashwood, T.; Hardemark, H.G.; Wasiewski, W.; et al. Additional outcomes and subgroup analyses of NXY-059 for acute ischemic stroke in the SAINT I trial. Stroke 2006, 37, 2970–2978. [Google Scholar] [CrossRef]
- Shuaib, A.; Lees, K.R.; Lyden, P.; Grotta, J.; Davalos, A.; Davis, S.M.; Diener, H.C.; Ashwood, T.; Wasiewski, W.W.; Emeribe, U. NXY-059 for the treatment of acute ischemic stroke. N. Engl. J. Med. 2007, 357, 562–571. [Google Scholar] [CrossRef]
- Koziol, J.A.; Feng, A.C. On the analysis and interpretation of outcome measures in stroke clinical trials: Lessons from the saint i study of NXY-059 for acute ischemic stroke. Stroke 2006, 37, 2644–2647. [Google Scholar] [CrossRef]
- Saver, J.L. Clinical impact of NXY-059 demonstrated in the saint I trial: Derivation of number needed to treat for benefit over entire range of functional disability. Stroke 2007, 38, 1515–1518. [Google Scholar] [CrossRef]
- Fisher, M.; Lees, K.; Papadakis, M.; Buchan, A.M. NXY-059: Brain or vessel protection. Stroke 2006, 37, 2189–2190. [Google Scholar] [CrossRef]
- Watanabe, T.; Tanaka, M.; Watanabe, K.; Takamatsu, Y.; Tobe, A. Research and development of the free radical scavenger edaravone as a neuroprotectant. Yakugaku Zasshi 2004, 124, 99–111. [Google Scholar] [CrossRef]
- Lapchak, P.A. A critical assessment of edaravone acute ischemic stroke efficacy trials: Is edaravone an effective neuroprotective therapy? Exp. Opin. Pharmacother. 2010, 11, 1753–1763. [Google Scholar] [CrossRef]
- Higashi, Y. Edaravone for the treatment of acute cerebral infarction: Role of endothelium-derived nitric oxide and oxidative stress. Exp. Opin. Pharmacother. 2009, 10, 323–331. [Google Scholar] [CrossRef]
- Watanabe, T.; Yuki, S.; Egawa, M.; Nishi, H. Protective effects of MCI-186 on cerebral-ischemia—Possible involvement of free-radical scavenging and antioxidant actions. J. Pharmacol. Exp. Ther. 1994, 268, 1597–1604. [Google Scholar] [PubMed]
- Wu, T.W.; Zeng, L.H.; Wu, J.; Fung, K.P. MCI-186: Further histochemical and biochemical evidence of neuroprotection. Life Sci. 2000, 67, 2387–2392. [Google Scholar] [CrossRef]
- Jin, Y.J.; Mima, T.; Raicu, V.; Park, K.C.; Shimizu, K. Combined argatroban and edaravone caused additive neuroprotection against 15 min of forebrain ischemia in gerbils. Neurosci. Res. 2002, 43, 75–79. [Google Scholar] [CrossRef]
- Ikeda, S.; Harada, K.; Ohwatashi, A.; Kamikawa, Y. Effects of edaravone, a free radical scavenger, on photochemically induced cerebral infarction in a rat hemiplegic model. Sci. World J. 2013, 2013, 175280. [Google Scholar]
- Otomo, E.; Tohgi, H.; Kogure, K.; Hirai, S.; Takakura, K.; Terashi, A.; Gotoh, F.; Maruyama, S.; Tazaki, Y.; Shinohara, Y.; et al. Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction—Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovas. Dis. 2003, 15, 222–229. [Google Scholar] [CrossRef]
- Inatomi, Y.; Takita, T.; Yonehara, T.; Fujioka, S.; Hashimoto, Y.; Hirano, T.; Uchino, M. Efficacy of edaravone in cardioembolic stroke. Int. Med. 2006, 45, 253–257. [Google Scholar] [CrossRef]
- Nakase, T.; Yoshioka, S.; Suzuki, A. Free radical scavenger, edaravone, reduces the lesion size of lacunar infarction in human brain ischemic stroke. BMC Neurol. 2011, 11, 39. [Google Scholar] [CrossRef]
- Warner, D.S.; Sheng, H.; Batinic-Haberle, I. Oxidants, antioxidants and the ischemic brain. J. Exp. Biol. 2004, 207, 3221–3231. [Google Scholar] [CrossRef]
- Gaspar, T.; Domoki, F.; Lenti, L.; Institoris, A.; Snipes, J.A.; Bari, F.; Busija, D.W. Neuroprotective effect of adenoviral catalase gene transfer in cortical neuronal cultures. Brain Res. 2009, 1270, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Jeong, H.J.; Kang, H.W.; Shin, M.J.; Sohn, E.J.; Kim, M.J.; Ahn, E.H.; An, J.J.; Jang, S.H.; Yoo, K.Y.; et al. Transduced human PEP-1-catalase fusion protein attenuates ischemic neuronal damage. Free Radic. Biol. Med. 2009, 47, 941–952. [Google Scholar] [CrossRef]
- Gu, W.; Zhao, H.; Yenari, M.A.; Sapolsky, R.M.; Steinberg, G.K. Catalase over-expression protects striatal neurons from transient focal cerebral ischemia. Neuroreport 2004, 15, 413–416. [Google Scholar] [CrossRef]
- Weisbrot-Lefkowitz, M.; Reuhl, K.; Perry, B.; Chan, P.H.; Inouye, M.; Mirochnitchenko, O. Overexpression of human glutathione peroxidase protects transgenic mice against focal cerebral ischemia/reperfusion damage. Brain Res. Mol. Brain Res. 1998, 53, 333–338. [Google Scholar] [CrossRef]
- Ishibashi, N.; Prokopenko, O.; Weisbrot-Lefkowitz, M.; Reuhl, K.R.; Mirochnitchenko, O. Glutathione peroxidase inhibits cell death and glial activation following experimental stroke. Brain Res. Mol. Brain Res. 2002, 109, 34–44. [Google Scholar] [CrossRef]
- Crack, P.J.; Taylor, J.M.; Flentjar, N.J.; de Haan, J.; Hertzog, P.; Iannello, R.C.; Kola, I. Increased infarct size and exacerbated apoptosis in the glutathione peroxidase-1 (GPX-1) knockout mouse brain in response to ischemia/reperfusion injury. J. Neurochem. 2001, 78, 1389–1399. [Google Scholar] [CrossRef]
- Fujimura, M.; Morita-Fujimura, Y.; Noshita, N.; Sugawara, T.; Kawase, M.; Chan, P.H. The cytosolic antioxidant copper/zinc-superoxide dismutase prevents the early release of mitochondrial cytochrome c in ischemic brain after transient focal cerebral ischemia in mice. J. Neurosci. 2000, 20, 2817–2824. [Google Scholar] [PubMed]
- Kondo, T.; Reaume, A.G.; Huang, T.T.; Murakami, K.; Carlson, E.; Chen, S.; Scott, R.W.; Epstein, C.J.; Chan, P.H. Edema formation exacerbates neurological and histological outcomes after focal cerebral ischemia in cuzn-superoxide dismutase gene knockout mutant mice. Brain Edema X 1997, 70, 62–64. [Google Scholar]
- Davis, A.S.; Zhao, H.; Sun, G.H.; Sapolsky, R.M.; Steinberg, G.K. Gene therapy using SOD1 protects striatal neurons from experimental stroke. Neurosci. Lett. 2007, 411, 32–36. [Google Scholar] [CrossRef]
- Sheng, H.; Bart, R.D.; Oury, T.D.; Pearlstein, R.D.; Crapo, J.D.; Warner, D.S. Mice overexpressing extracellular superoxide dismutase have increased resistance to focal cerebral ischemia. Neuroscience 1999, 88, 185–191. [Google Scholar] [CrossRef]
- Li, P.F. Oxidative modification of cupro-zinc superoxide dismutase by reactive oxygen species. Sheng Li Ke Xue Jin Zhan 1995, 26, 50–52. [Google Scholar] [PubMed]
- Kim, G.W.; Chan, P.H. Involvement of superoxide in excitotoxicity and DNA fragmentation in striatal vulnerability in mice after treatment with the mitochondrial toxin, 3-nitropropionic acid. J. Cereb. Blood Flow Metab. 2002, 22, 798–809. [Google Scholar] [PubMed]
- Maier, C.M.; Hsieh, L.; Crandall, T.; Narasinnhan, P.; Chan, P.H. A new approach for the investigation of reperfusion-related brain injury. Biochem. Soc. Trans. 2006, 34, 1366–1369. [Google Scholar] [CrossRef]
- Jung, J.E.; Kim, G.S.; Narasimhan, P.; Chan, P.H. STAT3 regulates the transcription of the mouse Mn-SOD gene as a neuroprotectant in cerebral ischemic reperfusion. J. Cereb. Blood Flow Metab. 2009, 29, S565–S566. [Google Scholar] [CrossRef]
- Jung, J.E.; Kim, G.S.; Chan, P.H. Neuroprotection by interleukin-6 is mediated by signal transducer and activator of transcription 3 and antioxidative signaling in ischemic stroke. Stroke 2011, 42, 3574–3579. [Google Scholar] [CrossRef]
- Namura, S.; Nagata, I.; Takami, S.; Masayasu, H.; Kikuchi, H. Ebselen reduces cytochrome c release from mitochondria and subsequent DNA fragmentation after transient focal cerebral ischemia in mice. Stroke 2001, 32, 1906–1911. [Google Scholar] [CrossRef]
- Imai, H.; Masayasu, H.; Dewar, D.; Graham, D.I.; Macrae, I.M. Ebselen protects both gray and white matter in a rodent model of focal cerebral ischemia. Stroke 2001, 32, 2149–2154. [Google Scholar] [CrossRef]
- Takasago, T.; Peters, E.E.; Graham, D.I.; Masayasu, H.; Macrae, I.M. Neuroprotective efficacy of ebselen, an anti-oxidant with anti-inflammatory actions, in a rodent model of permanent middle cerebral artery occlusion. Br. J. Pharmacol. 1997, 122, 1251–1256. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Sano, K.; Takakura, K.; Saito, I.; Shinohara, Y.; Asano, T.; Yasuhara, H. Ebselen in acute ischemic stroke: A placebo-controlled, double-blind clinical trial. Ebselen study group. Stroke 1998, 29, 12–17. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.-I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Singhal, A.B.; Wang, X.; Sumii, T.; Mori, T.; Lo, E.H. Effects of normobaric hyperoxia in a rat model of focal cerebral ischemia-reperfusion. J. Cereb. Blood Flow Metab. 2002, 22, 861–868. [Google Scholar] [PubMed]
- Singhal, A.B.; Dijkhuizen, R.M.; Rosen, B.R.; Lo, E.H. Normobaric hyperoxia reduces MRI diffusion abnormalities and infarct size in experimental stroke. Neurology 2002, 58, 945–952. [Google Scholar] [CrossRef]
- Esposito, E.; Mandeville, E.T.; Hayakawa, K.; Singhal, A.B.; Lo, E.H. Effects of normobaric oxygen on the progression of focal cerebral ischemia in rats. Exp. Neurol. 2013, 249, 33–38. [Google Scholar] [CrossRef]
- Kim, H.Y.; Singhal, A.B.; Lo, E.H. Normobaric hyperoxia extends the reperfusion window in focal cerebral ischemia. Ann. Neurol. 2005, 57, 571–575. [Google Scholar] [CrossRef]
- Geng, X.; Fu, P.; Ji, X.; Peng, C.; Fredrickson, V.; Sy, C.; Meng, R.; Ling, F.; Du, H.; Tan, X.; et al. Synergetic neuroprotection of normobaric oxygenation and ethanol in ischemic stroke through improved oxidative mechanism. Stroke 2013, 44, 1418–1425. [Google Scholar] [CrossRef]
- Geng, X.; Parmar, S.; Li, X.; Peng, C.; Ji, X.; Chakraborty, T.; Li, W.A.; Du, H.; Tan, X.; Ling, F.; et al. Reduced apoptosis by combining normobaric oxygenation with ethanol in transient ischemic stroke. Brain Res. 2013, 1531, 17–24. [Google Scholar] [CrossRef]
- Dalkara, T.; Moskowitz, M.A. The complex role of nitric oxide in the pathophysiology of focal cerebral ischemia. Brain Pathol. 1994, 4, 49–57. [Google Scholar] [CrossRef]
- Dalkara, T.; Yoshida, T.; Irikura, K.; Moskowitz, M.A. Dual role of nitric oxide in focal cerebral ischemia. Neuropharmacology 1994, 33, 1447–1452. [Google Scholar] [CrossRef]
- Huang, Y.; McNamara, J.O. Ischemic stroke: Acidotoxicity is a perpetrator. Cell 2004, 118, 665–666. [Google Scholar] [CrossRef]
- Charriaut-Marlangue, C.; Bonnin, P.; Gharib, A.; Leger, P.-L.; Villapol, S.; Pocard, M.; Gressens, P.; Renolleau, S.; Baud, O. Inhaled nitric oxide reduces brain damage by collateral recruitment in a neonatal stroke model. Stroke 2012, 43, 3078–3084. [Google Scholar] [CrossRef]
- Terpolilli, N.A.; Kim, S.-W.; Thal, S.C.; Kataoka, H.; Zeisig, V.; Nitzsche, B.; Klaesner, B.; Zhu, C.; Schwarzmaier, S.; Meissner, L.; et al. Inhalation of nitric oxide prevents ischemic brain damage in experimental stroke by selective dilatation of collateral arterioles. Circ. Res. 2012, 110, 727–738. [Google Scholar] [CrossRef]
- Lesage, A.S.; Peeters, L.; Leysen, J.E. Lubeluzole, a novel long-term neuroprotectant, inhibits the glutamate-activated nitric oxide synthase pathway. J. Pharmacol. Exp. Ther. 1996, 279, 759–766. [Google Scholar] [PubMed]
- Ashton, D.; Willems, R.; Wynants, J.; van Reempts, J.; Marrannes, R.; Clincke, G. Altered Na+-channel function as an in vitro model of the ischemic penumbra: Action of lubeluzole and other neuroprotective drugs. Brain Res. 1997, 745, 210–221. [Google Scholar] [CrossRef]
- Maiese, K.; TenBroeke, M.; Kue, I. Neuroprotection of lubeluzole is mediated through the signal transduction pathways of nitric oxide. J. Neurochem. 1997, 68, 710–714. [Google Scholar] [CrossRef]
- De Ryck, M.; Keersmaekers, R.; Duytschaever, H.; Claes, C.; Clincke, G.; Janssen, M.; van Reet, G. Lubeluzole protects sensorimotor function and reduces infarct size in a photochemical stroke model in rats. J. Pharmacol. Exp. Ther. 1996, 279, 748–758. [Google Scholar] [PubMed]
- Aronowski, J.; Strong, R.; Grotta, J.C. Treatment of experimental focal ischemia in rats with lubeluzole. Neuropharmacology 1996, 35, 689–693. [Google Scholar] [CrossRef]
- Diener, H.C.; Hacke, W.; Hennerici, M.; Radberg, J.; Hantson, L.; de Keyser, J. Lubeluzole in acute ischemic stroke. A double-blind, placebo-controlled phase II trial. Lubeluzole international study group. Stroke 1996, 27, 76–81. [Google Scholar] [CrossRef]
- Grotta, J. Lubeluzole treatment of acute ischemic stroke. The US and Canadian lubeluzole ischemic stroke study group. Stroke 1997, 28, 2338–2346. [Google Scholar] [CrossRef]
- Diener, H.C. Multinational randomised controlled trial of lubeluzole in acute ischaemic stroke. European and Australian lubeluzole ischaemic stroke study group. Cerebrovasc. Dis 1998, 8, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Diener, H.C.; Cortens, M.; Ford, G.; Grotta, J.; Hacke, W.; Kaste, M.; Koudstaal, P.J.; Wessel, T. Lubeluzole in acute ischemic stroke treatment: A double-blind study with an 8-h inclusion window comparing a 10-mg daily dose of lubeluzole with placebo. Stroke 2000, 31, 2543–2551. [Google Scholar] [CrossRef]
- Gandolfo, C.; Sandercock, P.; Conti, M. Lubeluzole for acute ischaemic stroke. Cochrane Database Syst. Rev. 2002. [Google Scholar] [CrossRef]
- Weisiger, R.A.; Fridovich, I. Mitochondrial superoxide simutase. Site of synthesis and intramitochondrial localization. J. Biol. Chem. 1973, 248, 4793–4796. [Google Scholar] [PubMed]
- Abramov, A.Y.; Scorziello, A.; Duchen, M.R. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J. Neurosci. 2007, 27, 1129–1138. [Google Scholar] [CrossRef]
- Murphy, M.P. Antioxidants as therapies: Can we improve on nature? Free Radic. Biol. Med. 2014, 66, 20–23. [Google Scholar] [CrossRef]
- Siler-Marsiglio, K.I.; Pan, Q.; Paiva, M.; Madorsky, F.; Khurana, N.C.; Heaton, M.B. Mitochondrially targeted vitamin E and vitamin E mitigate ethanol-mediated effects on cerebellar granule cell antioxidant defense systems. Brain Res. 2005, 1052, 202–211. [Google Scholar] [CrossRef]
- Dhanasekaran, A.; Kotamraju, S.; Kalivendi, S.V.; Matsunaga, T.; Shang, T.; Keszler, A.; Joseph, J.; Kalyanaraman, B. Supplementation of endothelial cells with mitochondria-targeted antioxidants inhibit peroxide-induced mitochondrial iron uptake, oxidative damage, and apoptosis. J. Biol. Chem. 2004, 279, 37575–37587. [Google Scholar] [CrossRef]
- Murphy, M.P.; Smith, R.A. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 629–656. [Google Scholar] [CrossRef]
- Kelso, G.F.; Porteous, C.M.; Hughes, G.; Ledgerwood, E.C.; Gane, A.M.; Smith, R.A.J.; Murphy, M.P. Prevention of mitochondrial oxidative damage using targeted antioxidants. Increasing Healthy Life Span 2002, 959, 263–274. [Google Scholar]
- Smith, R.A.; Murphy, M.P. Animal and human studies with the mitochondria-targeted antioxidant mitoq. Ann. N. Y. Acad.Sci. 2010, 1201, 96–103. [Google Scholar] [CrossRef]
- Adlam, V.J.; Harrison, J.C.; Porteous, C.M.; James, A.M.; Smith, R.A.; Murphy, M.P.; Sammut, I.A. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005, 19, 1088–1095. [Google Scholar] [CrossRef]
- Graham, D.; Huynh, N.N.; Hamilton, C.A.; Beattie, E.; Smith, R.A.; Cocheme, H.M.; Murphy, M.P.; Dominiczak, A.F. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension 2009, 54, 322–328. [Google Scholar] [CrossRef]
- McLachlan, J.; Beattie, E.; Murphy, M.P.; Koh-Tan, C.H.; Olson, E.; Beattie, W.; Dominiczak, A.F.; Nicklin, S.A.; Graham, D. Combined therapeutic benefit of mitochondria-targeted antioxidant, MitoQ10, and angiotensin receptor blocker, losartan, on cardiovascular function. J. Hypertens. 2014, 32, 555–564. [Google Scholar] [CrossRef]
- Wani, W.Y.; Gudup, S.; Sunkaria, A.; Bal, A.; Singh, P.P.; Kandimalla, R.J.; Sharma, D.R.; Gill, K.D. Protective efficacy of mitochondrial targeted antioxidant mitoq against dichlorvos induced oxidative stress and cell death in rat brain. Neuropharmacology 2011, 61, 1193–1201. [Google Scholar] [CrossRef]
- Hobbs, C.E.; Murphy, M.P.; Smith, R.A.; Oorschot, D.E. Neonatal rat hypoxia-ischemia: Effect of the anti-oxidant mitoquinol, and S-PBN. Pediatr. Int. 2008, 50, 481–488. [Google Scholar] [CrossRef]
- Miura, S.; Ishida-Nakajima, W.; Ishida, A.; Kawamura, M.; Ohmura, A.; Oguma, R.; Sato, Y.; Takahashi, T. Ascorbic acid protects the newborn rat brain from hypoxic-ischemia. Brain Dev. 2009, 31, 307–317. [Google Scholar] [CrossRef]
- Ducruet, A.F.; Mack, W.J.; Mocco, J.; Hoh, D.J.; Coon, A.L.; D’Ambrosio, A.L.; Winfree, C.J.; Pinsky, D.J.; Connolly, E.S., Jr. Preclinical evaluation of postischemic dehydroascorbic acid administration in a large-animal stroke model. Transl. Stroke Res. 2011, 2, 399–403. [Google Scholar] [CrossRef]
- Zhang, X.H.; Lei, H.; Liu, A.J.; Zou, Y.X.; Shen, F.M.; Su, D.F. Increased oxidative stress is responsible for severer cerebral infarction in stroke-prone spontaneously hypertensive rats. CNS Neurosci. Ther. 2011, 17, 590–598. [Google Scholar] [CrossRef]
- Yokoyama, T.; Date, C.; Kokubo, Y.; Yoshiike, N.; Matsumura, Y.; Tanaka, H. Serum vitamin C concentration was inversely associated with subsequent 20-year incidence of stroke in a japanese rural community. The shibata study. Stroke 2000, 31, 2287–2294. [Google Scholar] [CrossRef]
- Myint, P.K.; Luben, R.N.; Welch, A.A.; Bingham, S.A.; Wareham, N.J.; Khaw, K.T. Plasma vitamin C concentrations predict risk of incident stroke over 10 year in 20,649 participants of the european prospective investigation into cancer norfolk prospective population study. Am. J. Clin. Nutr. 2008, 87, 64–69. [Google Scholar] [PubMed]
- Kubota, Y.; Iso, H.; Date, C.; Kikuchi, S.; Watanabe, Y.; Wada, Y.; Inaba, Y.; Tamakoshi, A. Dietary intakes of antioxidant vitamins and mortality from cardiovascular disease: The Japan collaborative cohort study (JACC) study. Stroke 2011, 42, 1665–1672. [Google Scholar] [CrossRef]
- Heart Protection Study Collaborative Group. MRC/BHF heart protection study of antioxidant vitamin supplementation in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 2002, 360, 23–33. [Google Scholar] [CrossRef]
- Cook, N.R.; Albert, C.M.; Gaziano, J.M.; Zaharris, E.; MacFadyen, J.; Danielson, E.; Buring, J.E.; Manson, J.E. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: Results from the women’s antioxidant cardiovascular study. Arch. Intern. Med. 2007, 167, 1610–1618. [Google Scholar] [CrossRef]
- Sesso, H.D.; Buring, J.E.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Glynn, R.J.; Gaziano, J.M. Vitamins E and C in the prevention of cardiovascular disease in men: The physicians’ health study II randomized controlled trial. JAMA 2008, 300, 2123–2133. [Google Scholar] [CrossRef]
- Schurks, M.; Glynn, R.J.; Rist, P.M.; Tzourio, C.; Kurth, T. Effects of vitamin E on stroke subtypes: Meta-analysis of randomised controlled trials. Br. Med. J. 2010. [Google Scholar] [CrossRef]
- Bin, Q.; Hu, X.Y.; Cao, Y.; Gao, F. The role of vitamin E (tocopherol) supplementation in the prevention of stroke a meta-analysis of 13 randomised controlled trials. Thromb. Haemost. 2011, 105, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Semenza, G.L. Purification and characterization of hypoxia-inducible factor 1. J. Biol. Chem. 1995, 270, 1230–1237. [Google Scholar] [CrossRef]
- Bernaudin, M.; Nedelec, A.S.; Divoux, D.; MacKenzie, E.T.; Petit, E.; Schumann-Bard, P. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brain. J. Cereb. Blood Flow Metab. 2002, 22, 393–403. [Google Scholar] [PubMed]
- Jones, N.M.; Bergeron, M. Hypoxic preconditioning induces changes in HIF-1 target genes in neonatal rat brain. J. Cereb. Blood Flow Metab. 2001, 21, 1105–1114. [Google Scholar] [CrossRef]
- Prass, K.; Ruscher, K.; Karsch, M.; Isaev, N.; Megow, D.; Priller, J.; Scharff, A.; Dirnagl, U.; Meisel, A. Desferrioxamine induces delayed tolerance against cerebral ischemia in vivo and in vitro. J. Cereb. Blood Flow Metab. 2002, 22, 520–525. [Google Scholar] [PubMed]
- Freret, T.; Valable, S.; Chazalviel, L.; Saulnier, R.; Mackenzie, E.T.; Petit, E.; Bernaudin, M.; Boulouard, M.; Schumann-Bard, P. Delayed administration of deferoxamine reduces brain damage and promotes functional recovery after transient focal cerebral ischemia in the rat. Eur. J. Neurosci. 2006, 23, 1757–1765. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Zhu, H.; Tejima, E.; Tsuji, K.; Murata, Y.; Atochin, D.N.; Huang, P.L.; Zhang, C.; Lo, E.H. Effects of neuroglobin overexpression on acute brain injury and long-term outcomes after focal cerebral ischemia. Stroke 2008, 39, 1869–1874. [Google Scholar] [CrossRef]
- Li, R.C.; Guo, S.Z.; Lee, S.K.; Gozal, D. Neuroglobin protects neurons against oxidative stress in global ischemia. J. Cereb. Blood Flow Metab. 2010, 30, 1874–1882. [Google Scholar] [CrossRef]
- Sun, Y.; Jin, K.; Peel, A.; Mao, X.O.; Xie, L.; Greenberg, D.A. Neuroglobin protects the brain from experimental stroke in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 3497–3500. [Google Scholar] [CrossRef]
- Cai, B.; Lin, Y.; Xue, X.H.; Fang, L.; Wang, N.; Wu, Z.Y. Tat-mediated delivery of neuroglobin protects against focal cerebral ischemia in mice. Exp. Neurol. 2011, 227, 224–231. [Google Scholar] [CrossRef]
- Ord, E.N.; Shirley, R.; McClure, J.D.; McCabe, C.; Kremer, E.J.; Macrae, I.M.; Work, L.M. Combined antiapoptotic and antioxidant approach to acute neuroprotection for stroke in hypertensive rats. J. Cereb. Blood Flow Metab. 2013, 33, 1215–1224. [Google Scholar] [CrossRef]
- Hacke, W.; Kaste, M.; Bluhmki, E.; Brozman, M.; Davalos, A.; Guidetti, D.; Larrue, V.; Lees, K.R.; Medeghri, Z.; Machnig, T.; et al. Thrombolysis with alteplase 3 to 4.5 h after acute ischemic stroke. N. Engl. J. Med. 2008, 359, 1317–1329. [Google Scholar] [CrossRef]
- Liu, D.; Cheng, T.; Guo, H.; Fernandez, J.A.; Griffin, J.H.; Song, X.; Zlokovic, B.V. Tissue plasminogen activator neurovascular toxicity is controlled by activated protein c. Nat. Med. 2004, 10, 1379–1383. [Google Scholar] [CrossRef]
- Baker, A.H.; Sica, V.; Work, L.M.; Williams-Ignarro, S.; de Nigris, F.; Lerman, L.O.; Casamassimi, A.; Lanza, A.; Schiano, C.; Rienzo, M.; et al. Brain protection using autologous bone marrow cell, metalloproteinase inhibitors, and metabolic treatment in cerebral ischemia. Proc. Natl. Acad. Sci. USA 2007, 104, 3597–3602. [Google Scholar] [CrossRef]
- Asahi, M.; Asahi, K.; Wang, X.; Lo, E.H. Reduction of tissue plasminogen activator-induced hemorrhage and brain injury by free radical spin trapping after embolic focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 2000, 20, 452–457. [Google Scholar] [CrossRef]
- Barth, A.; Barth, L.; Newell, D.W. Combination therapy with MK-801 and alpha-phenyl-tert-butyl-nitrone enhances protection against ischemic neuronal damage in organotypic hippocampal slice cultures. Exp. Neurol. 1996, 141, 330–336. [Google Scholar] [CrossRef]
- Deguchi, K.; Miyazaki, K.; Tian, F.; Liu, N.; Liu, W.; Kawai, H.; Omote, Y.; Kono, S.; Yunoki, T.; Deguchi, S.; et al. Modifying neurorepair and neuroregenerative factors with TPA and edaravone after transient middle cerebral artery occlusion in rat brain. Brain Res. 2012, 1436, 168–177. [Google Scholar] [CrossRef] [PubMed]
- David, H.N.; Haelewyn, B.; Degoulet, M.; Colomb, D.G., Jr.; Risso, J.J.; Abraini, J.H. Prothrombolytic action of normobaric oxygen given alone or in combination with recombinant tissue-plasminogen activator in a rat model of thromboembolic stroke. J. Appl. Physiol. 2012, 112, 2068–2076. [Google Scholar] [CrossRef]
- Schmid-Elsaesser, R.; Hungerhuber, E.; Zausinger, S.; Baethmann, A.; Reulen, H.J. Neuroprotective efficacy of combination therapy with two different antioxidants in rats subjected to transient focal ischemia. Brain Res. 1999, 816, 471–479. [Google Scholar] [CrossRef]
- O’Collins, V.E.; Macleod, M.R.; Donnan, G.A.; Howells, D.W. Evaluation of combination therapy in animal models of cerebral ischemia. J. Cereb. Blood Flow Metab. 2012, 32, 585–597. [Google Scholar] [CrossRef]
- Dirnagl, U. Bench to bedside: The quest for quality in experimental stroke research. J. Cereb. Blood Flow Metab. 2006, 26, 1465–1478. [Google Scholar] [CrossRef]
- Stroke Therapy Academic Industry Roundtable (STAIR). Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 1999, 30, 2752–2758. [Google Scholar] [CrossRef]
- Fisher, M.; Feuerstein, G.; Howells, D.W.; Hurn, P.D.; Kent, T.A.; Savitz, S.I.; Lo, E.H.; Group, F.T.S. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 2009, 40, 2244–2250. [Google Scholar] [CrossRef]
- O’Collins, V.E.; Macleod, M.R.; Donnan, G.A.; Horky, L.L.; van der Worp, B.H.; Howells, D.W. 1026 Experimental treatments in acute stroke. Ann. Neurol. 2006, 59, 467–477. [Google Scholar] [CrossRef]
- Sena, E.S.; van der Worp, H.B.; Howells, D.; Macleod, M. How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci. 2007, 30, 433–439. [Google Scholar] [CrossRef]
- O’Collins, V.E.; Donnan, G.A.; Macleod, M.R.; Howells, D.W. Scope of preclinical testing versus quality control within experiments. Stroke 2009, 40, e497. [Google Scholar] [CrossRef]
- Macleod, M.R.; Fisher, M.; O’Collins, V.; Sena, E.S.; Dirnagl, U.; Bath, P.M.W.; Buchan, A.; van der Worp, H.B.; Traystman, R.; Minematsu, K.; et al. Good laboratory practice: Preventing introduction of bias at the bench. Stroke 2009, 40, e50–e52. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The arrive guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Dirnagl, U.; Hakim, A.; Macleod, M.; Fisher, M.; Howells, D.; Alan, S.M.; Steinberg, G.; Planas, A.; Boltze, J.; Savitz, S.; et al. A concerted appeal for international cooperation in preclinical stroke research. Stroke 2013, 44, 1754–1760. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shirley, R.; Ord, E.N.J.; Work, L.M. Oxidative Stress and the Use of Antioxidants in Stroke. Antioxidants 2014, 3, 472-501. https://doi.org/10.3390/antiox3030472
Shirley R, Ord ENJ, Work LM. Oxidative Stress and the Use of Antioxidants in Stroke. Antioxidants. 2014; 3(3):472-501. https://doi.org/10.3390/antiox3030472
Chicago/Turabian StyleShirley, Rachel, Emily N. J. Ord, and Lorraine M. Work. 2014. "Oxidative Stress and the Use of Antioxidants in Stroke" Antioxidants 3, no. 3: 472-501. https://doi.org/10.3390/antiox3030472



