Green Tea Extract Induces the Resistance of Caenorhabditis elegans against Oxidative Stress
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Plant Material
2.2. C. elegans Strains and Culture Conditions
2.3. Analysis of Catechins in GTE Using the LC/ESI-MS
2.4. DPPH• Free Radical Scavenging Activity
2.5. Superoxide Anion Radical Scavenging Activity
2.6. Quantitation of hsp-16.2/GFP Expression in C. elegans
2.7. Survival Assay
2.8. Statistical Analyses
3. Results and Discussion
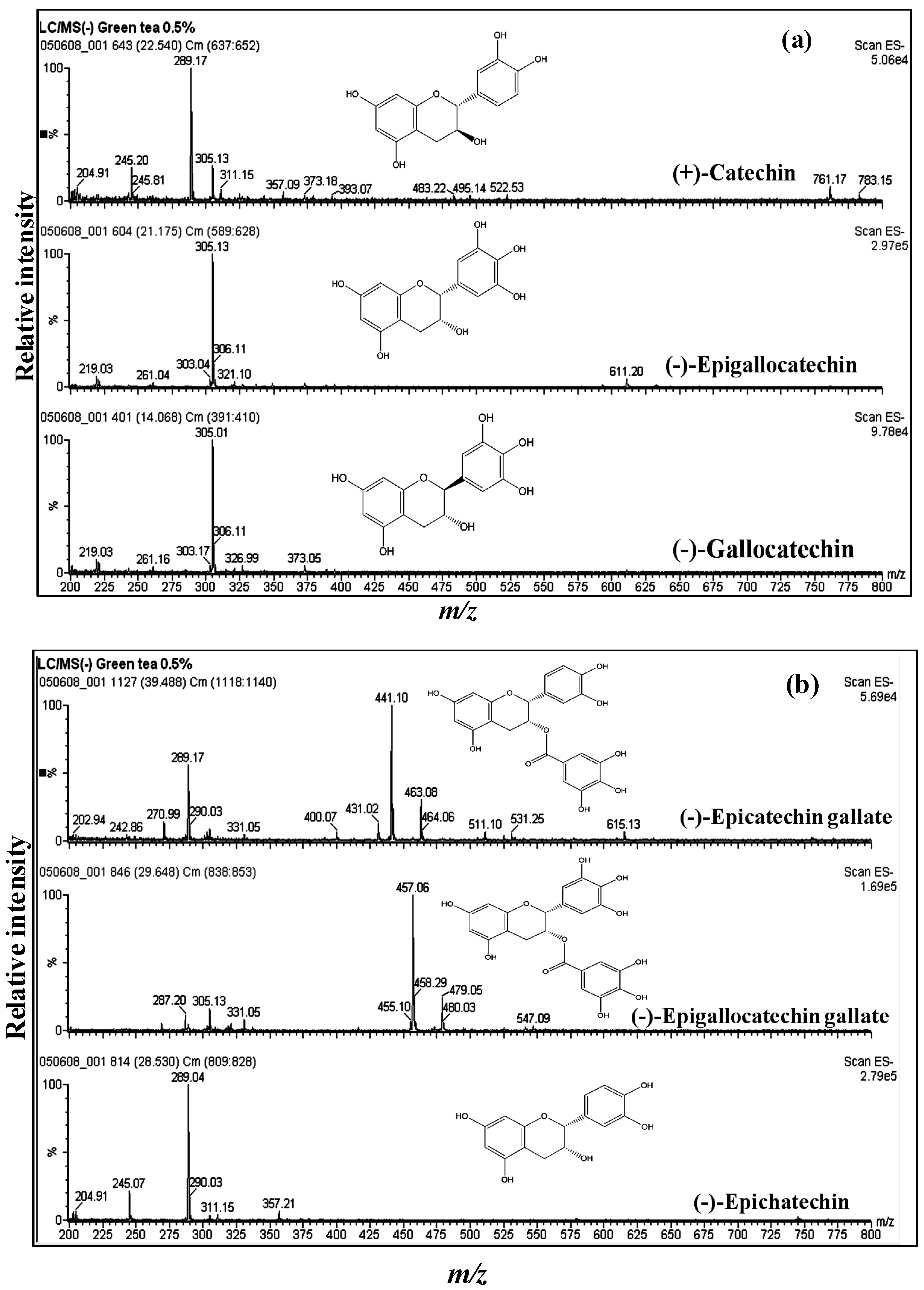
3.1. Catechins of GTE
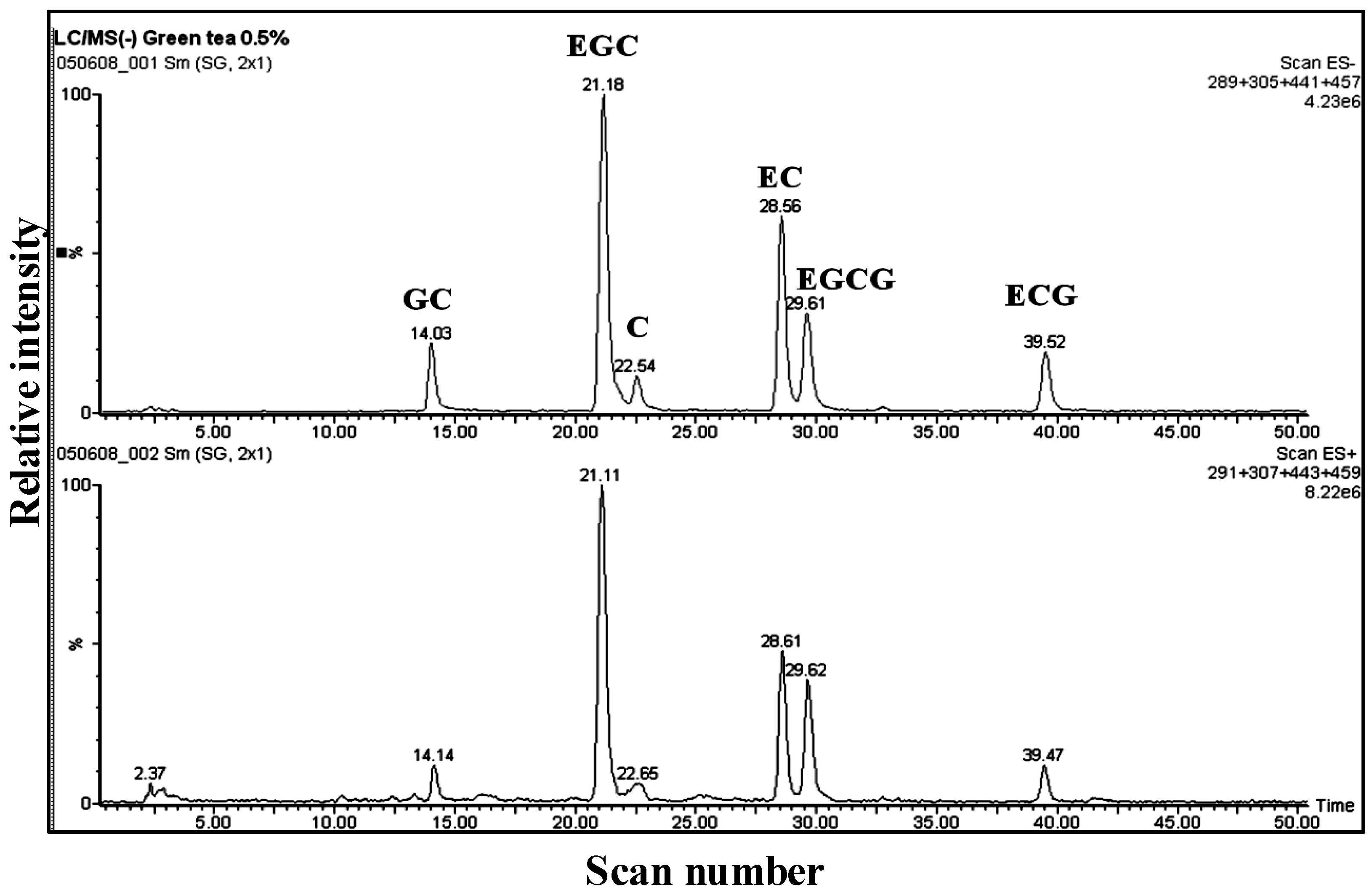
| Compound | MW | [M − H]− | Significant fragments |
|---|---|---|---|
| Catechin (C) | 290 | 289 | |
| Epicatechin (EC) | 290 | 289 | |
| Gallocatechin (GC) | 306 | 305 | |
| Epigallocatechin (EGC) | 306 | 305 | |
| Epicatechin gallate (ECG) | 442 | 441 | m/z 271 (EC without H2O) m/z 289 (EC) |
| Epigallocatechin gallate (EGCG) | 458 | 457 | m/z 287 (EGC without H2O) m/z 305 (EGC) |
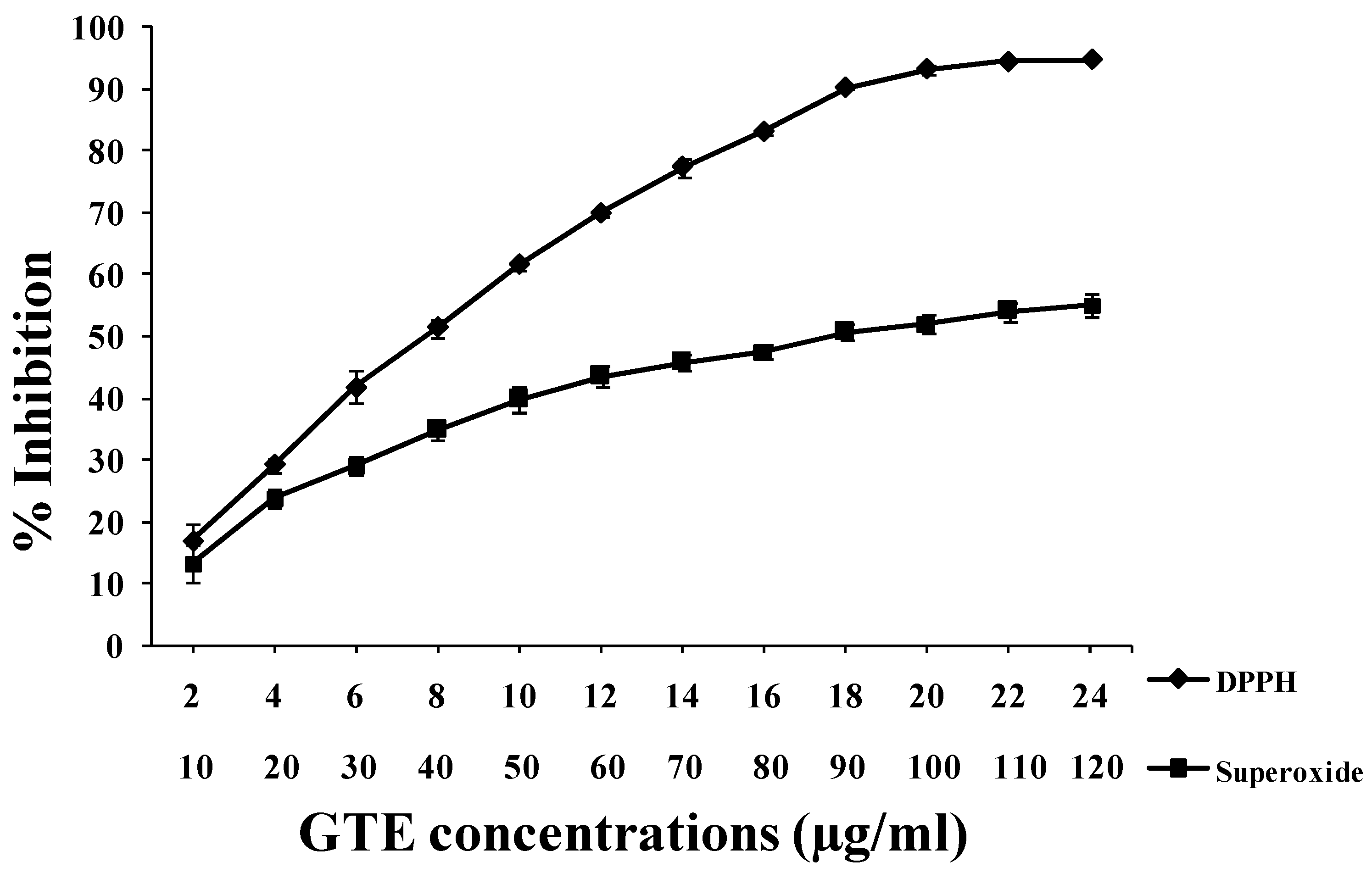
3.2. GTE Scavenges DPPH• and O2•− Free Radicals in Vitro
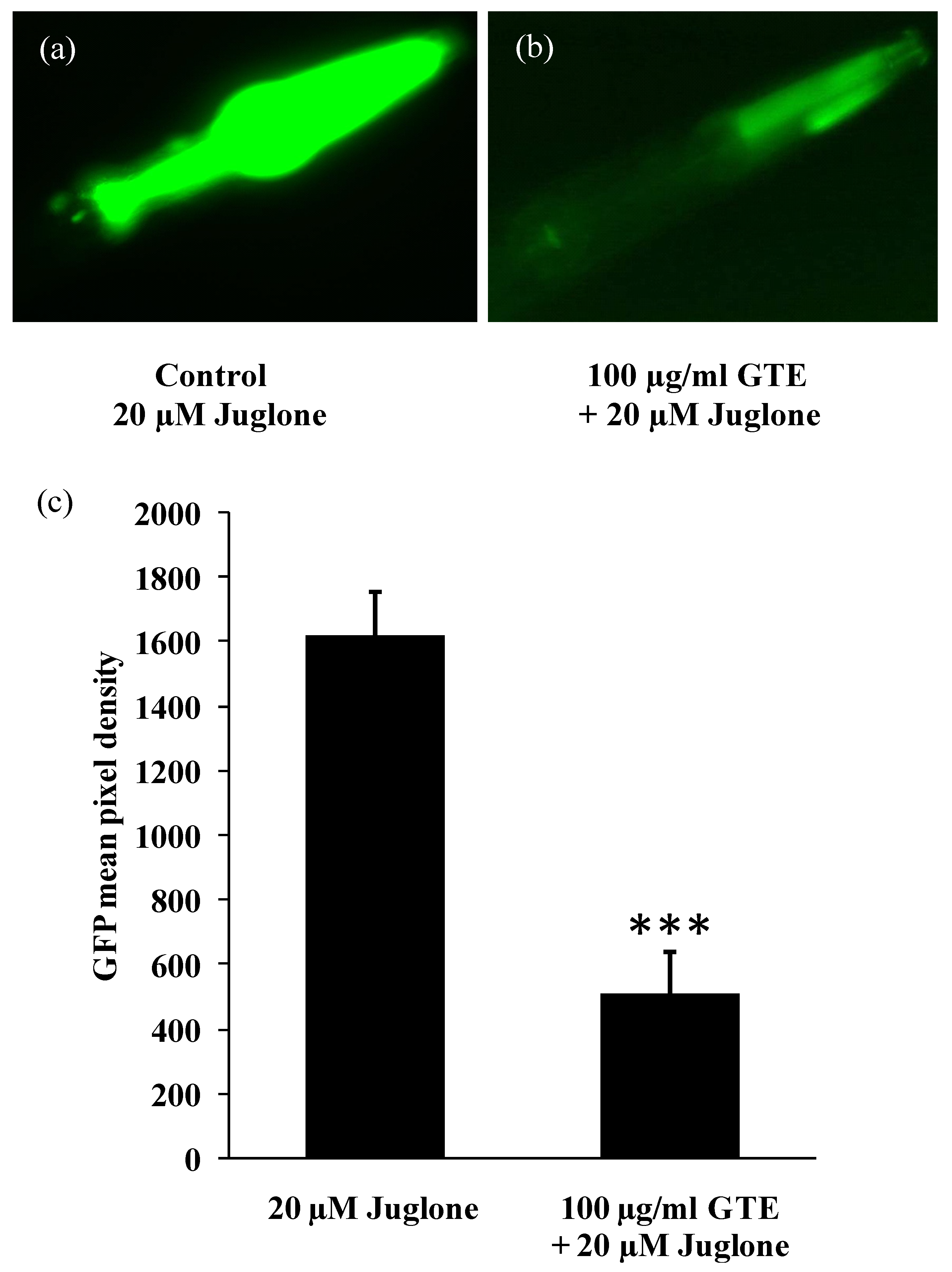
3.3. GTE Reduces the Stress-Induced hsp-16-2 Expression in C. elegans
3.4. GTE Increases Survival Rate in C. elegans

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef]
- Jenner, P. Oxidative stress in parkinson’s disease. Ann. Neurol. 2003, 53, 26–28. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell. Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Valko, M.; Izakovic, M.; Mazur, M.; Rhodes, C.J.; Telser, J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell. Biochem. 2004, 266, 37–56. [Google Scholar] [CrossRef]
- Van Wyk, B.A.; Wink, M. Medicinal Plants of the World; Timber Press: Portland, OR, USA, 2004. [Google Scholar]
- Miketova, P.; Schram, K.H.; Whitney, J.; Li, M.; Huang, R.; Kerns, E.; Valcic, S.; Timmermann, B.N.; Rourick, R.; Klohr, S. Tandem mass spectrometry studies of green tea catechins. Identification of three minor components in the polyphenolic extract of green tea. J. Mass Spectrom. 2000, 35, 860–869. [Google Scholar] [CrossRef]
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Jagtap, S.; Meganathan, K.; Wagh, V.; Winkler, J.; Hescheler, J.; Sachinidis, A. Chemoprotective mechanism of the natural compounds, epigallocatechin-3-o-gallate, quercetin and curcumin against cancer and cardiovascular diseases. Curr. Med. Chem. 2009, 16, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, S. Effects of green tea and EGCG on cardiovascular and metabolic health. J. Am. Coll. Nutr. 2007, 26, 373–388. [Google Scholar] [CrossRef]
- Nagle, D.G.; Ferreira, D.; Zhou, Y. Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef]
- Johnson, J.J.; Bailey, H.H.; Mukhtar, H. Green tea polyphenols for prostate cancer chemoprevention: A translational perspective. Phytomedicine 2010, 17, 3–13. [Google Scholar] [CrossRef]
- Chow, H.H.; Cai, Y.; Hakim, I.A.; Crowell, J.A.; Shahi, F.; Brooks, C.A.; Dorr, R.T.; Hara, Y.; Alberts, D.S. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin. Cancer Res. 2003, 9, 3312–3319. [Google Scholar] [PubMed]
- Chow, H.H.; Hakim, I.A.; Vining, D.R.; Crowell, J.A.; Ranger-Moore, J.; Chew, W.M.; Celaya, C.A.; Rodney, S.R.; Hara, Y.; Alberts, D.S. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of polyphenon E in healthy individuals. Clin. Cancer Res. 2005, 11, 4627–4633. [Google Scholar] [CrossRef]
- Hong, J.; Lu, H.; Meng, X.; Ryu, J.H.; Hara, Y.; Yang, C.S. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (−)-epigallocatechin-3-gallate in ht-29 human colon adenocarcinoma cells. Cancer Res. 2002, 62, 7241–7246. [Google Scholar] [PubMed]
- Zaveri, N.T. Green tea and its polyphenolic catechins: Medicinal uses in cancer and noncancer applications. Life Sci. 2006, 78, 2073–2080. [Google Scholar] [CrossRef]
- Cooper, R.; Morre, D.J.; Morre, D.M. Medicinal benefits of green tea: Part i. Review of noncancer health benefits. J. Altern. Complement. Med. 2005, 11, 521–528. [Google Scholar] [CrossRef]
- Bettuzzi, S.; Brausi, M.; Rizzi, F.; Castagnetti, G.; Peracchia, G.; Corti, A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: A preliminary report from a one-year proof-of-principle study. Cancer Res. 2006, 66, 1234–1240. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Cheng, S.J.; Zhou, Z.C.; Athar, M.; Khan, W.A.; Bickers, D.R.; Mukhtar, H. Antimutagenic activity of green tea polyphenols. Mutat. Res. 1989, 223, 273–285. [Google Scholar] [CrossRef]
- Santhosh, K.T.; Swarnam, J.; Ramadasan, K. Potent suppressive effect of green tea polyphenols on tobacco-induced mutagenicity. Phytomedicine 2005, 12, 216–220. [Google Scholar] [CrossRef]
- Mehrabian, S. The study of antioxidant and anticarcinogenic green tea and black tea. Pak. J. Biol. Sci. 2007, 10, 989–991. [Google Scholar] [CrossRef]
- Kuroda, Y.; Hara, Y. Antimutagenic and anticarcinogenic activity of tea polyphenols. Mutat. Res. 1999, 436, 69–97. [Google Scholar] [CrossRef]
- De Mejia, E.G.; Ramirez-Mares, M.V.; Puangpraphant, S. Bioactive components of tea: Cancer, inflammation and behavior. Brain Behav. Immun. 2009, 23, 721–731. [Google Scholar] [CrossRef]
- Stendell-Hollis, N.R.; Thomson, C.A.; Thompson, P.A.; Bea, J.W.; Cussler, E.C.; Hakim, I.A. Green tea improves metabolic biomarkers, not weight or body composition: A pilot study in overweight breast cancer survivors. J. Hum. Nutr. Diet. 2010, 23, 590–600. [Google Scholar] [CrossRef]
- Basu, A.; Sanchez, K.; Leyva, M.J.; Wu, M.; Betts, N.M.; Aston, C.E.; Lyons, T.J. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J. Am. Coll. Nutr. 2010, 29, 31–40. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, Z.Y. Tea and cancer. J. Natl. Cancer Inst. 1993, 85, 1038–1049. [Google Scholar] [CrossRef]
- Abbas, S.; Wink, M. Epigallocatechin gallate from green tea (Camellia sinensis) increases lifespan and stress resistance in Caenorhabditis elegans. Planta Med. 2009, 75, 216–221. [Google Scholar] [CrossRef]
- Wink, M.; Abbas, S. Epigallocatechin Gallate (EGCG) from Green Tea (Camellia sinensis) and Other Natural Products Mediate Stress Resistance and Slows down Aging Processes in Caenorhabditis elegans. In Tea in Health and Disease Prevention; Preedy, V.R., Ed.; Elsevier: London, UK, 2013; pp. 1105–1116. [Google Scholar]
- Wink, M. Evolutionary advantage and molecular modes of action of multi-component mixtures used in phytomedicine. Curr. Drug Metab. 2008, 9, 996–1009. [Google Scholar] [CrossRef]
- Wink, M.; Van Wyk, B.E. Mind-Altering and Poisonous Plants of the World; Timber Press: London, UK, 2008. [Google Scholar]
- Wink, M.; Schimmer, O. Molecular Modes of Action of Defensive Secondary Metabolites. In Annual Plant Reviews: Functions and Biotechnology of Plant Secondary Metabolites; Wink, M., Ed.; Wiley-Blackwell: Oxford, UK, 2009; Volume 39, pp. 21–161. [Google Scholar]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [PubMed]
- Sonnhammer, E.L.; Durbin, R. Analysis of protein domain families in Caenorhabditis elegans. Genomics 1997, 46, 200–216. [Google Scholar] [CrossRef]
- Kaletta, T.; Hengartner, M.O. Finding function in novel targets: C. elegans as a model organism. Nat. Rev. Drug Discov. 2006, 5, 387–398. [Google Scholar] [CrossRef]
- Brenner, S. Foreward. In The Nematode Caenorhabditis elegans; Wood, W.B., Ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1988; pp. ix–xiii. [Google Scholar]
- Nanjo, F.; Mori, M.; Goto, K.; Hara, Y. Radical scavenging activity of tea catechins and their related compounds. Biosci. Biotechnol. Biochem. 1999, 63, 1621–1623. [Google Scholar] [CrossRef]
- Abbas, S.; Wink, M. Epigallocatechin gallate inhibits beta amyloid oligomerization in Caenorhabditis elegans and affects the daf-2/insulin-like signaling pathway. Phytomedicine 2010, 17, 902–909. [Google Scholar] [CrossRef]
- Saul, N.; Pietsch, K.; Menzel, R.; Sturzenbaum, S.R.; Steinberg, C.E. Catechin induced longevity in C. elegans: From key regulator genes to disposable soma. Mech. Ageing Dev. 2009, 130, 477–486. [Google Scholar] [CrossRef]
- Kirkwood, T.B. Evolution of ageing. Nature 1977, 270, 301–304. [Google Scholar] [CrossRef]
- Chen, W.; Muller, D.; Richling, E.; Wink, M. Anthocyanin-rich purple wheat prolongs the life span of Caenorhabditis elegans probably by activating the daf-16/foxo transcription factor. J. Agric. Food Chem. 2013, 61, 3047–3053. [Google Scholar] [CrossRef]
- Chen, W.; Rezaizadehnajafi, L.; Wink, M. Influence of resveratrol on oxidative stress resistance and life span in Caenorhabditis elegans. J. Pharm. Pharmacol. 2013, 65, 682–688. [Google Scholar] [CrossRef]
- Chen, W.; Sudji, I.R.; Wang, E.; Joubert, E.; van Wyk, B.E.; Wink, M. Ameliorative effect of aspalathin from rooibos (Aspalathus linearis) on acute oxidative stress in Caenorhabditis elegans. Phytomedicine 2013, 20, 380–386. [Google Scholar] [CrossRef]
- Wilson, M.A.; Shukitt-Hale, B.; Kalt, W.; Ingram, D.K.; Joseph, J.A.; Wolkow, C.A. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell 2006, 5, 59–68. [Google Scholar] [CrossRef]
- Solomon, A.; Bandhakavi, S.; Jabbar, S.; Shah, R.; Beitel, G.J.; Morimoto, R.I. Caenorhabditis elegans osr-1 regulates behavioral and physiological responses to hyperosmotic environments. Genetics 2004, 167, 161–170. [Google Scholar] [CrossRef]
- Wu, Z.; Smith, J.V.; Paramasivam, V.; Butko, P.; Khan, I.; Cypser, J.R.; Luo, Y. Ginkgo biloba extract egb 761 increases stress resistance and extends life span of Caenorhabditis elegans. Cell. Mol. Biol. (Noisy-le-grand) 2002, 48, 725–731. [Google Scholar]
- Evason, K.; Collins, J.J.; Huang, C.; Hughes, S.; Kornfeld, K. Valproic acid extends Caenorhabditis elegans lifespan. Aging Cell 2008, 7, 305–317. [Google Scholar] [CrossRef]
- Sulston, J.; Hodgkin, J. The Nematode Caenorhabditis elegans; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1988; pp. 587–606. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 26, 1199–1200. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanisms of antioxidant activity using the DPPH• free radical method. LWT-Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Robak, J.; Gryglewski, R.J. Flavonoids are scavengers of superoxide anions. Biochem. Pharmacol. 1988, 37, 837–841. [Google Scholar] [CrossRef]
- Link, C.D.; Cypser, J.R.; Johnson, C.J.; Johnson, T.E. Direct observation of stress response in Caenorhabditis elegans using a reporter transgene. Cell Stress Chaperones 1999, 4, 235–242. [Google Scholar] [CrossRef]
- Miketova, P.; Schram, K.H.; Whitney, J.L.; Kerns, E.H.; Valcic, S.; Timmermann, B.N.; Volk, K.J. Mass spectrometry of selected components of biological interest in green tea extracts. J. Nat. Prod. 1998, 61, 461–467. [Google Scholar] [CrossRef]
- Poon, G.K. Analysis of catechins in tea extracts by liquid chromatography–electrospray ionization mass spectrometry. J. Chromatogr. A 1998, 794, 63–74. [Google Scholar] [CrossRef]
- Bastos, D.H.; Saldanha, L.A.; Catharino, R.R.; Sawaya, A.C.; Cunha, I.B.; Carvalho, P.O.; Eberlin, M.N. Phenolic antioxidants identified by esi-ms from yerba mate (Ilex paraguariensis) and green tea (Camellia sinensis) extracts. Molecules 2007, 12, 423–432. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef]
- Sayre, L.M.; Smith, M.A.; Perry, G. Chemistry and biochemistry of oxidative stress in neurodegenerative disease. Curr. Med. Chem. 2001, 8, 721–738. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. A Biol. Sci. Med. Sci. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Hu, C.; Kitts, D.D. Evaluation of antioxidant activity of epigallocatechin gallate in biphasic model systems in vitro. Mol. Cell. Biochem. 2001, 218, 147–155. [Google Scholar] [CrossRef]
- Nanjo, F.; Goto, K.; Seto, R.; Suzuki, M.; Sakai, M.; Hara, Y. Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picrylhydrazyl radical. Free Radic. Biol. Med. 1996, 21, 895–902. [Google Scholar] [CrossRef]
- Leung, L.K.; Su, Y.; Chen, R.; Zhang, Z.; Huang, Y.; Chen, Z.Y. Theaflavins in black tea and catechins in green tea are equally effective antioxidants. J. Nutr. 2001, 131, 2248–2251. [Google Scholar] [PubMed]
- Yokozawa, T.; Nakagawa, T.; Kitani, K. Antioxidative activity of green tea polyphenol in cholesterol-fed rats. J. Agric. Food Chem. 2002, 50, 3549–3552. [Google Scholar] [CrossRef]
- Leroux, M.R.; Melki, R.; Gordon, B.; Batelier, G.; Candido, E.P. Structure-function studies on small heat shock protein oligomeric assembly and interaction with unfolded polypeptides. J. Biol. Chem. 1997, 272, 24646–24656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jie, G.; Zhang, J.; Zhao, B. Significant longevity-extending effects of EGCG on Caenorhabditis elegans under stress. Free Radic. Biol. Med. 2009, 46, 414–421. [Google Scholar] [CrossRef]
- Henderson, S.T.; Johnson, T.E. Daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr. Biol. 2001, 11, 1975–1980. [Google Scholar] [CrossRef]
- Oh, S.W.; Mukhopadhyay, A.; Dixit, B.L.; Raha, T.; Green, M.R.; Tissenbaum, H.A. Identification of direct daf-16 targets controlling longevity, metabolism and diapause by chromatin immunoprecipitation. Nat. Genet. 2006, 38, 251–257. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Abbas, S.; Wink, M. Green Tea Extract Induces the Resistance of Caenorhabditis elegans against Oxidative Stress. Antioxidants 2014, 3, 129-143. https://doi.org/10.3390/antiox3010129
Abbas S, Wink M. Green Tea Extract Induces the Resistance of Caenorhabditis elegans against Oxidative Stress. Antioxidants. 2014; 3(1):129-143. https://doi.org/10.3390/antiox3010129
Chicago/Turabian StyleAbbas, Sami, and Michael Wink. 2014. "Green Tea Extract Induces the Resistance of Caenorhabditis elegans against Oxidative Stress" Antioxidants 3, no. 1: 129-143. https://doi.org/10.3390/antiox3010129






