Ten Key Insights into the Use of Spinal Cord fMRI
Abstract
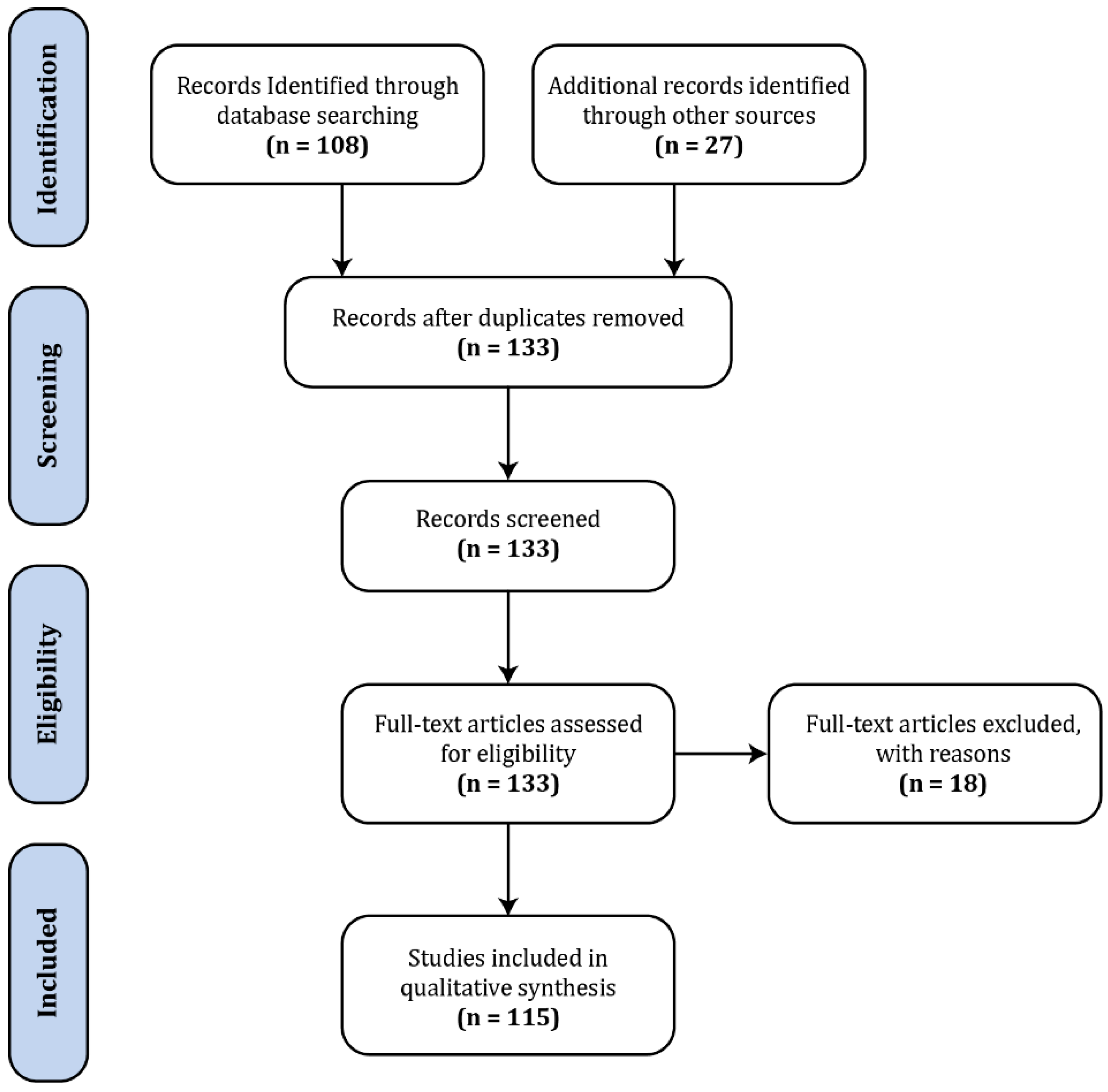
:1. Introduction
2. Animal Studies—Linking Neural Activity to BOLD Responses
3. Applications in Healthy People—Demonstrating Validity and Versatility
4. Applications in Patient Populations—Broadening Our Understanding
5. Resting-State-Confirming Activity in the Absence of a Task
6. Study Design—Adapting to the Environment
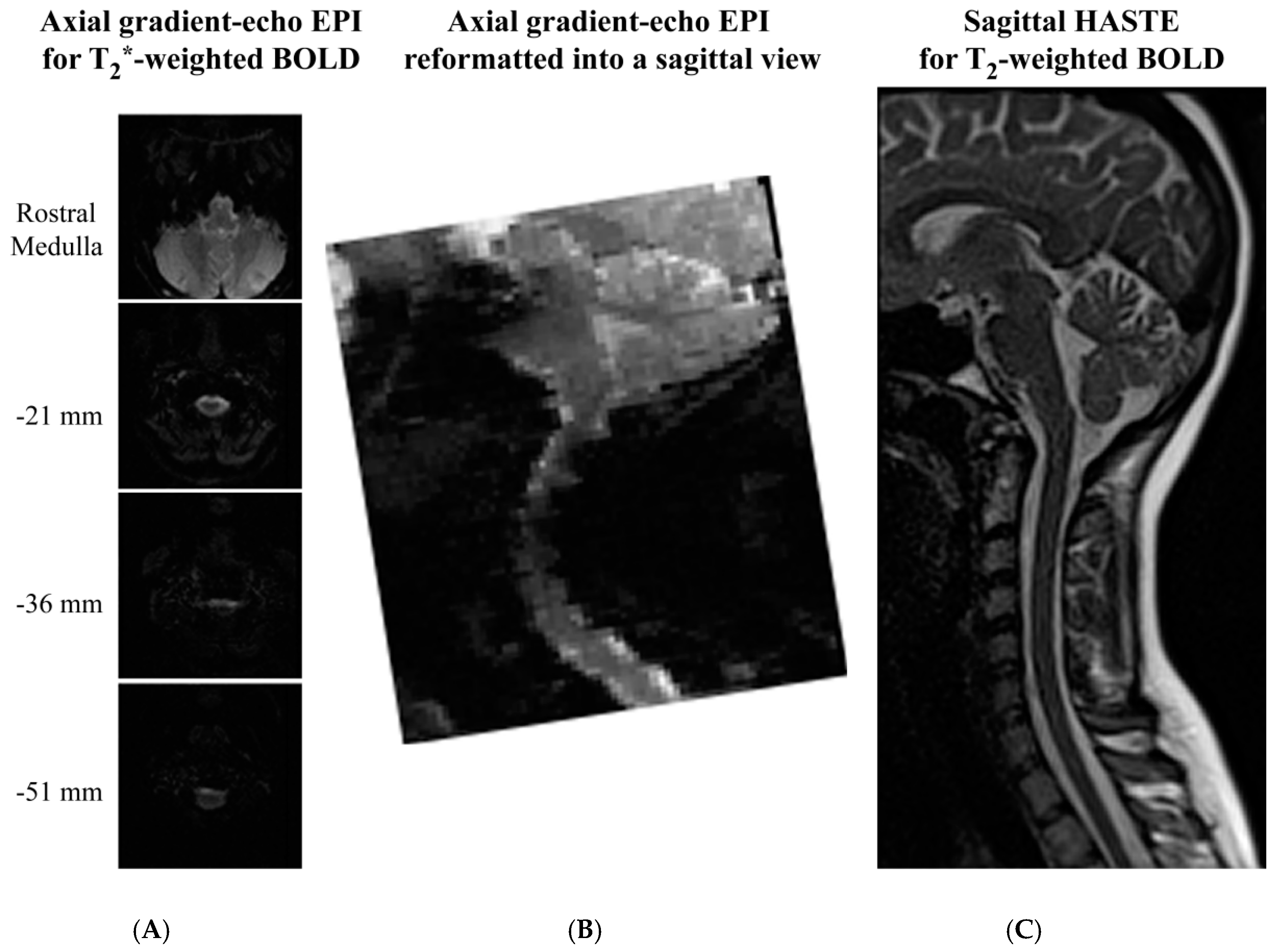
7. HASTE vs. EPI—Contrasting Quality and Quantity
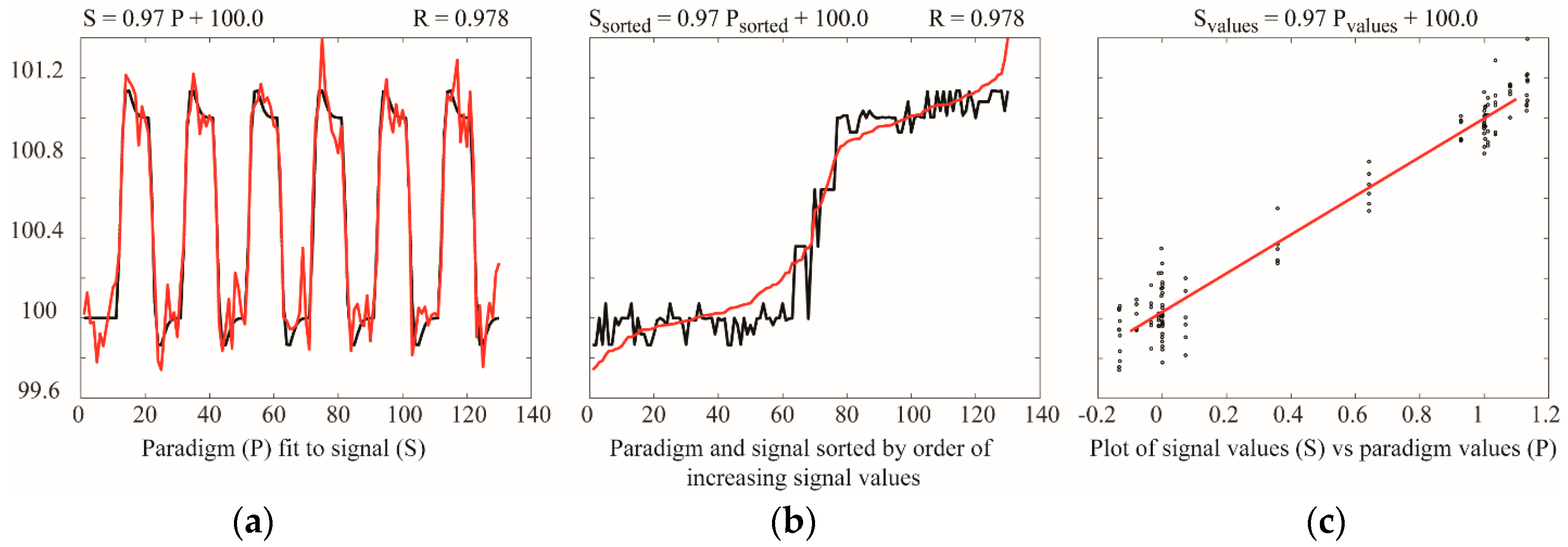
8. Physiological Motion—Putting the Noise into Perspective
9. Temporal Resolution—Balancing Sensitivity and Speed
10. Spatial Resolution—Establishing the Precision
11. Spatial Normalization and Anatomical Templates—Automating the Analysis
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Endo, T.; Spenger, C.; Westman, E.; Tominaga, T.; Olson, L. Reorganization of sensory processing below the level of spinal cord injury as revealed by fMRI. Exp. Neurol. 2008, 209, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.; Stroman, P.W.; Bascaramurty, S.; Jordan, L.M.; Malisza, K.L. Correlation of functional activation in the rat spinal cord with neuronal activation detected by immunohistochemistry. NeuroImage 2004, 22, 1802–1807. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.; Stroman, P.W.; Malisza, K.L. Functional MRI of the cervical spinal cord during noxious and innocuous thermal stimulation in the alpha-chloralose- and halothane-anesthetized rat. Magn. Reson. Imaging 2008, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.; Stroman, P.W.; Malisza, K.L. Comparison of functional activity in the rat cervical spinal cord during alpha-chloralose and halothane anesthesia. NeuroImage 2007, 34, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Majcher, K.; Tomanek, B.; Tuor, U.I.; Jasinski, A.; Foniok, T.; Rushforth, D.; Hess, G. Functional magnetic resonance imaging within the rat spinal cord following peripheral nerve injury. NeuroImage 2007, 38, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Majcher, K.; Tomanek, B.; Jasinski, A.; Foniok, T.; Stroman, P.W.; Tuor, U.I.; Kirk, D.; Hess, G. Simultaneous functional magnetic resonance imaging in the rat spinal cord and brain. Exp. Neurol. 2006, 197, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Malisza, K.L.; Jones, C.; Gruwel, M.L.; Foreman, D.; Fernyhough, P.; Calcutt, N.A. Functional magnetic resonance imaging of the spinal cord during sensory stimulation in diabetic rats. J. Magn. Reson. Imaging 2009, 30, 271–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malisza, K.L.; Stroman, P.W.; Turner, A.; Gregorash, L.; Foniok, T.; Wright, A. Functional MRI of the rat lumbar spinal cord involving painful stimulation and the effect of peripheral joint mobilization. J. Magn. Reson. Imagin 2003, 18, 152–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malisza, K.L.; Stroman, P.W. Functional imaging of the rat cervical spinal cord. J. Magn. Reson. Imaging 2002, 16, 553–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porszasz, R.; Beckmann, N.; Bruttel, K.; Urban, L.; Rudin, M. Signal changes in the spinal cord of the rat after injection of formalin into the hindpaw: Characterization using functional magnetic resonance imaging. Proc. Natl. Acad. Sci. USA 1997, 94, 5034–5039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.; Williams, M.; Meng, X.; Welsh, D.C.; Coimbra, A.; Crown, E.D.; Cook, J.J.; Urban, M.O.; Hargreaves, R.; Williams, D.S. Bold and blood volume-weighted fMRI of rat lumbar spinal cord during non-noxious and noxious electrical hindpaw stimulation. NeuroImage 2008, 40, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Williams, M.; Meng, X.; Welsh, D.C.; Grachev, I.D.; Hargreaves, R.; Williams, D.S. Pain fMRI in rat cervical spinal cord: An echo planar imaging evaluation of sensitivity of BOLD and blood volume-weighted fMRI. NeuroImage 2009, 44, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Williams, M.; Welsh, D.C.; Meng, X.; Ritter, A.; Abbadie, C.; Cook, J.J.; Reicin, A.S.; Hargreaves, R.; Williams, D.S. fMRI investigation of the effect of local and systemic lidocaine on noxious electrical stimulation-induced activation in spinal cord. Pain 2009, 145, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Stieltjes, B.; Klussmann, S.; Bock, M.; Umathum, R.; Mangalathu, J.; Letellier, E.; Rittgen, W.; Edler, L.; Krammer, P.H.; Kauczor, H.U.; et al. Manganese-enhanced magnetic resonance imaging for in vivo assessment of damage and functional improvement following spinal cord injury in mice. Magn. Reson. Med. 2006, 55, 1124–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.F.; Wang, F.; Chen, L.M. Differential fMRI activation patterns to noxious heat and tactile stimuli in the primate spinal cord. J. Neurosci. 2015, 35, 10493–10502. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Adad, J.; Hoge, R.D.; Leblond, H.; Xie, G.; Beaudoin, G.; Song, A.W.; Krueger, G.; Doyon, J.; Benali, H.; Rossignol, S. Investigations on spinal cord fMRI of cats under ketamine. NeuroImage 2009, 44, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Stroman, P.W.; Kornelsen, J.; Lawrence, J. An improved method for spinal functional MRI with large volume coverage of the spinal cord. J. Magn. Reson. Imaging 2005, 21, 520–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craig, A.D. Interoception: The sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003, 13, 500–505. [Google Scholar] [CrossRef]
- Yoshizawa, T.; Nose, T.; Moore, G.J.; Sillerud, L.O. Functional magnetic resonance imaging of motor activation in the human cervical spinal cord. NeuroImage 1996, 4, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Stroman, P.W.; Ryner, L.N. Functional MRI of motor and sensory activation in the human spinal cord. Magn. Reson. Imaging 2001, 19, 27–32. [Google Scholar] [CrossRef]
- Stroman, P.W.; Krause, V.; Malisza, K.L.; Frankenstein, U.N.; Tomanek, B. Functional magnetic resonance imaging of the human cervical spinal cord with stimulation of different sensory dermatomes. Magn. Reson. Imaging 2002, 20, 1–6. [Google Scholar] [CrossRef]
- Stroman, P.W.; Coe, B.C.; Munoz, D.P. Influence of attention focus on neural activity in the human spinal cord during thermal sensory stimulation. Magn. Reson. Imaging 2011, 29, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.M.; Stroman, P.W.; Kollias, S.S. Functional magnetic resonance imaging of the human spinal cord during vibration stimulation of different dermatomes. Neuroradiology 2008, 50, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Ghazni, N.F.; Cahill, C.M.; Stroman, P.W. Tactile sensory and pain networks in the human spinal cord and brain stem mapped by means of functional mr imaging. Am. J. Neuroradiol. 2010, 31, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Kornelsen, J.; Stroman, P.W. fMRI of the lumbar spinal cord during a lower limb motor task. Magn. Reson. Med. 2004, 52, 411–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maieron, M.; Iannetti, G.D.; Bodurka, J.; Tracey, I.; Bandettini, P.A.; Porro, C.A. Functional responses in the human spinal cord during willed motor actions: Evidence for side- and rate-dependent activity. J. Neurosci. 2007, 27, 4182–4190. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.C.; Wu, E.X.; Lau, H.F.; Hu, Y.; Lam, E.Y.; Luk, K.D. Cervical spinal cord BOLD fMRI study: Modulation of functional activation by dexterity of dominant and non-dominant hands. NeuroImage 2008, 39, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.H.; Kong, K.M.; Guan, J.T.; Chen, Y.X.; He, J.K.; Qi, W.L.; Wang, X.J.; Shen, Z.W.; Wu, R.H. Ssfse sequence functional MRI of the human cervical spinal cord with complex finger tapping. Eur. J. Radiol. 2009, 70, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vahdat, S.; Lungu, O.; Cohen-Adad, J.; Marchand-Pauvert, V.; Benali, H.; Doyon, J. Simultaneous brain-cervical cord fMRI reveals intrinsic spinal cord plasticity during motor sequence learning. PLoS Biol. 2015, 13, e1002186. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.A.; Chen, Y.; Wang, X.; Kahnt, T.; Parrish, T.B. Lateralization of cervical spinal cord activity during an isometric upper extremity motor task with functional magnetic resonance imaging. NeuroImage 2016, 125, 233–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stroman, P.W. Spinal fMRI investigation of human spinal cord function over a range of innocuous thermal sensory stimuli and study-related emotional influences. Magn. Reson. Imaging 2009, 27, 1333–1346. [Google Scholar] [CrossRef] [PubMed]
- Summers, P.E.; Ferraro, D.; Duzzi, D.; Lui, F.; Iannetti, G.D.; Porro, C.A. A quantitative comparison of BOLD fMRI responses to noxious and innocuous stimuli in the human spinal cord. NeuroImage 2010, 50, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Cahill, C.M.; Stroman, P.W. Mapping of neural activity produced by thermal pain in the healthy human spinal cord and brain stem: A functional magnetic resonance imaging study. Magn. Reson. Imaging 2011, 29, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Stroman, P.W.; Bosma, R.L.; Tsyben, A. Somatotopic arrangement of thermal sensory regions in the healthy human spinal cord determined by means of spinal cord functional MRI. Magn. Reson. Med. 2012, 68, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.C.; Kong, Y.; Lee, M.C.; Warnaby, C.E.; Wanigasekera, V.; Jenkinson, M.; Tracey, I. Stimulus site and modality dependence of functional activity within the human spinal cord. J. Neurosci. 2012, 32, 6231–6239. [Google Scholar] [CrossRef] [PubMed]
- Kornelsen, J.; Smith, S.D.; McIver, T.A.; Sboto-Frankenstein, U.; Latta, P.; Tomanek, B. Functional MRI of the thoracic spinal cord during vibration sensation. J. Magn. Reson. Imaging 2013, 37, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Rempe, T.; Wolff, S.; Riedel, C.; Baron, R.; Stroman, P.W.; Jansen, O.; Gierthmuhlen, J. Spinal and supraspinal processing of thermal stimuli: An fMRI study. J. Magn. Reson. Imaging 2015, 41, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Nash, P.; Wiley, K.; Brown, J.; Shinaman, R.; Ludlow, D.; Sawyer, A.M.; Glover, G.; Mackey, S. Functional magnetic resonance imaging identifies somatotopic organization of nociception in the human spinal cord. Pain 2013, 154, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, C.; Finsterbusch, J.; Buchel, C. Spinal cord-midbrain functional connectivity is related to perceived pain intensity: A combined spino-cortical fMRI study. J. Neurosci. 2015, 35, 4248–4257. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.A.; Chen, Y.; Wang, X.; Kahnt, T.; Parrish, T.B. Functional magnetic resonance imaging of the cervical spinal cord during thermal stimulation across consecutive runs. NeuroImage 2016, 143, 267–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rempe, T.; Wolff, S.; Riedel, C.; Baron, R.; Stroman, P.W.; Jansen, O.; Gierthmuhlen, J. Spinal fMRI reveals decreased descending inhibition during secondary mechanical hyperalgesia. PLoS ONE 2014, 9, e112325. [Google Scholar] [CrossRef] [PubMed]
- Bosma, R.L.; Ameli Mojarad, E.; Leung, L.; Pukall, C.; Staud, R.; Stroman, P.W. Neural correlates of temporal summation of second pain in the human brainstem and spinal cord. Hum. Brain Mapp. 2015, 36, 5038–5050. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D.; Andrew, D. Responses of spinothalamic lamina i neurons to repeated brief contact heat stimulation in the cat. J. Neurophysiol. 2002, 87, 1902–1914. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.S.; Stroman, P.W. Inter-individual differences in pain processing investigated by functional magnetic resonance imaging of the brainstem and spinal cord. Neuroscience 2015, 307, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Stroman, P.W. Investigating pain networks in the spinal cord using functional MRI. J. Imaging Med. 2011, 3, 141–143. [Google Scholar] [CrossRef]
- Sprenger, C.; Eippert, F.; Finsterbusch, J.; Bingel, U.; Rose, M.; Buchel, C. Attention modulates spinal cord responses to pain. Curr. Biol. CB 2012, 22, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Dobek, C.E.; Beynon, M.E.; Bosma, R.L.; Stroman, P.W. Music modulation of pain perception and pain-related activity in the brain, brain stem, and spinal cord: A functional magnetic resonance imaging study. J. Pain 2014, 15, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Stroman, P.W.; Bosma, R.L.; Cotoi, A.I.; Leung, R.H.; Kornelsen, J.; Lawrence-Dewar, J.M.; Pukall, C.F.; Staud, R. Continuous descending modulation of the spinal cord revealed by functional MRI. PLoS ONE 2016, 11, e0167317. [Google Scholar] [CrossRef] [PubMed]
- Stroman, P.W.; Ioachim, G.; Powers, J.M.; Staud, R.; Pukall, C. Pain processing in the human brainstem and spinal cord before, during and after the application of noxious heat stimuli. Pain 2018. [Google Scholar] [CrossRef] [PubMed]
- Eippert, F.; Finsterbusch, J.; Bingel, U.; Buchel, C. Direct evidence for spinal cord involvement in placebo analgesia. Science 2009, 326, 404. [Google Scholar] [CrossRef] [PubMed]
- Geuter, S.; Buchel, C. Facilitation of pain in the human spinal cord by nocebo treatment. J. Neurosci. 2013, 33, 13784–13790. [Google Scholar] [CrossRef] [PubMed]
- Tinnermann, A.; Geuter, S.; Sprenger, C.; Finsterbusch, J.; Buchel, C. Interactions between brain and spinal cord mediate value effects in nocebo hyperalgesia. Science 2017, 358, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Bosma, R.L.; Mojarad, E.A.; Leung, L.; Pukall, C.; Staud, R.; Stroman, P.W. fMRI of spinal and supra-spinal correlates of temporal pain summation in fibromyalgia patients. Hum. Brain Mapp. 2016, 37, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.S.; Kozyrev, N.; Bosma, R.L.; Figley, C.R.; Richards, J.S.; Stroman, P.W. fMRI localization of spinal cord processing underlying female sexual arousal. J. Sex Marital Ther. 2016, 42, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Kozyrev, N.; Figley, C.R.; Alexander, M.S.; Richards, J.S.; Bosma, R.L.; Stroman, P.W. Neural correlates of sexual arousal in the spinal cords of able-bodied men: A spinal fMRI investigation. J. Sex Marital Ther. 2012, 38, 418–435. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.D.; Kornelsen, J. Emotion-dependent responses in spinal cord neurons: A spinal fMRI study. NeuroImage 2011, 58, 269–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.D.; Kornelsen, J.; McIver, T.A. Generating facial expressions of disgust activates neurons in the thoracic spinal cord: A spinal fMRI study. Soc. Neurosci. 2018, 13, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Stroman, P.W.; Tomanek, B.; Krause, V.; Frankenstein, U.N.; Malisza, K.L. Mapping of neuronal function in the healthy and injured human spinal cord with spinal fMRI. NeuroImage 2002, 17, 1854–1860. [Google Scholar] [CrossRef] [PubMed]
- Stroman, P.W.; Kornelsen, J.; Bergman, A.; Krause, V.; Ethans, K.; Malisza, K.L.; Tomanek, B. Noninvasive assessment of the injured human spinal cord by means of functional magnetic resonance imaging. Spinal Cord 2004, 42, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kornelsen, J.; Stroman, P.W. Detection of the neuronal activity occurring caudal to the site of spinal cord injury that is elicited during lower limb movement tasks. Spinal Cord 2007, 45, 485–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadotte, D.W.; Bosma, R.; Mikulis, D.; Nugaeva, N.; Smith, K.; Pokrupa, R.; Islam, O.; Stroman, P.W.; Fehlings, M.G. Plasticity of the injured human spinal cord: Insights revealed by spinal cord functional MRI. PLoS ONE 2012, 7, e45560. [Google Scholar] [CrossRef] [PubMed]
- Stroman, P.W.; Khan, H.S.; Bosma, R.L.; Cotoi, A.I.; Leung, R.; Cadotte, D.W.; Fehlings, M.G. Changes in pain processing in the spinal cord and brainstem after spinal cord injury characterized by functional magnetic resonance imaging. J. Neurotrauma 2016, 33, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.P.; Chen, Y.X.; Li, Z.Y.; Shen, Z.W.; Kong, K.M.; Wu, R.H. Cervical spinal functional magnetic resonance imaging of the spinal cord injured patient during electrical stimulation. Eur. Spine J. 2017, 26, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.; Kozyrev, N.; Figley, C.R.; Richards, J.S. Altered spinal cord activity during sexual stimulation in women with sci: A pilot fMRI study. Spinal Cord Ser. Cases 2017, 3, 16041. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qian, W.; Jin, R.; Li, X.; Luk, K.D.; Wu, E.X.; Hu, Y. Amplitude of low frequency fluctuation (alff) in the cervical spinal cord with stenosis: A resting state fMRI study. PLoS ONE 2016, 11, e0167279. [Google Scholar] [CrossRef] [PubMed]
- Forstenpointner, J.; Wolff, S.; Stroman, P.W.; Jansen, O.; Rehm, S.; Baron, R.; Gierthmuhlen, J. “From ear to trunk”—MRI reveals referral of pain. Pain 2018, 159, 1900–1903. [Google Scholar] [PubMed]
- Wei, P.; Li, J.; Gao, F.; Ye, D.; Zhong, Q.; Liu, S. Resting state networks in human cervical spinal cord observed with fMRI. Eur. J. Appl. Physiol. 2010, 108, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Barry, R.L.; Smith, S.A.; Dula, A.N.; Gore, J.C. Resting state functional connectivity in the human spinal cord. eLife 2014, 3, e02812. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, M.C.; Ceko, M.; Low, L.A. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 2013, 14, 502–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harita, S.; Ioachim, G.; Powers, J.; Stroman, P. Investigation of Resting-State Bold Networks in the Human Brainstem and Spinal Cord. 2018; submitted. [Google Scholar]
- Barry, R.L.; Rogers, B.P.; Conrad, B.N.; Smith, S.A.; Gore, J.C. Reproducibility of resting state spinal cord networks in healthy volunteers at 7 tesla. NeuroImage 2016, 133, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Conrad, B.N.; Barry, R.L.; Rogers, B.P.; Maki, S.; Mishra, A.; Thukral, S.; Sriram, S.; Bhatia, A.; Pawate, S.; Gore, J.C.; et al. Multiple sclerosis lesions affect intrinsic functional connectivity of the spinal cord. Brain J. Neurol. 2018, 141, 1650–1664. [Google Scholar] [CrossRef] [PubMed]
- San Emeterio Nateras, O.; Yu, F.; Muir, E.R.; Bazan, C.; Franklin, C.G.; Li, W.; Li, J.; Lancaster, J.L.; Duong, T.Q. Intrinsic resting-state functional connectivity in the human spinal cord at 3.0 t. Radiology 2016, 279, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Eippert, F.; Kong, Y.; Winkler, A.M.; Andersson, J.L.; Finsterbusch, J.; Buchel, C.; Brooks, J.C.; Tracey, I. Investigating resting-state functional connectivity in the cervical spinal cord at 3t. NeuroImage 2017, 147, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, F.; Li, X.; Qian, W.; Cui, J.; Zhou, I.Y.; Luk, K.D.; Wu, E.X.; Hu, Y. Organization of the intrinsic functional network in the cervical spinal cord: A resting state functional MRI study. Neuroscience 2016, 336, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Figley, C.R.; Yau, D.; Stroman, P.W. Attenuation of lower-thoracic, lumbar, and sacral spinal cord motion: Implications for imaging human spinal cord structure and function. Am. J. Neuroradiol. 2008, 29, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Bosma, R.L.; Stroman, P.W. Assessment of data acquisition parameters, and analysis techniques for noise reduction in spinal cord fMRI data. Magn. Reson. Imaging 2014, 32, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Figley, C.R.; Stroman, P.W. Investigation of human cervical and upper thoracic spinal cord motion: Implications for imaging spinal cord structure and function. Magn. Reson. Med. 2007, 58, 185–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harita, S.; Stroman, P.W. Confirmation of resting-state BOLD fluctuations in the human brainstem and spinal cord after identification and removal of physiological noise. Magn. Reson. Med. 2017, 78, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Agosta, F.; Valsasina, P.; Caputo, D.; Stroman, P.W.; Filippi, M. Tactile-associated recruitment of the cervical cord is altered in patients with multiple sclerosis. NeuroImage 2008, 39, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Agosta, F.; Valsasina, P.; Rocca, M.A.; Caputo, D.; Sala, S.; Judica, E.; Stroman, P.W.; Filippi, M. Evidence for enhanced functional activity of cervical cord in relapsing multiple sclerosis. Magn. Reson. Med. 2008, 59, 1035–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosma, R.L.; Stroman, P.W. Spinal cord response to stepwise and block presentation of thermal stimuli: A functional MRI study. J. Magn. Reson. Imaging 2015, 41, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Piche, M.; Khoshnejad, M.; Perlbarg, V.; Chen, J.I.; Hoge, R.D.; Benali, H.; Rossignol, S.; Rainville, P.; Cohen-Adad, J. Reduction of physiological noise with independent component analysis improves the detection of nociceptive responses with fMRI of the human spinal cord. NeuroImage 2012, 63, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Bodurka, J.; Bandettini, P.A. How long to scan? The relationship between fMRI temporal signal to noise ratio and necessary scan duration. NeuroImage 2007, 34, 565–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figley, C.R.; Stroman, P.W. Measurement and characterization of the human spinal cord seep response using event-related spinal fMRI. Magn. Reson. Imaging 2012, 30, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Jenkinson, M.; Andersson, J.; Tracey, I.; Brooks, J.C. Assessment of physiological noise modelling methods for functional imaging of the spinal cord. NeuroImage 2012, 60, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.M.; Kornelsen, J.; Stroman, P.W. Noninvasive observation of cervical spinal cord activity in children by functional MRI during cold thermal stimulation. Magn. Reson. Imaging 2011, 29, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Kornelsen, J.; Smith, S.D.; McIver, T.A. A neural correlate of visceral emotional responses: Evidence from fMRI of the thoracic spinal cord. Soc. Cognit. Affect. Neurosci. 2015, 10, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Bandettini, P.A.; Wong, E.C.; Jesmanowicz, A.; Hinks, R.S.; Hyde, J.S. Spin-echo and gradient-echo EPI of human brain activation using BOLD contrast: A comparative study at 1.5 T. NMR Biomed. 1994, 7, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Norris, D.G.; Zysset, S.; Mildner, T.; Wiggins, C.J. An investigation of the value of spin-echo-based fMRI using a stroop color-word matching task and EPI at 3 T. Neuroimage 2002, 15, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Stroman, P.W.; Krause, V.; Frankenstein, U.N.; Malisza, K.L.; Tomanek, B. Spin-echo versus gradient-echo fMRI with short echo times. Magn. Reson. Imaging 2001, 19, 827–831. [Google Scholar] [CrossRef]
- Stroman, P.W. Essentials of Functional MRI; Taylor Francis Group, LLC: Boca Raton, FL, USA, 2011. [Google Scholar]
- Finsterbusch, J.; Eippert, F.; Buchel, C. Single, slice-specific z-shim gradient pulses improve T2*-weighted imaging of the spinal cord. NeuroImage 2012, 59, 2307–2315. [Google Scholar] [CrossRef] [PubMed]
- Eippert, F.; Kong, Y.; Jenkinson, M.; Tracey, I.; Brooks, J.C.W. Denoising spinal cord fMRI data: Approaches to acquisition and analysis. NeuroImage 2017, 154, 255–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, T.; Cohen-Adad, J. Effect of respiration on the B0 field in the human spinal cord at 3T. Magn. Reson. Med. 2014, 72, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Kolesar, T.A.; Fiest, K.M.; Smith, S.D.; Kornelsen, J. Assessing nociception by fMRI of the human spinal cord: A systematic review. Magn. Reson. Insights 2015, 8, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Figley, C.R.; Stroman, P.W. Development and validation of retrospective spinal cord motion time-course estimates (respite) for spin-echo spinal fMRI: Improved sensitivity and specificity by means of a motion-compensating general linear model analysis. NeuroImage 2009, 44, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.C.; Beckmann, C.F.; Miller, K.L.; Wise, R.G.; Porro, C.A.; Tracey, I.; Jenkinson, M. Physiological noise modelling for spinal functional magnetic resonance imaging studies. NeuroImage 2008, 39, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jin, R.; Li, G.; Luk, K.D.; Wu, E.X. Robust spinal cord resting-state fMRI using independent component analysis-based nuisance regression noise reduction. J. Magn. Reson. Imaging 2018. [Google Scholar] [CrossRef] [PubMed]
- Piche, M.; Cohen-Adad, J.; Nejad, M.K.; Perlbarg, V.; Xie, G.; Beaudoin, G.; Benali, H.; Rainville, P. Characterization of cardiac-related noise in fMRI of the cervical spinal cord. Magn. Reson. Imaging 2009, 27, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Stroman, P.W.; Filippi, M. Functional MRI of the spinal cord. In fMRI Techniques and Protocols, 2nd ed.; Filippi, M., Ed.; Humana Press: New York, NY, USA, 2016; pp. 871–892. [Google Scholar]
- Stroman, P.W.; Wheeler-Kingshott, C.; Bacon, M.; Schwab, J.M.; Bosma, R.; Brooks, J.; Cadotte, D.; Carlstedt, T.; Ciccarelli, O.; Cohen-Adad, J.; et al. The current state-of-the-art of spinal cord imaging: Methods. NeuroImage 2014, 84, 1070–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadotte, D.W.; Stroman, P.W.; Mikulis, D.; Fehlings, M.G. A systematic review of spinal fMRI research: Outlining the elements of experimental design. J. Neurosurg. Spine 2012, 17, 102–118. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Adad, J.; Gauthier, C.J.; Brooks, J.C.; Slessarev, M.; Han, J.; Fisher, J.A.; Rossignol, S.; Hoge, R.D. Bold signal responses to controlled hypercapnia in human spinal cord. NeuroImage 2010, 50, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J.; Jezzard, P.; Turner, R. Analysis of functional MRI time-series. Hum. Brain Mapp. 1994, 1, 153–171. [Google Scholar] [CrossRef] [Green Version]
- Biswal, B.; Yetkin, F.Z.; Haughton, V.M.; Hyde, J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995, 34, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Stroman, P.W. Discrimination of errors from neuronal activity in functional MRI of the human spinal cord by means of general linear model analysis. Magn. Reson. Med. 2006, 56, 452–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komisaruk, B.R.; Mosier, K.M.; Liu, W.C.; Criminale, C.; Zaborszky, L.; Whipple, B.; Kalnin, A. Functional localization of brainstem and cervical spinal cord nuclei in humans with fMRI. Am. J. Neuroradiol. 2002, 23, 609–617. [Google Scholar] [PubMed]
- Weber, K.A.; Sentis, A.I.; Bernadel-Huey, O.N.; Chen, Y.; Wang, X.; Parrish, T.B.; Mackey, S. Thermal stimulation alters cervical spinal cord functional connectivity in humans. Neuroscience 2018, 369, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Stroman, P.W.; Figley, C.R.; Cahill, C.M. Spatial normalization, bulk motion correction and coregistration for functional magnetic resonance imaging of the human cervical spinal cord and brainstem. Magn. Reson. Imaging 2008, 26, 809–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, J.; Bartram, C.T. Fila radicularia of the ventral and dorsal radices of the human spinal cord. Gegenbaurs. Morphol. Jahrb. 1982, 128, 417–462. [Google Scholar] [PubMed]
- Fonov, V.S.; Le Troter, A.; Taso, M.; De Leener, B.; Lévêque, G.; Benhamou, M.; Sdika, M.; Benali, H.; Pradat, P.F.; Collins, D.L. Framework for integrated MRI average of the spinal cord white and gray matter: The MNI-Poly-AMU template. Neuroimage 2014, 102, 817–827. [Google Scholar] [CrossRef]
- Taso, M.; Le Troter, A.; Sdika, M.; Ranjeva, J.P.; Guye, M.; Bernard, M.; Callot, V. Construction of an in vivo human spinal cord atlas based on high-resolution MR images at cervical and thoracic levels: Preliminary results. MAGMA 2014, 27, 257–267. [Google Scholar]
- Taso, M.; Le Troter, A.; Sdika, M.; Cohen-Adad, J.; Arnoux, P.J.; Guye, M.; Ranjeva, J.P.; Callot, V. A reliable spatially normalized template of the human spinal cord—Applications to automated white matter/gray matter segmentation and tensor-based morphometry (TBM) mapping of gray matter alterations occurring with age. Neuroimage 2015, 117, 20–28. [Google Scholar] [CrossRef]
- De Leener, B.; Fonov, V.S.; Collins, D.L.; Callot, V.; Stikov, N.; Cohen-Adad, J. Pam50: Unbiased multimodal template of the brainstem and spinal cord aligned with the icbm152 space. NeuroImage 2018, 165, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Cadotte, D.W.; Cadotte, A.; Cohen-Adad, J.; Fleet, D.; Livne, M.; Wilson, J.R.; Mikulis, D.; Nugaeva, N.; Fehlings, M.G. Characterizing the location of spinal and vertebral levels in the human cervical spinal cord. Am. J. Neuroradiol. 2015, 36, 803–810. [Google Scholar] [CrossRef] [PubMed]


| Gradient-Echo | Spin-Echo |
|---|---|
| Typical Brain fMRI | Gradient-Echo Spinal fMRI | Spin-Echo Spinal fMRI | |
|---|---|---|---|
| Imaging Parameters | 3.3 mm × 3.3 mm | 1 mm × 1 mm | 1.5 mm × 1.5 mm |
| 3.3 mm-thick slice | 5 mm-thick slice | 2 mm-thick slice | |
| 200 kHz bandwidth | 200 kHz bandwidth | 151 kHz bandwidth | |
| 64 × 64 matrix | 128 × 128 matrix | 192 × 144 matrix | |
| TE = T2* | TE: 30 ms (~1.2 T2*) | TE: 75 ms (T2) | |
| acceleration factor = 1 (no parallel imaging assumed) | acceleration factor = 2 | acceleration factor = 1 | |
| Estimated SNR | 150 | 24 | 56 |
| Acquisition time/volume | 3 s | 1.5 s | 6.75 s |
| Detectable effect size, p = 10−6, 12 min acquisition | 0.42% | 1.9% | 1.7% |
| Cadotte et al. 2015 [116] | Lang and Bartram 1982 [111] | |||
|---|---|---|---|---|
| Middle of Segment Position | Length (mm) | Middle of Segment Position | Length (mm) | |
| C1 | 25.0 | 8 | ||
| C2 | 35.3 | 12.5 | ||
| C3 | 51.5 | 10.5 | 46.7 | 10.4 |
| C4 | 65.7 | 9.9 | 57.7 | 11.5 |
| C5 | 81.1 | 10.5 | 70.9 | 15.0 |
| C6 | 95.4 | 9.7 | 85.4 | 14.0 |
| C7 | 109.3 | 9.4 | 98.6 | 12.4 |
| C8 | 122.8 | 9.6 | 111.3 | 13.0 |
| Distance from C3 to C8 | 71.3 mm | Distance from C3 to C8 | 64.6 mm | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Powers, J.M.; Ioachim, G.; Stroman, P.W. Ten Key Insights into the Use of Spinal Cord fMRI. Brain Sci. 2018, 8, 173. https://doi.org/10.3390/brainsci8090173
Powers JM, Ioachim G, Stroman PW. Ten Key Insights into the Use of Spinal Cord fMRI. Brain Sciences. 2018; 8(9):173. https://doi.org/10.3390/brainsci8090173
Chicago/Turabian StylePowers, Jocelyn M., Gabriela Ioachim, and Patrick W. Stroman. 2018. "Ten Key Insights into the Use of Spinal Cord fMRI" Brain Sciences 8, no. 9: 173. https://doi.org/10.3390/brainsci8090173





