Evidences from Rewarding System, FRN and P300 Effect in Internet-Addiction in Young People
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Procedure
2.3. Stimuli
2.4. IAT Scores
2.5. BIS/BAS Scores
2.6. EEG Recordings and Data Reduction
3. Data Analysis
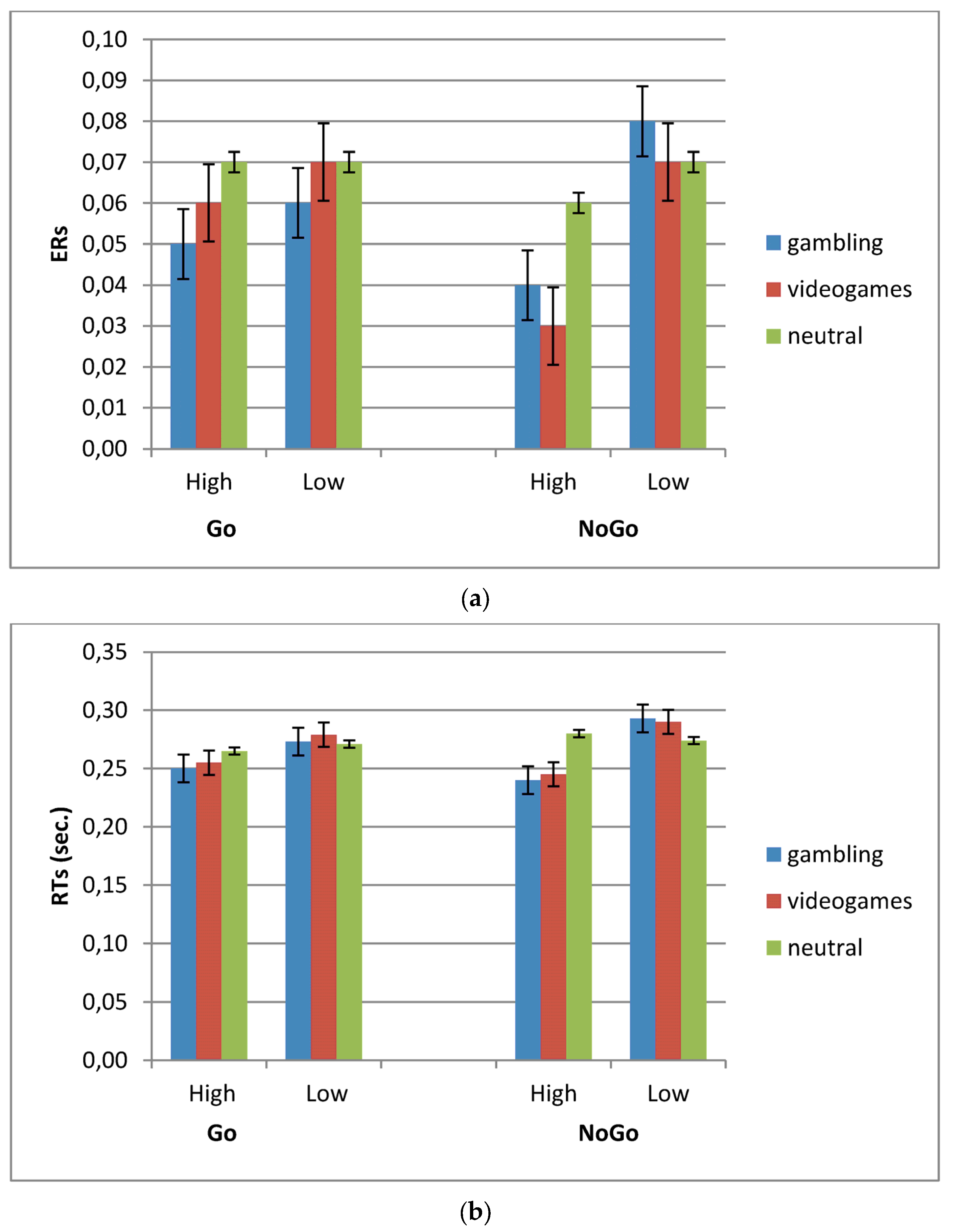
3.1. ERs
3.2. RTs
3.3. ERP Data
3.4. FRN
3.5. P300
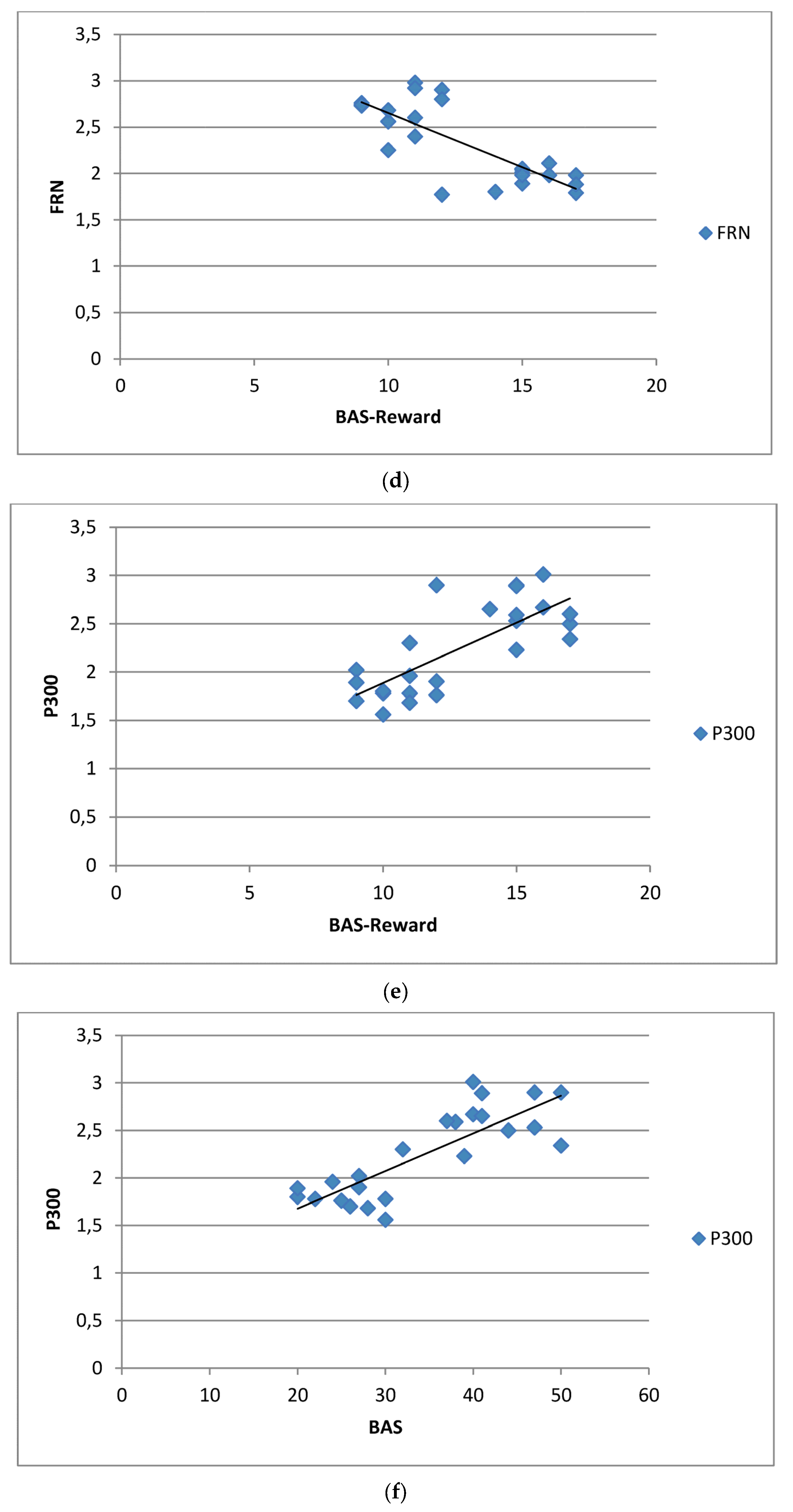
3.6. Correlational Analysis
4. Discussion
4.1. Cognitive Effects, Rewarding and Attentional Bias in IA
4.2. ERPs Effects: FRN and P300
4.3. BAS and BAS-Reward Subscale Contribution
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Zhou, Z.; Yu, R.; Zhou, X. To do or not to do? Action enlarges the FRN and P300 effects in outcome evaluation. Neuropsychologia 2010, 48, 3606–3613. [Google Scholar] [CrossRef] [PubMed]
- Han, D.H.; Lyoo, I.K.; Renshaw, P.F. Differential regional gray matter volumes in patients with on-line game addiction and professional gamers. J. Psychiatric Res. 2012, 46, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.E.; Potenza, M.N.; Weinstein, A.; Gorelick, D.A. Introduction to behavioral addictions. Am. J. Drug Alcohol Abus. 2010, 36, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Shapira, N.A.; Goldsmith, T.D.; Keck, P.E.; Khosla, U.M.; McElroy, S.L. Psychiatricfeatures of individuals with problematic internet use. J. Affect. Disord. 2000, 57, 267–272. [Google Scholar] [CrossRef]
- Dell’Osso, B.; Hadley, S.; Allen, A.; Baker, B.; Chaplin, W.F.; Hollander, E. Escitalopram in the treatment of impulsive-compulsive internet usage disorder: An open-label trial followed by a double-blind discontinuation phase. J. Clin. Psychiatry 2008, 69, 1–478. [Google Scholar] [CrossRef]
- Dong, G.; Zhou, H.; Zhao, X. Impulse inhibition in people with Internet addiction disorder: Electrophysiological evidence from a Go/NoGo study. Neurosci. Lett. 2010, 485, 138–142. [Google Scholar] [CrossRef] [PubMed]
- D’Hondt, F.; Maurage, P. Electrophysiological studies in Internet addiction: A review within the dual-process framework. Addict. Behav. 2015, 64, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Finocchiaro, R. Deficit in rewarding mechanisms and prefrontal left/right cortical effect in internet addiction. Acta Neuropsychiatr. 2016, 28, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Lee, J.H. How cognitive reappraisal of anger influences risktaking behavior. Soc. Behav. Pers.: Int. J. 2011, 29, 411–418. [Google Scholar] [CrossRef]
- Yen, J.Y.; Yen, C.-F.; Chen, C.S.; Chang, Y.H.; Yeh, Y.C.; Ko, C.H. The bidirectional interactions between addiction, behavior approach and behavior inhibition systems among adolescents in a prospective study. Psychiatry Res. 2012, 200, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Caseras, X.; Avila, C.; Torrubia, R. The measurement of individual differences in behavioural inhibition and behavioural activation systems: A comparison of personality scales. Pers. Individ. Differ. 2003, 34, 999–1013. [Google Scholar] [CrossRef]
- Beard, K.W.; Wolf, E.M. Modification in the proposed diagnostic criteria for Internet addiction. Cyberpsychol. Behav. 2001, 4, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Barnes, G.M.; Welte, J.W.; Hoffman, J.H.; Dintcheff, B.A. Shared predictors of youthful gambling, substance use, and delinquency. Psychol. Addict. Behav. 2005, 19, 165. [Google Scholar] [CrossRef] [PubMed]
- Dawe, S.; Loxton, N. The role of impulsivity in the development of substance use and eating disorders. Neurosci. Biobehav. Rev. 2004, 28, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Moeller, F.G.; Dougherty, D.M.; Barratt, E.S.; Schmitz, J.M.; Swann, A.C.; Grabowski, J. The impact of impulsivity on cocaine use and retention in treatment. J. Subst. Abus. Treat. 2001, 21, 193–198. [Google Scholar] [CrossRef]
- Vitaro, F.; Arseneault, L.; Tremblay, R.E. Impulsivity predicts problem gambling in low SES adolescent males. Addiction 1999, 94, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Patton, J.H.; Stanford, M.S.; Barratt, E.S. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 1995, 51, 768–774. [Google Scholar] [CrossRef]
- Cao, F.; Su, L.; Liu, T.; Gao, X. The relationship between impulsivity and Internet addiction in a sample of Chinese adolescents. Eur. Psychiatry 2007, 22, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Bechara, A.; Martin, E.M. Impaired Decision Making Related to Working Memory Deficits in Individuals With Substance Addictions. Neuropsychology 2004, 18, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Verdejo-García, A.; Bechara, A. Somatic marker theory of addiction. Neuropharmacology 2009, 56, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.L.; Joseph, J.E.; Jiang, Y.; Zimmerman, R.S.; Kelly, T.H.; Darna, M.; Huettl, P.; Dwoskin, L.P.; Bardo, M.T. Prefrontal Cortex and Drug Abuse Vulnerability: Translation to Prevention and Treatment Interventions. Brain Res. Rev. 2011, 65, 124–149. [Google Scholar] [CrossRef] [PubMed]
- Logan, G.D.; Cowan, W.B.; Davis, K.A. On the ability to inhibit simple and choice reaction time responses: A model and a method. J. Exp. Psychol. Hum. Percept. Perform. 1984, 10, 276. [Google Scholar] [CrossRef] [PubMed]
- Knyazev, G.G. Antero-posterior EEG spectral power gradient as a correlate of extraversion and behavioral inhibition. Open Neuroimaging J. 2010, 4, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Finocchiaro, R.; Canavesio, Y. Reward-system effect (BAS rating), left hemispheric “unbalance” (alpha band oscillations) and decisional impairments in drug addiction. Addict. Behav. 2014, 39, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Finocchiaro, R. Decisionalimpairments in cocaineaddiction, rewardbias, andcorticaloscillation “unbalance”. Neuropsychiatr. Dis. Treat. 2015, 11, 777. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Porjesz, B.; Rangaswamy, M.; Kamarajan, C.; Tang, Y.; Jones, K.A.; Chorlian, D.B.; Stimus, A.T.; Begleiter, H. Reduced frontal lobe activity in subjects with high impulsivity and alcoholism. Alcohol. Clin. Exp. Res. 2007, 31, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Baler, R.D.; Volkow, N.D. Drug addiction: The neurobiology of disrupted self-control. Trends Mol. Med. 2006, 12, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Bechara, A. Decision making, impulse control and loss of willpower to resist drugs: A neurocognitive perspective. Nat. Neurosci. 2005, 8, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Finocchiaro, R. Left Hemispheric “Imbalance” in Drug Addiction. Neuropathol. Drug Addict. Subst. Misuse 2016, 3, 229–237. [Google Scholar]
- Adinoff, B. Neurobiologic processes in drug reward and addiction. Harv. Rev. Psychiatry 2004, 12, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Limbrick-Oldfield, E.H.; van Holst, R.J.; Clark, L. Fronto-striatal dysregulation in drug addiction and pathological gambling: Consistent inconsistencies? NeuroImage Clin. 2013, 2, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Scheres, A.; Milham, M.P.; Knutson, B.; Castellanos, F.X. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol. Psychiatry 2007, 61, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Mazza, G. Consciousness and emotion: ERP modulation and attentive vs. pre-attentive elaboration of emotional facial expressions by backward masking. Motiv. Emot. 2009, 33, 113–124. [Google Scholar] [CrossRef]
- Balconi, M.; Mazza, G. Lateralisation effect in comprehension of emotional facial expression: A comparison between EEG alpha band power and behavioural inhibition (BIS) and activation (BAS) systems. Later. Asymmetr. Body Brain Cogn. 2010, 15, 361–384. [Google Scholar] [CrossRef] [PubMed]
- Harmon-Jones, E. Contributions from research on anger and cognitive dissonance to understanding the motivational functions of asymmetrical frontal brain activity. Biol. Psychol. 2004, 67, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Falbo, L.; Conte, V.A. BIS and BAS correlates with psychophysiological and cortical response systems during aversive and appetitive emotional stimuli processing. Motiv. Emot. 2012, 36, 218–231. [Google Scholar] [CrossRef]
- Balconi, M.; Brambilla, E.; Falbo, L. Appetitive vs. defensive responses to emotional cues. Autonomic measures and brain oscillation modulation. Brain Res. 2009, 1296, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Pozzoli, U. Event-related oscillations (ERO) and event-related potentials (ERP) in emotional face recognition. Int. J. Neurosci. 2008, 19, 1412–1424. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Brambilla, E.; Falbo, L. BIS/BAS, corticaloscillations and coherence in response to emotionalcues. Brain Res. Bull. 2009, 80, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Carver, C.S.; White, T.L. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. J. Pers. Soc. Psychol. 1994, 67, 319–333. [Google Scholar] [CrossRef]
- Fowles, D.C. Electrodermalhyporeactivity and antisocial behavior: Does anxiety mediate the relationship? J. Affect. Disord. 2000, 61, 177–189. [Google Scholar] [CrossRef]
- Fowles, D.C. The three arousal model: Implications of Gray’s two-factor learning theory for heart rate, electrodermal activity, and psychopathy. Psychophysiology 1980, 17, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.A. A critique of Eysenck’s theory of personality. In A Model for Personality; Eysenck, H.J., Ed.; Springer: Berlin, Germany, 1981; pp. 246–276. [Google Scholar]
- Yu, A.; Dayan, P. Uncertainty, neuromodulation and attention. Neuron 2005, 46, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Gaher, R.M.; Hahn, A.M.; Shishido, H.; Simons, J.S.; Gaster, S. Associations between sensitivity to punishment, sensitivity to reward and gambling. Addict. Behav. 2015, 42, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, R.; Balconi, M. Reward-system effect and “left hemispheric unbalance”: A comparison between drug addiction and high-BAS healthy subjects on gambling behavior. Neuropsychol. Trends 2015, 17, 37–45. [Google Scholar] [CrossRef]
- Balconi, M.; Finocchiaro, R.; Canavesio, Y.; Messina, R. Reward bias and lateralization in gambling behavior: BAS and alpha band analysis. Psychiatry Res. 2014, 219, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Crivelli, D. FRN and P300 ERP effect modulation in response to feedback sensitivity: The contribution of punishment-reward system (BIS/BAS) and Behaviour Identification of action. Neurosci. Res. 2010, 66, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Crivelli, D. Veridical and false feedback sensitivity and punishment-reward system (BIS/BAS): ERP amplitude and theta frequency band analysis. Clin. Neurophysiol. 2010, 121, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Holroyd, C.B.; Krigolson, O.E. Reward prediction error signals associated with a modified time estimation task. Psychophysiology 2007, 44, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Hewig, J.; Trippe, R.; Hecht, H.; Coles, M.G.H.; Holroyd, C.B.; Miltner, W.H.R. Decision-making in Blackjack: An elec-trophysiological analysis. Cereb. Cortex 2007, 17, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Holroyd, C.B.; Coles, M.G.H. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychol. Rev. 2002, 109, 679–709. [Google Scholar] [CrossRef] [PubMed]
- Hajcak, G.; Holroyd, C.B.; Moser, J.S.; Simons, R.F. Brain potentials associated with expected and unexpected good and bad outcomes. Psychophysiology 2005, 42, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Oberg, S.A.; Christie, G.J.; Tata, M.S. Problem gamblers exhibit reward hypersensitivity in medial frontal cortex during gambling. Neuropsychologia 2011, 49, 3768–3775. [Google Scholar] [CrossRef] [PubMed]
- Duncan-Johnson, C.C.; Donchin, E. On quantifying surprise: The variation of event-related potentials with subjective probability. Psychophysiology 1997, 14, 456–467. [Google Scholar] [CrossRef]
- Johnson, R.J.; Donchin, E. P300 and stimulus categorization: Two plus one is not so different from one plus one. Psychophysiology 1980, 17, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Israel, J.N.; Chesney, G.L.; Wickens, C.D.; Donchin, E. P300 and tracking difficulty: Evidence for multiple resources in dual-task performance. Psychophysiology 1980, 17, 259–273. [Google Scholar] [CrossRef]
- Pfabigan, D.M.; Alexopoulos, J.; Bauer, H.; Sailer, U. Manipulation of feedback expectancy and valence induces negative and positive reward prediction error signals manifest in event-related brain potentials. Psychophysiology 2011, 48, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Young, K.S. Internet addiction: The emergence of a new clinical disorder. Cyberpsychol. Behav. 1998, 1, 237–244. [Google Scholar] [CrossRef]
- Leone, L.; Pierro, A.; Mannetti, L. Validità della versione italiana delle scale BIS/BAS di Carver e White (1994): Generalizzabilità della struttura e relazioni con costrutti affini. G. Ital. Psicol. 2002, 29, 413–434. [Google Scholar]
- Jasper, H.H. The ten-twenty electrode system of International Federation EEG. Electroencephalography. Clin. Neurophysiol. 1958, 10, 371–375. [Google Scholar]
- Ludwig, A.; Miriani, R.M.; Langhals, N.B.; Joseph, M.D.; David, J. Neuron Recordings From Microelectrode Arrays Using a Common Average Reference to Improve Cortical. J. Neurophysiol. 2008, 101, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Semlitsch, H.V.; Anderer, P.; Schuster, P.; Presslich, O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology 1986, 23, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gu, R.; Wu, T.; Broster, L.S.; Luo, Y.; Jiang, Y.; Luo, Y.J. An electrophysiological index of changes in risk decision-making strategies. Neuropsychologia 2013, 51, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Schoenbaum, G.; Roesch, M.R.; Stalnaker, T.A. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 2006, 29, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; DeVito, E.; Huang, J.; Du, X. Diffusion tensor imaging reveals thalamus and posterior cingulate cortex abnormalities in internet gaming addicts. J. Psychiatry Res. 2012, 46, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Yeung, N.; Holroyd, C.B.; Cohen, J.D. ERP correlates of feedback and reward processing in the presence and absence of response choice. Cereb. Cortex 2005, 15, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Mazza, G. Brain oscillations and BIS/BAS (behavioral inhibition/activation system) effects on processing masked emotional cues. ERS/ERD and coherence measures of alpha band. Int. J. Psychophysiol. 2009, 74, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Braverman, E.R.; Holder, J.M.; Lubar, J.F.; Monastra, V.J.; Miller, D.; Lubar, J.O.; Chen, T.J.; Comings, D.E. Reward deficiency syndrome: A biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J. Psychoact. Drugs 2000, 32, 1–112. [Google Scholar] [CrossRef]
- Colder, C.R.; O’Connor, R. Attention biases and disinhibited behavior as predictors of alcohol use and enhancement reasons for drinking. Psychol. Addict. Behav. 2002, 16, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Franken, I.H.; Muris, P.; Georgieva, I. Gray’s model of personality and addiction. Addict. Behav. 2006, 31, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Pozzoli, U. Morphed facial expressions elicited a N400 ERP effect: A domain-specific semantic module? Scand. J. Psychol. 2005, 46, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Park, Y.A.; Lee, H.W.; Jung, H.Y.; Lee, J.Y.; Choi, J.S. The effects of behavioral inhibition/approach system as predictors of Internet addiction in adolescents. Pers. Individ. Differ. 2013, 54, 7–11. [Google Scholar] [CrossRef]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balconi, M.; Venturella, I.; Finocchiaro, R.
Evidences from Rewarding System, FRN and P300 Effect in Internet-Addiction in Young People
. Brain Sci. 2017, 7, 81.
https://doi.org/10.3390/brainsci7070081
Balconi M, Venturella I, Finocchiaro R.
Evidences from Rewarding System, FRN and P300 Effect in Internet-Addiction in Young People
. Brain Sciences. 2017; 7(7):81.
https://doi.org/10.3390/brainsci7070081
Balconi, Michela, Irene Venturella, and Roberta Finocchiaro.
2017. "Evidences from Rewarding System, FRN and P300 Effect in Internet-Addiction in Young People
" Brain Sciences 7, no. 7: 81.
https://doi.org/10.3390/brainsci7070081





