A Review of Issues Related to Data Acquisition and Analysis in EEG/MEG Studies
Abstract
:1. Introduction: The Evolution of EEG/MEG Studies
2. Idiosyncrasies of EEG and MEG Studies
3. Study Design
3.1. What Is and Is Not Possible?
3.2. Naturalistic Stimulation
3.3. Hyperscanning
3.4. Concurrent EEG-MEG Studies
3.5. Differences between EEG/MEG and fMRI Signals
3.6. Concurrent EEG-fMRI Studies
3.7. Planning the Study
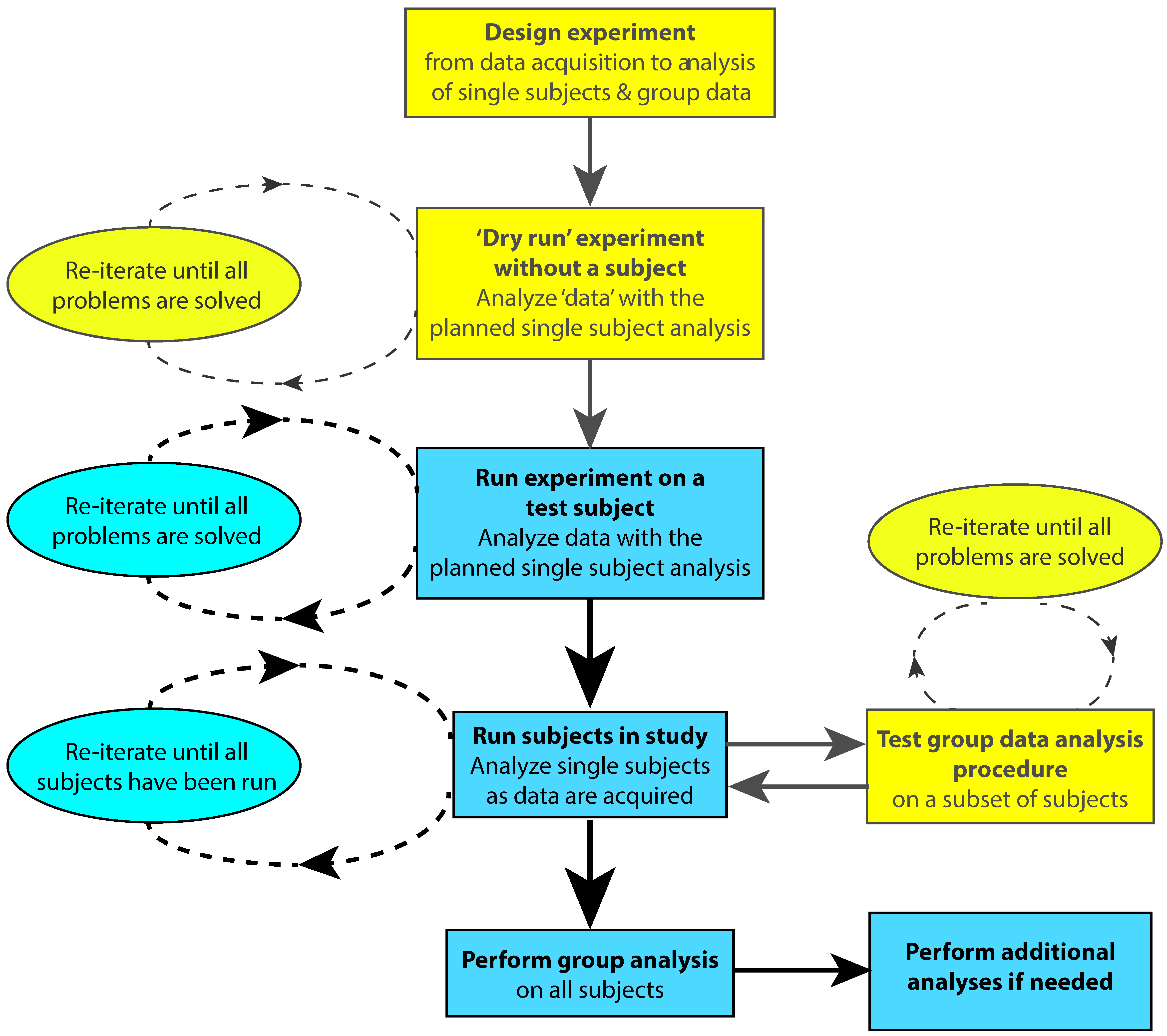
4. Testing the Experimental Design Prior to Data Acquisition
5. Data Acquisition
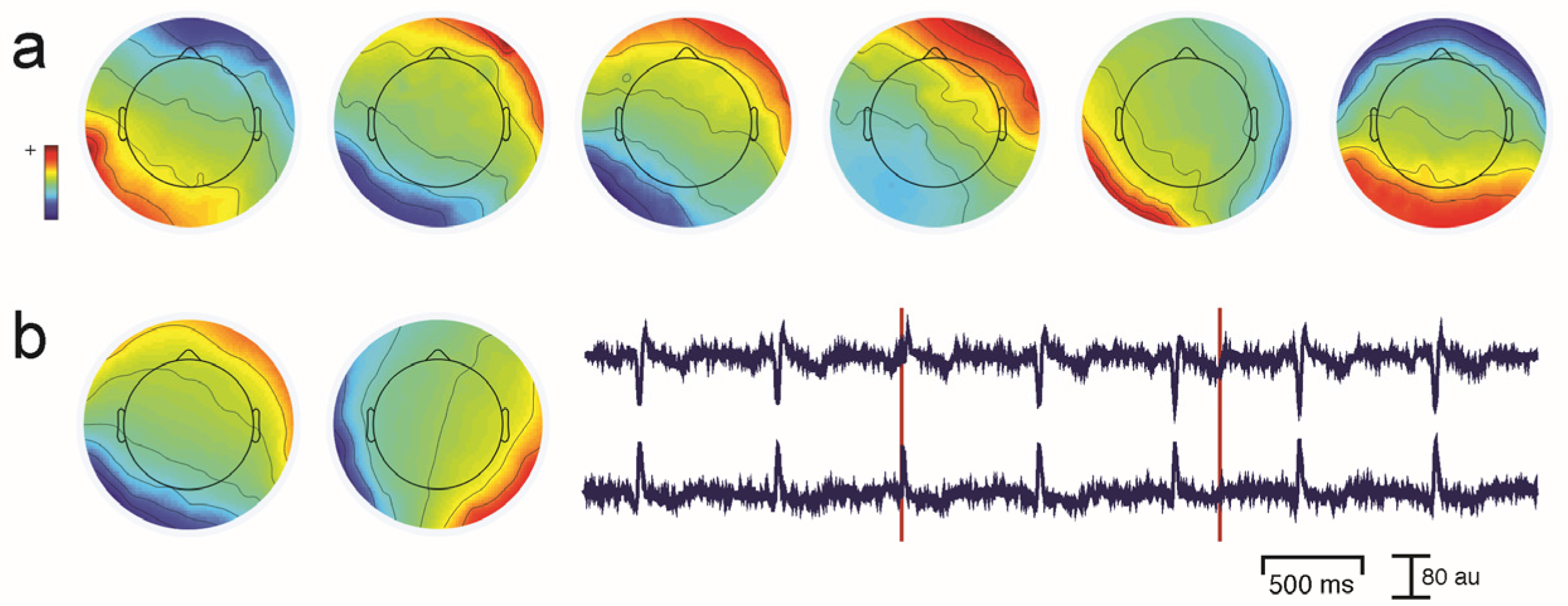
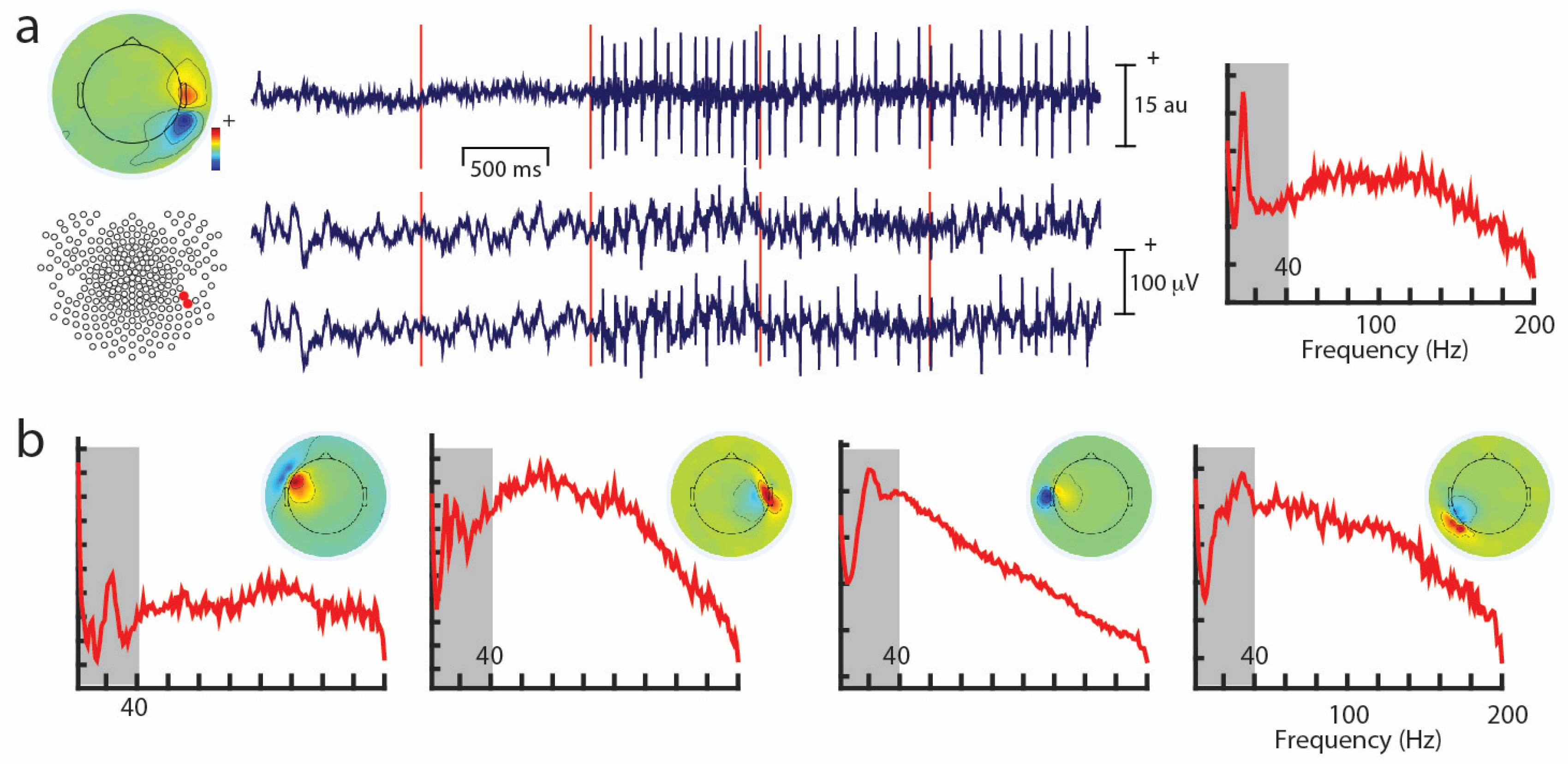
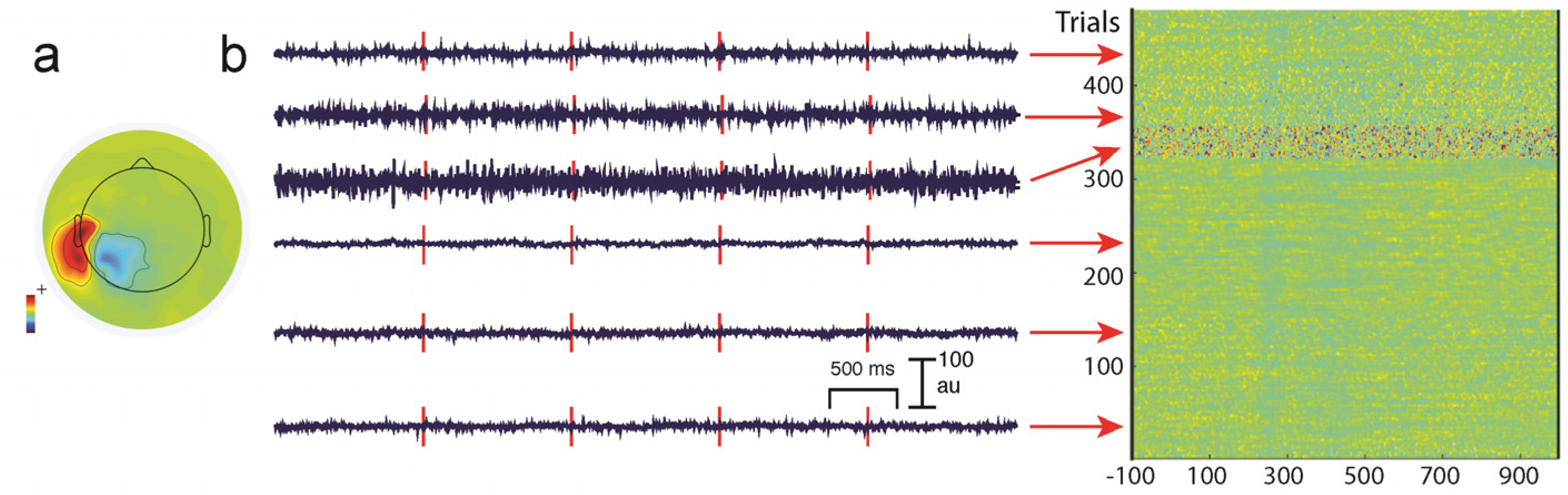
5.1. Common Types of Artifacts in EEG/MEG Recordings
5.2. Reducing Artifacts during Data Acquisition
5.3. Additional Issues with Head Movements during MEG Studies
5.4. Appropriate Filtering and Sampling Rates
5.5. EEG Reference Electrodes
5.6. Sensitivities of Different Types of MEG Sensors
6. Preprocessing and Analysis
6.1. Specialized MEG Artifact Removal
6.2. Removing Artifacts in MEG or EEG Data with Independent Component Analysis
6.3. Digital Re-Referencing of EEG Data
6.4. Sensor and Source Space Analyses
6.5. Source Estimation
7. Keeping Records
7.1. Cataloguing and Characterizing the Data
7.2. Data Storage and Accessibility
8. Data Sharing
9. Special Considerations for Studying Clinical or Pediatric Populations
9.1. Subject Comfort and Optimal Data Acquisition
9.2. Specialized Head Models for Pediatric Studies of EEG
10. Conclusions: Final Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Picton, T.W.; Bentin, S.; Berg, P.; Donchin, E.; Hillyard, S.A.; Johnson, R., Jr.; Miller, G.A.; Ritter, W.; Ruchkin, D.S.; Rugg, M.D.; et al. Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology 2000, 37, 127–152. [Google Scholar] [CrossRef] [PubMed]
- Pivik, R.T.; Broughton, R.J.; Coppola, R.; Davidson, R.J.; Fox, N.; Nuwer, M.R. Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology 1993, 30, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Duncan, C.C.; Barry, R.J.; Connolly, J.F.; Fischer, C.; Michie, P.T.; Naatanen, R.; Polich, J.; Reinvang, I.; van Petten, C. Event-related potentials in clinical research: Guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clin. Neurophysiol. 2009, 120, 1883–1908. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.; Baillet, S.; Barnes, G.R.; Henson, R.N.; Hillebrand, A.; Jensen, O.; Jerbi, K.; Litvak, V.; Maess, B.; Oostenveld, R.; et al. Good practice for conducting and reporting MEG research. NeuroImage 2013, 65, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Keil, A.; Debener, S.; Gratton, G.; Junghofer, M.; Kappenman, E.S.; Luck, S.J.; Luu, P.; Miller, G.A.; Yee, C.M. Committee report: Publication guidelines and recommendations for studies using electroencephalography and magnetoencephalography. Psychophysiology 2014, 51, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Hari, R.; Puce, A. MEG-EEG Primer; Oxford University Press: New York, NY, USA, 2017. [Google Scholar]
- Hämäläinen, M.; Hari, R.; Ilmoniemi, R.J.; Knuutila, J.E.T.; Lounasmaa, O.V. Magnetoencephalography—Theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev. Mod. Phys. 1993, 65, 413–497. [Google Scholar] [CrossRef]
- Hari, R.; Ilmoniemi, R.J. Cerebral magnetic fields. CRC Crit. Rev. Biomed. Eng. 1986, 14, 93–126. [Google Scholar]
- Stenroos, M.; Hauk, O. Minimum-norm cortical source estimation in layered head models is robust against skull conductivity error. NeuroImage 2013, 81, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, M.S.; Sarvas, J. Realistic conductivity geometry model of the human head for interpretation of neuromagnetic data. IEEE Trans. Biomed. Eng. 1989, 36, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Stenroos, M.; Hunold, A.; Haueisen, J. Comparison of three-shell and simplified volume conductor models in magnetoencephalography. NeuroImage 2014, 94, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Marin, G.; Guerin, C.; Baillet, S.; Garnero, L.; Meunier, G. Influence of skull anisotropy for the forward and inverse problem in EEG: Simulation studies using FEM on realistic head models. Hum. Brain Mapp. 1998, 6, 250–269. [Google Scholar] [CrossRef]
- Hallez, H.; Vanrumste, B.; Grech, R.; Muscat, J.; de Clercq, W.; Vergult, A.; D’Asseler, Y.; Camilleri, K.P.; Fabri, S.G.; van Huffel, S.; Lemahieu, I. Review on solving the forward problem in EEG source analysis. J. Neuroeng. Rehabil. 2007, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Montague, P.R.; Berns, G.S.; Cohen, J.D.; McClure, S.M.; Pagnoni, G.; Dhamala, M.; Wiest, M.C.; Karpov, I.; King, R.D.; Apple, N.; et al. Hyperscanning: Simultaneous fMRI during linked social interactions. NeuroImage 2002, 16, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Taheri, B.A.; Knight, R.T.; Smith, R.L. A dry electrode for EEG recording. Electroencephalogr. Clin. Neurophysiol. 1994, 90, 376–383. [Google Scholar]
- Fiedler, P.; Strohmeier, D.; Hunold, A.; Griebel, S.; Muhle, R.; Schreiber, M.; Pedrosa, P.; Vasconcelos, B.; Fonseca, C.; Vaz, F.; et al. Modular multipin electrodes for comfortable dry EEG. In Proceedings of the IEEE 38th Annual International Conference of the Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 5705–5708. [Google Scholar]
- Xu, J.; Mitra, S.; van Hoof, C.; Yazicioglu, R.; Makinwa, K.A. Active Electrodes for Wearable EEG Acquisition: Review and Electronics Design Methodology. IEEE Rev. Biomed. Eng. 2017. [Google Scholar] [CrossRef] [PubMed]
- Mathewson, K.E.; Harrison, T.J.; Kizuk, S.A. High and dry? Comparing active dry EEG electrodes to active and passive wet electrodes. Psychophysiology 2017, 54, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Jaaskelainen, I.P.; Belliveau, J.W.; Huang, S.; Hung, A.Y.; Rossi, S.; Ahveninen, J. Combined MEG and EEG show reliable patterns of electromagnetic brain activity during natural viewing. NeuroImage 2015, 114, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, P.; Parkkonen, L.; Hari, R.; Hyvarinen, A. Characterization of neuromagnetic brain rhythms over time scales of minutes using spatial independent component analysis. Hum. Brain Mapp. 2012, 33, 1648–1662. [Google Scholar] [CrossRef] [PubMed]
- Hasson, U.; Nir, Y.; Levy, I.; Fuhrmann, G.; Malach, R. Intersubject synchronization of cortical activity during natural vision. Science 2004, 303, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- Hasson, U.; Malach, R.; Heeger, D.J. Reliability of cortical activity during natural stimulation. Trends Cogn. Sci. 2010, 14, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Spiers, H.J.; Maguire, E.A. Decoding human brain activity during real-world experiences. Trends Cogn. Sci. 2007, 11, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, G.; Bifulco, P.; Cesarelli, M.; Jin, C.; McEwan, A.; van Schaik, A. Wearable dry sensors with bluetooth connection for use in remote patient monitoring systems. Stud. Health Technol. Inform. 2010, 161, 57–65. [Google Scholar] [PubMed]
- Mullen, T.R.; Kothe, C.A.; Chi, Y.M.; Ojeda, A.; Kerth, T.; Makeig, S.; Jung, T.P.; Cauwenberghs, G. Real-time neuroimaging and cognitive monitoring using wearable dry EEG. IEEE Trans. Biomed. Eng. 2015, 62, 2553–2567. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.D.; Chen, C.Y.; Wang, I.J.; Chen, S.F.; Li, S.Y.; Chen, B.W.; Chang, J.Y.; Lin, C.T. Gaming control using a wearable and wireless EEG-based brain-computer interface device with novel dry foam-based sensors. J. Neuroeng. Rehabil. 2012, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Stopczynski, A.; Stahlhut, C.; Larsen, J.E.; Petersen, M.K.; Hansen, L.K. The smartphone brain scanner: A portable real-time neuroimaging system. PLoS ONE 2014, 9, e86733. [Google Scholar] [CrossRef] [PubMed]
- Debener, S.; Minow, F.; Emkes, R.; Gandras, K.; de Vos, M. How about taking a low-cost, small, and wireless EEG for a walk? Psychophysiology 2012, 49, 1617–1621. [Google Scholar] [CrossRef] [PubMed]
- Boto, E.; Meyer, S.S.; Shah, V.; Alem, O.; Knappe, S.; Kruger, P.; Fromhold, T.M.; Lim, M.; Glover, P.M.; Morris, P.G.; et al. A new generation of magnetoencephalography: Room temperature measurements using optically-pumped magnetometers. NeuroImage 2017, 149, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Bigdely-Shamlo, N.; Cockfield, J.; Makeig, S.; Rognon, T.; la Valle, C.; Miyakoshi, M.; Robbins, K.A. Hierarchical Event Descriptors (HED): Semi-Structured Tagging for Real-World Events in Large-Scale EEG. Front. Neuroinform. 2016, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Dumas, G.; Nadel, J.; Soussignan, R.; Martinerie, J.; Garnero, L. Inter-brain synchronization during social interaction. PLoS ONE 2010, 5, e12166. [Google Scholar] [CrossRef] [PubMed]
- Konvalinka, I.; Vuust, P.; Roepstorff, A.; Frith, C.D. Follow you, follow me: Continuous mutual prediction and adaptation in joint tapping. Q. J. Exp. Psychol. (Hove) 2010, 63, 2220–2230. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Bourguignon, M.; Parkkonen, L.; Hari, R. Neural signatures of hand kinematics in leaders vs. followers: A dual-MEG study. NeuroImage 2016, 125, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Lachat, F.; Hugueville, L.; Lemarechal, J.D.; Conty, L.; George, N. Oscillatory brain correlates of live joint attention: A dual-EEG study. Front. Hum. Neurosci. 2012, 6, 156. [Google Scholar] [CrossRef] [PubMed]
- Hirata, M.; Ikeda, T.; Kikuchi, M.; Kimura, T.; Hiraishi, H.; Yoshimura, Y.; Asada, M. Hyperscanning MEG for understanding mother–child cerebral interactions. Front. Hum. Neurosci. 2014, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, L.; Toppi, J.; Fallani, F.D.; Vecchiato, G.; Salinari, S.; Mattia, D.; Cincotti, F.; Babiloni, F. Neuroelectrical hyperscanning measures simultaneous brain activity in humans. Brain Topogr. 2010, 23, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, F.; Astolfi, L. Social neuroscience and hyperscanning techniques: Past, present and future. Neurosci. Biobehav. Rev. 2014, 44, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.P. On the interpretation of synchronization in EEG hyperscanning studies: A cautionary note. Front. Hum. Neurosci. 2013, 7, 881. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Yuan, H. Simultaneous EEG and MEG source reconstruction in sparse electromagnetic source imaging. Hum. Brain Mapp. 2013, 34, 775–795. [Google Scholar] [CrossRef] [PubMed]
- Chatrian, G.E.; Lettich, E.; Nelson, P.L. Ten percent electrode system for topographic studies of spontaneous and evoked EEG activity. Am. J. EEG Technol. 1985, 25, 83–92. [Google Scholar]
- Jasper, H. The ten-twenty electrode system of the International Federation, Electroencephalogr. Clin. Neurophysiol. 1958, 10, 371–375. [Google Scholar]
- Klem, G.H.; Luders, H.O.; Jasper, H.H.; Elger, C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 3–6. [Google Scholar] [PubMed]
- Tucker, D.M. Spatial sampling of head electrical fields: The geodesic sensor net. Electroencephalogr. Clin. Neurophysiol. 1993, 87, 154–163. [Google Scholar] [CrossRef]
- Fuchs, M.; Wagner, M.; Wischmann, H.A.; Kohler, T.; Theissen, A.; Drenckhahn, R.; Buchner, H. Improving source reconstructions by combining bioelectric and biomagnetic data. Electroencephalogr. Clin. Neurophysiol. 1998, 107, 93–111. [Google Scholar] [CrossRef]
- Henson, R.N.; Mouchlianitis, E.; Friston, K.J. MEG and EEG data fusion: Simultaneous localisation of face-evoked responses. NeuroImage 2009, 47, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.A.; Zerouali, Y.; Hedrich, T.; Heers, M.; Kobayashi, E.; Lina, J.M.; Grova, C. MEG-EEG Information Fusion and Electromagnetic Source Imaging: From Theory to Clinical Application in Epilepsy. Brain Topogr. 2015, 28, 785–812. [Google Scholar] [CrossRef] [PubMed]
- Ebersole, J.S.; Ebersole, S.M. Combining MEG and EEG source modeling in epilepsy evaluations. J. Clin. Neurophysiol. 2010, 27, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Agam, Y.; Hamalainen, M.S.; Lee, A.K.; Dyckman, K.A.; Friedman, J.S.; Isom, M.; Makris, N.; Manoach, D.S. Multimodal neuroimaging dissociates hemodynamic and electrophysiological correlates of error processing. Proc. Natl. Acad. Sci. USA 2011, 108, 17556–17561. [Google Scholar] [CrossRef] [PubMed]
- Heilbronner, S.R.; Platt, M.L. Causal evidence of performance monitoring by neurons in posterior cingulate cortex during learning. Neuron 2013, 80, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, H.E.; Mantle, M.; Nagarajan, S.S. Concordance between routine interictal magnetoencephalography and simultaneous scalp electroencephalography in a sample of patients with epilepsy. J. Clin. Neurophysiol. 2007, 24, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Aydin, U.; Vorwerk, J.; Dumpelmann, M.; Kupper, P.; Kugel, H.; Heers, M.; Wellmer, J.; Kellinghaus, C.; Haueisen, J.; Rampp, S.; et al. Combined EEG/MEG can outperform single modality EEG or MEG source reconstruction in presurgical epilepsy diagnosis. PLoS ONE 2015, 10, e0118753. [Google Scholar] [CrossRef] [PubMed]
- Hunold, A.; Funke, M.E.; Eichardt, R.; Stenroos, M.; Haueisen, J. EEG and MEG: Sensitivity to epileptic spike activity as function of source orientation and depth. Physiol. Meas. 2016, 37, 1146–1162. [Google Scholar] [CrossRef] [PubMed]
- Bast, T.; Wright, T.; Boor, R.; Harting, I.; Feneberg, R.; Rupp, A.; Hoechstetter, K.; Rating, D.; Baumgartner, U. Combined EEG and MEG analysis of early somatosensory evoked activity in children and adolescents with focal epilepsies. Clin. Neurophysiol. 2007, 118, 1721–1735. [Google Scholar] [CrossRef] [PubMed]
- Henson, R.N.; Flandin, G.; Friston, K.J.; Mattout, J. A Parametric Empirical Bayesian framework for fMRI-constrained MEG/EEG source reconstruction. Hum. Brain Mapp. 2010, 31, 1512–1531. [Google Scholar] [CrossRef] [PubMed]
- Puce, A.; Allison, T.; Spencer, S.S.; Spencer, D.D.; McCarthy, G. Comparison of cortical activation evoked by faces measured by intracranial field potentials and functional MRI: Two case studies. Hum. Brain Mapp. 1997, 5, 298–305. [Google Scholar] [CrossRef]
- Ahlfors, S.P.; Han, J.; Lin, F.H.; Witzel, T.; Belliveau, J.W.; Hamalainen, M.S.; Halgren, E. Cancellation of EEG and MEG signals generated by extended and distributed sources. Hum. Brain Mapp. 2010, 31, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Spironelli, C.; Busenello, J.; Angrilli, A. Supine posture inhibits cortical activity: Evidence from Delta and Alpha EEG bands. Neuropsychologia 2016, 89, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Lifshitz, M.; Thibault, R.T.; Roth, R.R.; Raz, A. Source Localization of Brain States Associated with Canonical Neuroimaging Postures. J. Cogn. Neurosci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ozgoren, M.; Tetik, M.; Izzetoglu, K.; Oniz, A.; Onaral, B. Effect of body position on NIRS based hemodynamic measures from prefrontal cortex; In BICS 2012: Lecture Notes in Computer Science, Proceedings of the Advances in Brain Inspired Cognitive Systems, Shenyang, China, 11–14 July 2012; Zhang, H., Hussain, A., Liu, D., Wang, Z., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 138–146. [Google Scholar]
- Huster, R.J.; Debener, S.; Eichele, T.; Herrmann, C.S. Methods for simultaneous EEG-fMRI: An introductory review. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 6053–6060. [Google Scholar] [CrossRef] [PubMed]
- Ullsberger, M.; Debener, S. Simultaneous EEG and fMRI; Oxford University Press: New York, NY, USA, 2010. [Google Scholar]
- Scheeringa, R.; Koopmans, P.J.; van Mourik, T.; Jensen, O.; Norris, D.G. The relationship between oscillatory EEG activity and the laminar-specific BOLD signal. Proc. Natl. Acad. Sci. USA 2016, 113, 6761–6766. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, S.D.; Ostwald, D.; Porcaro, C.; Bagshaw, A.P. Spontaneous EEG alpha oscillation interacts with positive and negative BOLD responses in the visual-auditory cortices and default-mode network. NeuroImage 2013, 76, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Liu, Y.; Huang, H.; Ding, M. Coupling between visual alpha oscillations and default mode activity. NeuroImage 2013, 68, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Aladjalova, N. Infra-slow rhythmic osciallations of the steady potential of the cerebral cortex. Nature 1957, 179, 957–959. [Google Scholar] [CrossRef] [PubMed]
- Achermann, P.; Borbely, A.A. Low-frequency (<1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience 1997, 81, 213–222. [Google Scholar] [CrossRef]
- Monto, S.; Palva, S.; Voipio, J.; Palva, J.M. Very slow EEG fluctuations predict the dynamics of stimulus detection and oscillation amplitudes in humans. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 8268–8272. [Google Scholar] [CrossRef] [PubMed]
- Damoiseaux, J.S.; Rombouts, S.A.; Barkhof, F.; Scheltens, P.; Stam, C.J.; Smith, S.M.; Beckmann, C.F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. USA 2006, 103, 13848–13853. [Google Scholar] [CrossRef] [PubMed]
- Omata, K.; Hanakawa, T.; Morimoto, M.; Honda, M. Spontaneous Slow Fluctuation of EEG Alpha Rhythm Reflects Activity in Deep-Brain Structures: A Simultaneous EEG-fMRI Study. PLoS ONE 2013, 8, e66869. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.D.; Setsompop, K.; Rosen, B.R.; Polimeni, J.R. Fast fMRI can detect oscillatory neural activity in humans. Proc. Natl. Acad. Sci. USA 2016, 113, E6679–E6685. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, D. Multichannel topography of human alpha EEG fields. Electroencephalogr. Clin. Neurophysiol. 1971, 31, 439–449. [Google Scholar] [CrossRef]
- Lehmann, D.; Skrandies, W. Reference-free identification of components of checkerboard-evoked multichannel potential fields. Electroenceph. Clin. Neurophysiol. 1980, 48, 609–621. [Google Scholar] [CrossRef]
- Britz, J.; van de Ville, D.; Michel, C.M. BOLD correlates of EEG topography reveal rapid resting-state network dynamics. NeuroImage 2010, 52, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Musso, F.; Brinkmeyer, J.; Mobascher, A.; Warbrick, T.; Winterer, G. Spontaneous brain activity and EEG microstates. A novel EEG/fMRI analysis approach to explore resting-state networks. NeuroImage 2010, 52, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Weisend, M.P.; Hanlon, F.M.; Montaño, R.; Ahlfors, S.P.; Leuthold, A.C.; Pantazis, D.; Mosher, J.C.; Georgopoulos, A.P.; Hämäläinen, M.S.; Aine, C. Paving the way for cross-site pooling of magnetoencephalography (MEG) data. Int. Congr. Ser. 2007, 1300, 615–618. [Google Scholar] [CrossRef]
- Ou, W.; Golland, P.; Hamalainen, M. Sources of variability in MEG. Med. Image Comput. Comput. Assist. Interv. 2007, 10, 751–759. [Google Scholar] [PubMed]
- White, D.M.; van Cott, A.C. EEG artifacts in the intensive care unit setting. Am. J. Electroneurodiagnostic Technol. 2010, 50, 8–25. [Google Scholar] [CrossRef] [PubMed]
- Fisch, B. Fisch and Spehlmann’s EEG Primer, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Goncharova, I.I.; McFarland, D.J.; Vaughan, T.M.; Wolpaw, J.R. EMG contamination of EEG: Spectral and topographical characteristics. Clin. Neurophysiol. 2003, 114, 1580–1593. [Google Scholar] [CrossRef]
- Taulu, S.; Kajola, M.; Simola, J. Suppression of interference and artifacts by the Signal Space Separation Method. Brain Topogr. 2004, 16, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Taulu, S.; Simola, J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys. Med. Biol. 2006, 51, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.S.; Bonaiuto, J.; Lim, M.; Rossiter, H.; Waters, S.; Bradbury, D.; Bestmann, S.; Brookes, M.; Callaghan, M.F.; Weiskopf, N.; et al. Flexible head-casts for high spatial precision MEG. J Neurosci. Methods 2016, 276, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.; Panizza, M.; Hallett, M. Principles of digital sampling of a physiologic signal. Electroencephalogr. Clin. Neurophysiol. 1993, 89, 349–358. [Google Scholar] [CrossRef]
- Widmann, A.; Schröger, E.; Maess, B. Digital filter design for electrophysiological data—A practical approach. J. Neurosci. Methods 2015, 250, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Katznelson, R.D. EEG Recording, Electrode Placement, and Aspects of Generator Localization. In Electric Fields of the Brain: The Neurophysics of EEG; Nunez, P., Ed.; Oxford University Press: New York, NY, USA, 1981; pp. 176–213. [Google Scholar]
- Uusitalo, M.; Ilmoniemi, R.J. Signal-space projection method for separating MEG and EEG into components. Med. Biol. Eng. Comput. 1997, 35, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, M.; Ilmoniemi, R. Interpreting Magnetic Fields of the Brain: Minimum Norm Estimates. Med. Biol. Eng.Comput. 1994, 32, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.A.; Strohmeier, D.; Brodbeck, C.; Parkkonen, L.; Hamalainen, M.S. MNE software for processing MEG and EEG data. NeuroImage 2014, 86, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.J.; Sejnowski, T.J. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995, 7, 1129–1259. [Google Scholar] [CrossRef] [PubMed]
- Onton, J.; Makeig, S. Information-based modeling of event-related brain dynamics. Prog. Brain Res. 2006, 159, 99–120. [Google Scholar] [PubMed]
- Chaumon, M.; Bishop, D.V.; Busch, N.A. A practical guide to the selection of independent components of the electroencephalogram for artifact correction. J. Neurosci. Methods 2015, 250, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Onton, J.; Westerfield, M.; Townsend, J.; Makeig, S. Imaging human EEG dynamics using independent component analysis. Neurosci. Biobehav. Rev. 2006, 30, 808–822. [Google Scholar] [CrossRef] [PubMed]
- Latinus, M.; Love, S.A.; Rossi, A.; Parada, F.J.; Huang, L.; Conty, L.; George, N.; James, K.; Puce, A. Social decisions affect neural activity to perceived dynamic gaze. Soc. Cogn. Affect. Neurosci. 2015, 10, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Parada, F.J.; Kolchinsky, A.; Puce, A. Neural correlates of apparent motion perception of impoverished facial stimuli: A comparison of ERP and ERSP activity. NeuroImage 2014, 98, 442–459. [Google Scholar] [CrossRef] [PubMed]
- Litvak, V.; Mattout, J.; Kiebel, S.; Phillips, C.; Henson, R.; Kilner, J.; Barnes, G.; Oostenveld, R.; Daunizeau, J.; Flandin, G.; et al. EEG and MEG data analysis in SPM8. Comput. Intell. Neurosci. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.M.; Murray, M.M.; Lantz, G.; Gonzalez, S.; Spinelli, L.; de Peralta, R.G. EEG source imaging. Clin. Neurophysiol. 2004, 115, 2195–2222. [Google Scholar] [CrossRef] [PubMed]
- Scherg, M.; Ille, N.; Weckesser, D.; Ebert, A.; Ostendorf, A.; Boppel, T.; Schubert, S.; Larsson, P.G.; Henning, O.; Bast, T. Fast evaluation of interictal spikes in long-term EEG by hyper-clustering. Epilepsia 2012, 53, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Winter, W.R.; Ding, J.; Nunez, P.L. EEG and MEG coherence: Measures of functional connectivity at distinct spatial scales of neocortical dynamics. J. Neurosci. Methods 2007, 166, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Fein, G.; Raz, J.; Brown, F.F.; Merrin, E.L. Common reference coherence data are confounded by power and phase effects. Electroencephalogr. Clin. Neurophysiol. 1988, 69, 581–584. [Google Scholar] [CrossRef]
- Guevara, R.; Velazquez, J.L.; Nenadovic, V.; Wennberg, R.; Senjanovic, G.; Dominguez, L.G. Phase synchronization measurements using electroencephalographic recordings: What can we really say about neuronal synchrony? Neuroinformatics 2005, 3, 301–314. [Google Scholar] [CrossRef]
- Bertrand, O.; Perrin, F.; Pernier, J. A theoretical justification of the average reference in topographic evoked potential studies. Electroencephalogr. Clin. Neurophysiol. 1985, 62, 462–464. [Google Scholar] [CrossRef]
- Desmedt, J.E.; Chalklin, V.; Tomberg, C. Emulation of somatosensory evoked potential (SEP) components with the 3-shell head model and the problem of ‘ghost potential fields’ when using an average reference in brain mapping. Electroencephalogr. Clin. Neurophysiol. 1990, 77, 243–258. [Google Scholar] [CrossRef]
- Nunez, P.L.; Srinivasan, R. Electric Fields of the Brain. The Neurophysics of EEG, 2nd ed.; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Srinivasan, R.; Tucker, D.M.; Murias, M. Estimating the spatial Nyquist of the human EEG. Behav. Res. Methods Instrum. Comput. 1998, 30, 8–19. [Google Scholar] [CrossRef]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Nunez, P.L. REST: A good idea but not the gold standard. Clin. Neurophysiol. 2010, 121, 2177–2180. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, L.; della Penna, S.; Snyder, A.Z.; Pizzella, V.; Nolte, G.; de Pasquale, F.; Romani, G.L.; Corbetta, M. Frequency specific interactions of MEG resting state activity within and across brain networks as revealed by the multivariate interaction measure. NeuroImage 2013, 79, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Yao, D. A method to standardize a reference of scalp EEG recordings to a point at infinity. Physiol. Meas. 2001, 22, 693–711. [Google Scholar] [CrossRef] [PubMed]
- Scherg, M. Fundamentals of dipole source potential analysis. In Auditory Evoked Magnetic Fields and Potentials; Grandori, F., Hoke, M., Romani, G.L., Eds.; Karger: Basel, Switzerland, 1990. [Google Scholar]
- Raij, T.; Karhu, J.; Kicic, D.; Lioumis, P.; Julkunen, P.; Lin, F.H.; Ahveninen, J.; Ilmoniemi, R.J.; Makela, J.P.; Hamalainen, M.; et al. Parallel input makes the brain run faster. NeuroImage 2008, 40, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Forss, N.; Hari, R.; Salmelin, R.; Ahonen, A.; Hamalainen, M.; Kajola, M.; Knuutila, J.; Simola, J. Activation of the human posterior parietal cortex by median nerve stimulation. Exp. Brain Res. 1994, 99, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Dale, A.M.; Liu, A.K.; Fischl, B.R.; Buckner, R.L.; Belliveau, J.W.; Lewine, J.D.; Halgren, E. Dynamic statistical parametric mapping: Combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron 2000, 26, 55–67. [Google Scholar] [CrossRef]
- Dale, A.M.; Sereno, M.I. Improved localizadon of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. J. Cogn. Neurosci. 1993, 5, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Parkkonen, L.; Andersson, J.; Hämäläinen, M.; Hari, R. Early visual brain areas reflect the percept of an ambiguous scene. Proc. Natl. Acad. Sci. USA 2008, 105, 20500–20504. [Google Scholar] [CrossRef] [PubMed]
- Ahveninen, J.; Jaaskelainen, I.P.; Raij, T.; Bonmassar, G.; Devore, S.; Hamalainen, M.; Levanen, S.; Lin, F.H.; Sams, M.; Shinn-Cunningham, B.G.; et al. Task-modulated “what” and “where” pathways in human auditory cortex. Proc. Natl. Acad. Sci. USA 2006, 103, 14608–14613. [Google Scholar] [CrossRef] [PubMed]
- Van Veen, B.; Buckley, K. Beamforming: A versatile approach to spatial filtering. IEEE ASSP Mag. 1988. [Google Scholar] [CrossRef]
- Sekihara, K.; Nagarajan, S.S. Adaptive Spatial Filters for Electromagnetic Brain Imaging; Springer: Berlin, Germany, 2008. [Google Scholar]
- Mosher, J.C.; Lewis, P.S.; Leahy, R. Multiple dipole modeling and localization from spatio-temporal MEG data. IEEE Trans. Biomed. Eng. 1992, 39, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Mosher, J.C.; Leahy, R.M. Source localization using recursively applied and projected (RAP) MUSIC. IEEE Trans. Signal Proc. 1999, 47, 332–340. [Google Scholar] [CrossRef]
- Steinstrater, O.; Sillekens, S.; Junghoefer, M.; Burger, M.; Wolters, C.H. Sensitivity of beamformer source analysis to deficiencies in forward modeling. Hum. Brain Mapp. 2010, 31, 1907–1927. [Google Scholar] [CrossRef] [PubMed]
- Brookes, M.J.; Vrba, J.; Robinson, S.E.; Stevenson, C.M.; Peters, A.M.; Barnes, G.R.; Hillebrand, A.; Morris, P.G. Optimising experimental design for MEG beamformer imaging. NeuroImage 2008, 39, 1788–1802. [Google Scholar] [CrossRef] [PubMed]
- Hauk, O.; Stenroos, M. A framework for the design of flexible cross-talk functions for spatial filtering of EEG/MEG data: DeFleCT. Hum. Brain Mapp. 2014, 35, 1642–1653. [Google Scholar] [CrossRef] [PubMed]
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.A.; Strohmeier, D.; Brodbeck, C.; Goj, R.; Jas, M.; Brooks, T.; Parkkonen, L.; et al. MEG and EEG data analysis with MNE-Python. Front. Neurosci. 2013, 7, 267. [Google Scholar] [CrossRef] [PubMed]
- Brain Imaging Data Structure. Available online: http://bids.neuroimaging.io/ (accessed on 30 May 2017).
- van Essen, D.C.; Smith, S.M.; Barch, D.M.; Behrens, T.E.; Yacoub, E.; Ugurbil, K.; Consortium, W.U.-M.H. The WU-Minn Human Connectome Project: An overview. NeuroImage 2013, 80, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Niso, G.; Rogers, C.; Moreau, J.T.; Chen, L.Y.; Madjar, C.; Das, S.; Bock, E.; Tadel, F.; Evans, A.C.; Jolicoeur, P.; et al. OMEGA: The Open MEG Archive. NeuroImage 2016, 124, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Gorgolewski, K.J.; Poldrack, R.A. A Practical Guide for Improving Transparency and Reproducibility in Neuroimaging Research. PLoS Biol. 2016, 14, e1002506. [Google Scholar] [CrossRef] [PubMed]
- Gorgolewski, K.J.; Varoquaux, G.; Rivera, G.; Schwartz, Y.; Sochat, V.V.; Ghosh, S.S.; Maumet, C.; Nichols, T.E.; Poline, J.B.; Yarkoni, T.; et al. NeuroVault.org: A repository for sharing unthresholded statistical maps, parcellations, and atlases of the human brain. NeuroiImage 2016, 124, 1242–1244. [Google Scholar] [CrossRef] [PubMed]
- Frishkoff, G.; Sydes, J.; Mueller, K.; Frank, R.; Curran, T.; Connolly, J.; Kilborn, K.; Molfese, D.; Perfetti, C.; Malony, A. Minimal Information for Neural Electromagnetic Ontologies (MINEMO): A standards-compliant method for analysis and integration of event-related potentials (ERP) data. Stand. Genomic. Sci. 2011, 5, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Test datasets. Available online: http://megcommunity.org/analysis/testdatasets (accessed on 30 May 2017).
- Cambridge Centre for Ageing and Neuroscience. Available online: http://www.cam-can.org/ (accessed on 30 May 2017).
- Larson-Prior, L.J.; Oostenveld, R.; della Penna, S.; Michalareas, G.; Prior, F.; Babajani-Feremi, A.; Schoffelen, J.M.; Marzetti, L.; de Pasquale, F.; di Pompeo, F.; et al. Adding dynamics to the Human Connectome Project with MEG. NeuroImage 2013, 80, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Aine, C.J.; Sanfratello, L.; Ranken, D.; Best, E.; MacArthur, J.A.; Wallace, T.; Gilliam, K.; Donahue, C.H.; Montano, R.; Bryant, J.E.; et al. MEG-SIM: A web portal for testing MEG analysis methods using realistic simulated and empirical data. Neuroinformatics 2012, 10, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, E.B.; Weiner, H.L. Skull deformities. Pediatr. Clin. N. Am. 2004, 51, 359–387. [Google Scholar] [CrossRef] [PubMed]
- Lew, S.; Sliva, D.D.; Choe, M.S.; Grant, P.E.; Okada, Y.; Wolters, C.H.; Hamalainen, M.S. Effects of sutures and fontanels on MEG and EEG source analysis in a realistic infant head model. NeuroImage 2013, 76, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.E.; Richards, J.E.; Almli, C.R. Neurodevelopmental MRI brain templates for children from 2 weeks to 4 years of age. Dev. Psychobiol. 2012, 54, 77–91. [Google Scholar] [CrossRef] [PubMed]



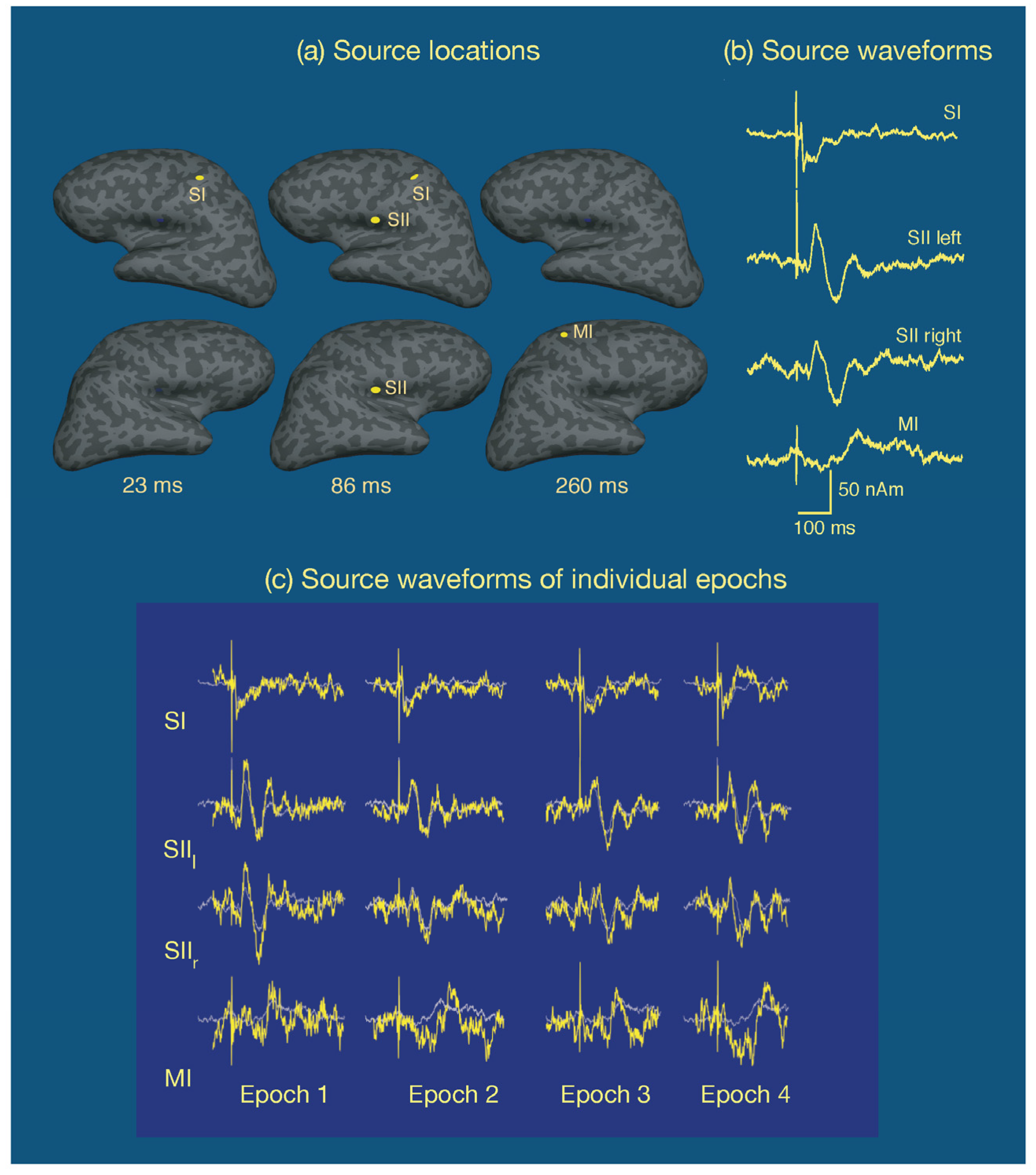
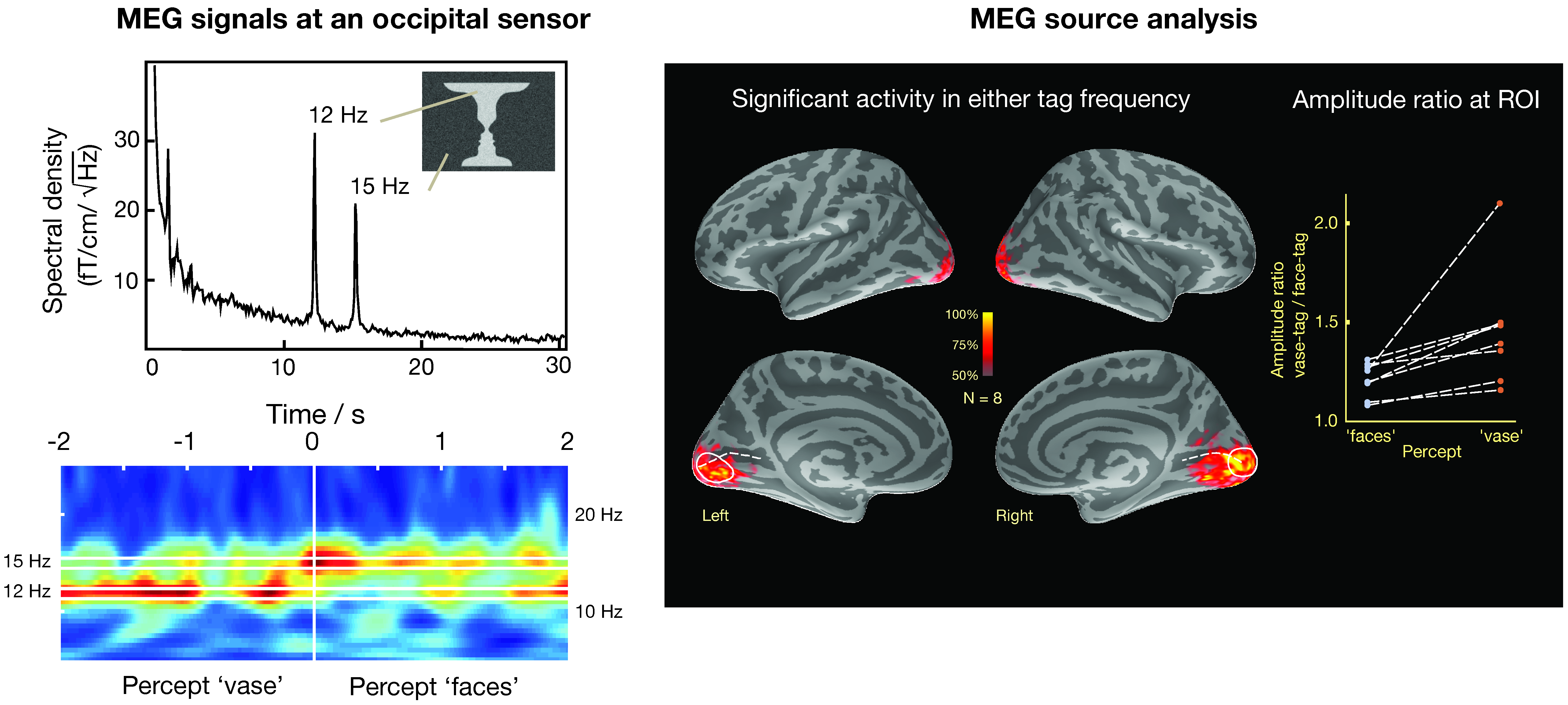
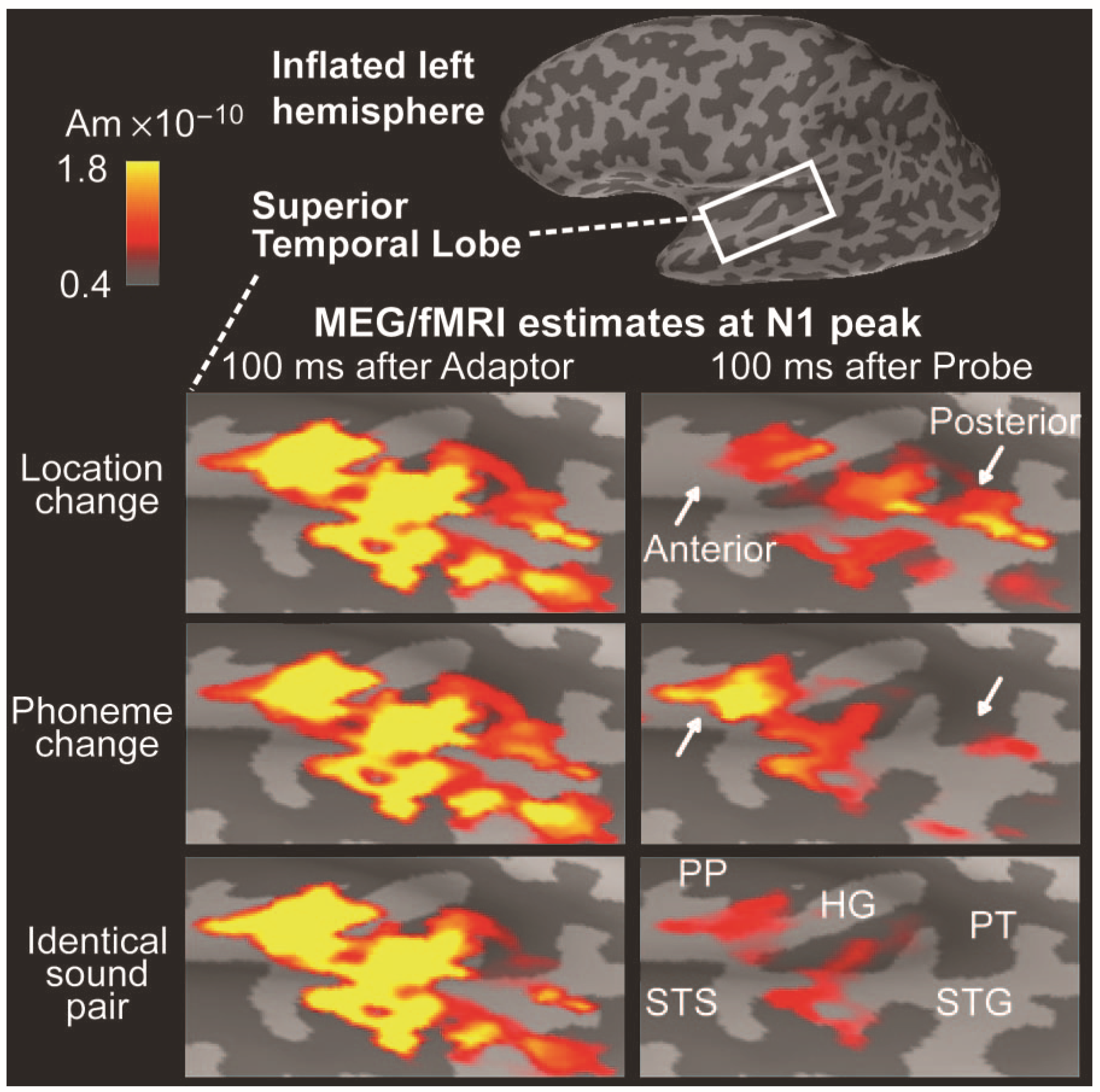
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puce, A.; Hämäläinen, M.S. A Review of Issues Related to Data Acquisition and Analysis in EEG/MEG Studies. Brain Sci. 2017, 7, 58. https://doi.org/10.3390/brainsci7060058
Puce A, Hämäläinen MS. A Review of Issues Related to Data Acquisition and Analysis in EEG/MEG Studies. Brain Sciences. 2017; 7(6):58. https://doi.org/10.3390/brainsci7060058
Chicago/Turabian StylePuce, Aina, and Matti S. Hämäläinen. 2017. "A Review of Issues Related to Data Acquisition and Analysis in EEG/MEG Studies" Brain Sciences 7, no. 6: 58. https://doi.org/10.3390/brainsci7060058





