Hyperarousal and Beyond: New Insights to the Pathophysiology of Insomnia Disorder through Functional Neuroimaging Studies
Abstract
:1. Introduction
2. Spectroscopy Neuroimaging Studies of Insomnia
3. Functional Neuroimaging Studies of Insomnia
4. Summary of Neuroimaging Studies of Insomnia
5. Heuristic Model of Sleep-Wake States
6. Insomnia
7. Treatment
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Perlis, M.L.; Smith, M.T.; Pigeon, W.R. Etiology and pathophysiology of insomnia. In Principles and Practice of Sleep Medicine, 4th ed.; Kryger, M.H., Roth, T., Dement, W.C., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2006; pp. 714–725. [Google Scholar]
- Lushington, K.; Dawson, D.; Lack, L. Core body temperature is elevated during constant wakefulness in elderly poor sleepers. Sleep 2000, 23, 504–510. [Google Scholar] [PubMed]
- Bonnet, M.H.; Arand, D.L. 24-Hour metabolic rate in insomniacs and matched normal sleepers. Sleep 1995, 18, 581–588. [Google Scholar] [PubMed]
- Bonnet, M.H.; Arand, D.L. Heart rate variability in insomniacs and matched normal sleepers. Psychosom. Med. 1998, 60, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Rodenbeck, A.; Huether, G.; Ruther, E.; Hajak, G. Interactions between evening and nocturnal cortisol secretion and sleep parameters in patients with severe chronic primary insomnia. Neurosci. Lett. 2002, 324, 159–163. [Google Scholar] [CrossRef]
- Seelig, E.; Keller, U.; Klarhofer, M.; Scheffler, K.; Brand, S.; Holsboer-Trachsler, E.; Hatzinger, M.; Bilz, S. Neuroendocrine regulation and metabolism of glucose and lipids in primary chronic insomnia: A prospective case-control study. PLoS ONE 2013, 8, e61780. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Bixler, E.O.; Lin, H.M.; Prolo, P.; Mastorakos, G.; Vela-Bueno, A.; Kales, A.; Chrousos, G.P. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: Clinical implications. J. Clin. Endocrinol. Metab. 2001, 86, 3787–3794. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Chen, G.H.; Li, Z.H.; Jiang, S.; Shen, J. Alterations in hypothalamus-pituitary-adrenal/thyroid axes and gonadotropin-releasing hormone in the patients with primary insomnia: A clinical research. PLoS ONE 2013, 8, e71065. [Google Scholar] [CrossRef] [PubMed]
- Backhaus, J.; Junghanns, K.; Hohagen, F. Sleep disturbances are correlated with decreased morning awakening salivary cortisol. Psychoneuroendocrinology 2004, 29, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.C.; Huang, C.J.; Yang, T.T.; Tsai, P.S. Heart rate variability and daytime functioning in insomniacs and normal sleepers: Preliminary results. J. Psychosom. Res. 2008, 65, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Freedman, R.R.; Sattler, H.L. Physiological and psychological factors in sleep-onset insomnia. J. Abnorm. Psychol. 1982, 91, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Lattova, Z.; Keckeis, M.; Maurovich-Horvat, E.; Wetter, T.C.; Wilde-Frenz, J.; Schuld, A.; Pollmacher, T. The stress hormone system in various sleep disorders. J. Psychiatr. Res. 2011, 45, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, W.B.; Garnett, D.; Gillin, J.C.; Weingartner, H. The experience of insomnia and daytime and nighttime functioning. Psychiatry Res. 1984, 12, 235–250. [Google Scholar] [CrossRef]
- Riemann, D.; Klein, T.; Rodenbeck, A.; Feige, B.; Horny, A.; Hummel, R.; Weske, G.; Al-Shajlawi, A.; Voderholzer, U. Nocturnal cortisol and melatonin secretion in primary insomnia. Psychiatry Res. 2002, 113, 17–27. [Google Scholar] [CrossRef]
- Shaver, J.L.; Johnston, S.K.; Lentz, M.J.; Landis, C.A. Stress exposure, psychological distress, and physiological stress activation in midlife women with insomnia. Psychosom. Med. 2002, 64, 793–802. [Google Scholar] [PubMed]
- Varkevisser, M.; van Dongen, H.P.; Kerkhof, G.A. Physiologic indexes in chronic insomnia during a constant routine: Evidence for general hyperarousal? Sleep 2005, 28, 1588–1596. [Google Scholar] [PubMed]
- Zhang, J.; Lam, S.P.; Li, S.X.; Ma, R.C.; Kong, A.P.; Chan, M.H.; Ho, C.S.; Li, A.M.; Wing, Y.K. A community-based study on the association between insomnia and hypothalamic-pituitary-adrenal axis: Sex and pubertal influences. J. Clin. Endocrinol. Metab. 2014, 99, 2277–2287. [Google Scholar] [CrossRef] [PubMed]
- Castro-Diehl, C.; Diez Roux, A.V.; Redline, S.; Seeman, T.; Shrager, S.E.; Shea, S. Association of sleep duration and quality with alterations in the hypothalamic-pituitary adrenocortical axis: The multi-ethnic study of atherosclerosis (MESA). J. Clin. Endocrinol. Metab. 2015, 100, 3149–3158. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.N.; Blackwell, T.; Redline, S.; Punjabi, N.M.; Barrett-Connor, E.; Neylan, T.C.; Stone, K.L. Association between sleep duration and 24-hour urine free cortisol in the MrOS Sleep Study. PLoS ONE 2013, 8, e75205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spath-Schwalbe, E.; Scholler, T.; Kern, W.; Fehm, H.L.; Born, J. Nocturnal adrenocorticotropin and cortisol secretion depends on sleep duration and decreases in association with spontaneous awakening in the morning. J. Clin. Endocrinol. Metab. 1992, 75, 1431–1435. [Google Scholar] [PubMed]
- Monroe, L.J. Psychological and physiological differences between good and poor sleepers. J. Abnorm. Psychol. 1967, 72, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Freedman, R.R. EEG power spectra in sleep-onset insomnia. Electroencephalogr. Clin. Neurophysiol. 1986, 63, 408–413. [Google Scholar] [CrossRef]
- Lamarche, C.H.; Ogilvie, R.D. Electrophysiological changes during the sleep onset period of psychophysiological insomniacs, psychiatric insomniacs, and normal sleepers. Sleep 1997, 20, 724–733. [Google Scholar] [PubMed]
- Merica, H.; Gaillard, J.M. The EEG of the sleep onset period in insomnia: A discriminant analysis. Physiol. Behav. 1992, 52, 199–204. [Google Scholar] [CrossRef]
- Staner, L.; Cornette, F.; Maurice, D.; Viardot, G.; Le Bon, O.; Haba, J.; Staner, C.; Luthringer, R.; Muzet, A.; Macher, J.P. Sleep microstructure around sleep onset differentiates major depressive insomnia from primary insomnia. J. Sleep Res. 2003, 12, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Germain, A.; Hall, M.L.; Moul, D.E.; Nofzinger, E.A.; Begley, A.; Ehlers, C.L.; Thompson, W.; Kupfer, D.J. EEG spectral analysis in primary insomnia: NREM period effects and sex differences. Sleep 2008, 31, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Krystal, A.D.; Edinger, J.D.; Wohlgemuth, W.K.; Marsh, G.R. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep 2002, 25, 630–640. [Google Scholar]
- Merica, H.; Blois, R.; Gaillard, J.M. Spectral characteristics of sleep EEG in chronic insomnia. Eur. J. Neurosci. 1998, 10, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Perlis, M.L.; Smith, M.T.; Andrews, P.J.; Orff, H.; Giles, D.E. Beta/Gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep 2001, 24, 110–117. [Google Scholar] [PubMed]
- Meyerhoff, D.J.; Mon, A.; Metzler, T.; Neylan, T.C. Cortical gamma-aminobutyric acid and glutamate in posttraumatic stress disorder and their relationships to self-reported sleep quality. Sleep 2014, 37, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Pennington, D.L.; Abe, C.; Batki, S.L.; Meyerhoff, D.J. A preliminary examination of cortical neurotransmitter levels associated with heavy drinking in posttraumatic stress disorder. Psychiatry Res. 2014, 224, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.T.; Pace-Schott, E.F.; Mason, G.F.; Forselius, E.; Fasula, M.; Valentine, G.W.; Sanacora, G. Cortical GABA levels in primary insomnia. Sleep 2012, 35, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Plante, D.T.; Jensen, J.E.; Schoerning, L.; Winkelman, J.W. Reduced gamma-aminobutyric acid in occipital and anterior cingulate cortices in primary insomnia: A link to major depressive disorder? Neuropsychopharmacology 2012, 37, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Winkelman, J.W.; Buxton, O.M.; Jensen, J.E.; Benson, K.L.; O’Connor, S.P.; Wang, W.; Renshaw, P.F. Reduced brain GABA in primary insomnia: Preliminary data from 4T proton magnetic resonance spectroscopy (1H-MRS). Sleep 2008, 31, 1499–1506. [Google Scholar] [PubMed]
- Agosto, J.; Choi, J.C.; Parisky, K.M.; Stilwell, G.; Rosbash, M.; Griffith, L.C. Modulation of GABAA receptor desensitization uncouples sleep onset and maintenance in Drosophila. Nat. Neurosci. 2008, 11, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Muthukumaraswamy, S.D.; Edden, R.A.; Jones, D.K.; Swettenham, J.B.; Singh, K.D. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8356–8361. [Google Scholar] [CrossRef] [PubMed]
- Porjesz, B.; Almasy, L.; Edenberg, H.J.; Wang, K.; Chorlian, D.B.; Foroud, T.; Goate, A.; Rice, J.P.; O’Connor, S.J.; Rohrbaugh, J.; et al. Linkage disequilibrium between the beta frequency of the human EEG and a GABAA receptor gene locus. Proc. Natl. Acad. Sci. USA 2002, 99, 3729–3733. [Google Scholar] [CrossRef] [PubMed]
- Saper, C.B.; Chou, T.C.; Scammell, T.E. The sleep switch: Hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001, 24, 726–731. [Google Scholar] [CrossRef]
- Steriade, M. The corticothalamic system in sleep. Front. Biosci. 2003, 8, d878–d899. [Google Scholar] [CrossRef] [PubMed]
- McGinty, D.J.; Sterman, M.B. Sleep suppression after basal forebrain lesions in the cat. Science 1968, 160, 1253–1255. [Google Scholar] [CrossRef] [PubMed]
- Gottesmann, C. GABA mechanisms and sleep. Neuroscience 2002, 111, 231–239. [Google Scholar] [CrossRef]
- Wilt, T.J.; MacDonald, R.; Brasure, M.; Olson, C.M.; Carlyle, M.; Fuchs, E.; Khawaja, I.S.; Diem, S.; Koffel, E.; Ouellette, J.; et al. Pharmacologic treatment of insomnia disorder: An evidence report for a clinical practice guideline by the American college of physicians. Ann. Intern. Med. 2016, 165, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Plante, D.T.; Jensen, J.E.; Winkelman, J.W. The role of GABA in primary insomnia. Sleep 2012, 35, 741–742. [Google Scholar] [CrossRef] [PubMed]
- Bastien, C.H.; St-Jean, G.; Morin, C.M.; Turcotte, I.; Carrier, J. Chronic psychophysiological insomnia: Hyperarousal and/or inhibition deficits? An erps investigation. Sleep 2008, 31, 887–898. [Google Scholar]
- Colombo, M.A.; Ramautar, J.R.; Wei, Y.; Gomez-Herrero, G.; Stoffers, D.; Wassing, R.; Benjamins, J.S.; Tagliazucchi, E.; van der Werf, Y.D.; Cajochen, C.; et al. Wake high-density electroencephalographic spatiospectral signatures of insomnia. Sleep 2016, 39, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Corsi-Cabrera, M.; Figueredo-Rodriguez, P.; del Rio-Portilla, Y.; Sanchez-Romero, J.; Galan, L.; Bosch-Bayard, J. Enhanced frontoparietal synchronized activation during the wake-sleep transition in patients with primary insomnia. Sleep 2012, 35, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Riedner, B.A.; Goldstein, M.R.; Plante, D.T.; Rumble, M.E.; Ferrarelli, F.; Tononi, G.; Benca, R.M. Regional patterns of elevated alpha and high-frequency electroencephalographic activity during nonrapid eye movement sleep in chronic insomnia: A pilot study. Sleep 2016, 39, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Perico, C.A.; Skaf, C.R.; Yamada, A.; Duran, F.; Buchpiguel, C.A.; Castro, C.C.; Soares, J.C.; Busatto, G.F. Relationship between regional cerebral blood flow and separate symptom clusters of major depression: A single photon emission computed tomography study using statistical parametric mapping. Neurosci. Lett. 2005, 384, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.T.; Perlis, M.L.; Chengazi, V.U.; Pennington, J.; Soeffing, J.; Ryan, J.M.; Giles, D.E. Neuroimaging of NREM sleep in primary insomnia: A Tc-99-HMPAO single photon emission computed tomography study. Sleep 2002, 25, 325–335. [Google Scholar] [PubMed]
- Drummond, S.P.; Smith, M.T.; Orff, H.J.; Chengazi, V.; Perlis, M.L. Functional imaging of the sleeping brain: Review of findings and implications for the study of insomnia. Sleep Med. Rev. 2004, 8, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Nofzinger, E.A.; Buysse, D.J.; Germain, A.; Price, J.C.; Miewald, J.M.; Kupfer, D.J. Functional neuroimaging evidence for hyperarousal in insomnia. Am. J. Psychiatry 2004, 161, 2126–2128. [Google Scholar] [CrossRef] [PubMed]
- Kay, D.B.; Karim, H.T.; Soehner, A.M.; Hasler, B.P.; Wilckens, K.A.; James, J.A.; Aizenstein, H.J.; Price, J.C.; Rosario, B.L.; Kupfer, D.J.; et al. Sleep-wake differences in relative regional cerebral metabolic rate for glucose among patients with insomnia compared with good sleepers. Sleep 2016, 39, 1779–1794. [Google Scholar] [CrossRef] [PubMed]
- AFNI Program: 3dClustSim. Available online: https://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html (accessed on 22 February 2017).
- Altena, E.; van der Werf, Y.D.; Sanz-Arigita, E.J.; Voorn, T.A.; Rombouts, S.A.; Kuijer, J.P.; van Someren, E.J. Prefrontal hypoactivation and recovery in insomnia. Sleep 2008, 31, 1271–1276. [Google Scholar] [PubMed]
- Drummond, S.P.; Walker, M.; Almklov, E.; Campos, M.; Anderson, D.E.; Straus, L.D. Neural correlates of working memory performance in primary insomnia. Sleep 2013, 36, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Wang, E.; Zhang, H.; Dou, S.; Tong, L.; Cheng, J.; Chen, C.; Shi, D. Abnormal neural network of primary insomnia: Evidence from spatial working memory task fMRI. Eur. Neurol. 2016, 75, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, C.; Spiegelhalder, K.; Regen, W.; Feige, B.; Nissen, C.; Lombardo, C.; Violani, C.; Hennig, J.; Riemann, D. Insomnia disorder is associated with increased amygdala reactivity to insomnia-related stimuli. Sleep 2014, 37, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Jiang, T.; Lu, Y.; He, Y.; Tian, L. Regional homogeneity approach to fMRI data analysis. Neuroimage 2004, 22, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.F.; He, Y.; Zhu, C.Z.; Cao, Q.J.; Sui, M.Q.; Liang, M.; Tian, L.X.; Jiang, T.Z.; Wang, Y.F. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007, 29, 83–91. [Google Scholar] [PubMed]
- Liu, H.; Liu, Z.; Liang, M.; Hao, Y.; Tan, L.; Kuang, F.; Yi, Y.; Xu, L.; Jiang, T. Decreased regional homogeneity in schizophrenia: A resting state functional magnetic resonance imaging study. Neuroreport 2006, 17, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.J.; Peng, D.C.; Gong, H.H.; Wan, A.L.; Nie, X.; Li, H.J.; Wang, Y.X. Altered intrinsic regional brain spontaneous activity and subjective sleep quality in patients with chronic primary insomnia: A resting-state fMRI study. Neuropsychiatr. Dis. Treat. 2014, 10, 2163–2175. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, S.; Jiang, G.; Lin, C.; Li, M.; Ma, X.; Zhan, W.; Fang, J.; Li, L.; Li, C.; et al. Regional homogeneity changes in patients with primary insomnia. Eur. Radiol. 2016, 26, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.J.; Nie, X.; Liu, X.; Pei, L.; Jiang, J.; Peng, D.C.; Gong, H.H.; Zeng, X.J.; Wang, Y.X.; Zhan, Y. Gender differences in regional brain activity in patients with chronic primary insomnia: Evidence from a resting-state fMRI Study. J. Clin. Sleep Med. 2016, 12, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Liu, C.Z.; Zhang, J.; Yuan, Z.; Tang, L.R.; Tie, C.L.; Fan, J.; Liu, Q.Q. Reduced spontaneous neuronal activity in the insular cortex and thalamus in healthy adults with insomnia symptoms. Brain Res. 2016, 1648, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, E.; Laufs, H. Decoding wakefulness levels from typical fMRI resting-state data reveals reliable drifts between wakefulness and sleep. Neuron 2014, 82, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L.; Krienen, F.M.; Yeo, B.T. Opportunities and limitations of intrinsic functional connectivity MRI. Nat. Neurosci. 2013, 16, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Damoiseaux, J.S.; Rombouts, S.A.; Barkhof, F.; Scheltens, P.; Stam, C.J.; Smith, S.M.; Beckmann, C.F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. USA 2006, 103, 13848–13853. [Google Scholar] [CrossRef]
- Downar, J.; Crawley, A.P.; Mikulis, D.J.; Davis, K.D. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. J. Neurophysiol. 2002, 87, 615–620. [Google Scholar] [PubMed]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Chang, C.; Glover, G.H.; Gotlib, I.H. Increased insula coactivation with salience networks in insomnia. Biol. Psychol. 2014, 97, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liang, P.; Jia, X.; Zhan, S.; Li, N.; Ding, Y.; Lu, J.; Wang, Y.; Li, K. Abnormal amygdala connectivity in patients with primary insomnia: Evidence from resting state fmri. Eur. J. Radiol. 2012, 81, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Huang, S.; Gao, L.; Zhuang, Y.; Ding, S.; Gong, H. Temporal regularity of intrinsic cerebral activity in patients with chronic primary insomnia: A brain entropy study using resting-state fMRI. Brain Behav. 2016, 6, e00529. [Google Scholar] [CrossRef] [PubMed]
- Killgore, W.D.; Schwab, Z.J.; Kipman, M.; Deldonno, S.R.; Weber, M. Insomnia-related complaints correlate with functional connectivity between sensory-motor regions. Neuroreport 2013, 24, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Andrews-Hanna, J.R.; Reidler, J.S.; Sepulcre, J.; Poulin, R.; Buckner, R.L. Functional-anatomic fractionation of the brain's default network. Neuron 2010, 65, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Shao, Y.; Liu, S.Y.; Li, H.J.; Wan, A.L.; Nie, S.; Peng, D.C.; Dai, X.J. Functional connectivity of paired default mode network subregions in primary insomnia. Neuropsychiatr. Dis. Treat. 2015, 11, 3085–3093. [Google Scholar] [PubMed]
- Li, Y.; Wang, E.; Zhang, H.; Dou, S.; Liu, L.; Tong, L.; Lei, Y.; Wang, M.; Xu, J.; Shi, D.; et al. Functional connectivity changes between parietal and prefrontal cortices in primary insomnia patients: Evidence from resting-state fMRI. Eur. J. Med. Res. 2014, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Perrier, J.; Chavoix, C.; Bocca, M.L. Functioning of the three attentional networks and vigilance in primary insomnia. Sleep Med. 2015, 16, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Dzierzewski, J.M.; O’Brien, E.M.; Kay, D.; McCrae, C.S. Tackling sleeplessness: Psychological treatment options for insomnia in older adults. Nat. Sci. Sleep 2010, 2, 47–61. [Google Scholar] [PubMed]
- McGinty, D.; Szymusiak, R. Sleep mechanisms and phylogeny. In Principles and Practices of Sleep Medicine, 5th ed.; Siegel, J.M., Ed.; Elsevier, Saunders: St. Louis, MO, USA, 2011; pp. 76–91. [Google Scholar]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; van Essen, D.C.; Raichle, M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Germain, A.; Hall, M.; Monk, T.H.; Nofzinger, E.A. A Neurobiological model of insomnia. Drug Discov. Today Dis. Models 2011, 8, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Borbely, A.A.; Daan, S.; Wirz-Justice, A.; Deboer, T. The two-process model of sleep regulation: A reappraisal. J. Sleep Res. 2016, 25, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Landolt, H.P.; Retey, J.V.; Tonz, K.; Gottselig, J.M.; Khatami, R.; Buckelmuller, I.; Achermann, P. Caffeine attenuates waking and sleep electroencephalographic markers of sleep homeostasis in humans. Neuropsychopharmacology 2004, 29, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.R.; Balkin, T.J.; Wesenten, N.J.; Carson, R.E.; Varga, M.; Baldwin, P.; Selbie, S.; Belenky, G.; Herscovitch, P. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain 1997, 120 Pt 7, 1173–1197. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.H.; Moore, S.E. The threshold of sleep: Perception of sleep as a function of time asleep and auditory threshold. Sleep 1982, 5, 267–276. [Google Scholar] [PubMed]
- Webb, W.B. Sleep, the Gentle Tyrant; Prentice-Hall: Englewood Cliffs, NJ, USA, 1975. [Google Scholar]
- Siclari, F.; Bernardi, G.; Riedner, B.A.; LaRocque, J.J.; Benca, R.M.; Tononi, G. Two distinct synchronization processes in the transition to sleep: A high-density electroencephalographic study. Sleep 2014, 37, 1621–1637. [Google Scholar] [CrossRef] [PubMed]
- Bush, G.; Luu, P.; Posner, M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000, 4, 215–222. [Google Scholar] [CrossRef]
- Cavanna, A.E.; Trimble, M.R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 2006, 129 Pt 3, 564–583. [Google Scholar] [CrossRef] [PubMed]
- Del Cul, A.; Dehaene, S.; Reyes, P.; Bravo, E.; Slachevsky, A. Causal role of prefrontal cortex in the threshold for access to consciousness. Brain 2009, 132 Pt 9, 2531–2540. [Google Scholar] [CrossRef] [PubMed]
- Koubeissi, M.Z.; Bartolomei, F.; Beltagy, A.; Picard, F. Electrical stimulation of a small brain area reversibly disrupts consciousness. Epilepsy Behav. 2014, 37, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.D.; Reiman, E.M.; Axelrod, B.; Yun, L.S.; Holmes, A.; Schwartz, G.E. Neural correlates of levels of emotional awareness. Evidence of an interaction between emotion and attention in the anterior cingulate cortex. J. Cogn. Neurosci. 1998, 10, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Boveroux, P.; Vanhaudenhuyse, A.; Bruno, M.A.; Noirhomme, Q.; Lauwick, S.; Luxen, A.; Degueldre, C.; Plenevaux, A.; Schnakers, C.; Boly, M.; et al. Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology 2010, 113, 1038–1053. [Google Scholar] [CrossRef]
- Vanhaudenhuyse, A.; Noirhomme, Q.; Tshibanda, L.J.; Bruno, M.A.; Boveroux, P.; Schnakers, C.; Soddu, A.; Perlbarg, V.; Ledoux, D.; Brichant, J.F.; et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain 2010, 133 Pt 1, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Massimini, M.; Ferrarelli, F.; Huber, R.; Esser, S.K.; Singh, H.; Tononi, G. Breakdown of cortical effective connectivity during sleep. Science 2005, 309, 2228–2232. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, X.; Gu, H.; Yang, Y. Resting-state glutamate and GABA concentrations predict task-induced deactivation in the default mode network. J. Neurosci. 2013, 33, 18566–18573. [Google Scholar] [CrossRef]
- Andersson, J.L.; Onoe, H.; Hetta, J.; Lidstrom, K.; Valind, S.; Lilja, A.; Sundin, A.; Fasth, K.J.; Westerberg, G.; Langstrom, B.; et al. Brain networks affected by synchronized sleep visualized by positron emission tomography. J. Cereb. Blood Flow Metab. 1998, 18, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Nofzinger, E.A.; Buysse, D.J.; Miewald, J.M.; Meltzer, C.C.; Price, J.C.; Sembrat, R.C.; Ombao, H.; Reynolds, C.F.; Monk, T.H.; Hall, M.; et al. Human regional cerebral glucose metabolism during non-rapid eye movement sleep in relation to waking. Brain 2002, 125 Pt 5, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Haimov, I.; Shatil, E. Cognitive training improves sleep quality and cognitive function among older adults with insomnia. PLoS ONE 2013, 8, e61390. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.C.; Manber, R.; Segal, Z.; Xia, Y.; Shapiro, S.; Wyatt, J.K. A randomized controlled trial of mindfulness meditation for chronic insomnia. Sleep 2014, 37, 1553–1563. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.G.; Zhang, T.; Yue, F.G.; Yi, M.L.; Gao, D. Efficacy of repetitive transcranial magnetic stimulation in the treatment of patients with chronic primary insomnia. Cell Biochem. Biophys. 2013, 67, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Perrier, J.; Clochon, P.; Bertran, F.; Couque, C.; Bulla, J.; Denise, P.; Bocca, M.L. Specific EEG sleep pattern in the prefrontal cortex in primary insomnia. PLoS ONE 2015, 10, e0116864. [Google Scholar] [CrossRef] [PubMed]
- Feige, B.; Al-Shajlawi, A.; Nissen, C.; Voderholzer, U.; Hornyak, M.; Spiegelhalder, K.; Kloepfer, C.; Perlis, M.; Riemann, D. Does REM sleep contribute to subjective wake time in primary insomnia? A comparison of polysomnographic and subjective sleep in 100 patients. J. Sleep Res. 2008, 17, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Kansagara, D.; Forciea, M.A.; Cooke, M.; Denberg, T.D.; Barry, M.J.; Boyd, C.; Chow, R.D.; Fitterman, N.; Harris, R.P.; et al. Management of chronic insomnia disorder in adults: A clinical practice guideline from the American college of physicians. Ann. Intern. Med. 2016, 165, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Schutte-Rodin, S.; Broch, L.; Buysse, D.; Dorsey, C.; Sateia, M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J. Clin. Sleep Med. 2008, 4, 487–504. [Google Scholar] [PubMed]
- Smith, M.T.; Perlis, M.L.; Chengazi, V.U.; Soeffing, J.; McCann, U. NREM sleep cerebral blood flow before and after behavior therapy for chronic primary insomnia: Preliminary single photon emission computed tomography (SPECT) data. Sleep Med. 2005, 6, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.B.; Kyle, S.D.; Gordon, C.J.; Espie, C.A.; Grunstein, R.R.; Mullins, A.E.; Postnova, S.; Bartlett, D.J. Physiological markers of arousal change with psychological treatment for insomnia: A preliminary investigation. PLoS ONE 2015, 10, e0145317. [Google Scholar] [CrossRef] [PubMed]
- Jarrin, D.C.; Chen, I.Y.; Ivers, H.; Lamy, M.; Vallieres, A.; Morin, C.M. Nocturnal heart rate variability in patients treated with cognitive-behavioral therapy for insomnia. Health Psychol. 2016, 35, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Kay, D.B.; Buysse, D.J.; Germain, A.; Hall, M.; Monk, T.H. Subjective-objective sleep discrepancy among older adults: Associations with insomnia diagnosis and insomnia treatment. J. Sleep Res. 2015, 24, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Khalsa, S.; Mayhew, S.D.; Przezdzik, I.; Wilson, R.; Hale, J.; Goldstone, A.; Bagary, M.; Bagshaw, A.P. Variability in cumulative habitual sleep duration predicts waking functional connectivity. Sleep 2016, 39, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Economo, C.V. Sleep as a problem of localization. J. Nerv. Ment. Dis. 1930, 71, 249–259. [Google Scholar] [CrossRef]
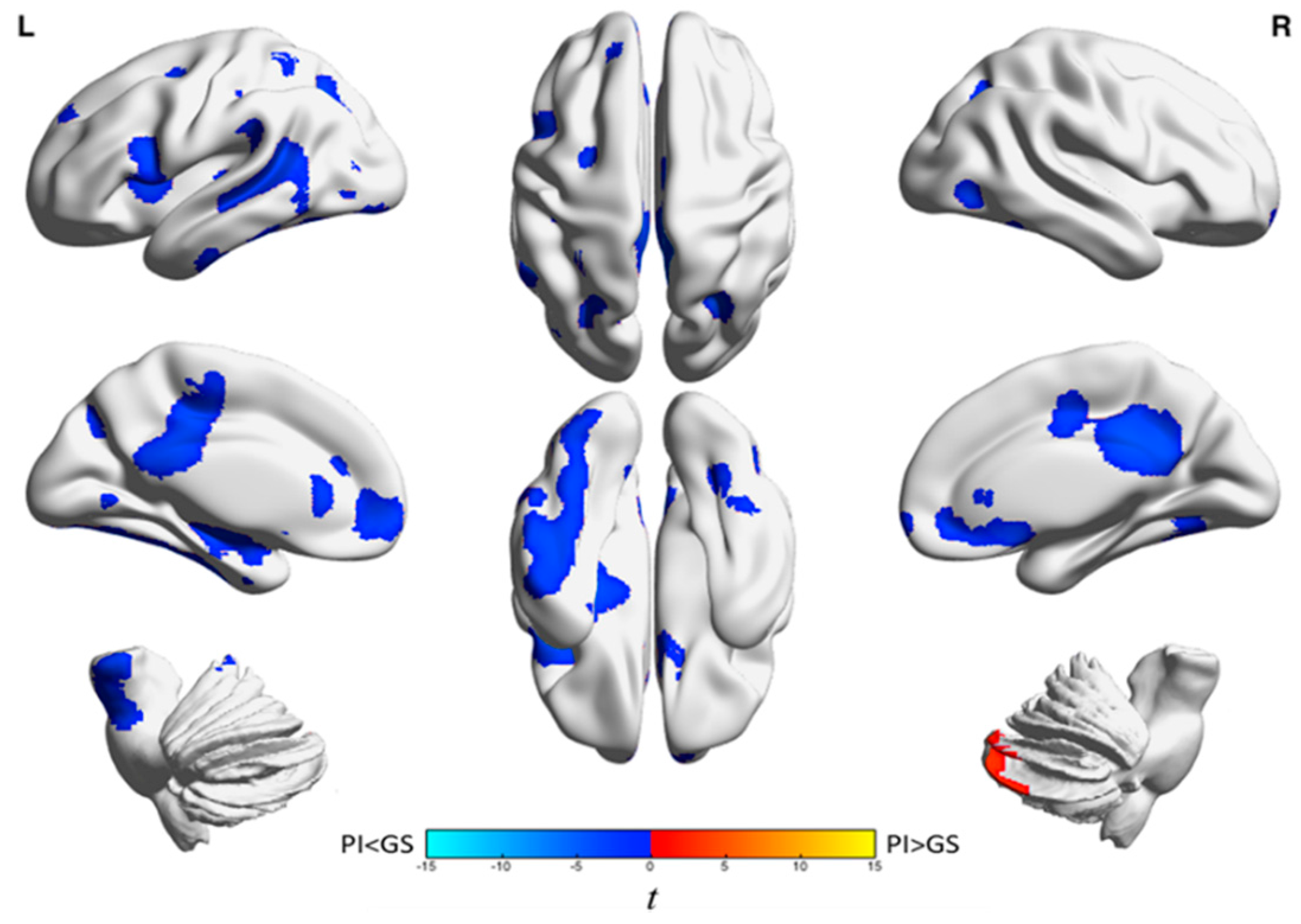
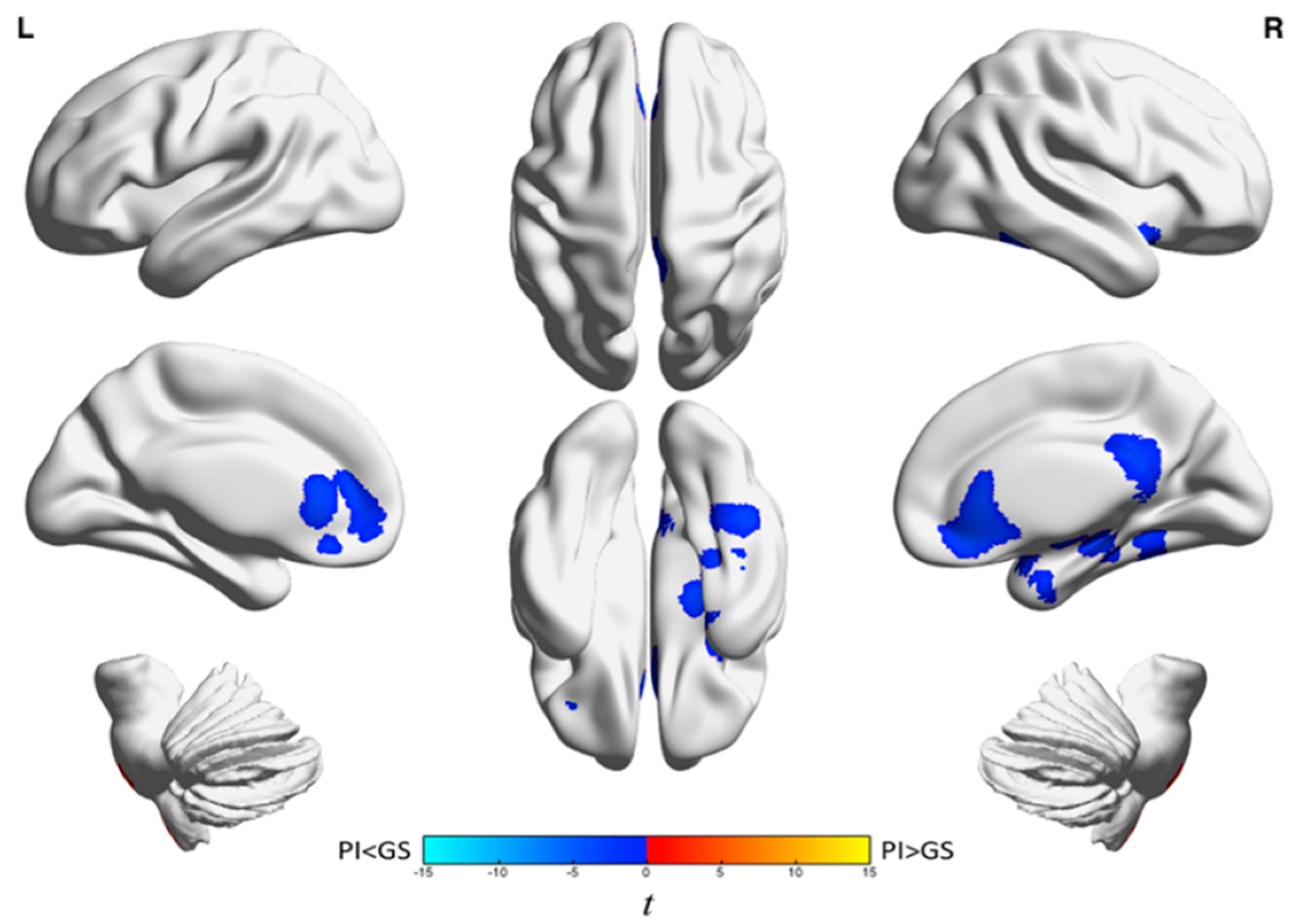

| Analysis | Brain Region | k A | t-Statistic (Max) B | x | y | z |
|---|---|---|---|---|---|---|
| Wake | Left frontal cortex and anterior cingulate gyrus | 1439 | −4.0 | −18 | 38 | −4 |
| Left inferior frontal gyrus and left insula | 947 | −44 | −44 | 14 | 14 | |
| Right medial frontal gyrus, anterior cingulate, frontal-orbital gyrus, superior frontal gyrus, and caudate | 1128 | −4.7 | 14 | 28 | −10 | |
| Temporal lobe, parietal lobe, precuneus, middle and posterior cingulate gyri, frontal lobe, occipital lobe, left hippocampus, putamen, insula, left brainstem, and left amygdala | 11,925 | −5.4 | 26 | −60 | −36 | |
| Right cerebellum | 729 | 3.8 | 16 | −88 | −32 | |
| NREM | Anterior cingulate, medial frontal gyrus, orbitofrontal cortex, inferior frontal gyrus, and right caudate | 2335 | −4.6 | 14 | 30 | −10 |
| Right posterior cingulate, bilateral precuneus, and middle cingulum | 1100 | −5.3 | 12 | −40 | 20 | |
| Right fusiform gyrus, parahippocampus, superior and inferior temporal gyri, hippocampus, and amygdala | 2076 | −5.1 | 38 | 2 | −24 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kay, D.B.; Buysse, D.J. Hyperarousal and Beyond: New Insights to the Pathophysiology of Insomnia Disorder through Functional Neuroimaging Studies. Brain Sci. 2017, 7, 23. https://doi.org/10.3390/brainsci7030023
Kay DB, Buysse DJ. Hyperarousal and Beyond: New Insights to the Pathophysiology of Insomnia Disorder through Functional Neuroimaging Studies. Brain Sciences. 2017; 7(3):23. https://doi.org/10.3390/brainsci7030023
Chicago/Turabian StyleKay, Daniel B., and Daniel J. Buysse. 2017. "Hyperarousal and Beyond: New Insights to the Pathophysiology of Insomnia Disorder through Functional Neuroimaging Studies" Brain Sciences 7, no. 3: 23. https://doi.org/10.3390/brainsci7030023





