Effects of Three Lipidated Oxytocin Analogs on Behavioral Deficits in CD38 Knockout Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of LOTs
2.2. Animals
2.3. Parental Retrieval Test
2.4. Rapid Test of Social Acquisition and Recognition
2.5. Tail Suspension Test
2.6. Elevated plus Maze
2.7. Sucrose Preference
2.8. Pup Locomotion Test
2.9. Statistical Analysis
3. Results
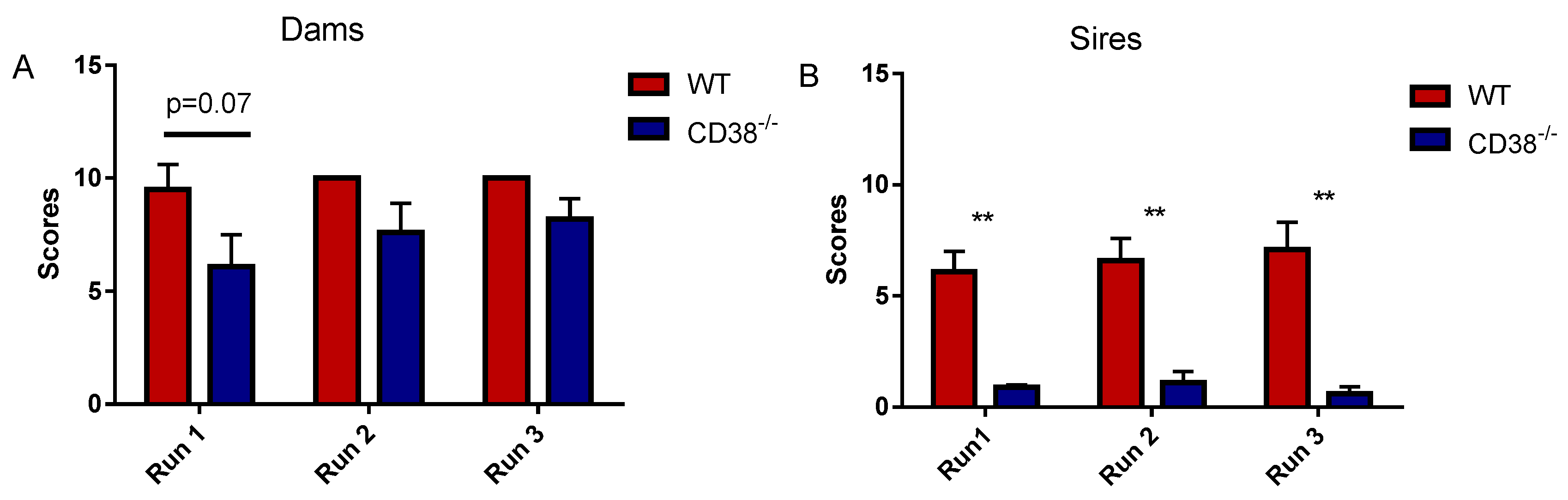
3.1. Parental Retrieval Test
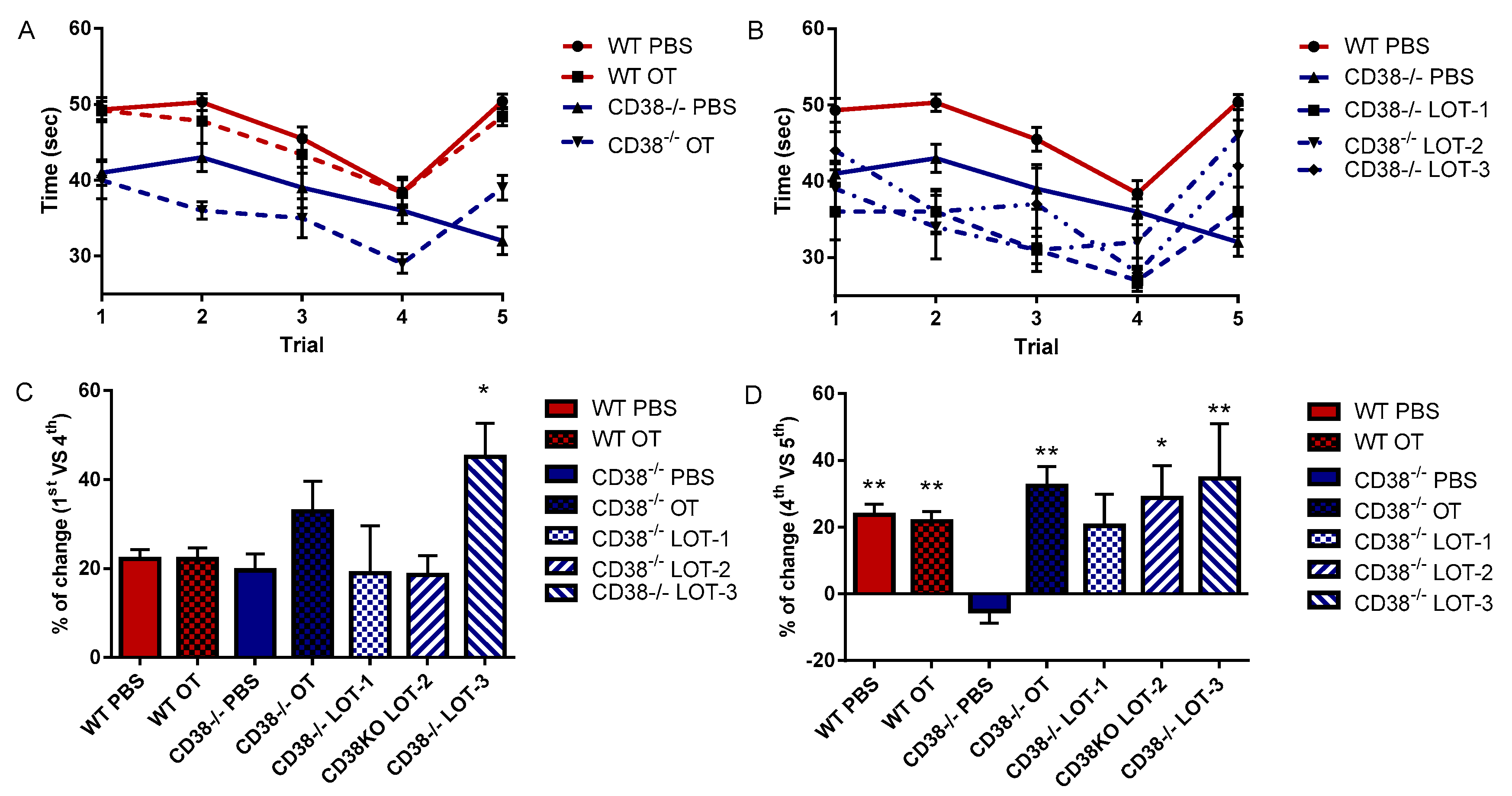
3.2. Rapid Tests of Social Acquisition and Recognition
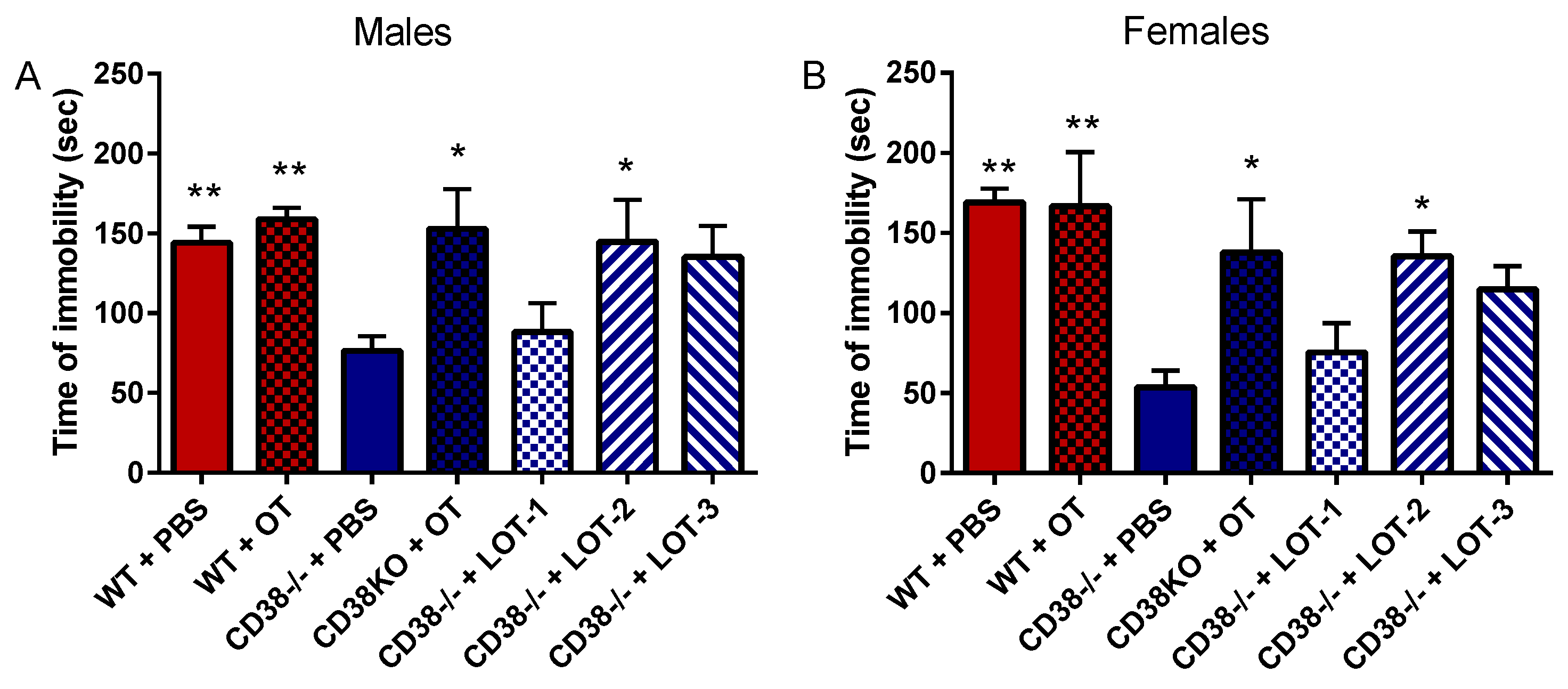
3.3. Tail Suspension Test
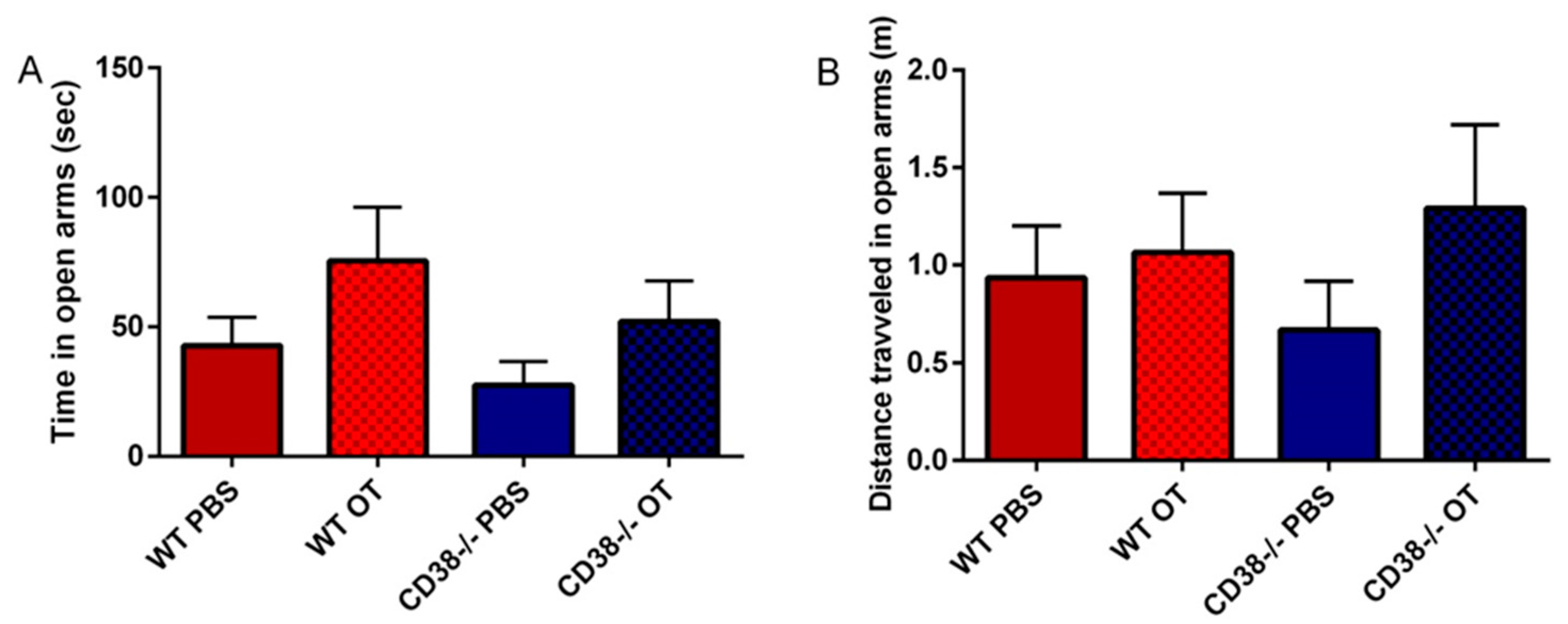
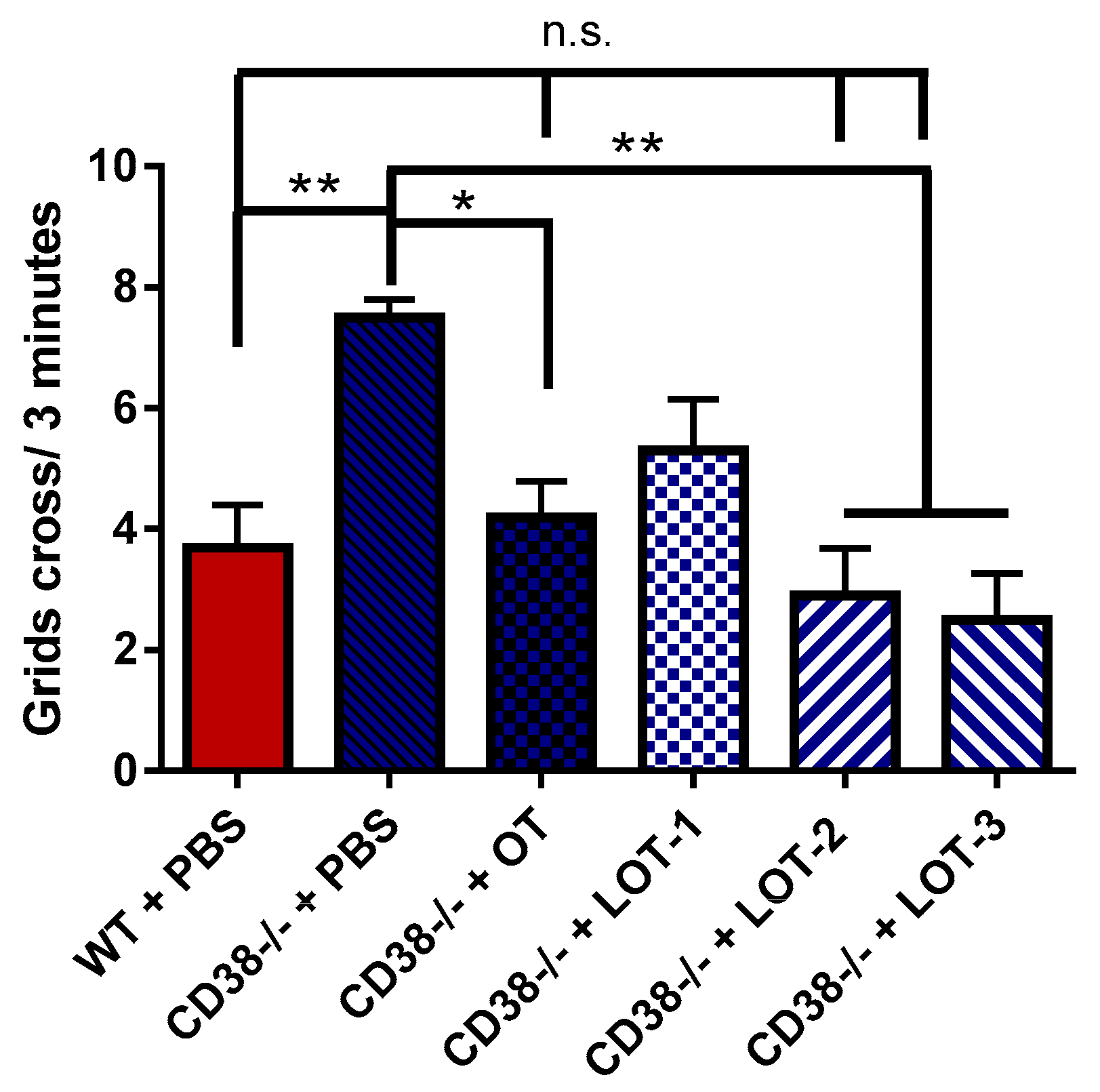
3.4. Elevated plus Maze
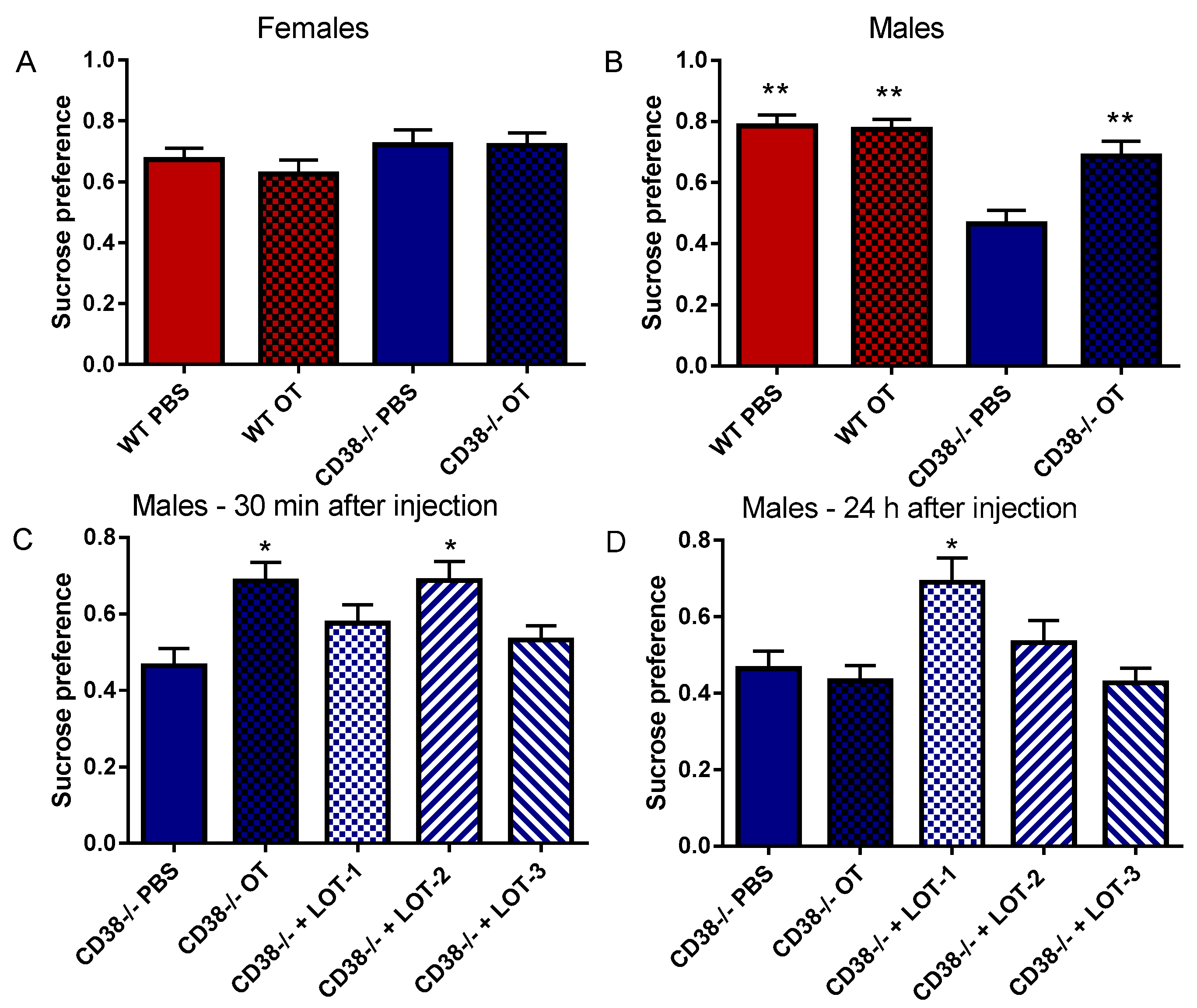
3.5. Sucrose Preference
3.6. Pup Locomotion Test
4. Discussion
5. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lai, M.C.; Lombardo, M.V.; Baron-Cohen, S. Autism. Lancet 2014, 383, 896–910. [Google Scholar] [CrossRef]
- Peñagarikano, O. New Therapeutic Options for Autism Spectrum Disorder: Experimental Evidences. Exp. Neurobiol. 2015, 24, 301–311. [Google Scholar] [PubMed]
- Bishop-Fitzpatrick, L.; Minshew, N.J.; Eack, S.M. A systematic review of psychosocial interventions for adults with autism spectrum disorders. J. Autism Dev. Disord. 2013, 43, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Berggren, S.; Fletcher-Watson, S.; Milenkovic, N.; Marschik, P.B.; Bölte, S.; Jonsson, U. Emotion recognition training in autism spectrum disorder: A systematic review of challenges related to generalizability. Dev. Neurorehabil. 2017, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Baribeau, D.A.; Anagnostou, E. An update on medication management of behavioral disorders in autism. Curr. Psychiatry Rep. 2014, 16, 437. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, P. Oxytocin in the socioemotional brain: Implications for psychiatric disorders. Dialogues Clin. Neurosci. 2015, 17, 463–476. [Google Scholar] [PubMed]
- Hofmann, S.G.; Fang, A.; Brager, D.N. Effect of intranasal oxytocin administration on psychiatric symptoms: A metaanalysis of placebo-controlled studies. Psychiatry Res. 2015, 228, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Liu, H.X.; Hirai, H.; Torashima, T.; Nagai, T.; Lopatina, O.; Shnayder, N.A.; Yamada, K.; Noda, M.; Seike, T.; et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 2007, 446, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, M.; Leng, G. Dendritic peptide release and peptide-dependent behaviours. Nat. Rev. Neurosci. 2006, 7, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Higashida, H. Somato-axodendritic release of oxytocin into the brain due to calcium amplification is essential for social memory. J. Physiol. Sci. 2016, 66, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Kavaliers, M.; Matta, R.; Choleris, E. Mate-choice copying, social information processing, and the roles of oxytocin. Neurosci. Biobehav. Rev. 2017, 72, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Marlin, B.J.; Froemke, R.C. Oxytocin modulation of neural circuits for social behavior. Dev. Neurobiol. 2017, 77, 169–189. [Google Scholar] [CrossRef] [PubMed]
- Kosfeld, M.; Heinrichs, M.; Zak, P.J.; Fischbacher, U.; Fehr, E. Oxytocin increases trust in humans. Nature 2005, 435, 673–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arakawa, H.; Blanchard, D.C.; Blanchard, R.J. Central oxytocin regulates social familiarity and scent marking behavior that involves amicable odor signals between male mice. Physiol. Behav. 2015, 146, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Ebstein, R.P.; Israel, S.; Lerer, E. Arginine vasopressin andoxytocin modulate human social behavior. Ann. N. Y. Acad. Sci. 2009, 167, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Lukas, M.; Toth, I.; Reber, S.O.; Slattery, D.A.; Veenema, A.H.; Neumann, I.D. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology 2011, 36, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Hirosawa, T.; Kikuchi, M.; Okumura, E.; Yoshimura, Y.; Hiraishi, H.; Munesue, T.; Takesaki, N.; Furutani, N.; Ono, Y.; Higashida, H.; et al. Attentional control and interpretation of facial expression after oxytocin administration to typically developed male adults. PLoS ONE 2015, 10, e0116918. [Google Scholar] [CrossRef] [PubMed]
- Elmadih, A.; Wan, M.W.; Numan, M.; Elliott, R.; Downey, D.; Abel, K.M. Does oxytocin modulate variation in maternal caregiving in healthy new mothers? Brain Res. 2014, 1580, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Neumann, I.D. Brain oxytocin: A key regulator of emotional and social behaviours in both females and males. J. Neuroendocrinol. 2008, 20, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Bartz, J.A.; Zaki, J.; Bolger, N.; Ochsner, K.N. Social effects of oxytocin in humans: Context and person matter. Trends Cogn. Sci. 2011, 15, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Lischke, A.; Berger, C.; Prehn, K.; Heinrichs, M.; Herpertz, S.C.; Domes, G. Intranasal oxytocin enhances emotion recognition from dynamic facial expressions and leaves eye-gaze unaffected. Psychoneuroendocrinology 2012, 37, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Fonagy, P.; Koos, O.; Dorsett, K.; Strathearn, L. Maternal oxytocin response predicts mother-to-infant gaze. Brain Res. 2014, 11, 1580. [Google Scholar] [CrossRef] [PubMed]
- Bernaerts, S.; Berra, E.; Wenderoth, N.; Alaerts, K. Influence of oxytocin on emotion recognition from body language: A randomized placebo-controlled trial. Psychoneuroendocrinology 2016, 72, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Scheele, D.; Schwering, C.; Elison, J.T.; Spunt, R.; Maier, W.; Hurlemann, R. A human tendency to anthropomorphize is enhanced by oxytocin. Eur. Neuropsychopharmacol. 2015, 25, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Bakermans-Kranenburg, M.J.; van I. Jzendoorn, M.H. Sniffing around oxytocin: Review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Transl. Psychiatry 2013, 3, e258. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Abe, O.; Kuwabara, H.; Yahata, N.; Takano, Y.; Iwashiro, N.; Natsubori, T.; Aoki, Y.; Takao, H.; Kawakubo, Y.; et al. Mitigation of sociocommunicational deficits of autism through oxytocin-induced recovery of medial prefrontal activity: A randomized trial. JAMA Psychiatry 2014, 71, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Guastella, A.J.; Gray, K.M.; Rinehart, N.J.; Alvares, G.A.; Tonge, B.J.; Hickie, I.B.; Keating, C.M.; Cacciotti-Saija, C.; Einfeld, S.L. The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: A randomized controlled trial. J. Child Psychol. Psychiatry 2015, 56, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, H.; Okamoto, Y.; Munesue, T.; Yamasue, H.; Inohara, K.; Fujioka, T.; Anme, T.; Orisaka, M.; Ishitobi, M.; Jung, M.; et al. Oxytocin efficacy is modulated by dosage and oxytocin receptor genotype in young adults with high-functioning autism: A 24-week randomized clinical trial. Transl. Psychiatry 2016, 6, e872. [Google Scholar] [CrossRef] [PubMed]
- Ooi, Y.P.; Weng, S.J.; Kossowsky, J.; Gerger, H.; Sung, M. Oxytocin and Autism Spectrum Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmacopsychiatry 2017, 50, 5–13. [Google Scholar] [CrossRef] [PubMed]
- De Groot, A.N.; Vree, T.B.; Hekster, Y.A.; Pesman, G.J.; Sweep, F.C.; van Dongen, P.J.; van Roosmalen, J. Bioavailability and pharmacokinetics of sublingual oxytocin in male volunteers. J. Pharm. Pharmacol. 1995, 47, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Schramme, A.R.; Pinto, C.R.; Davis, J.; Whisnant, C.S.; Whitacre, M.D. Pharmacokinetics of carbetocin, a long-acting oxytocin analogue, following intravenous administration in horses. Equine Vet. J. 2008, 40, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Varamini, P.; Toth, I. Lipid- and sugar-modified endomorphins 4. Novel targets for the treatment of neuropathic pain. Front. Pharmacol. 2013, 4, 155. [Google Scholar] [CrossRef] [PubMed]
- Mikulášková, B.; Zemenová, J.; Pirník, Z.; Pražienková, V.; Bednárová, L.; Železná, B.; Maletínská, L.; Kuneš, J. Effect of palmitoylated prolactin-releasing peptide on food intake and neural activation after different routes of peripheral administration in rats. Peptides 2016, 75, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Egleton, R.D.; Davis, R.D. Development of neuropeptide drugs that cross the blood-brain barrier. NeuroRx 2005, 2, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, A.; Cherepanov, S.M.; Kikuchi, Y.; Fakhrul, A.A.; Akther, S.; Deguchi, K.; Yoshihara, T.; Ishihara, K.; Shuto, S.; Higashida, H. Lipo-oxytocin-1, a novel oxytocin analog conjugated with two palmitoyl groups, has long-lasting effects on anxiety-related behavior and social avoidance in CD157 knockout mice. Brain Sci. 2015, 5, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Cherepanov, S.M.; Yokoyama, S.; Mizuno, A.; Ichinose, W.; Lopatina, O.; Shabalova, A.; Salmina, A.B.; Yamamoto, Y.; Okamoto, H.; Shuto, S.; et al. Structure-specific effects of lipidated oxytocin analogs on intracellular calcium levels, parental behavior, and oxytocin concentrations in the plasma and cerebrospinal fluid in mice. Pharmacol. Res. Perspect. 2017, 5, e00290. [Google Scholar] [CrossRef] [PubMed]
- Lopatina, O.; Yoshihara, T.; Nishimura, T.; Zhong, J.; Akther, S.; Fakhrul, A.A.K.M.; Liang, M.; Higashida, C.; Sumi, K.; Furuhara, K.; et al. Anxiety- and depression-like behavior in mice lacking the CD157/BST1 gene, a risk factor for Parkinson’s disease. Front. Behav. Neurosci. 2014, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Lopatina, O.; Inzhutova, A.; Pichugina, Y.A.; Okamoto, H.; Salmina, A.B.; Higashida, H. Reproductive experience affects parental retrieval behaviour associated with increased plasma oxytocin levels in wild-type and CD38-knockout mice. J. Neuroendocrinol. 2011, 23, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Higashida, H.; Yokoyama, S.; Huang, J.J.; Liu, L.; Ma, W.J.; Akther, S.; Higashida, C.; Kikuchi, M.; Minabe, Y.; Munesue, T. Social memory, amnesia, and autism: brain oxytocin secretion is regulated by NAD+ metabolites and single nucleotide polymorphisms of CD38. Neurochem. Int. 2012, 61, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Higashida, H.; Yokoyama, S.; Kikuchi, M.; Munesue, T. CD38 and its role in oxytocin secretion and social behavior. Horm. Behav. 2012, 61, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.X.; Lopatina, O.; Higashida, C.; Tsuji, T.; Kato, I.; Takasawa, S.; Okamoto, H.; Yokoyama, S.; Higashida, H. Locomotor activity, ultrasonic vocalization and oxytocin levels in infant CD38 knockout mice. Neurosci. Lett. 2008, 448, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Higashida, H.; Yokoyama, S.; Munesue, T.; Kikuchi, M.; Minabe, Y.; Lopatina, O. CD38 gene knockout juvenile mice: A model of oxytocin signal defects in autism. Biol. Pharm. Bull. 2011, 34, 1369–1372. [Google Scholar] [CrossRef] [PubMed]
- Kato, I.; Yamamoto, Y.; Fujimura, M.; Noguchi, N.; Takasawa, S.; Okamoto, H. CD38 disruption impairs glucose-induced increases in cyclic ADP-ribose, [Ca2+]i, and insulin secretion. J. Biol. Chem. 1999, 274, 1869–1872. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.X.; Lopatina, O.; Higashida, C.; Fujimoto, H.; Akther, S.; Inzhutova, A.; Liang, M.; Zhong, J.; Tsuji, T.; Yoshihara, T.; et al. Displays of paternal mouse pup retrieval following communicative interaction with maternal mates. Nat. Commun. 2013, 1346, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Winslow, J.T.; Camacho, E. Cholinergic modulation of a decrement in social investigation following repeated contacts between mice. Psychopharmacology 1995, 121, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The Tail Suspension Test. J. Vis. Exp. 2012. [Google Scholar] [CrossRef] [PubMed]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open: Closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Choi, C.S.; Gonzales, E.L.; Kim, K.C.; Yang, S.M.; Kim, J.; Mabunga, D.F.; Cheong, J.H.; Han, S.; Bahn, G.H.; Shin, C.Y. The transgenerational inheritance of autism-like phenotypes in mice exposed to valproic acid during pregnancy. Sci. Rep. 2016, 6, 36250. [Google Scholar] [CrossRef] [PubMed]
- Akther, S.; Korshnova, N.; Zhong, J.; Liang, M.; Cherepanov, S.M.; Lopatina, O.; Komleva, Y.K.; Salmina, A.B.; Nishimura, T.; Fakhrul, A.A.K.M.; et al. CD38 in the nucleus accumbens and oxytocin are related to paternal behavior in mice. Mol. Brain 2013, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.N.; Young, L.J.; Hearn, E.F.; Matzuk, M.M.; Insel, T.R.; Winslow, J.T. Social amnesia in mice lacking the oxytocin gene. Nat. Genet. 2000, 25, 284–288. [Google Scholar] [PubMed]
- Higashida, H.; Liang, M.; Yoshihara, T.; Akther, S.; Fakhrul, A.; Stanislav, C.; Nam, T.; Kim, U.; Kasai, S.; Nishimura, T.; et al. An immunohistochemical, enzymatic, and behavioral study of CD157/BST-1 as a neuroregulator. BMC Neurosci. 2017, 18, 35. [Google Scholar] [CrossRef] [PubMed]
- Willner, P.; Muscat, R.; Papp, M. Chronic mild stress-induced anhedonia: A realistic animal model of depression. Neurosci. Biobehav. Rev. 1992, 16, 525–534. [Google Scholar] [CrossRef]
- Zhong, J.; Amina, S.; Liang, M.; Akther, S.; Yuhi, T.; Nishimura, T.; Tsuji, C.; Tsuji, T.; Liu, H.X.; Hashii, M.; et al. Cyclic ADP-Ribose and Heat Regulate Oxytocin Release via CD38 and TRPM2 in the Hypothalamus during Social or Psychological Stress in Mice. Front. Neurosci. 2016, 10, 304. [Google Scholar] [CrossRef] [PubMed]
- Lopatina, O.; Liu, H.; Amina, S.; Hashii, M.; Higashida, H. Oxytocin-induced elevation of ADP-ribosyl cyclase activity, cyclic ADP-ribose or Ca2+ concentrations is involved in autoregulation of oxytocin secretion in the hypothalamus and posterior pituitary in male mice. Neuropharmacology 2010, 58, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; McCann, K.E.; McNeill, J.K.; Larkin, T.E.; Huhman, K.L.; Albers, H.E. Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology 2014, 50, 14–19. [Google Scholar] [CrossRef] [PubMed]

| 30 min after Injection | CD38−/− | +OT | +LOT-1 | +LOT-2 | +LOT-3 |
| Parental scores | ↓ | + | 0 | ++ | ++ |
| Social recognition | ↓ | ++ | 0 | + | ++ |
| Depression-like behavior | ↓ | + | 0 | + | 0 |
| Anxiety-like behavior | 0 | 0 | n.t. | n.t. | n.t. |
| Adhedonic | ↓ | + | 0 | + | 0 |
| Pup locomotion | ↑ | + | 0 | ++ | ++ |
| 24 h after Injection | CD38−/− | +OT | +LOT-1 | +LOT-2 | +LOT-3 |
| Parental scores | ↓ | 0 | ++ | 0 | 0 |
| Adhedonic | ↓ | 0 | + | 0 | 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherepanov, S.M.; Akther, S.; Nishimura, T.; Shabalova, A.A.; Mizuno, A.; Ichinose, W.; Shuto, S.; Yamamoto, Y.; Yokoyama, S.; Higashida, H. Effects of Three Lipidated Oxytocin Analogs on Behavioral Deficits in CD38 Knockout Mice. Brain Sci. 2017, 7, 132. https://doi.org/10.3390/brainsci7100132
Cherepanov SM, Akther S, Nishimura T, Shabalova AA, Mizuno A, Ichinose W, Shuto S, Yamamoto Y, Yokoyama S, Higashida H. Effects of Three Lipidated Oxytocin Analogs on Behavioral Deficits in CD38 Knockout Mice. Brain Sciences. 2017; 7(10):132. https://doi.org/10.3390/brainsci7100132
Chicago/Turabian StyleCherepanov, Stanislav M., Shirin Akther, Tomoko Nishimura, Anna A. Shabalova, Akira Mizuno, Wataru Ichinose, Satoshi Shuto, Yasuhiko Yamamoto, Shigeru Yokoyama, and Haruhiro Higashida. 2017. "Effects of Three Lipidated Oxytocin Analogs on Behavioral Deficits in CD38 Knockout Mice" Brain Sciences 7, no. 10: 132. https://doi.org/10.3390/brainsci7100132




