Impact of Prenatal and Subsequent Adult Alcohol Exposure on Pro-Inflammatory Cytokine Expression in Brain Regions Necessary for Simple Recognition Memory
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rats
2.2. Breeding and Treatment
2.3. Adulthood Ethanol Exposure
2.4. Euthanasia, Perfusion, and Tissue Collection
2.5. Quantitative Real-Time PCR (qPCR)
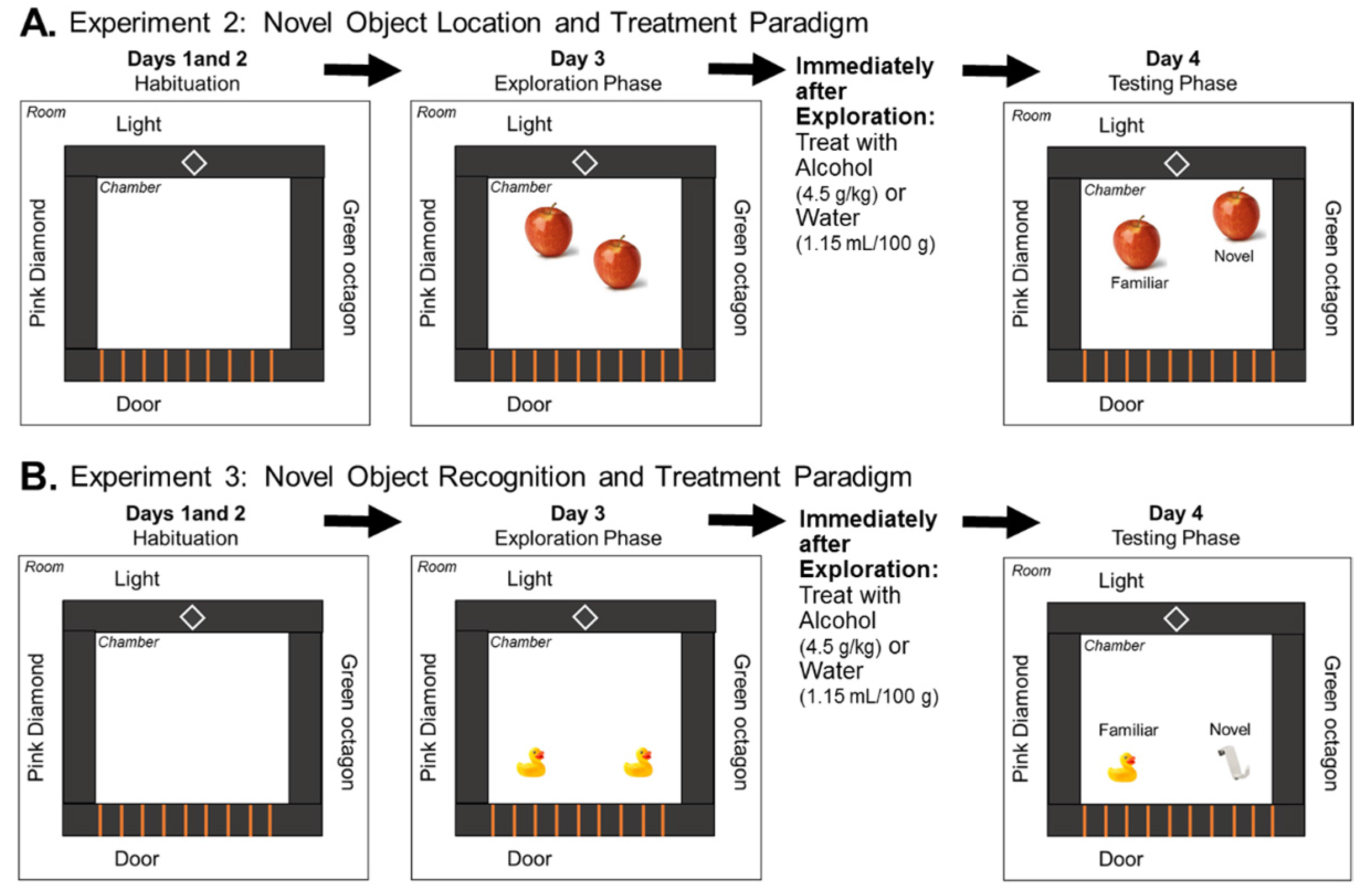
2.6. Novel Object Location Task
Examination of NOL Memory Following Acute Alcohol Exposure during a 24 h Delay
2.7. Novel Object Recognition Task
Examination of NOR Memory Following Acute Alcohol Exposure during a 24 h Delay
2.8. Statistical Analyses
3. Results
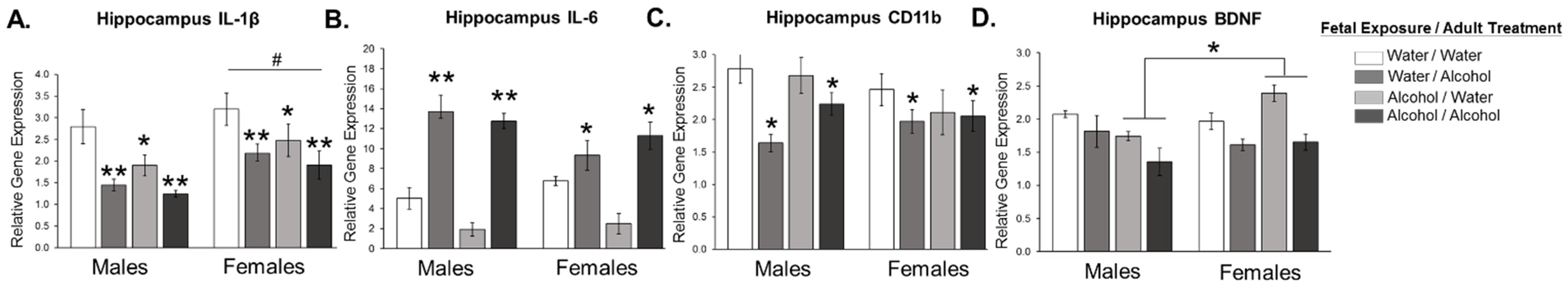
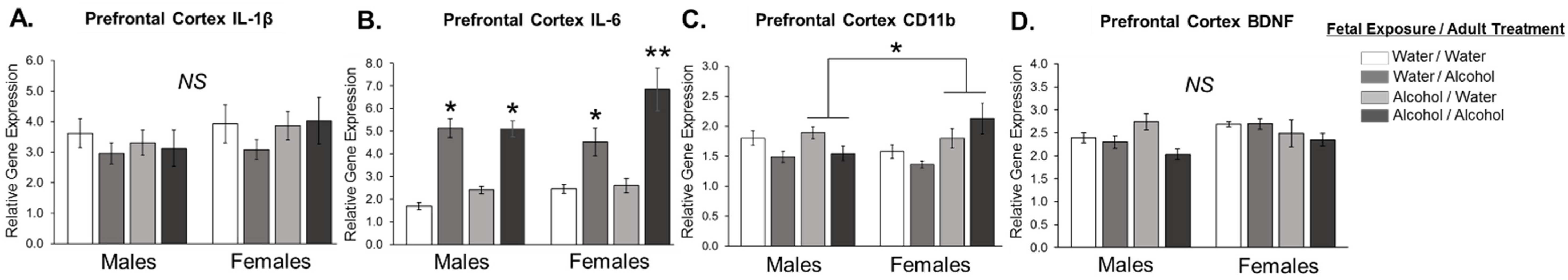
3.1. Experiment 1: Effect of Low Dose Prenatal Alcohol Exposure and Acute Adulthood Alcohol Exposure on Neuroinflammation in Male and Female Adult Offspring across Various Cognitive Brain Regions
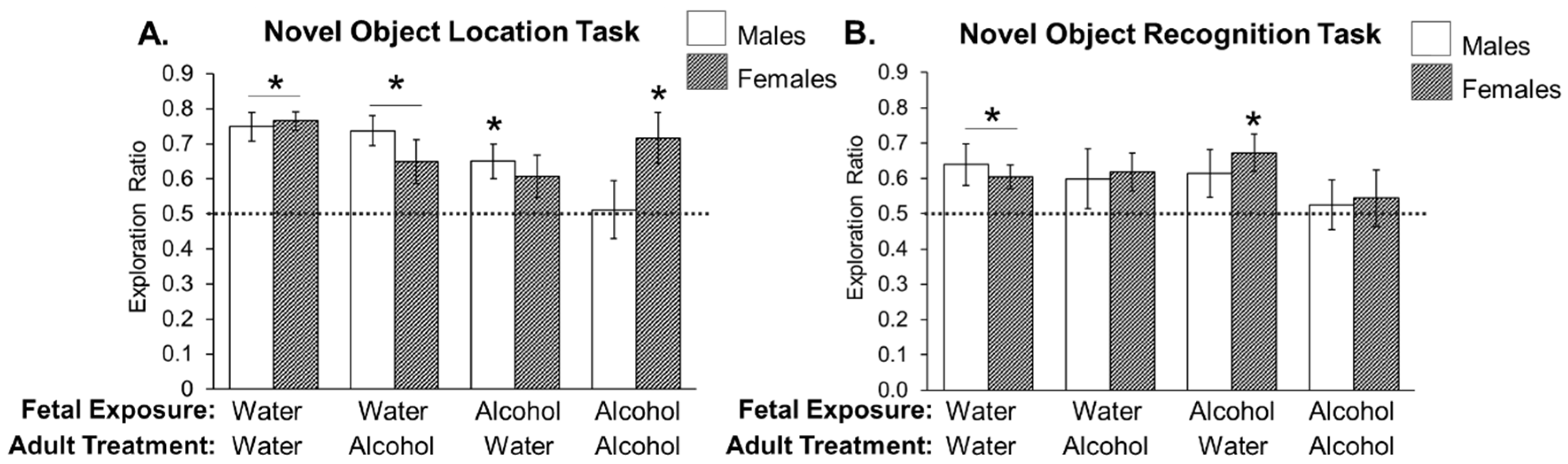
3.2. Experiment 2: Effects of Low Prenatal Alcohol Exposure and Acute Adulthood Alcohol Exposure on a Spatial Memory Task
3.3. Experiment 3: Effects of Low Prenatal Alcohol Exposure and Acute Adulthood Alcohol Exposure on Recognition Memory Task
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bilbo, S.D.; Schwarz, J.M. Early-life programming of later-life brain and behavior: A critical role for the immune system. Front. Behav. Neurosci. 2009, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Bilbo, S.D.; Schwarz, J.M. The immune system and developmental programming of brain and behavior. Front. Neuroendocrinol. 2012, 33, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Monji, A.; Kato, T.A.; Mizoguchi, Y.; Horikawa, H.; Seki, Y.; Kasai, M.; Yamauchi, Y.; Yamada, S.; Kanba, S. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 42, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Estes, M.L.; McAllister, A.K. Maternal immune activation: Implications for neuropsychiatric disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Estes, M.L.; McAllister, A.K. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat. Rev. Neurosci. 2015, 16, 469–486. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K. Can minocycline prevent the onset of Alzheimer’s disease? Ann. Neurol. 2011, 69, 739. [Google Scholar] [CrossRef] [PubMed]
- Fassbender, K.; Walter, S.; Kuhl, S.; Landmann, R.; Ishii, K.; Bertsch, T.; Stalder, A.K.; Muehlhauser, F.; Liu, Y.; Ulmer, A.J.; et al. The LPS receptor (CD14) links innate immunity with Alzheimer’s disease. FASEB J. 2004, 18, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, M. Microglia and astrocytes in Alzheimer’s disease: Implications for therapy. Curr. Neuropharmacol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.T. NF-kB as a mediator of brain inflammation in AD. CNS Neurol. Disord. Drug Targets 2017. [Google Scholar] [CrossRef]
- Crews, F.T.; Zou, J.; Qin, L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav. Immun. 2011, 25 (Suppl. S1), S4–S12. [Google Scholar] [CrossRef] [PubMed]
- Crews, F.T.; Vetreno, R.P. Mechanisms of neuroimmune gene induction in alcoholism. Psychopharmacology 2016, 233, 1543–1557. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, J.; Alfonso-Loeches, S.; Guerri, C. Impact of the Innate Immune Response in the Actions of Ethanol on the Central Nervous System. Alcohol. Clin. Exp. Res. 2016, 40, 2260–2270. [Google Scholar] [CrossRef] [PubMed]
- Chastain, L.G.; Sarkar, D.K. Role of microglia in regulation of ethanol neurotoxic action. Int. Rev. Neurobiol. 2014, 118, 81–103. [Google Scholar] [PubMed]
- Cook, R.T. Alcohol abuse, alcoholism, and damage to the immune system—A review. Alcohol. Clin. Exp. Res. 1998, 22, 1927–1942. [Google Scholar] [PubMed]
- Drew, P.D.; Johnson, J.W.; Douglas, J.C.; Phelan, K.D.; Kane, C.J.M. Pioglitazone Blocks Ethanol Induction of Microglial Activation and Immune Responses in the Hippocampus, Cerebellum, and Cerebral Cortex in a Mouse Model of Fetal Alcohol Spectrum Disorders. Alcohol. Clin. Exp. Res. 2015, 39, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Kane, C.J.; Phelan, K.D.; Han, L.; Smith, R.R.; Xie, J.; Douglas, J.C.; Drew, P.D. Protection of neurons and microglia against ethanol in a mouse model of fetal alcohol spectrum disorders by peroxisome proliferator-activated receptor-gamma agonists. Brain Behav. Immun. 2011, 25 (Suppl. S1), S137–S145. [Google Scholar] [CrossRef] [PubMed]
- Kane, C.J.M.; Phelan, K.D.; Drew, P.D. Neuroimmune mechanisms in fetal alcohol spectrum disorder. Dev. Neurobiol. 2012, 72, 1302–1316. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, L.S.; Schwarz, J.M. Effects of Moderate Prenatal Alcohol Exposure during Early Gestation in Rats on Inflammation across the Maternal-Fetal-Immune Interface and Later-Life Immune Function in the Offspring. J. Neuroimmune Pharmacol. 2016, 11, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Green, P.P.; McKnight-Eily, L.R.; Tan, C.H.; Mejia, R.; Denny, C.H. Vital Signs: Alcohol-Exposed Pregnancies—United States, 2011–2013. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Kesmodel, U.; Eriksen, H.F.; Underbjerg, M.; Kilburn, T.; Støvring, H.; Wimberley, T.; Mortensen, E. The effect of alcohol binge drinking in early pregnancy on general intelligence in children. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Kesmodel, U.; Bertrand, J.; Støvring, H.; Skarpness, B.; Denny, C.; Mortensen, E. The lifestyle during Pregnancy Study Group the effect of different alcohol drinking patterns in early to mid pregnancy on the child’s intelligence, attention, and executive function. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Skogerbø, Å.; Kesmodel, U.; Wimberley, T.; Støvring, H.; Bertrand, J.; Landrø, N.; Mortensen, E. The effects of low to moderate alcohol consumption and binge drinking in early pregnancy on executive function in 5-year-old children. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 1201–1210. [Google Scholar]
- Falgreen Eriksen, H.; Mortensen, E.; Kilburn, T.; Underbjerg, M.; Bertrand, J.; Støvring, H.; Wimberley, T.; Grove, J.; Kesmodel, U. The effects of low to moderate prenatal alcohol exposure in early pregnancy on IQ in 5-year-old children. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.Y.; Kohsaka, S.; Rezaie, P. The origin and cell lineage of microglia: New concepts. Brain Res. Rev. 2007, 53, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Gauvin, D.V. Blood Alcohol Concentrations in Rats Drinking or Intubated with Ethanol. Alcohol. Clin. Exp. Res. 1999, 23, 1945–1946. [Google Scholar] [CrossRef] [PubMed]
- Livy, D.J.; Parnell, S.E.; West, J.R. Blood ethanol concentration profiles: A comparison between rats and mice. Alcohol 2003, 29, 165–171. [Google Scholar] [CrossRef]
- Walker, B.M.; Ehlers, C.L. Age-related differences in the blood alcohol levels of Wistar rats. Pharmacol. Biochem. Behav. 2009, 91, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Bielawski, D.M.; Abel, E.L. The effect of administering ethanol as single vs. divided doses on blood alcohol levels in the rat. Neurotoxicol. Teratol. 2002, 24, 559–562. [Google Scholar] [CrossRef]
- Mendez, M.; Arias, N.; Uceda, S.; Arias, J.L. c-Fos expression correlates with performance on novel object and novel place recognition tests. Brain Res. Bull. 2015, 117, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Morici, J.F.; Ciccia, L.; Malleret, G.; Gingrich, J.A.; Bekinschtein, P.; Weisstaub, N.V. Serotonin 2a Receptor and Serotonin 1a Receptor Interact within the Medial Prefrontal Cortex During Recognition Memory in Mice. Front. Pharmacol. 2015, 6, 298. [Google Scholar] [CrossRef] [PubMed]
- Gaffan, D.; Parker, A. Interaction of perirhinal cortex with the fornix-fimbria: Memory for objects and “object-in-place” memory. J. Neurosci. 1996, 16, 5864–5869. [Google Scholar] [PubMed]
- Winters, B.D.; Saksida, L.M.; Bussey, T.J. Object recognition memory: Neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci. Biobehav. Rev. 2008, 32, 1055–1070. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.W.; Aggleton, J.P. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat. Rev. Neurosci. 2001, 2, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, S.R.; Brennan, L.E.; Stanton, M.E. Ontogeny of object versus location recognition in the rat: acquisition and retention effects. Dev. Psychobiol. 2014, 56, 1492–1506. [Google Scholar] [CrossRef] [PubMed]
- Ennaceur, A. One-trial object recognition in rats and mice: Methodological and theoretical issues. Behav. Brain Res. 2010, 215, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Blaser, R.; Heyser, C. Spontaneous object recognition: A promising approach to the comparative study of memory. Front. Behav. Neurosci. 2015, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, S.; Prickaerts, J.; Steinbusch, H.W.; Blokland, A. Object recognition testing: Statistical considerations. Behav. Brain Res. 2012, 232, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Crews, F.T.; Vetreno, R.P. Addiction, adolescence, and innate immune gene induction. Front. Psychiatry 2011, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Boschen, K.E.; Ruggiero, M.J.; Klintsova, A.Y. Neonatal binge alcohol exposure increases microglial activation in the developing rat hippocampus. Neuroscience 2016, 324, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Cuadros, M.A.; Navascues, J. The origin and differentiation of microglial cells during development. Prog. Neurobiol. 1998, 56, 173–189. [Google Scholar] [CrossRef]
- Fowler, A.K.; Thompson, J.; Chen, L.; Dagda, M.; Dertien, J.; Dossou, K.S.; Moaddel, R.; Bergeson, S.E.; Kruman, I.I. Differential sensitivity of prefrontal cortex and hippocampus to alcohol-induced toxicity. PLoS ONE 2014, 9, e106945. [Google Scholar] [CrossRef] [PubMed]
- Deviere, J.; Content, J.; Denys, C.; Vandenbussche, P.; Schandene, L.; Wybran, J.; Dupont, E. High interleukin-6 serum levels and increased production by leucocytes in alcoholic liver cirrhosis. Correlation with IgA serum levels and lymphokines production. Clin. Exp. Immunol. 1989, 77, 221–225. [Google Scholar] [PubMed]
- Khoruts, A.; Stahnke, L.; McClain, C.J.; Logan, G.; Allen, J.I. Circulating tumor necrosis factor, interleukin-1 and interleukin-6 concentrations in chronic alcoholic patients. Hepatology 1991, 13, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Quintela, A.; Dominguez-Santalla, M.J.; Perez, L.F.; Vidal, C.; Lojo, S.; Barrio, E. Influence of acute alcohol intake and alcohol withdrawal on circulating levels of IL-6, IL-8, IL-10 and IL-12. Cytokine 2000, 12, 1437–1440. [Google Scholar] [CrossRef] [PubMed]
- Williamson, L.L.; Sholar, P.W.; Mistry, R.S.; Smith, S.H.; Bilbo, S.D. Microglia and memory: Modulation by early-life infection. J. Neurosci. 2011, 31, 15511–15521. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Geil Nickell, C.R.; Chen, K.Y.; McClain, J.A.; Nixon, K. Increased expression of M1 and M2 phenotypic markers in isolated microglia after four-day binge alcohol exposure in male rats. Alcohol 2017, 62, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D. M1 and M2 Macrophages: Oracles of Health and Disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, S.A.; Stanton, M.E. Neonatal alcohol impairs the context preexposure facilitation effect in juvenile rats: Dose-response and post-training consolidation effects. Alcohol 2014, 48, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, M.J.; Lindquist, D.H. Significant long-term, but not short-term, hippocampal-dependent memory impairment in adult rats exposed to alcohol in early postnatal life. Dev. Psychobiol. 2014, 56, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Howell, K.K.; Lynch, M.E.; Platzman, K.A.; Smith, G.H.; Coles, C.D. Prenatal alcohol exposure and ability, academic achievement, and school functioning in adolescence: A longitudinal follow-up. J. Pediatr. Psychol. 2006, 31, 116–126. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terasaki, L.S.; Schwarz, J.M. Impact of Prenatal and Subsequent Adult Alcohol Exposure on Pro-Inflammatory Cytokine Expression in Brain Regions Necessary for Simple Recognition Memory. Brain Sci. 2017, 7, 125. https://doi.org/10.3390/brainsci7100125
Terasaki LS, Schwarz JM. Impact of Prenatal and Subsequent Adult Alcohol Exposure on Pro-Inflammatory Cytokine Expression in Brain Regions Necessary for Simple Recognition Memory. Brain Sciences. 2017; 7(10):125. https://doi.org/10.3390/brainsci7100125
Chicago/Turabian StyleTerasaki, Laurne S., and Jaclyn M. Schwarz. 2017. "Impact of Prenatal and Subsequent Adult Alcohol Exposure on Pro-Inflammatory Cytokine Expression in Brain Regions Necessary for Simple Recognition Memory" Brain Sciences 7, no. 10: 125. https://doi.org/10.3390/brainsci7100125



