Insomnia Phenotypes Based on Objective Sleep Duration in Adolescents: Depression Risk and Differential Behavioral Profiles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Sleep Laboratory Evaluation
2.3. Insomnia Symptoms and Other Self-Reports
2.4. Child and Adult Behavior Checklist
2.5. Pediatric Behavior Scale
2.6. Statistical Analyses
3. Results
3.1. Demographic and Clinical Characteristics of the Sample
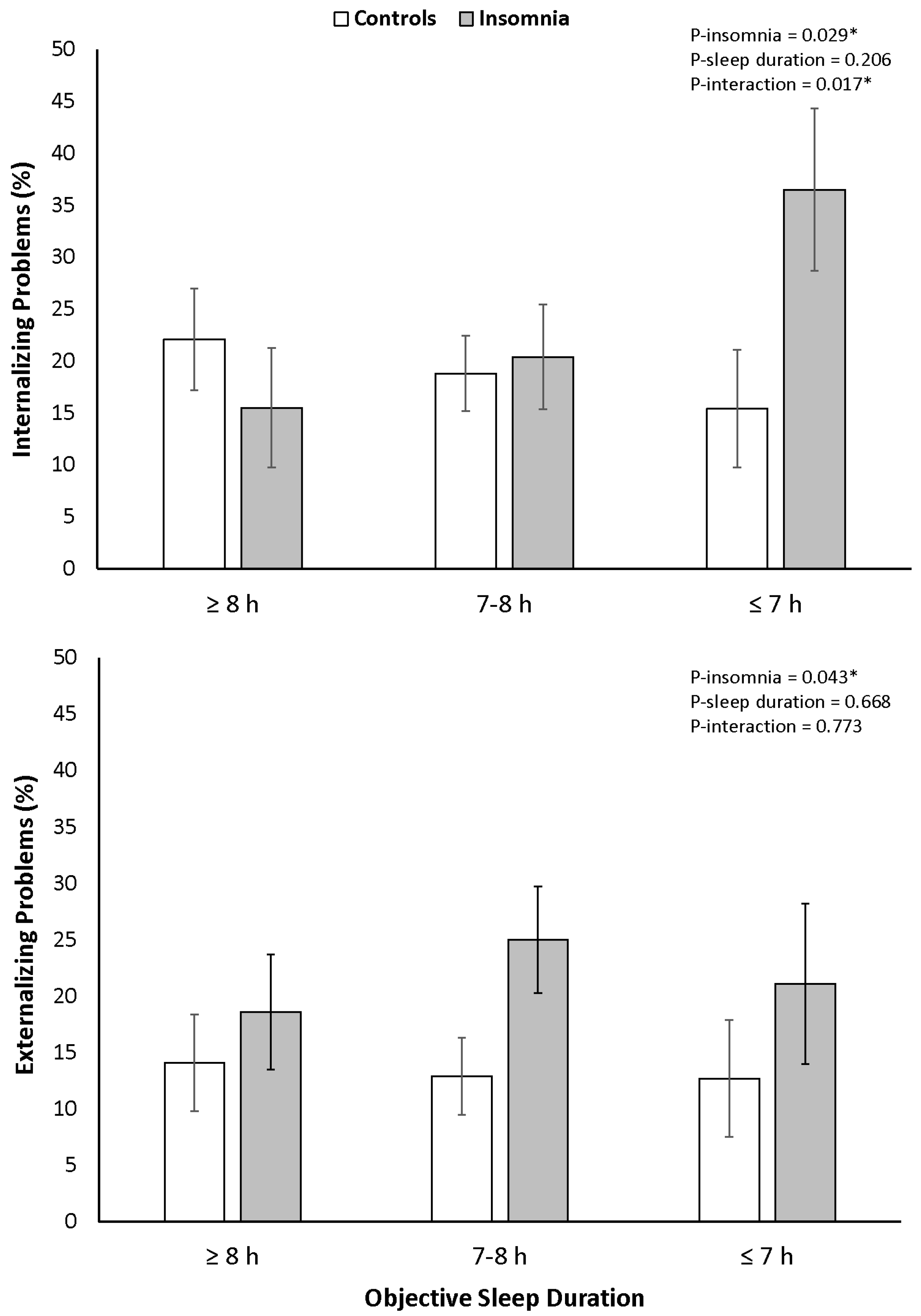
3.2. Internalizing and Externalizing Problems
3.3. Behavioral Profiles
3.4. Rumination, Internalization and Acting-Out
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gradisar, M.; Gardner, G.; Dohnt, H. Recent worldwide sleep patterns and problems during adolescence: A review and meta-analysis of age, region, and sleep. Sleep Med. 2011, 12, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Archbold, K.H.; Pituch, K.J.; Panahi, P.; Chervin, R.D. Symptoms of sleep disturbances among children at two general pediatric clinics. J. Pediatr. 2002, 140, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, H. Sleep duration, insomnia and behavioral problems among Chinese adolescents. Psychiatry Res. 2002, 111, 75–85. [Google Scholar] [CrossRef]
- Russo, P.M.; Bruni, O.; Lucidi, F.; Ferri, R.; Violani, C. Sleep habits and circadian preference in Italian children and adolescents. J. Sleep Res. 2007, 16, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.E.; Roberts, C.R.; Duong, H.T. Chronic insomnia and its negative consequences for health and functioning of adolescents: A 12-month prospective study. J. Adolesc. Health 2008, 42, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Fernandez-Mendoza, J.; Bixler, E.O.; Singareddy, R.; Shaffer, M.L.; Calhoun, S.L.; Liao, D.; Basta, M.; Chrousos, G.P. Persistent insomnia: The role of objective short sleep duration and mental health. Sleep 2012, 35, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Mendoza, J.; Vgontzas, A.N.; Bixler, E.O.; Singareddy, R.; Shaffer, M.L.; Calhoun, S.L.; Karataraki, M.; Vela-Bueno, A.; Liao, D. Clinical and polysomnographic predictors of the natural history of poor sleep in the general population. Sleep 2012, 35, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.M.; Caspi, A.; Eley, T.C.; Moffitt, T.E.; Oconnor, T.G.; Poulton, R. Prospective longitudinal associations between persistent sleep problems in childhood and anxiety and depression disorders in adulthood. J. Abnorm. Child Psychol. 2005, 33, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, S.L.; Fernandez-Mendoza, J.; Vgontzas, A.N.; Mayes, S.D.; Liao, D.; Bixler, E.O. Behavioral profiles associated with objective sleep duration in young children with insomnia symptoms. J. Abnorm. Child Psychol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Paavonen, E.J.; Aronen, E.T.; Moilanen, I.; Piha, J.; Räsänen, E.; Tamminen, T.; Almqvist, F. Sleep problems of school-aged children: A complementary view. Acta Paediatr. 2000, 89, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Smedje, H.; Broman, J.E.; Hetta, J. Associations between disturbed sleep and behavioural difficulties in 635 children aged six to eight years: A study based on parents’ perceptions. Eur. Child Adolesc. Psychiatry 2001, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mayes, S.D.; Calhoun, S.L.; Bixler, E.O.; Vgontzas, A.N.; Mahr, F.; Hillwig-Garcia, J.; Elamir, B.; Edhere-Ekezie, L.; Parvin, M. ADHD subtypes and comorbid anxiety, depression, and oppositional-defiant disorder: Differences in sleep problems. J. Pediatr. Psychol. 2009, 34, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Mayes, S.D.; Calhoun, S.L.; Bixler, E.O.; Vgontzas, A.N. Sleep problems in children withautism, ADHD, anxiety, depression, acquired brain injury, and typical development. Sleep Med. Clin. 2009, 4, 19–25. [Google Scholar] [CrossRef]
- Shochat, T.; Cohen-Zion, M.; Tzischinsky, O. Functional consequences of inadequate sleep in adolescents: A systematic review. Sleep Med. Rev. 2014, 18, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.M.; O’Connor, T.G. Sleep problems in childhood: A longitudinal study of developmental change and association with behavioral problems. J. Am. Acad. Child Adolesc. Psychiatry 2002, 41, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.M.; Eley, T.C.; O’Connor, T.G.; Plomin, R. Etiologies of associations between childhood sleep and behavioral problems in a large twin sample. J. Am. Acad. Child Adolesc. Psychiatry 2004, 43, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.M.; Rijsdijk, F.V.; Lau, J.Y.; Dahl, R.E.; Eley, T.C. The direction of longitudinal associations between sleep problems and depression symptoms: A study of twins aged 8 and 10 years. Sleep 2009, 32, 189–199. [Google Scholar] [PubMed]
- Pieters, S.; Burk, W.J.; Van der Vorst, H.; Dahl, R.E.; Wiers, R.W.; Engels, R.C. Prospective relationships between sleep problems and substance use, internalizing and externalizing problems. J. Youth Adolesc. 2015, 44, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Fernandez-Mendoza, J.; Liao, D.; Bixler, E.O. Insomnia with objective short sleep duration: The most biologically severe phenotype of the disorder. Sleep Med. Rev. 2013, 17, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Mendoza, J.; Shea, S.; Vgontzas, A.N.; Calhoun, S.L.; Liao, D.; Bixler, E.O. Insomnia and incident depression: Role of objective sleep duration and natural history. J. Sleep Res. 2015, 24, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Mendoza, J.; Vgontzas, A.N.; Calhoun, S.L.; Vgontzas, A.; Tsaoussoglou, M.; Gaines, J.; Liao, D.; Chrousos, G.P.; Bixler, E.O. Insomnia symptoms, objective sleep duration and hypothalamic-pituitary-adrenal activity in children. Eur. J. Clin. Investig. 2014, 44, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Hayley, A.C.; Skogen, J.C.; Sivertsen, B.; Wold, B.; Berk, M.; Pasco, J.A.; Øverland, S. Symptoms of depression and difficulty initiating sleep from early adolescence to early adulthood: A longitudinal study. Sleep 2015, 38, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.M.; Brower, K.J.; Craun, E.A. Insomnia symptoms and suicidality in the National Comorbidity Survey—Adolescent Supplement. J. Psychiatr. Res. 2016, 81, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.; Wang, G.; Lewin, D.; Skora, E.; Baylor, A. Association between short sleep duration and risk behavior factors in middle school students. Sleep 2016, in press. [Google Scholar]
- Sivertsen, B.; Harvey, A.G.; Lundervold, A.J.; Hysing, M. Sleep problems and depression in adolescence: Results from a large population-based study of Norwegian adolescents aged 16–18 years. Eur. Child Adolesc. Psychiatry 2014, 23, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Bixler, E.O.; Fernandez-Mendoza, J.; Liao, D.; Calhoun, S.; Rodriguez-Colon, S.M.; Gaines, J.; He, F.; Vgontzas, A.N. Natural history of sleep disordered breathing in prepubertal children transitioning to adolescence. Eur. Respir. J. 2016, 47, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Kuczmarski, R.J.; Ogden, C.L.; Guo, S.S. 2000 CDC Growth Charts for The United States: Methods and development. Vital Health Stat. 11 2002, 246, 1–190. [Google Scholar]
- Rechtschaffen, A.; Kales, A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Clin. Neurophysiol. 1969, 26, 644. [Google Scholar]
- Grigg-Damberger, M.; Gozal, D.; Marcus, C.L.; Quan, S.F.; Rosen, C.L.; Chervin, R.D.; Wise, M.; Picchietti, D.L.; Sheldon, S.H.; Iber, C. The visual scoring of sleep and arousal in infants and children. J. Clin. Sleep Med. 2007, 3, 201–240. [Google Scholar] [PubMed]
- Ohayon, M.M.; Carskadon, M.A.; Guilleminault, C.; Vitiello, M.V. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep 2004, 27, 1255–1273. [Google Scholar] [PubMed]
- Paruthi, S.; Brooks, L.J.; D’Ambrosio, C.; Hall, W.A.; Kotagal, S.; Lloyd, R.M.; Malow, B.A.; Maski, K.; Nichols, C.; Quan, S.F.; et al. Recommended amount of sleep for pediatric populations: A consensus statement of the american academy of sleep medicine. J. Clin. Sleep Med. 2016, 12, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Chervin, R.D.; Hedger, K.; Dillon, J.E.; Pituch, K.J. Pediatric sleep questionnaire (PSQ): Validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000, 1, 21–32. [Google Scholar] [CrossRef]
- Carskadon, M.A.; Acebo, C. A self-administered rating scale for pubertal development. J. Adolesc. Health 1993, 14, 190–195. [Google Scholar] [CrossRef]
- Carskadon, M.A.; Vieira, C.; Acebo, C. Association between puberty and delayed phase preference. Sleep 1993, 16, 258–262. [Google Scholar] [PubMed]
- Achenbach, T.M. Integrative Guide for the 1991 CBCL/4–8, YSR, and TRF Profiles; University of Vermont Department of Psychiatry: Burlington, VT, USA, 1991. [Google Scholar]
- Achenbach, T.M. Manual for the ASEBA Adult Forms and Profiles; University of Vermont, Research Center for Children, Youth and Families: Burlington, VT, USA, 2003. [Google Scholar]
- Lindgren, S.D.; Koeppl, G.K. Assessing child behavior problems in a medical setting: Development of the pediatric behavior scale. In Advances in Behavioral Assessment of Children and Families; Prinz, R.J., Ed.; JAI Press: Greenwich, CT, USA, 1987; Volume 3, pp. 57–90. [Google Scholar]
- Singareddy, R.; Moole, S.; Calhoun, S.; Vocalan, P.; Tsaoussoglou, M.; Vgontzas, A.N.; Bixler, E.O. Medical complaints are more common in young school-aged children with parent reported insomnia symptoms. J. Clin. Sleep Med. 2009, 5, 549–553. [Google Scholar] [PubMed]
- Fernandez-Mendoza, J.; Calhoun, S.L.; Bixler, E.O.; Karataraki, M.; Liao, D.; Vela-Bueno, A.; Jose Ramos-Platon, M.; Sauder, K.A.; Basta, M.; Vgontzas, A.N. Sleep misperception and chronic insomnia in the general population: Role of objective sleep duration and psychological profiles. Psychosom. Med. 2011, 73, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.E.; Harvey, A.G.; van der Linden, M. Cognitive and affective control in insomnia. Front. Psychol. 2011, 2, 349. [Google Scholar] [CrossRef] [PubMed]
- Carney, C.E.; Harris, A.L.; Moss, T.G.; Edinger, J.D. Distinguishing rumination from worry in clinical insomnia. Behav. Res. Ther. 2010, 48, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Mendoza, J.; Li, Y.; Vgontzas, A.N.; Fang, J.; Gaines, J.; Calhoun, S.L.; Liao, D.; Bixler, E.O. Insomnia is associated with cortical hyperarousal as early as adolescence. Sleep 2016, 39, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Kales, A.; Caldwell, A.B.; Preston, T.A.; Healey, S.; Kales, J.D. Personality patterns in insomnia: Theoretical implications. Arch. Gen. Psychiatry 1976, 33, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Gaines, J.; Vgontzas, A.N.; Fernandez-Mendoza, J.; Basta, M.; Pejovic, S.; He, F.; Bixler, E.O. Short- and long-term sleep stability in insomniacs and healthy controls. Sleep 2015, 38, 1727–1734. [Google Scholar] [CrossRef] [PubMed]

| Insomnia Symptoms | p | Objective Sleep Duration | p | ||||
|---|---|---|---|---|---|---|---|
| No | Yes | ≥8 h | 7–8 h | ≤7 h | |||
| (n = 249) | (n = 148) | (n = 120) | (n = 181) | (n = 96) | |||
| Female, % | 37.8 | 58.1 | <0.001 | 54.2 | 43.6 | 37.5 | 0.042 |
| Ethnic-Minority, % | 19.3 | 24.3 | 0.234 | 20.8 | 22.7 | 18.8 | 0.747 |
| Low SES, % | 60.9 | 73.6 | 0.010 | 69.7 | 61.3 | 68.8 | 0.247 |
| Age, years | 16.9 ± 2.3 | 17.1 ± 2.2 | 0.368 | 16.7 ± 2.2 | 17.1 ± 2.4 | 17.1 ± 2.1 | 0.190 |
| Tanner, stage | 4.1 ± 0.8 | 4.25 ± 0.81 | 0.195 | 4.2 ± 0.8 | 4.2 ± 0.8 | 4.1 ± 0.8 | 0.883 |
| BMI percentile | 64.4 ± 28.6 | 68.16 ± 27.9 | 0.198 | 65.9 ± 29.4 | 66.6 ± 26.0 | 64.1 ± 31.3 | 0.788 |
| MEQ, total score | 26.7 ± 4.7 | 24.4 ± 5.3 | <0.001 | 25.9 ± 4.7 | 26.2 ± 5.2 | 25.2 ± 5.3 | 0.246 |
| M-type | 37.9 | 22.3 | <0.001 | 28.3 | 36.5 | 28.4 | 0.514 |
| I-type | 37.9 | 35.1 | 39.2 | 35.4 | 36.8 | ||
| E-type | 24.2 | 42.6 | 32.5 | 28.2 | 34.7 | ||
| SOL, minutes | 23.7 ± 19.1 | 28.6 ± 30.4 | 0.080 | 12.7 ± 6.5 | 23.9 ± 14.2 | 44.5 ± 37.3 | <0.001 |
| Awakenings, number | 36.5 ± 11.3 | 36.7 ± 13.6 | 0.565 | 32.9 ± 8.6 | 39.0 ± 12.1 | 36.4 ± 14.9 | <0.001 |
| WASO, minutes | 71.4 ± 44.9 | 66.0 ± 41.5 | 0.238 | 33.0 ± 10.0 | 63.9 ± 19.6 | 125.2 ± 47.2 | <0.001 |
| TWT, minutes | 92.9 ± 51.8 | 92.8 ± 59.1 | 0.982 | 44.1 ± 11.3 | 86.0 ± 18.3 | 166.9 ± 54.6 | <0.001 |
| TST, minutes | 447.2 ± 52.4 | 448.2 ± 60.0 | 0.866 | 497.1 ± 10.5 | 454.7 ± 18.1 | 372.2 ± 55.4 | <0.001 |
| Sleep efficiency, % | 82.8 ± 9.6 | 82.3 ± 11.0 | 0.961 | 91.8 ± 2.1 | 84.1 ± 3.4 | 68.9 ± 10.2 | <0.001 |
| Stage 1, % | 1.0 ± 1.3 | 1.0 ± 1.9 | 0.712 | 0.49 ± 0.5 | 0.9 ± 1.0 | 1.9 ± 2.5 | <0.001 |
| Stage 2, % | 53.5 ± 9.8 | 53.4 ± 10.3 | 0.736 | 52.72 ± 9.5 | 53.7 ± 9.7 | 54.0 ± 10.9 | 0.614 |
| Stage 3, % | 26.9 ± 9.4 | 27.0 ± 8.1 | 0.936 | 27.0 ± 8.7 | 26.4 ± 9.2 | 28.0 ± 9.6 | 0.399 |
| Stage R, % | 18.6 ± 4.7 | 18.6 ± 5.6 | 0.913 | 19.8 ± 4.4 | 19.1 ± 4.5 | 16.1 ± 6.0 | <0.001 |
| PLMI, events/hour | 4.4 ± 6.5 | 3.0 ± 4.8 | 0.014 | 3.4 ± 5.7 | 3.8 ± 5.8 | 4.7 ± 6.6 | 0.299 |
| AHI, events/hour | 2.4 ± 3.1 | 2.7 ± 4.3 | 0.379 | 2.1 ± 3.5 | 2.5 ± 2.7 | 2.9 ± 5.0 | 0.271 |
| Internalizing problems | |||||||
| T-score, total | 49.6 ± 10.1 | 52.8 ± 11.1 | 0.004 | 51.1 ± 9.5 | 50.5 ± 10.9 | 50.9 ± 11.4 | 0.907 |
| Clinically elevated, % | 17.3 | 25.0 | 0.064 | 19.2 | 19.3 | 22.9 | 0.741 |
| Externalizing problems | |||||||
| T-score, total | 47.5 ± 9.3 | 51.5 ± 11.0 | <0.001 | 49.1 ± 9.8 | 49.0 ± 10.1 | 48.9 ± 10.7 | 0.990 |
| Clinically elevated, % | 12.0 | 23.6 | 0.002 | 15.8 | 17.1 | 15.6 | 0.933 |
| 1. Controls >7 h | 2. Controls ≤7 h | 3. Insomnia >7 h | 4. Insomnia ≤7 h | p | ||||
|---|---|---|---|---|---|---|---|---|
| (n = 188) | (n = 61) | (n = 113) | (n = 35) | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | 3 vs. 4 | |
| Internalizing problems | ||||||||
| Anxious depressed | ||||||||
| T-score, total | 53.7 ± 0.4 | 53.8 ± 0.8 | 54.3 ± 0.6 | 54.6 ± 1.0 | 0.892 | 0.369 | 0.401 | 0.796 |
| Clinically elevated, % | 14.9 | 17.2 | 20.7 | 18.4 | 0.675 | 0.207 | 0.621 | 0.759 |
| Withdrawn depressed | ||||||||
| T-score, total | 54.8 ± 0.5 | 54.1 ± 0.9 | 55.6 ± 0.8 | 58.4 ± 1.2 | 0.553 | 0.318 | 0.008 | 0.049 |
| Clinically elevated, % | 20.4 | 20.3 | 24.3 | 37.7 | 0.990 | 0.445 | 0.034 | 0.110 |
| Somatic complaints | ||||||||
| T-score, total | 54.9 ± 0.5 | 54.1 ± 0.9 | 56.7 ± 0.6 | 57.9 ± 1.2 | 0.392 | 0.035 | 0.020 | 0.342 |
| Clinically elevated, % | 18.4 | 19.3 | 24.1 | 30.5 | 0.881 | 0.251 | 0.122 | 0.427 |
| Externalizing problems | ||||||||
| Thought problems | ||||||||
| T-score, total | 54.1 ± 0.5 | 53.6 ± 1.0 | 56.8 ± 0.6 | 56.8 ± 1.1 | 0.607 | <0.01 | 0.019 | 0.981 |
| Clinically elevated, % | 13.9 | 15.3 | 30.5 | 29.8 | 0.809 | <0.01 | 0.032 | 0.934 |
| Attention problems | ||||||||
| T-score, total | 55.2 ± 0.5 | 55.1 ± 0.9 | 56.1 ± 0.7 | 56.3 ± 1.2 | 0.975 | 0.281 | 0.410 | 0.896 |
| Clinically elevated, % | 18.8 | 16.0 | 23.3 | 25.6 | 0.650 | 0.353 | 0.367 | 0.764 |
| Rule-breaking behaviors | ||||||||
| T-score, total | 53.4 ± 0.9 | 53.1 ± 0.7 | 55.6 ± 0.5 | 53.5 ± 0.3 | 0.727 | <0.01 | 0.883 | 0.051 |
| Clinically elevated, % | 12.8 | 10.0 | 23.2 | 19.7 | 0.598 | 0.018 | 0.314 | 0.621 |
| Aggressive behaviors | ||||||||
| T-score, total | 52.7 ± 0.4 | 53.6 ± 0.7 | 54.9 ± 0.5 | 55.4 ± 1.0 | 0.195 | <0.01 | 0.010 | 0.604 |
| Clinically elevated, % | 9.5 | 17.5 | 19.1 | 26.1 | 0.123 | 0.024 | 0.013 | 0.302 |
| 1. Controls | 2. Insomnia >7 h | 3. Insomnia ≤7 h | p | |||
|---|---|---|---|---|---|---|
| 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | ||||
| C/ABCL | (n = 247) | (n = 113) | (n = 35) | |||
| Aggressive behavior | ||||||
| “fights” | 4.8% | 12.4% | 5.7% | 0.009 | 0.846 | 0.176 |
| “screams a lot” | 8.4% | 17.7% | 14.3% | 0.011 | 0.309 | 0.580 |
| “mood changes” | 25.3% | 38.9% | 45.7% | 0.009 | 0.014 | 0.445 |
| “loses temper” | 20.9% | 30.1% | 37.1% | 0.060 | 0.037 | 0.398 |
| Thought problems | ||||||
| “can’t get mind off of certain thoughts” | 33.3% | 47.8% | 54.3% | 0.009 | 0.017 | 0.488 |
| “strange ideas” | 6.4% | 10.6% | 14.3% | 0.181 | 0.115 | 0.492 |
| “strange behavior” | 7.6% | 13.3% | 14.3% | 0.095 | 0.216 | 0.861 |
| PBS | (n = 217) | (n = 93) | (n = 29) | |||
| Aggression | 0.81 ± 0.16 | 1.54 ± 0.24 | 0.88 ± 0.43 | 0.011 | 0.871 | 0.177 |
| Inappropriate Behavior | 1.82 ± 0.26 | 3.36 ± 0.39 | 2.73 ± 0.72 | 0.001 | 0.242 | 0.432 |
| Social Isolation | 0.71 ± 0.13 | 0.93 ± 0.19 | 1.97 ± 0.35 | 0.288 | 0.001 | 0.011 |
| Perseverative Thinking | 0.66 ± 0.11 | 1.04 ± 0.17 | 1.43 ± 0.31 | 0.062 | 0.019 | 0.256 |
| Thought Disorder | 0.09 ± 0.04 | 0.20 ± 0.06 | 0.07 ± 0.10 | 0.119 | 0.801 | 0.246 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez-Mendoza, J.; Calhoun, S.L.; Vgontzas, A.N.; Li, Y.; Gaines, J.; Liao, D.; Bixler, E.O. Insomnia Phenotypes Based on Objective Sleep Duration in Adolescents: Depression Risk and Differential Behavioral Profiles. Brain Sci. 2016, 6, 59. https://doi.org/10.3390/brainsci6040059
Fernandez-Mendoza J, Calhoun SL, Vgontzas AN, Li Y, Gaines J, Liao D, Bixler EO. Insomnia Phenotypes Based on Objective Sleep Duration in Adolescents: Depression Risk and Differential Behavioral Profiles. Brain Sciences. 2016; 6(4):59. https://doi.org/10.3390/brainsci6040059
Chicago/Turabian StyleFernandez-Mendoza, Julio, Susan L. Calhoun, Alexandros N. Vgontzas, Yun Li, Jordan Gaines, Duanping Liao, and Edward O. Bixler. 2016. "Insomnia Phenotypes Based on Objective Sleep Duration in Adolescents: Depression Risk and Differential Behavioral Profiles" Brain Sciences 6, no. 4: 59. https://doi.org/10.3390/brainsci6040059





