Reliability and Variability of tDCS Induced Changes in the Lower Limb Motor Cortex
Abstract
:1. Introduction
2. Experimental Section
2.1. Selection Criteria
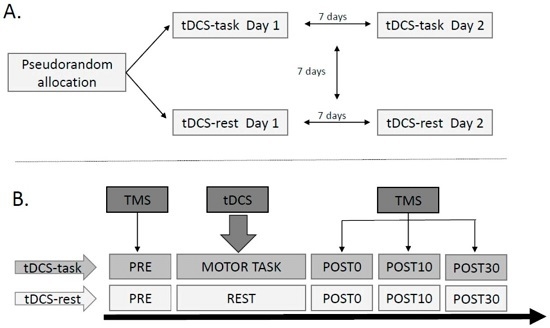
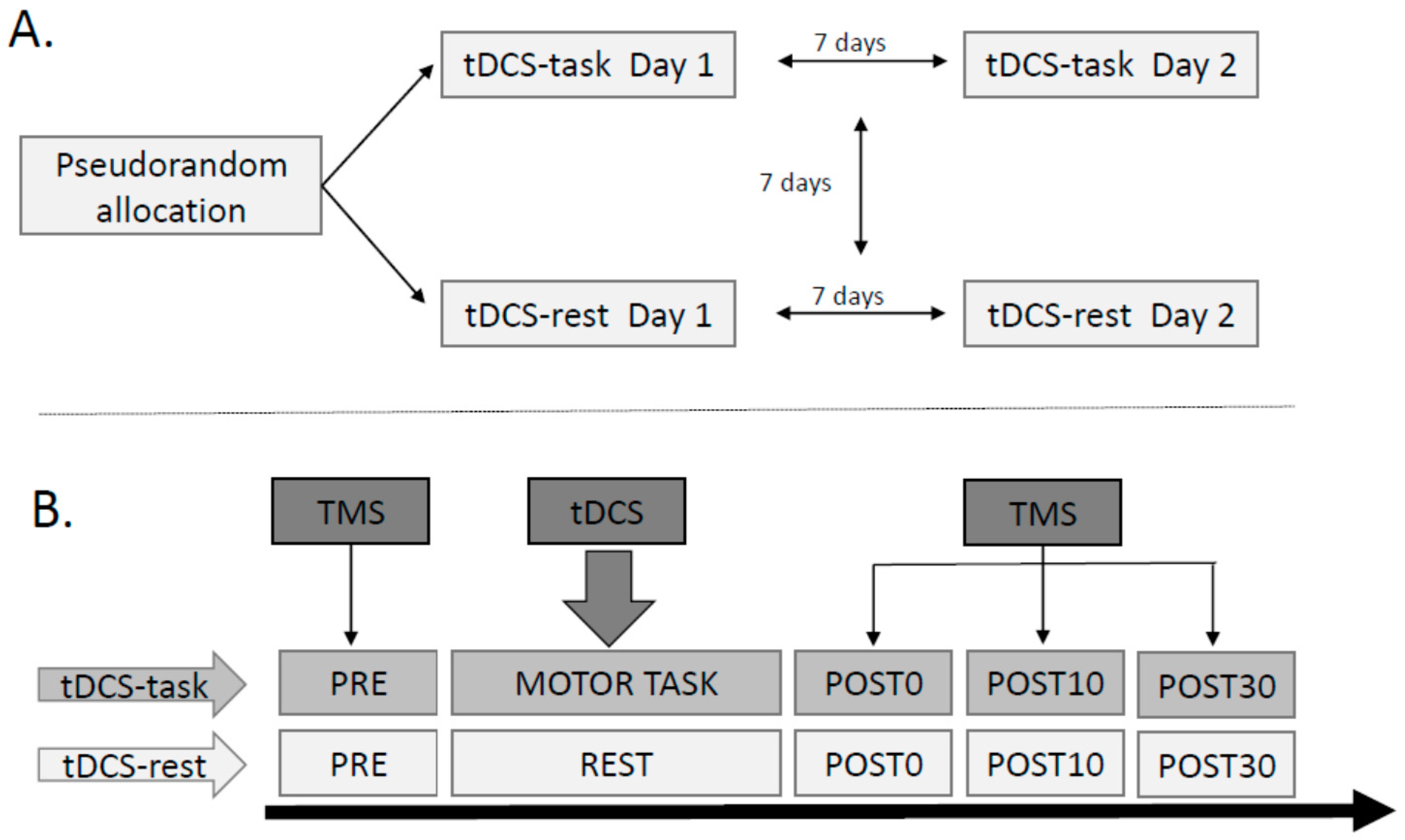
2.2. Study Design
2.3. Instrumentation
2.3.1. Electromyography (EMG)
2.3.2. Transcranial Direct Current Stimulation (tDCS)
2.3.3. Transcranial Magnetic Stimulation (TMS)
2.3.4. Ankle Motor Task
2.4. Data Analyses
2.5. Statistical Analyses
2.5.1. Effects of Anodal tDCS
2.5.2. Reliability of Anodal tDCS-Induced Changes
2.5.3. Intra-and Inter-Individual Variability
3. Results
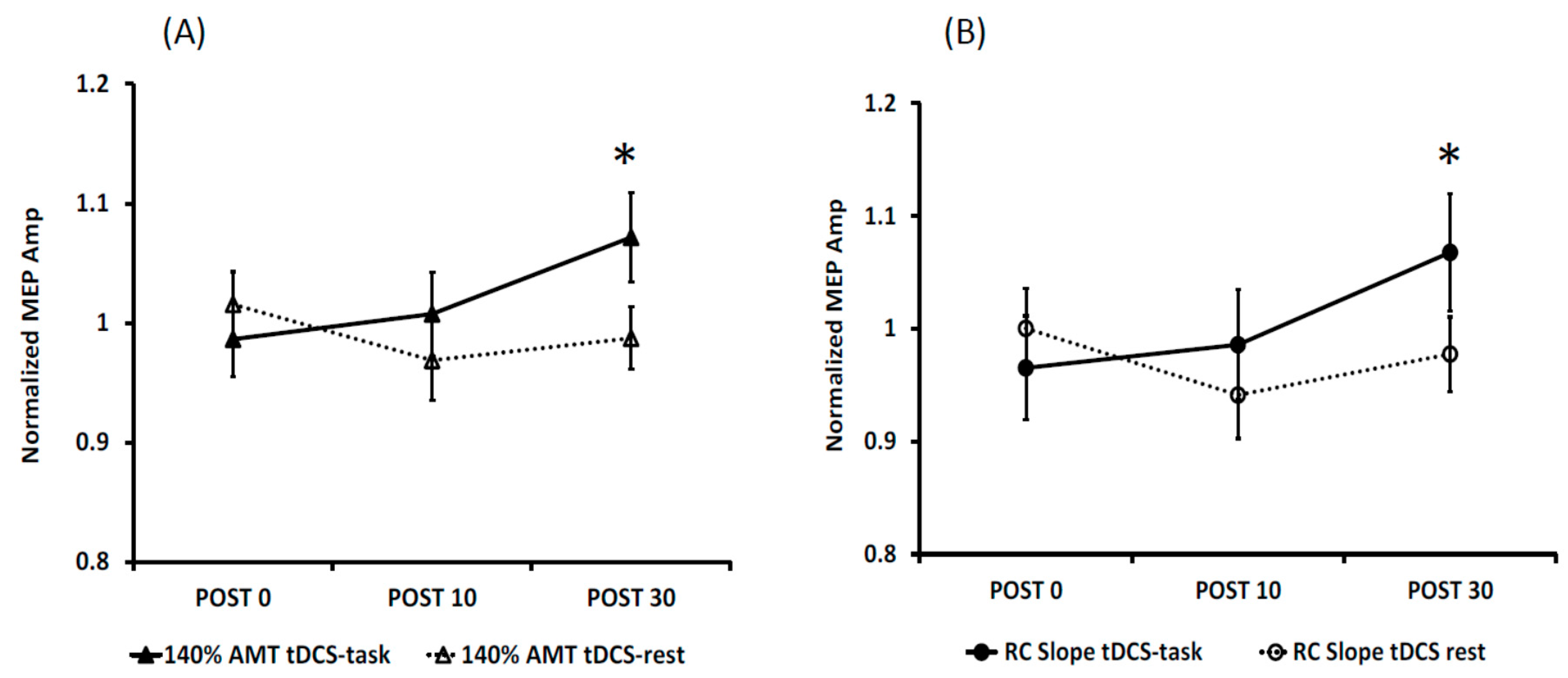
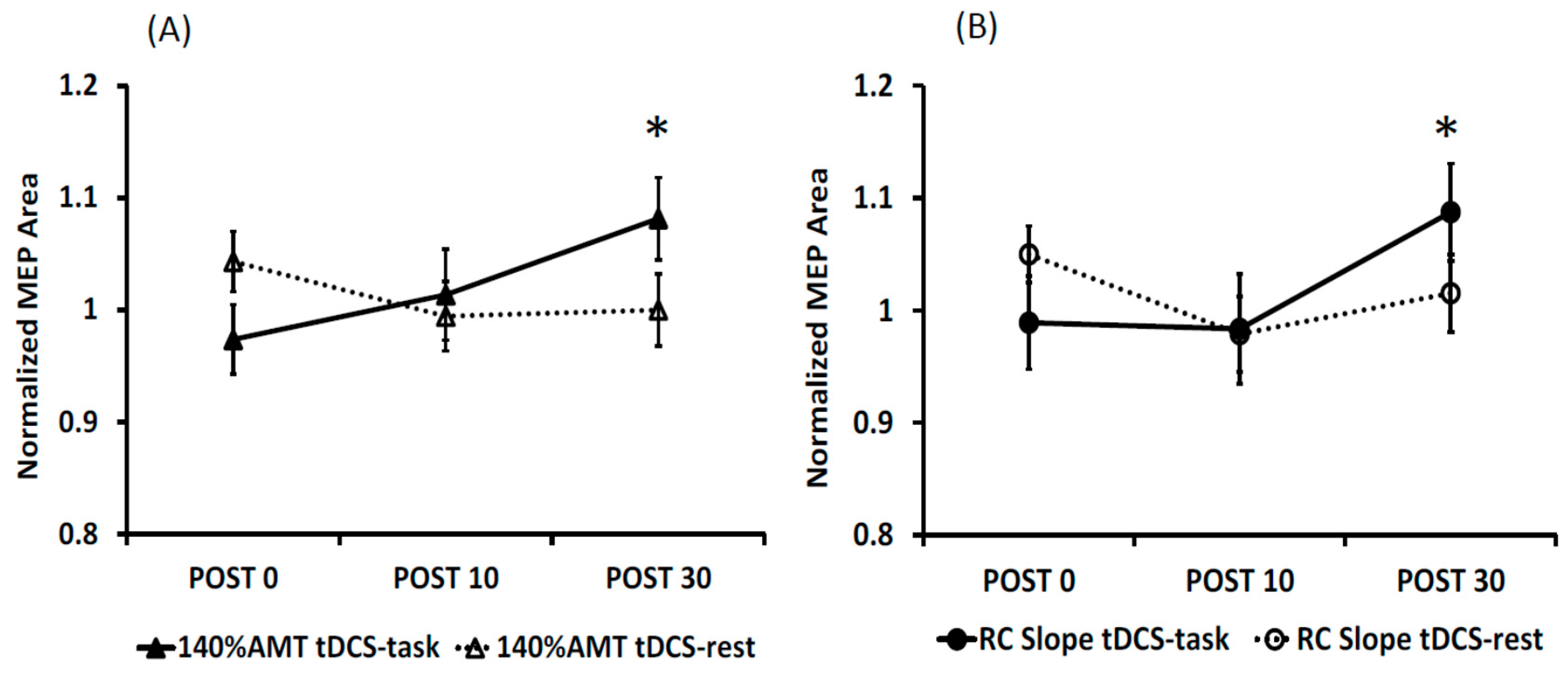
3.1. Effects of Anodal tDCS
3.2. Reliability of tDCS-Induced Changes
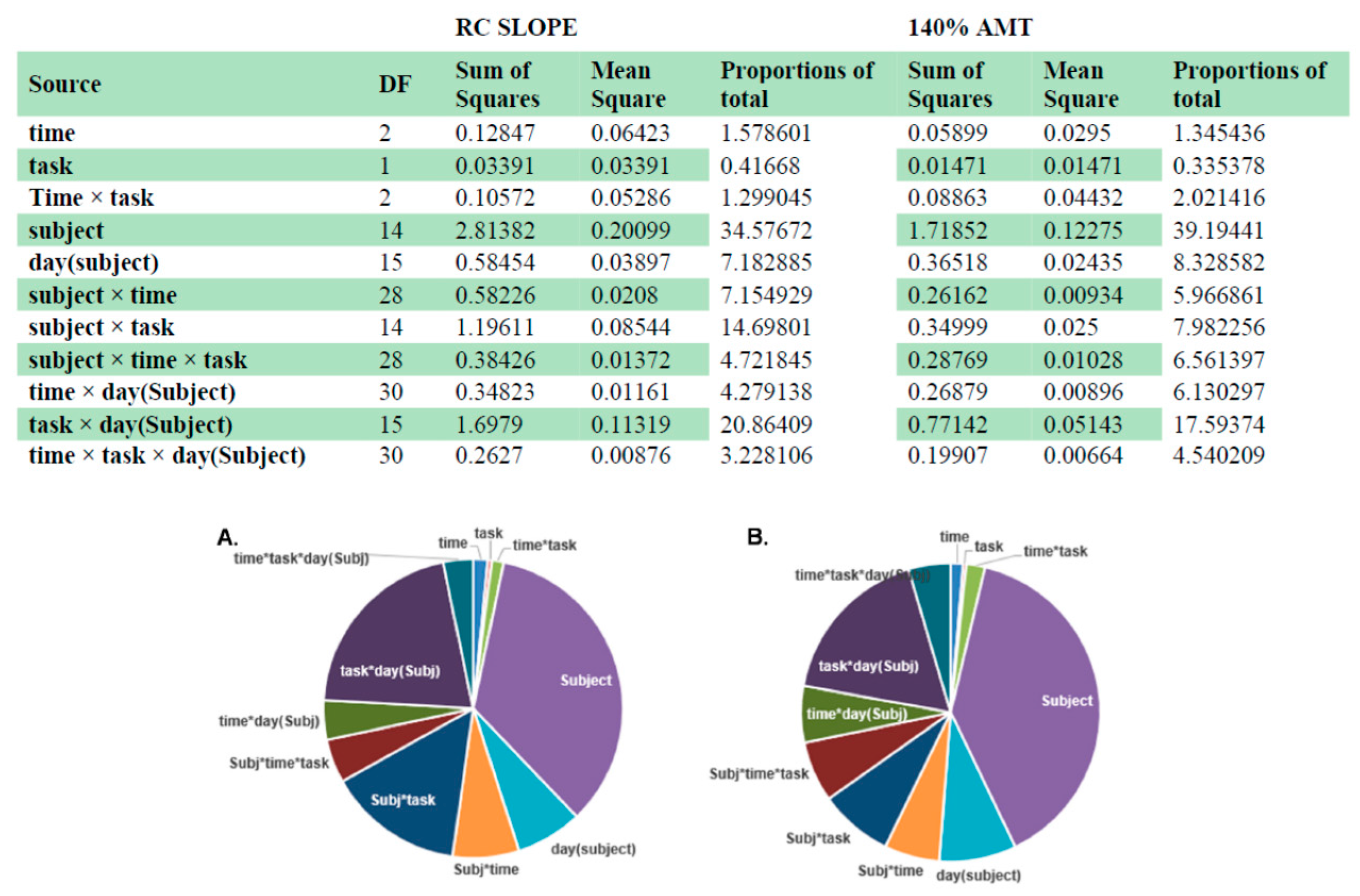
3.3. Intra- and Inter-Individual Variability
4. Discussion
4.1. Reliability of TMS as an Experimental Tool
4.2. Reliability of tDCS
4.3. Variability of tDCS
4.4. Comparison of Corticomotor Changes between the Two Task Conditions
4.5. Is tDCS a Clinically Effective Tool?
4.6. Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F.; et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, S.; Shah, B. Enhancing motor skill learning with transcranial direct current stimulation—A concise review with applications to stroke. Front. Psychiatry 2012. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Schauenburg, A.; Lang, N.; Liebetanz, D.; Exner, C.; Paulus, W.; Tergau, F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J. Cogn. Neurosci. 2003, 15, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Schambra, H.M.; Cohen, L.G.; Buch, E.R.; Fritsch, B.; Zarahn, E.; Celnik, P.A.; Krakauer, J.W. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc. Natl. Acad. Sci. USA 2009, 106, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Sriraman, A.; Oishi, T.; Madhavan, S. Timing-dependent priming effects of tDCS on ankle motor skill learning. Brain Res. 2014, 581, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Stagg, C.J.; Jayaram, G.; Pastor, D.; Kincses, Z.T.; Matthews, P.M.; Johansen-Berg, H. Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia 2011, 49, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Bastani, A.; Jaberzadeh, S. a-tDCS differential modulation of corticospinal excitability: The effects of electrode size. Brain Stimul. 2013, 6, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Kidgell, D.J.; Daly, R.M.; Young, K.; Lum, J.; Tooley, G.; Jaberzadeh, S.; Zoghi, M.; Pearce, A.J. Different current intensities of anodal transcranial direct current stimulation do not differentially modulate motor cortex plasticity. Neural Plast. 2013. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.F.; Paulus, W.; Nitsche, M.A. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. Neuroimage 2014, 85, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Floel, A. tDCS-enhanced motor and cognitive function in neurological diseases. Neuroimage 2014, 85, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Horvath, J.C.; Forte, J.D.; Carter, O. Evidence that transcranial direct current stimulation (tDCS) generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: A systematic review. Neuropsychologia 2015, 66, 213–236. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Alonso, V.; Cheeran, B.; Río-Rodríguez, D.; Fernández-Del-Olmo, M. Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul. 2014, 7, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Wiethoff, S.; Hamada, M.; Rothwell, J.C. Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul. 2014, 7, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Alonso, V.; Fernández-Del-Olmo, M.; Costantini, A.; Gonzalez-Henriquez, J.J.; Cheeran, B. Intra-individual variability in the response to anodal transcranial direct current stimulation. Clin. Neurophysiol. 2015, 126, 2342–2347. [Google Scholar] [CrossRef] [PubMed]
- Chew, T.; Ho, K.A.; Loo, C.K. Inter- and intra-individual variability in response to transcranial direct current stimulation (tDCS) at varying current intensities. Brain Stimul. 2015. [Google Scholar] [CrossRef] [PubMed]
- Lemon, R.N. Descending pathways in motor control. Annu. Rev. Neurosci. 2008, 31, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Labruna, L.; Jamil, A.; Fresnoza, S.; Batsikadze, G.; Kuo, M.F.; Vanderschelden, B.; Ivry, R.B.; Nitsche, M.A. Efficacy of anodal transcranial direct current stimulation is related to sensitivity to transcranial magnetic stimulation. Brain Stimul. 2016, 9, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Marquez, J.; Conley, A.; Karayanidis, F.; Lagopoulos, J.; Parsons, M. Anodal direct current stimulation in the healthy aged: Effects determined by the hemisphere stimulated. Restor. Neurol. Neurosci. 2015, 33, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Nguyen, T.T.; Madhavan, S. Polarity independent effects of cerebellar tDCS on short term ankle visuomotor learning. Brain Stimul. 2013, 6, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, S.; Stinear, J.W. Focal and bidirectional modulation of lower limb motor cortex using anodal transcranial direct current stimulation. Brain Stimul. 2010, 3, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Herwig, U.; Padberg, F.; Unger, J.; Spitzer, M.; Schönfeldt-Lecuona, C. Transcranial magnetic stimulation in therapy studies: Examination of the reliability of “standard” coil positioning by neuronavigation. Biol. Psychiatry 2001, 50, 58–61. [Google Scholar] [CrossRef]
- Sparing, R.; Buelte, D.; Meister, I.G.; Paus, T.; Fink, G.R. Transcranial magnetic stimulation and the challenge of coil placement: A comparison of conventional and stereotaxic neuronavigational strategies. Hum. Brain Mapp. 2008, 29, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Bartko, J.J. The intraclass correlation coefficient as a measure of reliability. Psychol. Rep. 1966, 19, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Sommer, M.; Wu, T.; Tergau, F.; Paulus, W. Intra- and interindividual variability of motor responses to repetitive transcranial magnetic stimulation. Clin. Neurophysiol. 2002, 113, 265–269. [Google Scholar] [CrossRef]
- Malcolm, M.P.; Triggs, W.J.; Light, K.E.; Shechtman, O.; Khandekar, G.; Gonzalez Rothi, L.J. Reliability of motor cortex transcranial magnetic stimulation in four muscle representations. Clin. Neurophysiol. 2006, 117, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Christie, A.; Fling, B.; Crews, R.T.; Mulwitz, L.A.; Kamen, G. Reliability of motor-evoked potentials in the ADM muscle of older adults. J. Neurosci. Methods 2007, 164, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Carroll, T.J.; Riek, S.; Carson, R.G. Reliability of the input-output properties of the cortico-spinal pathway obtained from transcranial magnetic and electrical stimulation. J. Neurosci. Methods 2001, 112, 193–202. [Google Scholar] [CrossRef]
- Van Hedel, H.J.; Murer, C.; Dietz, V.; Curt, A. The amplitude of lower leg motor evoked potentials is a reliable measure when controlled for torque and motor task. J. Neurol. 2007, 254, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Cacchio, A.; Cimini, N.; Alosi, P.; Santilli, V.; Marrelli, A. Reliability of transcranial magnetic stimulation-related measurements of tibialis anterior muscle in healthy subjects. Clin. Neurophysiol. 2009, 120, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, A.; Brassil, J.; Taylor, J.L.; Martin, D.; Loo, C.K. Daily transcranial direct current stimulation (tDCS) leads to greater increases in cortical excitability than second daily transcranial direct current stimulation. Brain Stimul. 2012, 5, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Galvez, V.; Alonzo, A.; Martin, D.; Loo, C.K. Transcranial direct current stimulation treatment protocols: Should stimulus intensity be constant or incremental over multiple sessions? Int. J. Neuropsychopharmacol. 2013, 16, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Chaieb, L.; Moliadze, V.; Monte-Silva, K.; Poreisz, C.; Thirugnanasambandam, N.; Nitsche, M.A.; Shoukier, M.; Ludwig, H.; Paulus, W. Brain-derived neurotrophic factor (BDNF) gene polymorphisms shape cortical plasticity in humans. Brain Stimul. 2010, 3, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Inghilleri, M.; Conte, A.; Currà, A.; Frasca, V.; Lorenzano, C.; Berardelli, A. Ovarian hormones and cortical excitability. An rTMS study in humans. Clin. Neurophysiol. 2004, 115, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Li, L.M.; Uehara, K.; Hanakawa, T. The contribution of interindividual factors to variability of response in transcranial direct current stimulation studies. Front. Cell. Neurosci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ridding, M.C.; Ziemann, U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J. Physiol. 2010, 588 Pt 13, 2291–2304. [Google Scholar] [CrossRef] [PubMed]
- Tecchio, F.; Zappasodi, F.; Assenza, G.; Tombini, M.; Vollaro, S.; Barbati, G.; Rossini, P.M. Anodal transcranial direct current stimulation enhances procedural consolidation. J. Neurophysiol. 2010, 104, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Osman, J.; Field-Fote, E.C. Bihemispheric anodal corticomotor stimulation using transcranial direct current stimulation improves bimanual typing task performance. J. Mot. Behav. 2013, 45, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, S.; Weber, K.A., II; Stinear, J.W. Non-invasive brain stimulation enhances fine motor control of the hemiparetic ankle: Implications for rehabilitation. Exp. Brain Res. 2011, 209, 9–17. [Google Scholar] [PubMed]
- Jayaram, G.; Stinear, J.W. The effects of transcranial stimulation on paretic lower limb motor excitability during walking. J. Clin. Neurophysiol. 2009, 26, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Horvath, J.C.; Carter, O.; Forte, J.D. Transcranial direct current stimulation: Five important issues we aren’t discussing (but probably should be). Front. Syst. Neurosci. 2014. [Google Scholar] [CrossRef] [PubMed]
- Vines, B.W.; Cerruti, C.; Schlaug, G. Dual-hemisphere tDCS facilitates greater improvements for healthy subjects’ non-dominant hand compared to uni-hemisphere stimulation. BMC Neurosci. 2008. [Google Scholar] [CrossRef] [PubMed]
- Darling, W.G.; Wolf, S.L.; Butler, A.J. Variability of motor potentials evoked by transcranial magnetic stimulation depends on muscle activation. Exp. Brain Res. 2006, 174, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Devanathan, D.; Madhavan, S. Effects of anodal tDCS of the lower limb M1 on ankle reaction time in young adults. Exp. Brain Res. 2015, 234, 377–385. [Google Scholar] [CrossRef] [PubMed]
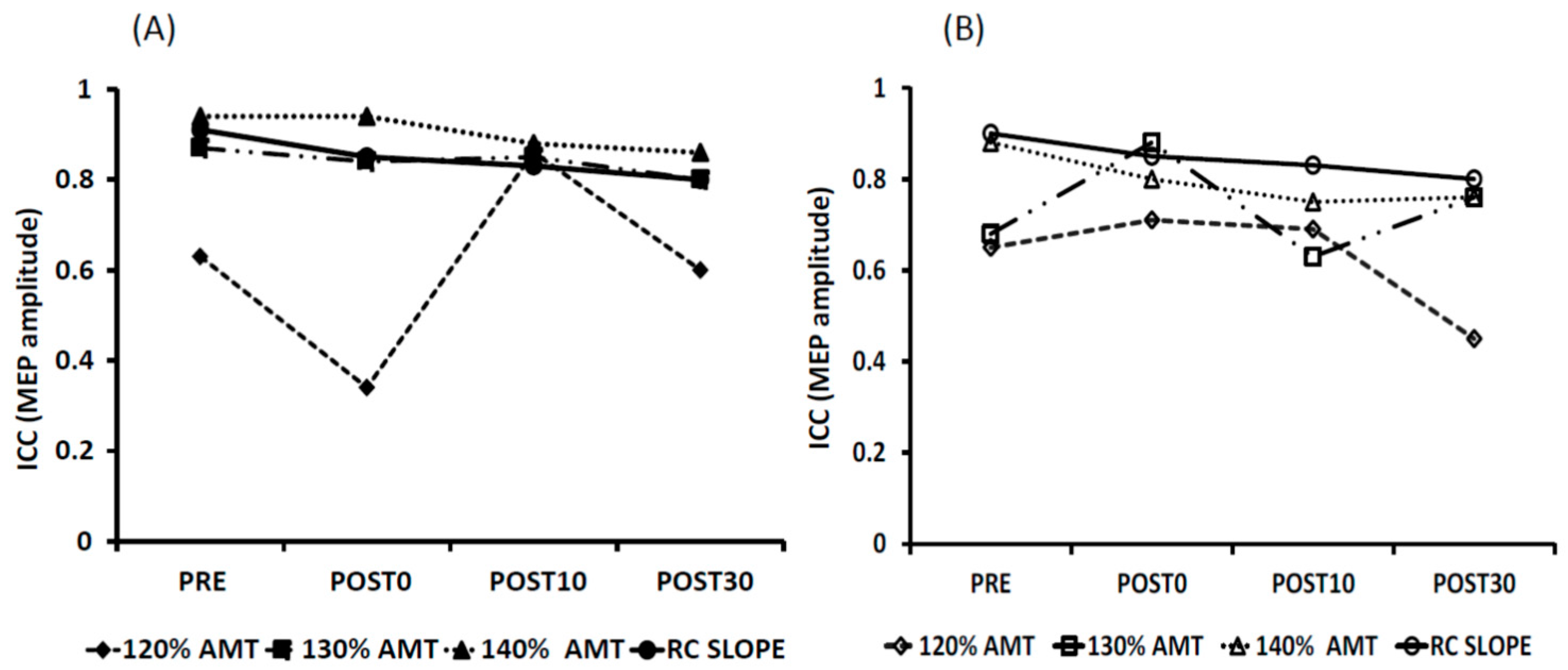

| Day 1 vs. Day 2 | PRE | POST0 | POST10 | POST30 | ||||
|---|---|---|---|---|---|---|---|---|
| tDCS-Task | tDCS-Rest | tDCS-Task | tDCS-Rest | tDCS-Task | tDCS-Rest | tDCS-Task | tDCS-Rest | |
| 120% AMT | 0.63 * | 0.65 * | 0.34 | 0.71 * | 0.86 * | 0.69 * | 0.60 * | 0.45 |
| 130% AMT | 0.87 * | 0.68 * | 0.84 * | 0.88 * | 0.85 * | 0.63 * | 0.76 * | 0.76 * |
| 140% AMT | 0.94 * | 0.88 * | 0.94 * | 0.80 * | 0.88 * | 0.73 * | 0.86 * | 0.76 * |
| RC SLOPE | 0.91 * | 0.81 * | 0.85 * | 0.80 * | 0.83 * | 0.63 * | 0.80 * | 0.72 * |
| Day 1 vs. Day 2 | PRE | POST0 | POST10 | POST30 | ||||
|---|---|---|---|---|---|---|---|---|
| tDCS-Task | tDCS-Rest | tDCS-Task | tDCS-Rest | tDCS-Task | tDCS-Rest | tDCS-Task | tDCS-Rest | |
| 120% AMT | 0.66 * | 0.85 * | 0.62 * | 0.82 * | 0.79 * | 0.86 * | 0.61 | 0.71 * |
| 130% AMT | 0.68 * | 0.69 * | 0.68 * | 0.89 * | 0.76 * | 0.67 * | 0.77 * | 0.81 * |
| 140% AMT | 0.88 * | 0.87 * | 0.87 * | 0.78 * | 0.85 * | 0.75 * | 0.87 * | 0.89 * |
| RC SLOPE | 0.85 * | 0.93 * | 0.76 * | 0.80 * | 0.78 * | 0.55 | 0.80 * | 0.86 * |
| tDCS-task | tDCS-rest | ||||
|---|---|---|---|---|---|
| R | NR | R | NR | ||
| 140% AMT | Day 1 | 8 | 7 | 8 | 7 |
| Day 2 | 10 | 5 | 6 | 9 | |
| Facilitation for both task conditions | 7 | ||||
| RC SLOPE | Day 1 | 11 | 4 | 6 | 9 |
| Day 2 | 9 | 6 | 7 | 8 | |
| Facilitation for both task conditions | 7 | ||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madhavan, S.; Sriraman, A.; Freels, S. Reliability and Variability of tDCS Induced Changes in the Lower Limb Motor Cortex. Brain Sci. 2016, 6, 26. https://doi.org/10.3390/brainsci6030026
Madhavan S, Sriraman A, Freels S. Reliability and Variability of tDCS Induced Changes in the Lower Limb Motor Cortex. Brain Sciences. 2016; 6(3):26. https://doi.org/10.3390/brainsci6030026
Chicago/Turabian StyleMadhavan, Sangeetha, Aishwarya Sriraman, and Sally Freels. 2016. "Reliability and Variability of tDCS Induced Changes in the Lower Limb Motor Cortex" Brain Sciences 6, no. 3: 26. https://doi.org/10.3390/brainsci6030026