The Scalp Confounds Near-Infrared Signal from Rat Brain Following Innocuous and Noxious Stimulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
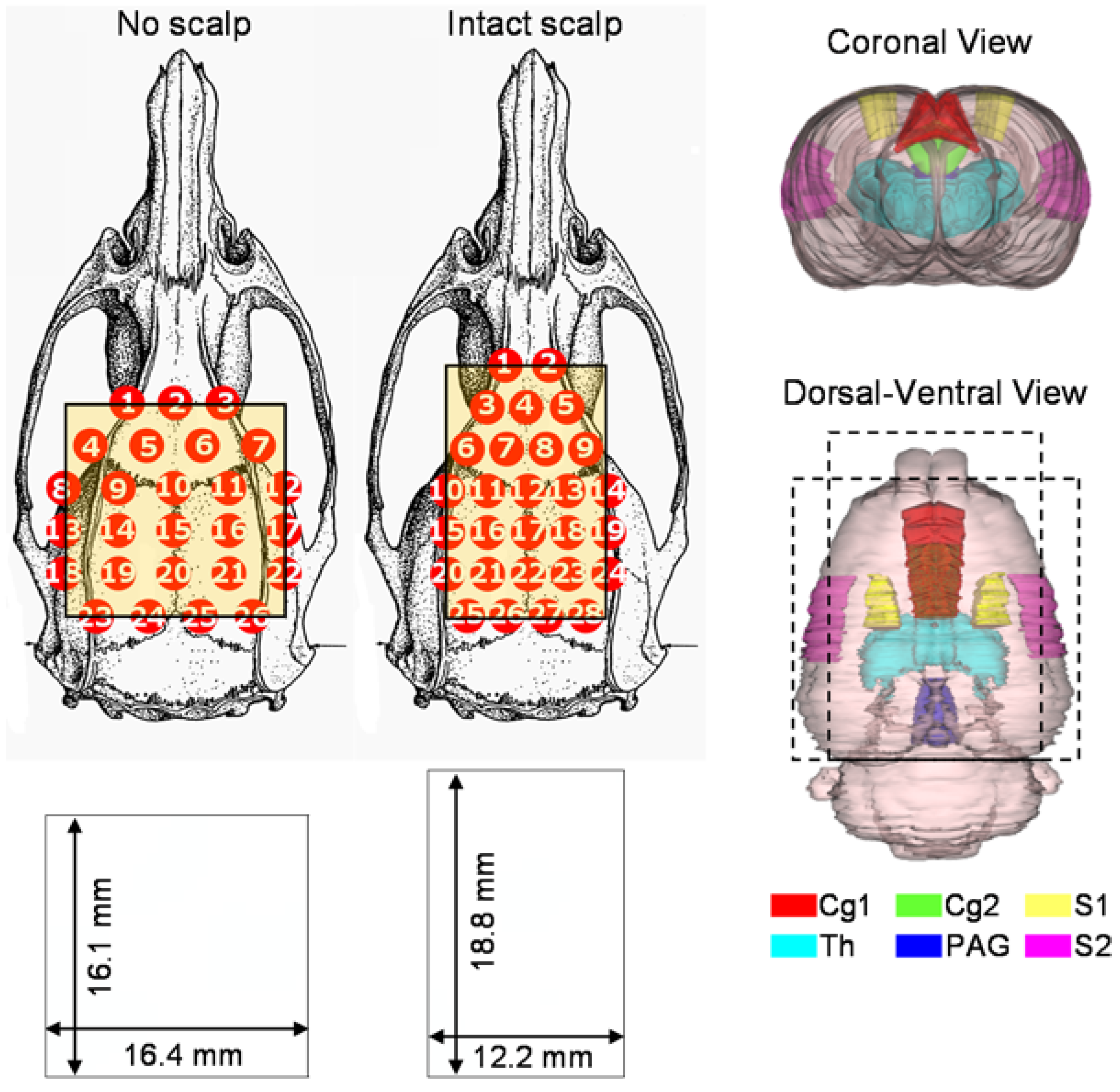
2.2. Animal Preparations
2.3. Brief Mechanical Stimulations
2.4. A Long-Lasting Noxious Stimulation by Formalin Injection
2.5. Statistical Analysis
3. Results
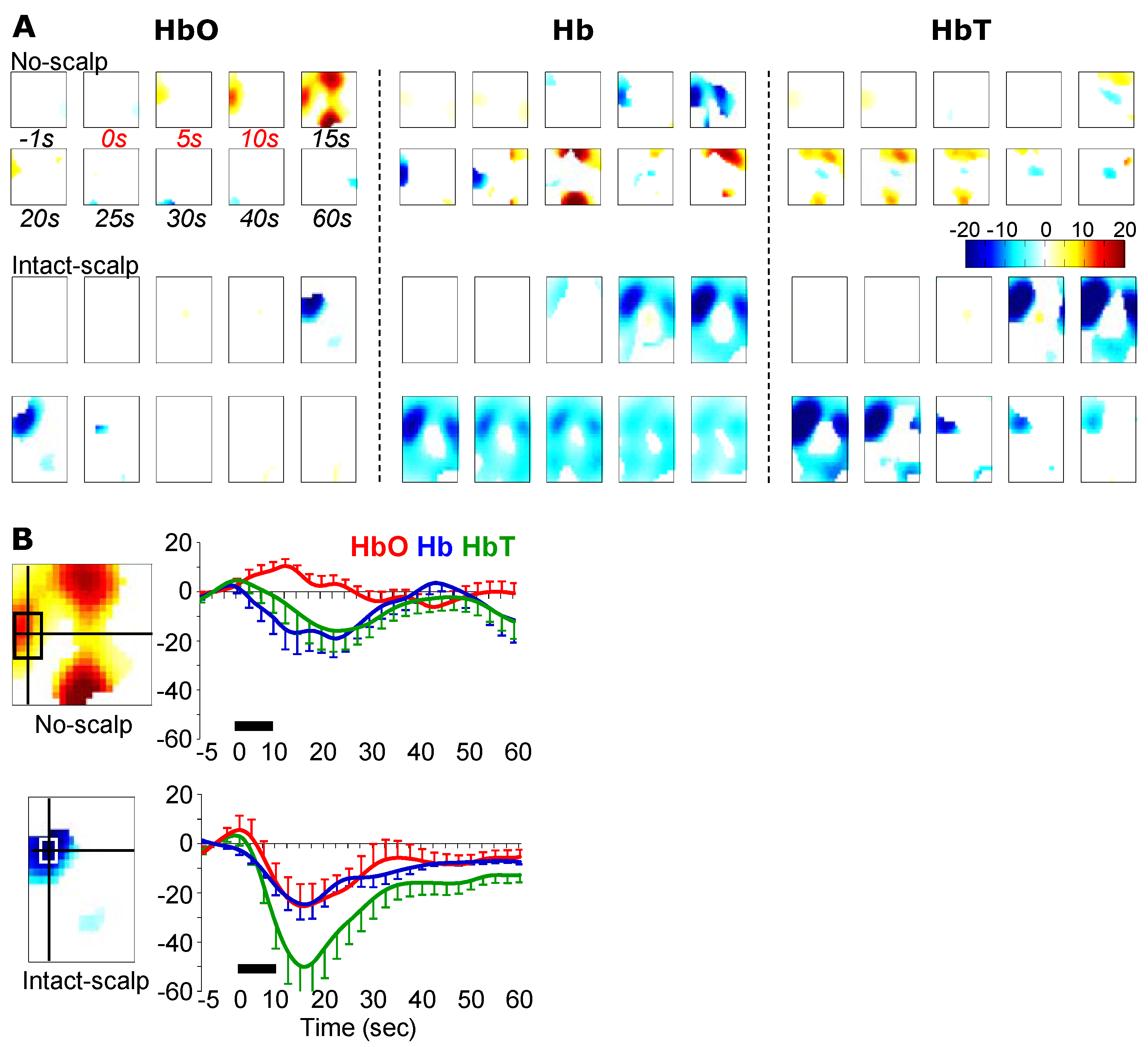
3.1. Formalin-Induced Hemodynamic Changes

3.2. Pinching-Induced Hemodynamic Changes
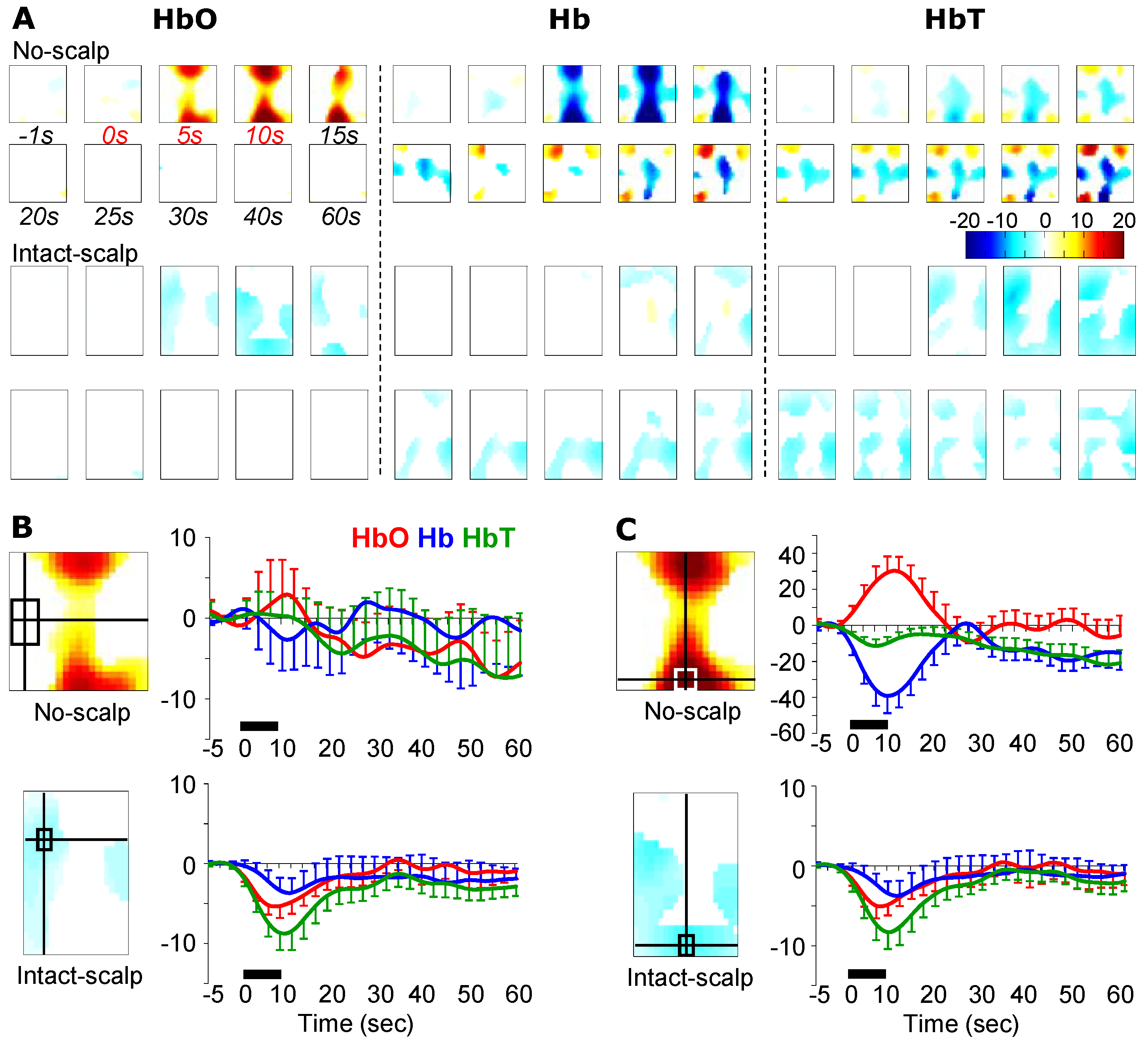
3.3. Brushing-Induced Hemodynamic Changes
4. Discussion
4.1. Limitations
4.2. A Potential Mechanism: Autonomic Arousal
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jobsis, F.F. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 1977, 198, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Kreisman, N.R.; Sick, T.J.; Rosenthal, M. Concepts of brain oxygen sufficiency during seizures. Adv. Exp. Med. Biol. 1984, 180, 381–392. [Google Scholar]
- Brazy, J.E.; Lewis, D.V.; Mitnick, M.H.; Jobsis vander Vliet, F.F. Noninvasive monitoring of cerebral oxygenation in preterm infants: Preliminary observations. Pediatrics 1985, 75, 217–225. [Google Scholar] [PubMed]
- Villringer, A.; Planck, J.; Hock, C.; Schleinkofer, L.; Dirnagl, U. Near infrared spectroscopy (nirs): A new tool to study hemodynamic changes during activation of brain function in human adults. Neurosci. Lett. 1993, 154, 101–104. [Google Scholar] [CrossRef]
- Mehagnoul-Schipper, D.J.; van der Kallen, B.F.; Colier, W.N.; van der Sluijs, M.C.; van Erning, L.J.; Thijssen, H.O.; Oeseburg, B.; Hoefnagels, W.H.; Jansen, R.W. Simultaneous measurements of cerebral oxygenation changes during brain activation by near-infrared spectroscopy and functional magnetic resonance imaging in healthy young and elderly subjects. Hum. Brain Mapp. 2002, 16, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, Y. Functional near-infrared optical imaging: Utility and limitations in human brain mapping. Psychophysiology 2003, 40, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Ranger, M.; Johnston, C.C.; Limperopoulos, C.; Rennick, J.E.; du Plessis, A.J. Cerebral near-infrared spectroscopy as a measure of nociceptive evoked activity in critically ill infants. Pain Res. Manag. 2011, 16, 331–336. [Google Scholar] [PubMed]
- Becerra, L.; Harris, W.; Joseph, D.; Huppert, T.; Boas, D.A.; Borsook, D. Diffuse optical tomography of pain and tactile stimulation: Activation in cortical sensory and emotional systems. NeuroImage 2008, 41, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Funane, T.; Atsumori, H.; Katura, T.; Obata, A.N.; Sato, H.; Tanikawa, Y.; Okada, E.; Kiguchi, M. Quantitative evaluation of deep and shallow tissue layers’ contribution to fnirs signal using multi-distance optodes and independent component analysis. NeuroImage 2014, 85, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Germon, T.J.; Evans, P.D.; Barnett, N.J.; Wall, P.; Manara, A.R.; Nelson, R.J. Cerebral near infrared spectroscopy: Emitter-detector separation must be increased. Br. J. Anaesth. 1999, 82, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Arridge, S.R. Optical tomography in medical imaging. Inverse Probl. 1999, 15, R41–R93. [Google Scholar] [CrossRef]
- He, J.W.; Tian, F.; Liu, H.; Peng, Y.B. Cerebrovascular responses of the rat brain to noxious stimuli as examined by functional near-infrared whole brain imaging. J. Neurophysiol. 2012, 107, 2853–2865. [Google Scholar] [CrossRef] [PubMed]
- Iversen, S.; Kupfermann, I.; Kandel, E.R. Emotional states and feelings. In Principles of Neural Science; Kandel, E.R., Schwartz, J.H., Jessell, T.M., Eds.; McGraw-Hill: New York, NY, USA, 2000; pp. 982–997. [Google Scholar]
- Chapman, C.R. Pain perception and assessment. Minerva Anestesiol. 2005, 71, 413–417. [Google Scholar] [PubMed]
- Terkelsen, A.J.; Molgaard, H.; Hansen, J.; Andersen, O.K.; Jensen, T.S. Acute pain increases heart rate: Differential mechanisms during rest and mental stress. Auton. Neurosci. Basic Clin. 2005, 121, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.N.; Bailey, S.M. Near infrared spectroscopy in adults. Does the invos 3100 really measure intracerebral oxygenation? Anaesthesia 1993, 48, 694–696. [Google Scholar] [CrossRef]
- Germon, T.J.; Young, A.E.; Manara, A.R.; Nelson, R.J. Extracerebral absorption of near infrared light influences the detection of increased cerebral oxygenation monitored by near infrared spectroscopy. J. Neurol. Neurosurg. Psychiatry 1995, 58, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Yucel, M.A.; Aasted, C.M.; Petkov, M.P.; Borsook, D.; Boas, D.A.; Becerra, L. Specificity of hemodynamic brain responses to painful stimuli: A functional near-infrared spectroscopy study. Sci. Rep. 2015, 5, 9469. [Google Scholar] [CrossRef]
- Li, A.; Zhang, Q.; Culver, J.P.; Miller, E.L.; Boas, D.A. Reconstructing chromosphere concentration images directly by continuous-wave diffuse optical tomography. Opt. Lett. 2004, 29, 256–258. [Google Scholar] [CrossRef]
- Tian, F.; Chance, B.; Liu, H. Investigation of the prefrontal cortex in response to duration-variable anagram tasks using functional near-infrared spectroscopy. J. Biomed. Opt. 2009, 14, 054016. [Google Scholar] [CrossRef]
- Huppert, T.J.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. Homer: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt. 2009, 48, D280–D298. [Google Scholar] [CrossRef] [PubMed]
- Kavuri, V.C.; Lin, Z.J.; Tian, F.; Liu, H. Sparsity enhanced spatial resolution and depth localization in diffuse optical tomography. Biomed. Opt. Express 2012, 3, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Xia, M.; Liu, H. Extinction coefficients of hemoglobin for near-infrared spectroscopy of tissue. IEEE Eng. Med. Biol. Mag. 2005, 24, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Anon R. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Choi, J.; Wolf, M.; Toronov, V.; Wolf, U.; Polzonetti, C.; Hueber, D.; Safonova, L.P.; Gupta, R.; Michalos, A.; Mantulin, W.; et al. Noninvasive determination of the optical properties of adult brain: Near-infrared spectroscopy approach. J. Biomed. Opt. 2004, 9, 221–229. [Google Scholar] [PubMed]
- Franceschini, M.A.; Gratton, E.; Hueber, D.; Fantini, S. Near-infrared absorption and scattering spectra of tissues in vivo. P. Soc. Photo-Opt. Ins. 1999, 3597, 526–531. [Google Scholar]
- Nowak, K.; Mix, E.; Gimsa, J.; Strauss, U.; Sriperumbudur, K.K.; Benecke, R.; Gimsa, U. Optimizing a rodent model of parkinson’s disease for exploring the effects and mechanisms of deep brain stimulation. Parkinson Dis. 2011, 2011, 414682. [Google Scholar] [CrossRef] [PubMed]
- Tremoleda, J.L.; Kerton, A.; Gsell, W. Anaesthesia and physiological monitoring during in vivo imaging of laboratory rodents: Considerations on experimental outcomes and animal welfare. EJNMMI Res. 2012, 2, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Masamoto, K.; Kanno, I. Anesthesia and the quantitative evaluation of neurovascular coupling. J. Cereb. Blood Flow Metab. 2012, 32, 1233–1247. [Google Scholar] [CrossRef] [PubMed]
- Bereiter, D.A.; Benetti, A.P.; Thrivikraman, K.V. Thermal nociception potentiates the release of acth and norepinephrine by blood loss. Am. J. Physiol. 1990, 259, R1236–R1242. [Google Scholar] [PubMed]
- Menendez, L.; Bester, H.; Besson, J.M.; Bernard, J.F. Parabrachial area: Electrophysiological evidence for an involvement in cold nociception. J. Neurophysiol. 1996, 75, 2099–2116. [Google Scholar] [PubMed]
- Allen, G.V.; Pronych, S.P. Trigeminal autonomic pathways involved in nociception-induced reflex cardiovascular responses. Brain Res. 1997, 754, 269–278. [Google Scholar] [CrossRef]
- Magerl, W.; Koltzenburg, M.; Schmitz, J.M.; Handwerker, H.O. Asymmetry and time-course of cutaneous sympathetic reflex responses following sustained excitation of chemosensitive nociceptors in humans. J. Auton. Nerv. Syst. 1996, 57, 63–72. [Google Scholar] [CrossRef]
- Collet, C.; Vernet-Maury, E.; Delhomme, G.; Dittmar, A. Autonomic nervous system response patterns specificity to basic emotions. J. Auton. Nerv. Syst. 1997, 62, 45–57. [Google Scholar] [CrossRef]
- Rainville, P.; Bechara, A.; Naqvi, N.; Damasio, A.R. Basic emotions are associated with distinct patterns of cardiorespiratory activity. Int. J. Psychophysiol. 2006, 61, 5–18. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.-W.; Liu, H.; Peng, Y.B. The Scalp Confounds Near-Infrared Signal from Rat Brain Following Innocuous and Noxious Stimulation. Brain Sci. 2015, 5, 387-399. https://doi.org/10.3390/brainsci5040387
He J-W, Liu H, Peng YB. The Scalp Confounds Near-Infrared Signal from Rat Brain Following Innocuous and Noxious Stimulation. Brain Sciences. 2015; 5(4):387-399. https://doi.org/10.3390/brainsci5040387
Chicago/Turabian StyleHe, Ji-Wei, Hanli Liu, and Yuan Bo Peng. 2015. "The Scalp Confounds Near-Infrared Signal from Rat Brain Following Innocuous and Noxious Stimulation" Brain Sciences 5, no. 4: 387-399. https://doi.org/10.3390/brainsci5040387




