Removal of Ionic Liquids from Oil Sands Processing Solution by Ion-Exchange Resin
Abstract
:Featured Application
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Ionic Liquid Solution Samples
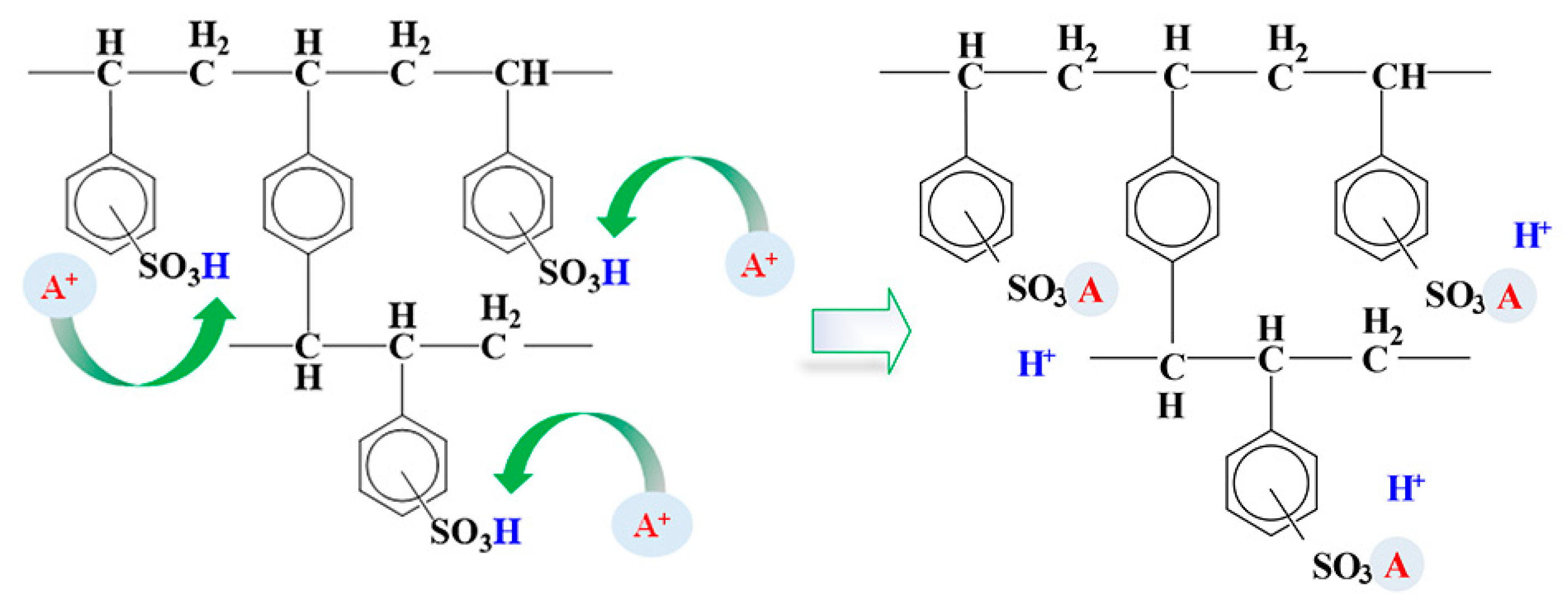
2.3. Adsorption of ILs onto Ion-Exchange Resins
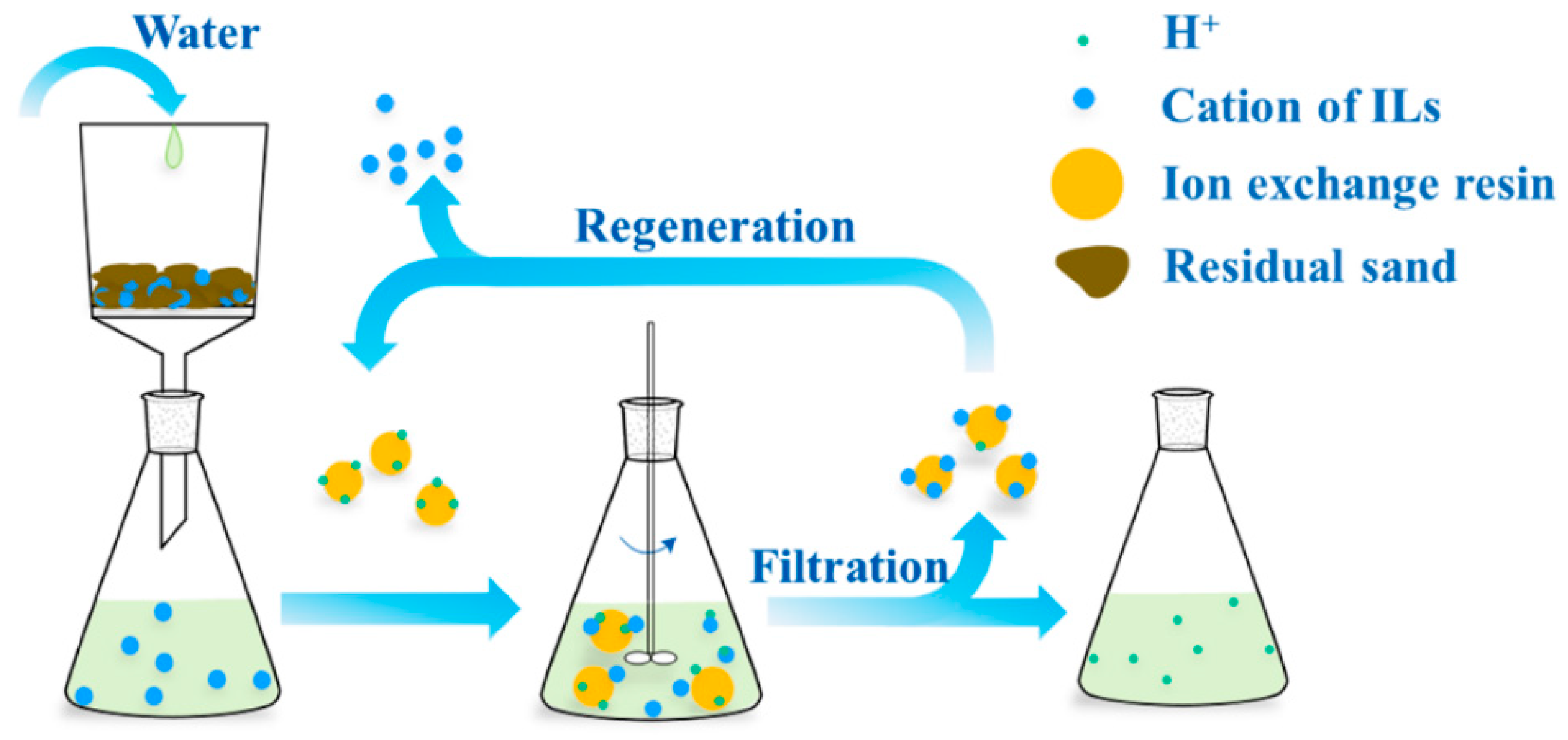
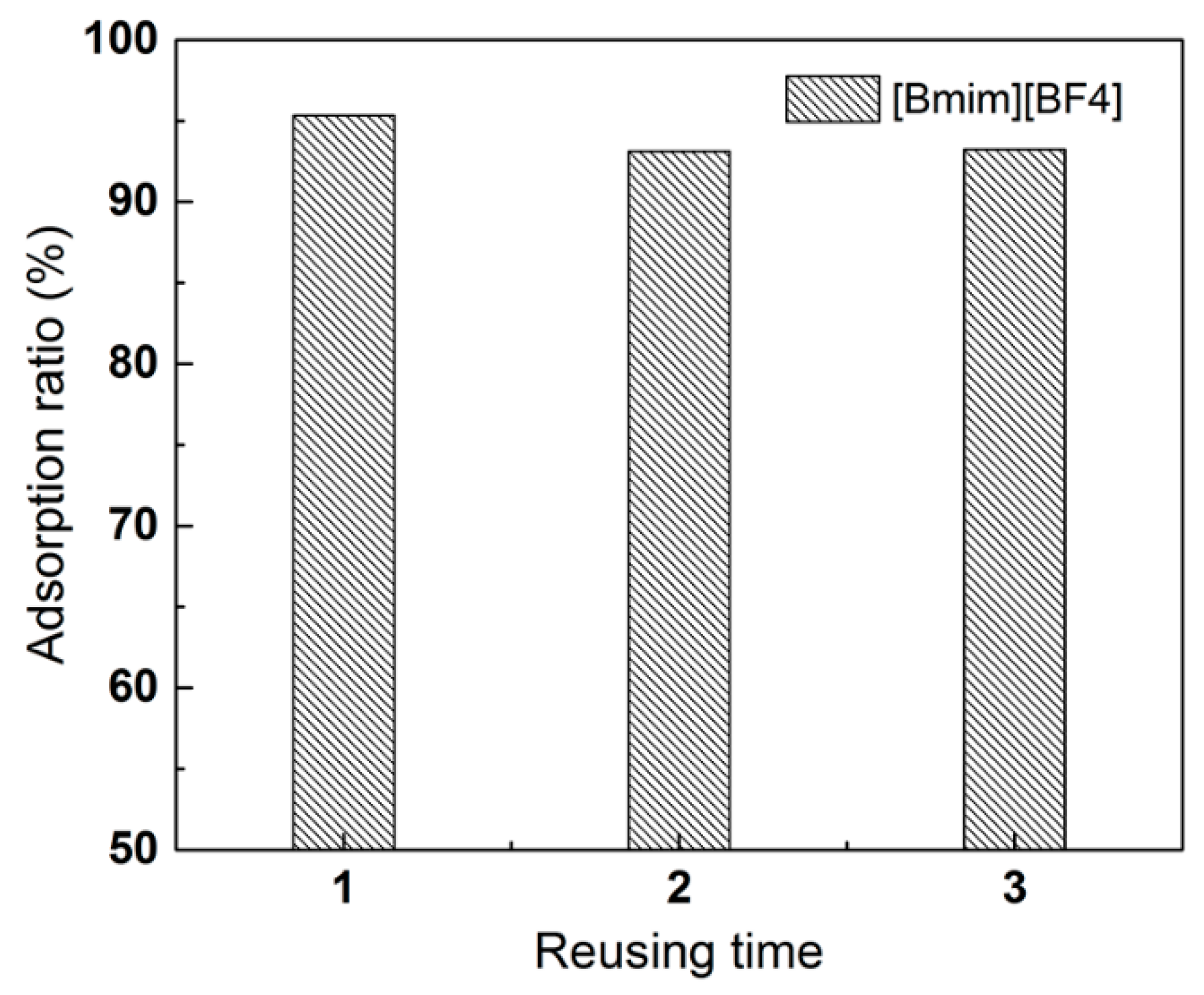
2.4. Regeneration and Reusing of Resins
2.5. Instrumental Analysis
3. Results and Discussion
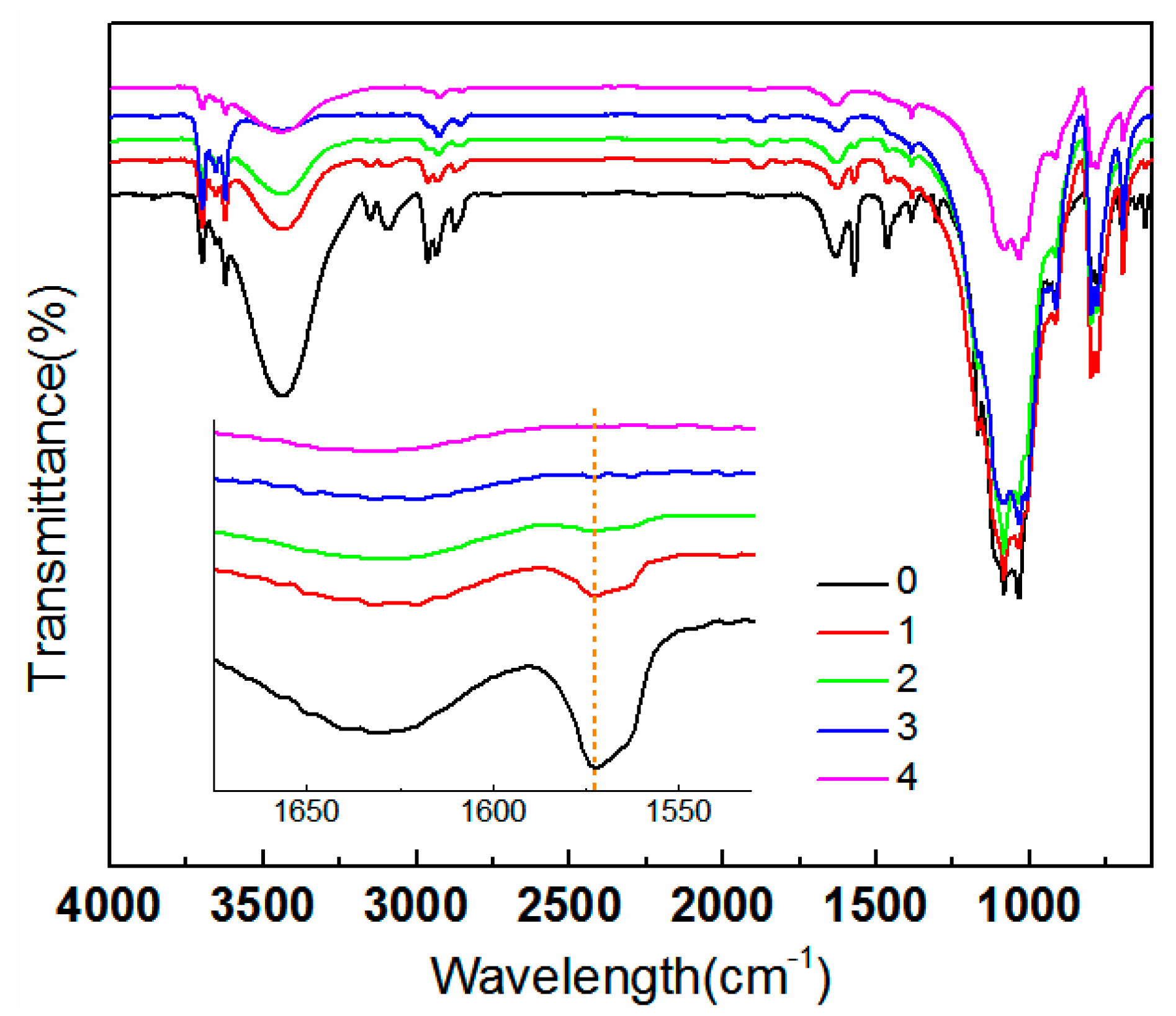
3.1. Effect of Washing Times
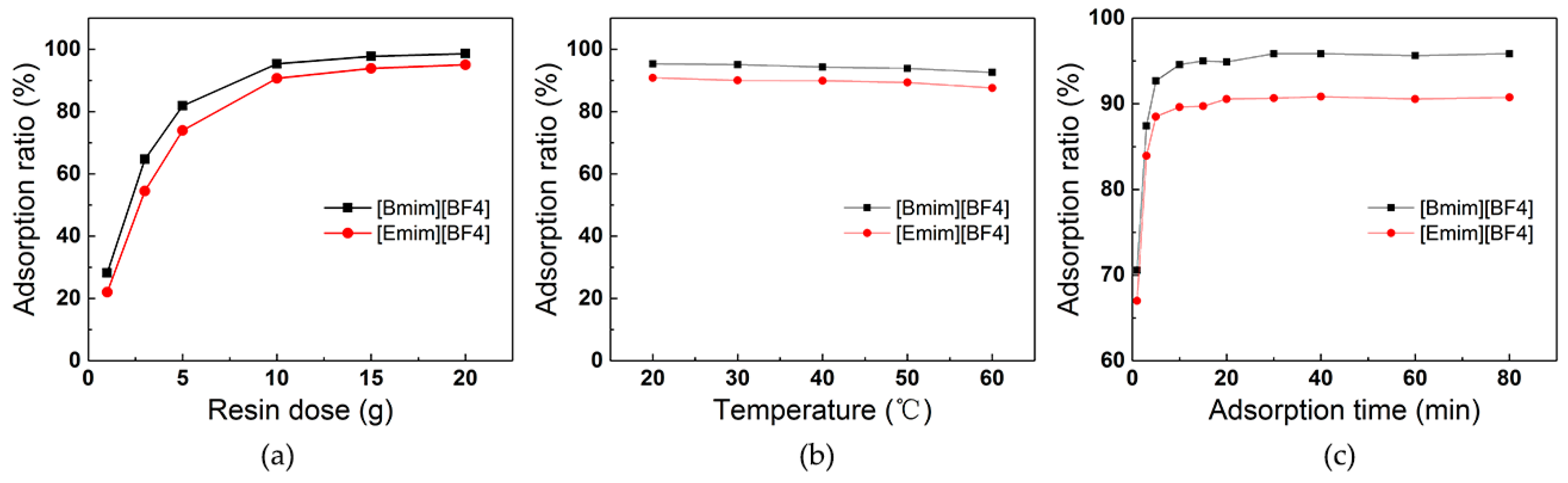
3.2. Effect of Operation Parameters on ILs Adsorption
3.3. Adsorption Isotherms
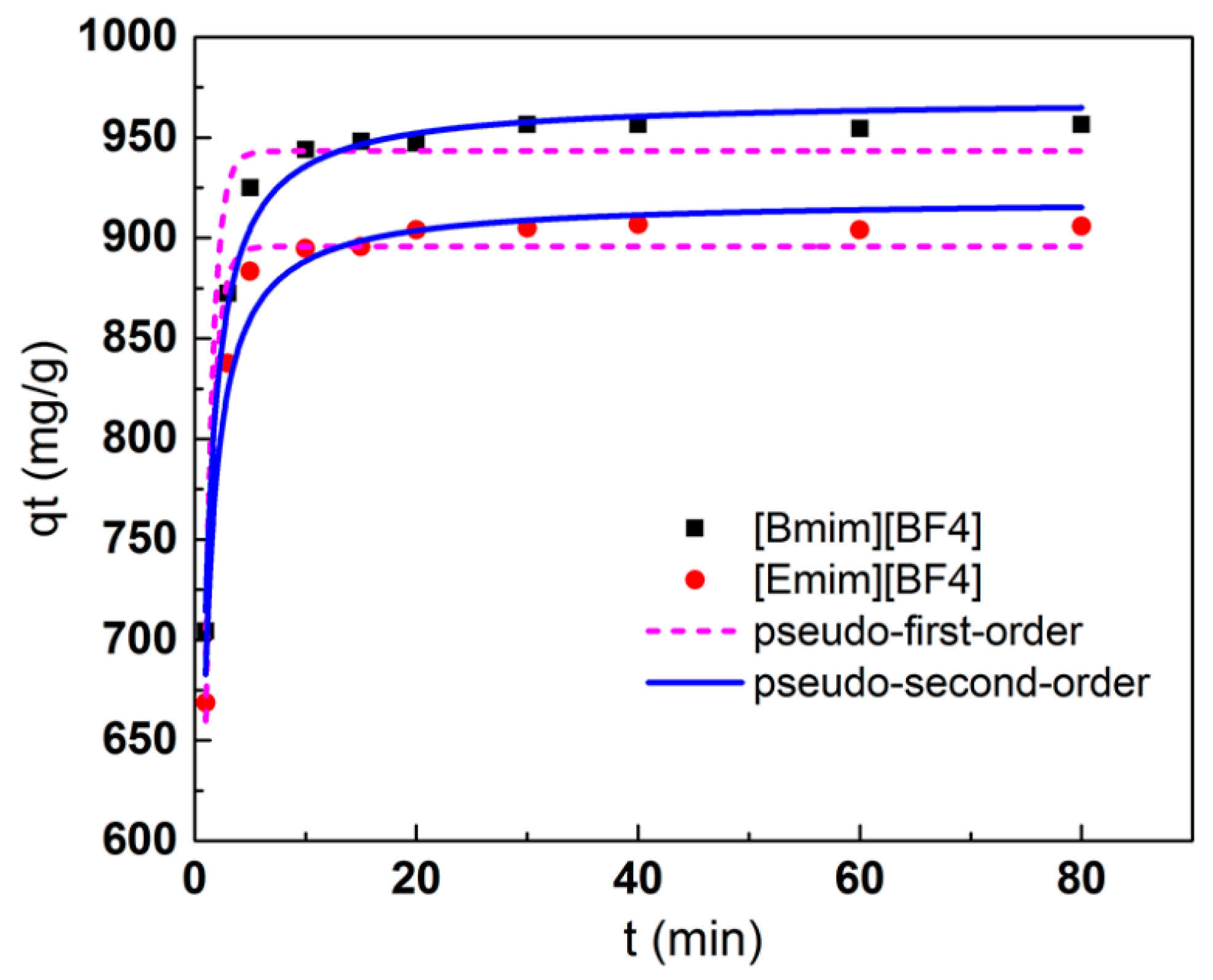
3.4. Adsorption Kinetics
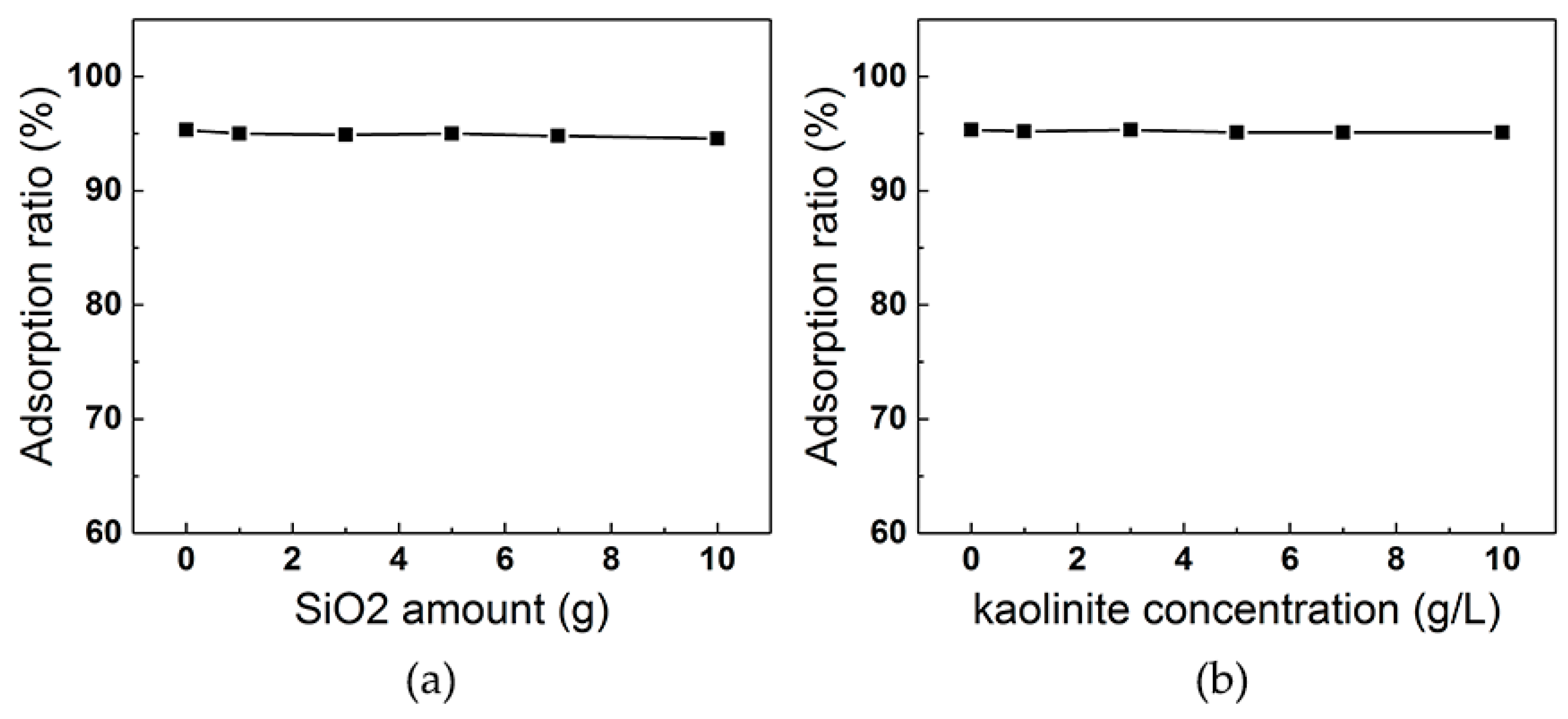
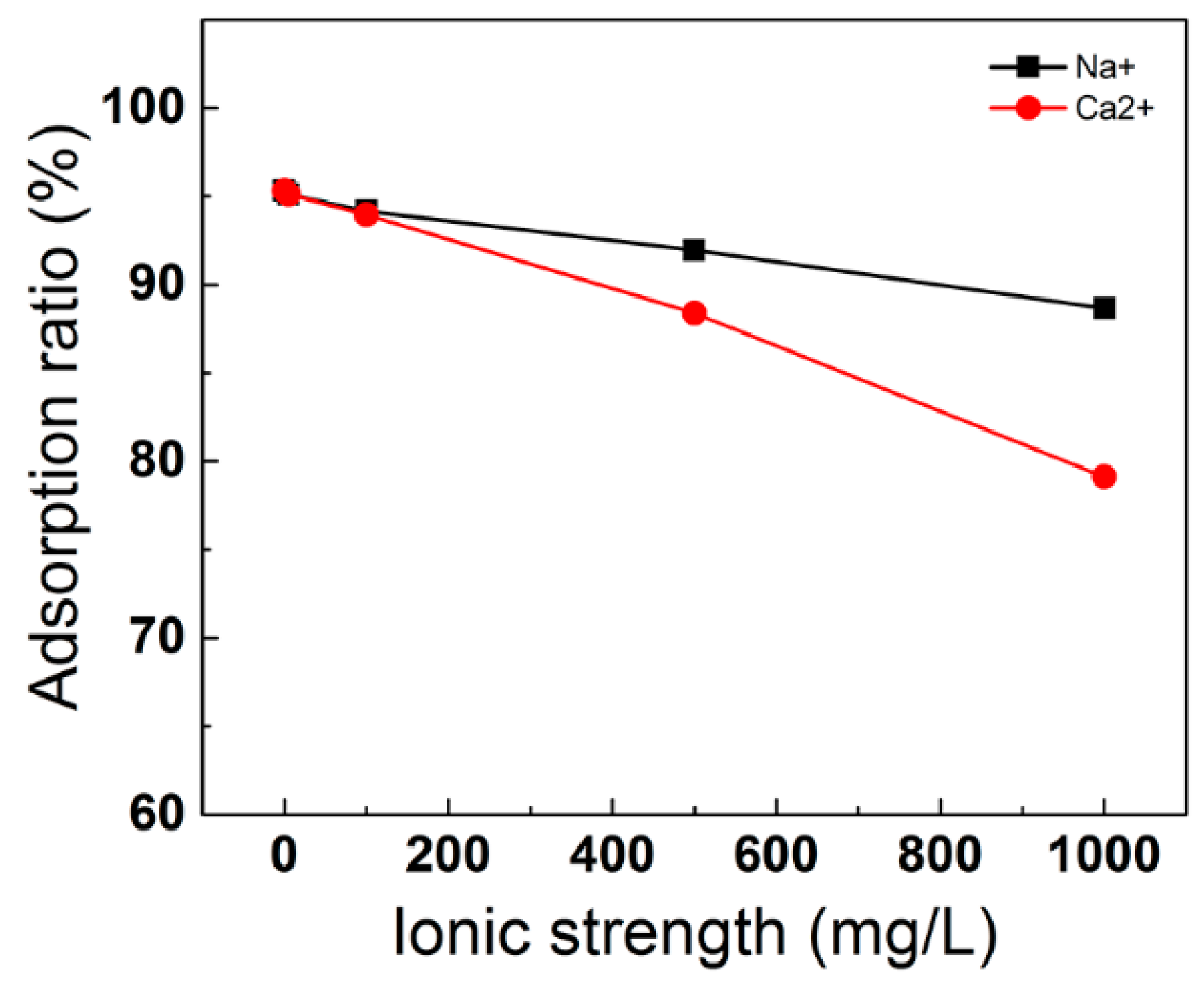
3.5. Effect of Background Chemicals on ILs Adsorption
3.5.1. Effect of Silica and Kaolinite
3.5.2. Effect of Ionic Strength
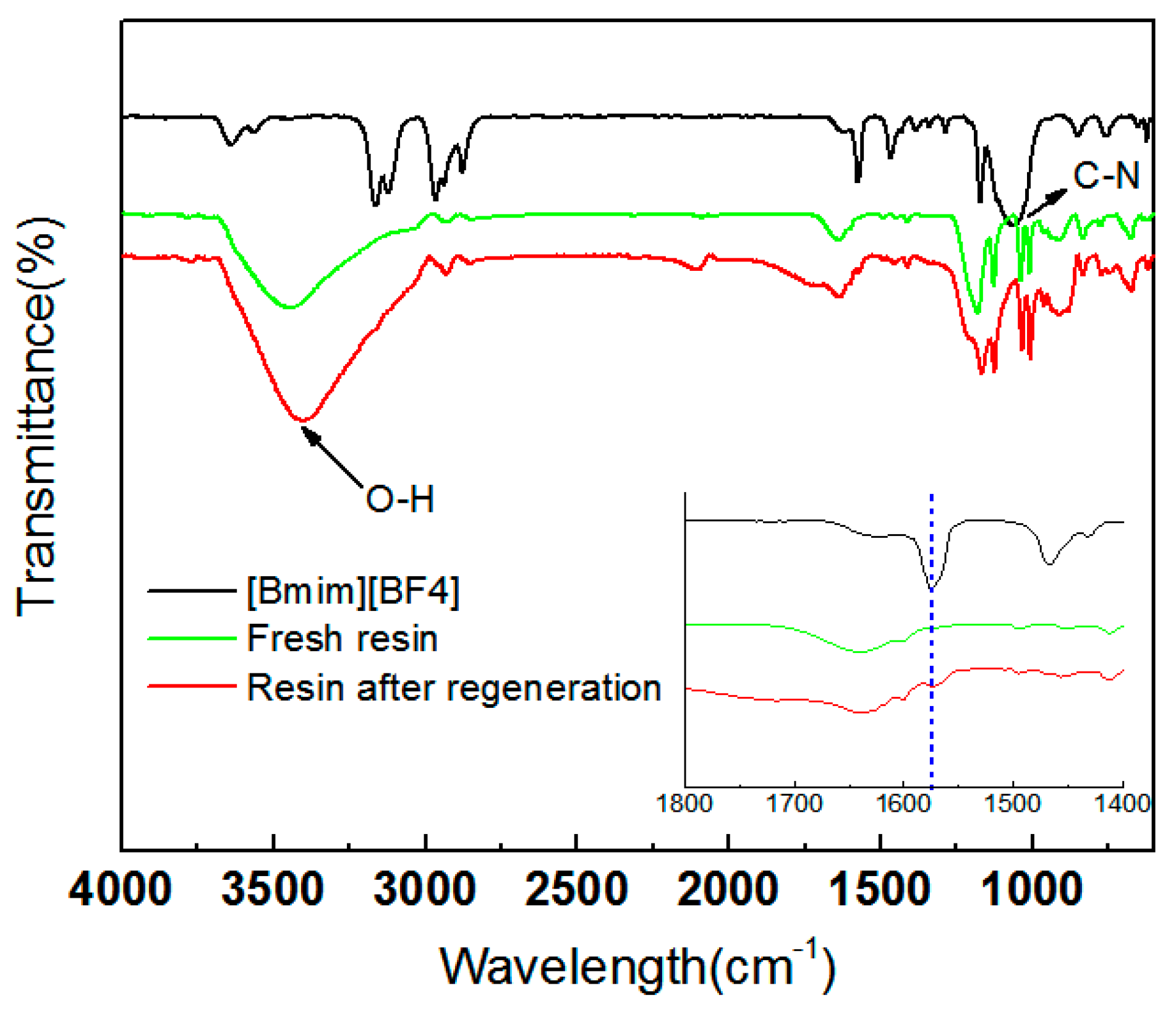
3.6. Recyclability of Ion-Exchange Resins
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Buszewski, B.; Studzińska, S. A review of ionic liquids in chromatographic and electromigration techniques. Chromatographia 2008, 68, 1–10. [Google Scholar] [CrossRef]
- Sheldon, R. Catalytic reactions in ionic liquids. Chem. Commun. 2001, 23, 2399–2407. [Google Scholar] [CrossRef]
- Li, X.; Sun, W.; Wu, G.; He, L.; Li, H.; Sui, H. Ionic liquid enhanced solvent extraction for bitumen recovery from oil sands. Energy Fuel 2011, 25, 5224–5231. [Google Scholar] [CrossRef]
- Painter, P.; Williams, P.; Lupinsky, A. Recovery of bitumen from utah tar sands using ionic liquids. Energy Fuel 2010, 24, 5081–5088. [Google Scholar] [CrossRef]
- Painter, P.; Williams, P.; Mannebach, E. Recovery of bitumen from oil or tar sands using ionic liquids. Energy Fuel 2010, 24, 1094–1098. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; He, L.; Sui, H.; Yin, W. Ionic liquid-assisted solvent extraction for unconventional oil recovery: Computational simulation and experimental tests. Energy Fuel 2016, 30, 7074–7081. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Grimes, S.; Cai, H.; Zhang, M. Study on the absorbability, regeneration characteristics and thermal stability of ionic liquids for vocs removal. Chem. Eng. J. 2017, 328, 353–359. [Google Scholar] [CrossRef]
- Ahmad, W.; Ostonen, A.; Jakobsson, K.; Uusi-Kyyny, P.; Alopaeus, V.; Hyväkkö, U.; King, A.W.T. Feasibility of thermal separation in recycling of the distillable ionic liquid [DBNH][OAc] in cellulose fiber production. Chem. Eng. Res. Des. 2016, 114, 287–298. [Google Scholar] [CrossRef]
- Blanchard, L.A.; Brennecke, J.F. Recovery of organic products from ionic liquids using supercritical carbon dioxide. Ind. Eng. Chem. Res. 2001, 40, 287–292. [Google Scholar] [CrossRef]
- Mukesh, C.; Mondal, D.; Sharma, M.; Prasad, K. Rapid dissolution of DNA in a novel bio-based ionic liquid with long-term structural and chemical stability: Successful recycling of the ionic liquid for reuse in the process. Chem. Commun. 2013, 49, 6849–6851. [Google Scholar] [CrossRef] [PubMed]
- Cláudio, A.F.M.; Marques, C.F.C.; Boal-Palheiros, I.; Freire, M.G.; Coutinho, J.A.P. Development of back-extraction and recyclability routes for ionic-liquid-based aqueous two-phase systems. Green Chem. 2014, 16, 259–268. [Google Scholar] [CrossRef]
- Gutowski, K.E.; Broker, G.A.; Willauer, H.D.; Huddleston, J.G.; Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Controlling the aqueous miscibility of ionic liquids: Aqueous biphasic systems of water-miscible ionic liquids and water-structuring salts for recycle, metathesis, and separations. J. Am. Chem. Soc. 2003, 125, 6632–6633. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Luo, J.; Zhang, X.; Wan, Y. Concentration of ionic liquids by nanofiltration for recycling: Filtration behavior and modeling. Sep. Purif. Technol. 2016, 165, 18–26. [Google Scholar] [CrossRef]
- Wu, H.; Shen, F.; Wang, J.; Luo, J.; Liu, L.; Khan, R.; Wan, Y. Separation and concentration of ionic liquid aqueous solution by vacuum membrane distillation. J. Membr. Sci. 2016, 518, 216–228. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Fu, H.; Zhang, H.; Liu, H.; Wan, Y.; Zheng, S.; Xu, Z. Microporous zeolite-templated carbon as an adsorbent for the removal of long alkyl-chained imidazolium-based ionic liquid from aqueous media. Microporous Microporous Mater. 2018, 260, 59–69. [Google Scholar] [CrossRef]
- Lemus, J.; Moya, C.; Gilarranz, M.A.; Rodriguez, J.J.; Palomar, J. Fixed-bed adsorption of ionic liquids onto activated carbon from aqueous phase. J. Environ. Chem. Eng. 2017, 5, 5347–5351. [Google Scholar] [CrossRef]
- Lemus, J.; Palomar, J.; Gilarranz, M.A.; Rodriguez, J.J. On the kinetics of ionic liquid adsorption onto activated carbons from aqueous solution. Ind. Eng. Chem. Res. 2013, 52, 2969–2976. [Google Scholar] [CrossRef]
- Palomar, J.; Lemus, J.; Gilarranz, M.A.; Rodriguez, J.J. Adsorption of ionic liquids from aqueous effluents by activated carbon. Carbon 2009, 47, 1846–1856. [Google Scholar] [CrossRef]
- Min, Y.; Zhou, Y.; Zhang, M.; Qiao, H.; Huang, Q.; Ma, T. Removal of ionic liquid by engineered bentonite from aqueous solution. J. Taiwan Inst. Chem. E 2015, 53, 153–159. [Google Scholar] [CrossRef]
- Won, S.W.; Choi, S.B.; Mao, J.; Yun, Y.S. Removal of 1-ethyl-3-methylimidazolium cations with bacterial biosorbents from aqueous media. J. Hazard. Mater. 2013, 244–245, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.B.; Won, S.W.; Yun, Y.-S. Use of ion-exchange resins for the adsorption of the cationic part of ionic liquid, 1-ethyl-3-methylimidazolium. Chem. Eng. J. 2013, 214, 78–82. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Qi, X. Adsorption of imidazolium-based ionic liquids with different chemical structures onto various resins from aqueous solutions. RSC Adv. 2015, 5, 41352–41358. [Google Scholar] [CrossRef]
- Ma, C.H.; Zu, Y.G.; Yang, L.; Li, J. Two solid-phase recycling method for basic ionic liquid [C4mim]AC by macroporous resin and ion exchange resin from schisandra chinensis fruits extract. J. Chromatogr. B 2015, 976–977, 1–5. [Google Scholar] [CrossRef] [PubMed]
- He, A.; Dong, B.; Feng, X.; Yao, S. Recovery of benzothiazolium ionic liquids from the coexisting glucose by ion-exchange resins. J. Mol. Liq. 2017, 227, 178–183. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, T.; Zhang, Y.; Wang, H.; Yu, M. The physical properties of aqueous solutions of the ionic liquid [Bmim][BF4]. J. Solut. Chem. 2006, 35, 1337–1346. [Google Scholar] [CrossRef]
- Vasiliu, S.; Bunia, I.; Racovita, S.; Neagu, V. Adsorption of cefotaxime sodium salt on polymer coated ion exchange resin microparticles: Kinetics, equilibrium and thermodynamic studies. Carbohydr. Polym. 2011, 85, 376–387. [Google Scholar] [CrossRef]
- Shek, T.; Ma, A.; Lee, V.; McKay, G. Kinetics of zinc ions removal from effluents using ion exchange resin. Chem. Eng. J. 2009, 146, 63–70. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Altintas, B.; Arica, M.Y. Adsorption kinetics and thermodynamic parameters of cationic dyes from aqueous solutions by using a new strong cation-exchange resin. Chem. Eng. J. 2009, 152, 339–346. [Google Scholar] [CrossRef]
- Pehlivan, E.; Altun, T. The study of various parameters affecting the ion exchange of Cu2+, Zn2+, Ni2+, Cd2+, and Pb2+ from aqueous solution on dowex 50w synthetic resin. J. Hazard. Mater. 2006, 134, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A.; Pehlivan, E.; Gode, F.; Altun, T.; Arslan, G. Adsorption of Cu(ii), Zn(ii), Ni(ii), Pb(ii), and Cd(ii) from aqueous solution on Amberlite IR-120 synthetic resin. J. Colloid Interface Sci. 2005, 282, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Naushad, M.; Alothman, Z.A.; Sharma, G. Kinetics, isotherm and thermodynamic investigations for the adsorption of Co(ii) ion onto crystal violet modified Amberlite IR-120 resin. Ionics 2014, 21, 1453–1459. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Uber die adsorption in losungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Ushiki, I.; Tashiro, M.; Smith, R.L. Measurement and modeling of adsorption equilibria of imidazolium-based ionic liquids on activated carbon from aqueous solutions. Fluid Phase Equilib. 2017, 441, 17–23. [Google Scholar] [CrossRef]
- Stepnowski, P.; Mrozik, W.; Nichthauser, J. Adsorption of alkylimidazolium and alkylpyridinium ionic liquids onto natural soils. Environ. Sci. Technol. 2007, 41, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Sips, R. Combined form of langmuir and freundlich equations. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Umpleby, R.J.; Baxter, S.C.; Chen, Y.; Shah, R.N.; Shimizu, K.D. Characterization of molecularly imprinted polymers with the langmuir−freundlich isotherm. Anal. Chem. 2001, 73, 4584–4591. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Sun, L.; Zhou, Y.; Gao, B.; Gao, W.; Bao, C.; Feng, C.; Li, Y. Biosorbents based on agricultural wastes for ionic liquid removal: An approach to agricultural wastes management. Chemosphere 2016, 165, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-S. Second-order kinetic model for the sorption of cadmium onto tree fern: A comparison of linear and non-linear methods. Water Res. 2006, 40, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, F.; Jing, X.; Ling, P.; Li, A. Displacement mechanism of binary competitive adsorption for aqueous divalent metal ions onto a novel ida-chelating resin: Isotherm and kinetic modeling. Water Res. 2011, 45, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Hepler, L.G.; Smith, R.G. The Alberta Oil Sands: Industrial Procedures for Extraction and Some Recent Fundamental Research; AOSTRA Technical Publication Series #14; Alberta Oil Sands Technology and Research Authority: Edmonton, AB, Canada, 1994. [Google Scholar]
- Dang-Vu, T.; Jha, R.; Wu, S.-Y.; Tannant, D.D.; Masliyah, J.; Xu, Z. Wettability determination of solids isolated from oil sands. Colloid Surf. A 2009, 337, 80–90. [Google Scholar] [CrossRef]
- Matzke, M.; Thiele, K.; Muller, A.; Filser, J. Sorption and desorption of imidazolium based ionic liquids in different soil types. Chemosphere 2009, 74, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, W.; Alvarez, P.J.J.; Qu, X.; Fu, H.; Zheng, S.; Xu, Z.; Zhu, D. Oxidized template-synthesized mesoporous carbon with ph-dependent adsorption activity: A promising adsorbent for removal of hydrophilic ionic liquid. Appl. Surf. Sci. 2018, 440, 821–829. [Google Scholar] [CrossRef]
- Lan, Q.; Bassi, A.S.; Zhu, J.-X.; Margaritis, A. A modified langmuir model for the prediction of the effects of ionic strength on the equilibrium characteristics of protein adsorption onto ion exchange/affinity adsorbents. Chem. Eng. J. 2001, 81, 179–186. [Google Scholar] [CrossRef]
- Wu, J.; Wang, M.-J.; Stark, J.P.W. Evaluation of band structure and concentration of ionic liquid bmimbf4 in molecular mixtures by using second derivatives of ftir spectra. J. Quant. Spectrosc. Radait. Transf. 2006, 102, 228–235. [Google Scholar] [CrossRef]
- Elabd, A.A.; Zidan, W.I.; Abo-Aly, M.M.; Bakier, E.; Attia, M.S. Uranyl ions adsorption by novel metal hydroxides loaded Amberlite IR 120. J. Environ. Radioact. 2014, 134, 99–108. [Google Scholar] [CrossRef] [PubMed]

| Matrix | Type | Functional Group | Total Exchange Capacity | Ionic Form as Shipped | Particle Size | Moisture Content | Shipping Weight | Physical Form |
|---|---|---|---|---|---|---|---|---|
| Styrene divinylbenzene copolymer | Gel | Sulfonic acid | ≥2.0 eq/L (Na+ form) | Na+ | 0.6~0.8 mm | 45~50% | 840 g/L | Amber spherical beads |
| Ionic Liquids | 1-Butyl-3-Methylimidazolium Tetrafluororate | 1-Ethyl-3-Methylimidazolium Tetrafluororate |
|---|---|---|
| Molecular structure | 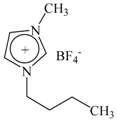 | 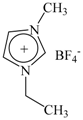 |
| Acronym | [Bmim][BF4] | [Emim][BF4] |
| Chemical formula | C8H15BF4N2 | C6H11BF4N2 |
| Molecular weight (g/mol) | 226.02 | 197.97 |
| Viscosity (cP) | 153.8 (298.1 K) | 66.5 (298.1 K) |
| Density (g/mL) | 1.171 (298.1 K) [28] | 1.248 (298.1 K) [6] |
| ILs | Washing Times | Residual Sands (w/w %) | ||||
|---|---|---|---|---|---|---|
| C | O | Si | Al | F | ||
| [Bmim][BF4] | 2 | 5.6 | 51.6 | 38.5 | 3.0 | 1.3 |
| 3 | 7.4 | 46.2 | 43.1 | 3.3 | - | |
| 4 | 10.5 | 43.1 | 39.4 | 7.0 | - | |
| [Emim][BF4] | 2 | 6.5 | 47.3 | 38.9 | 6.0 | 1.3 |
| 3 | 11.2 | 46.3 | 36.0 | 6.5 | - | |
| 4 | 12.1 | 42.5 | 43.5 | 1.9 | - | |
| Ionic Liquids | Langmuir Model | Freundlich Model | Sips Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| qm (mg/g) | KL (mL/mg) | RL | R2 | KF | 1/n | R2 | qm (mg/g) | KS (10−2) | 1/p | R2 | |
| [Bmim][BF4] | 1577.7 | 4.24 | 0.105 | 0.96 | 144.3 | 0.300 | 0.98 | 2524.1 | 2.67 | 0.512 | 0.99 |
| [Emim][BF4] | 1365.8 | 2.31 | 0.178 | 0.98 | 81.5 | 0.341 | 0.98 | 2100.9 | 1.47 | 0.569 | 0.99 |
| Ionic Liquids | Pseudo-First-Order Model | Pseudo-Second-Order Model | |||||
|---|---|---|---|---|---|---|---|
| qe,cal (mg/g) | k1 (min–1) | R2 | qe,cal (mg/g) | k2 (10−3g/(mg·min)) | h | R2 | |
| [Bmim][BF4] | 943.3 | 3.05 | 0.921 | 969.0 | 2.90 | 2722.9 | 0.984 |
| [Emim][BF4] | 895.8 | 3.07 | 0.945 | 919.3 | 3.14 | 2653.7 | 0.972 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, H.; Zhou, J.; Ma, G.; Niu, Y.; Cheng, J.; He, L.; Li, X. Removal of Ionic Liquids from Oil Sands Processing Solution by Ion-Exchange Resin. Appl. Sci. 2018, 8, 1611. https://doi.org/10.3390/app8091611
Sui H, Zhou J, Ma G, Niu Y, Cheng J, He L, Li X. Removal of Ionic Liquids from Oil Sands Processing Solution by Ion-Exchange Resin. Applied Sciences. 2018; 8(9):1611. https://doi.org/10.3390/app8091611
Chicago/Turabian StyleSui, Hong, Jingjing Zhou, Guoqiang Ma, Yaqi Niu, Jing Cheng, Lin He, and Xingang Li. 2018. "Removal of Ionic Liquids from Oil Sands Processing Solution by Ion-Exchange Resin" Applied Sciences 8, no. 9: 1611. https://doi.org/10.3390/app8091611





