1. Introduction
Polyurethanes (PUs) are polymers composed of a soft segment and a hard segment prepared by the reaction between hard isocyanates and soft polyols. Polypeptides of the hard segments form strong hydrogen interactions with each other. In contrast, soft segments comprising linear long-chain polyols have flexible properties. The physical properties of synthesized polyurethanes depend on the reaction ratio between isocyanate and polyol and the types of reactants, such as polyols, chain extenders, or isocyanates. Therefore, a variety of synthetic polyurethanes prepared with various combinations of reactants have been widely adopted in automotive interior materials, adhesives, coatings, shoes, clothing, etc.
However, the low thermal stabilities of polyurethanes limit their use in high-temperature processes or applications. Furthermore, polyurethanes begin to decompose at temperatures >180 °C, resulting in the production of toxic gases, such as HCN or CO, that are harmful to the human body or environment [
1,
2,
3,
4].
Recently, researchers have been interested in introducing flame retardants into polyurethanes to compensate for the low thermal stability. The flame retardants can be classified into two types: an additive type, in which a flame retardant is physically added to the polymeric resin, and a reactive type, in which a flame retardant reacts with reactant monomers, forming polymers. In general, the most often used additive flame retardants need to be highly miscible with the raw materials and not deteriorate the mechanical properties of the produced polymers nor produce any toxic gases.
Since environmental regulations prohibit the use of halogenated flame retardants, phosphoric flame retardants have attracted attention as one alternative. In particular, the flame retardancy of polyurethane is improved by introducing a monomer having flame retardancy into the main chain, using a method of encapsulating the flame retardant at the end of the polyurethane or by using organic–inorganic additives [
5,
6,
7,
8].
Epoxy resins are thermosetting polymers constructed of three-dimensional network structures after a curing reaction with hardeners in the presence of catalysts. Epoxy resin derivatives can exhibit various physical properties by reacting with different hardeners, and they are widely used in coatings, adhesives, and moldings because the polymerized matrices provide excellent thermal and mechanical properties and chemical resistance [
9,
10]. However, the high brittleness of cured epoxy polymers promotes easy crack propagation. Therefore, numerous scientists have reported methods to reduce this brittleness by adding thermoplastic resin, rubber resins, or elastomeric polyurethanes [
11,
12]. Specifically, polyurethanes in epoxy compositions, which are known to be phase separated after curing, have been added to absorb external impact [
13,
14].
In this report, a phosphoric monomer was reacted with an epoxy resin to prepare a reactive polyol. The polyol mixed with a polyether polyol in a 1:1 ratio was reacted with 2 mol of isophorone diisocyanate to enhance thermal resistance. In addition, the prepared polyurethane was added to epoxy compositions to investigate physical changes, where bisphenol A epoxy resin and dicyandiamide (dicy) were stoichiometrically blended. The effects of the prepared polyurethane addition to the epoxy compositions were further analyzed with tensile and flexural strength measurements, the Izod impact method, thermogravimetric analysis, and scanning electron microscopy.
2. Materials and Methods
2.1. Materials
The phosphorous flame retardant 10-(2,5-dihydroxyphenyl)-10H-9-oxa-10-phospha-phenanthrene-10-oxide (DOPO) was obtained from Pharmicell Co., Ltd. (Ulsan, Korea) and 1,6-Hexanediol glycidyl ether (HDGE) was purchased from HJ Chemco (Ulsan, Korea). Tetrabutylammonium iodide (TBAI), polytetrahydrofuran (PolyTHF) (Mn = 2000 g/mol), isophorone diisocyanate (IPDI), and 2-allyl phenol (2-AP, 98%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dibutyltindilaurylmercaptide (DBTDL) was obtained from Gelest Co (Morrisville, PA, USA). Bisphenol A epoxy resin (DGEBA, 187 g/eq) was purchased from Momentive Co (USA). The hardener, dicyandiamide, and 1,1-dimethyl-3-phenyl urea (DPU) were obtained from Air Products (Arlington, PA, USA).
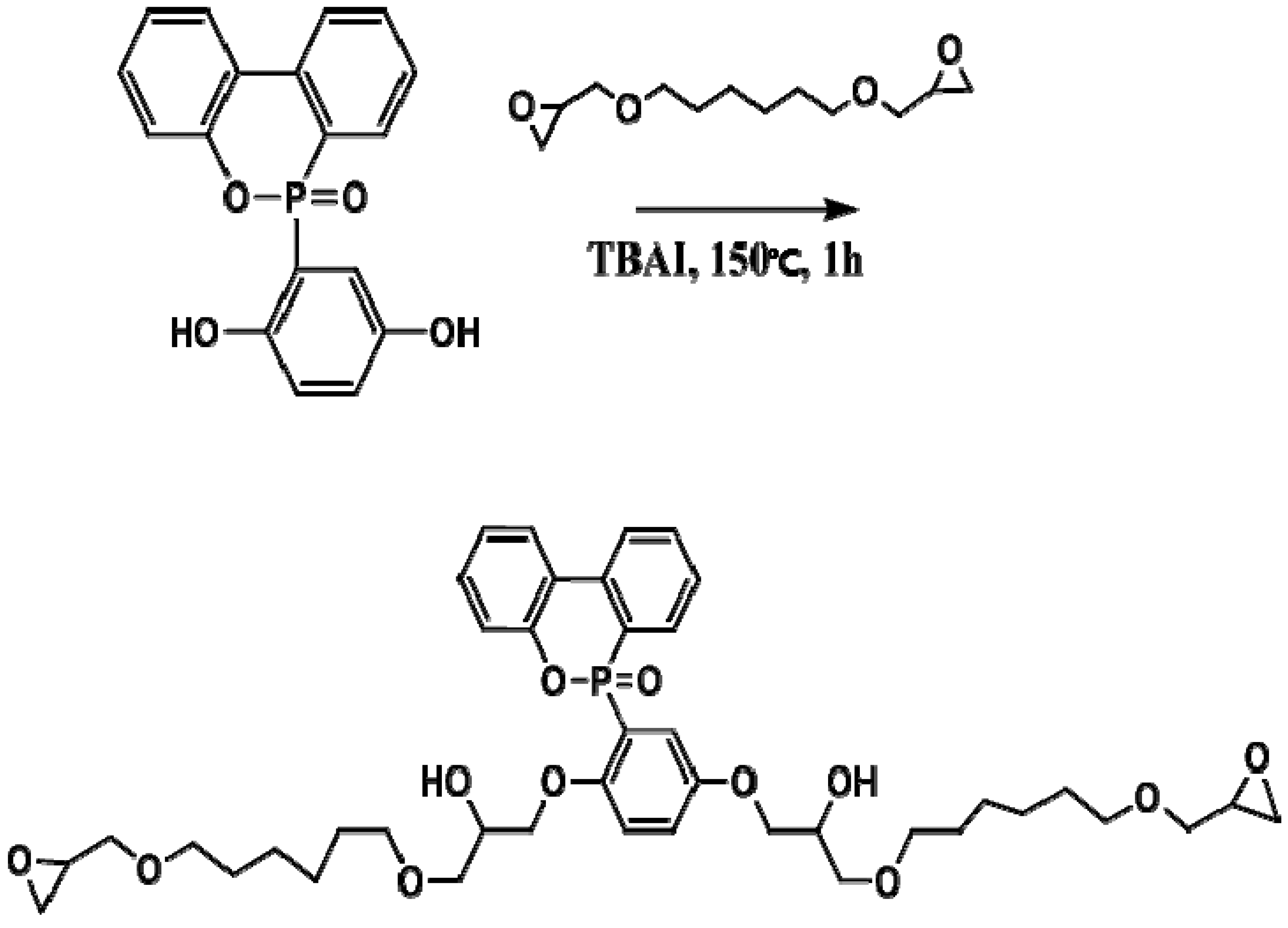
2.2. Preparation of Polyol (EP-DOPO)
DOPO (64.8 g) and HDGE (92 g) were poured into a 500 mL beaker and stirred at 60 °C until the DOPO completely dissolved. TBAI, a catalyst, was added to the beaker, which was heated at 150 °C for 1 h (
Figure 1). The product was cooled to room temperature and characterized (
Figure 2).
2.3. Synthesis of Polyurethane
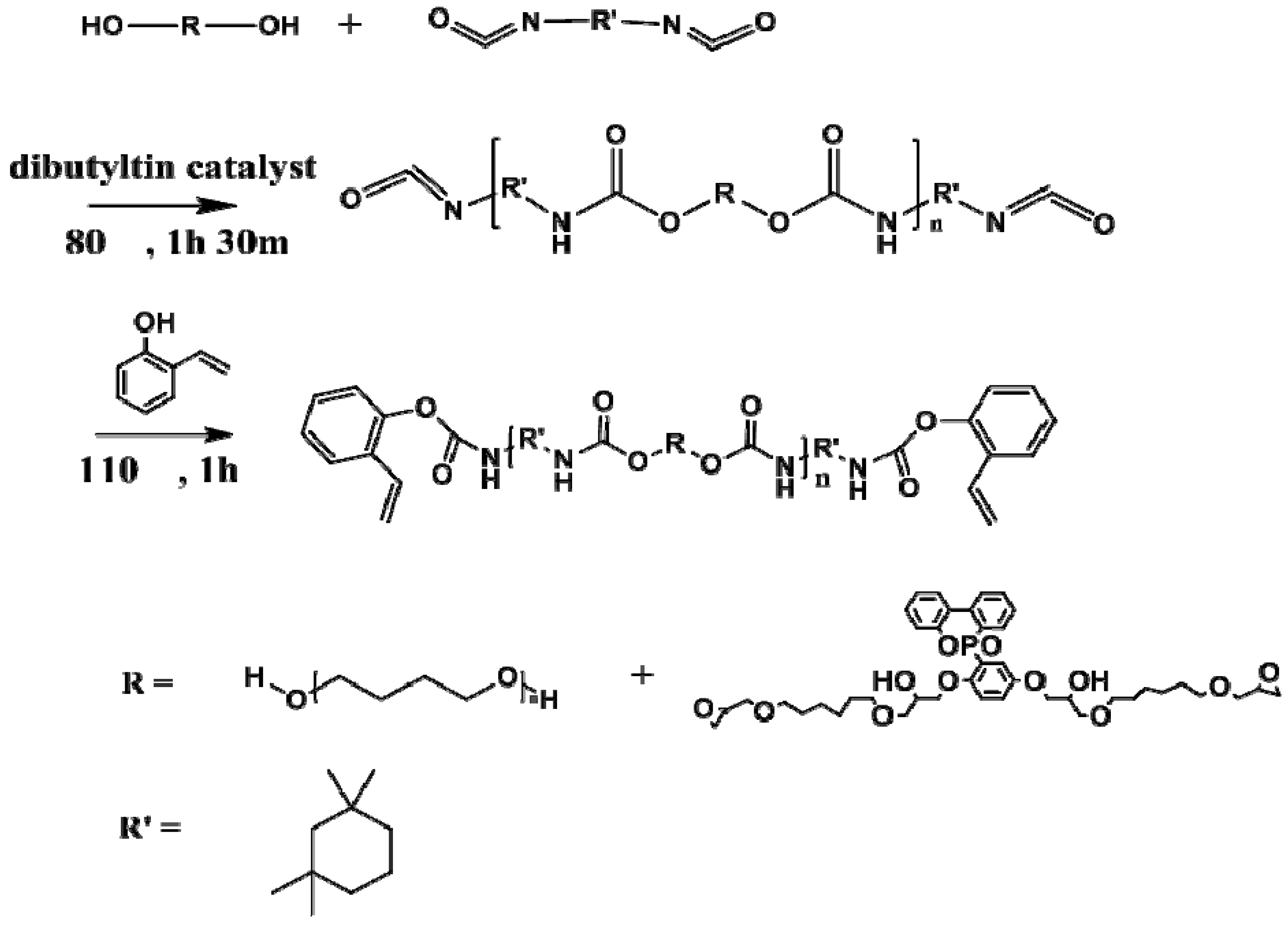
Reference polyurethane (Ref PU) was prepared by reacting PolyTHF (80 g, 0.04 mol) with IPDI (34 mL, 0.08 mol) at 90 °C for 90 min under nitrogen in the presence of DBTDL catalyst. Then, 2-AP (23 mL, 0.18 mol) was added and the mixture was heated at 120 °C for 1 h [
15]. The product was cooled to room temperature. Modified polyurethane (EPPU) was prepared by reacting IPDI (34 mL, 0.08 mol) with the mixture of PolyTHF (40 g, 0.02 mol) and EP-DOPO (15.6 g, 0.02 mol) at 90 °C for 2.5 h under nitrogen. After the addition of 2-AP (26 mL, 0.2 mol), the solution was stirred at 120 °C for 1 h. Two types of flame retardant polyurethanes (EPPU, and EPPU-2) were synthesized based on the molar ratio of PTMG, EP-DOPO, and IPDI. EPPU had a molar ratio of 0.2:0.2:0.8, while EPPU-2 had a ratio of 0:0.4:0.8, as shown in
Figure 3.
2.4. Preparation of Epoxy Compositions and Curing Process
DGEBA and dicy (1:1 mole ratio) with DPU (0.2%) were stirred with a mechanical stirrer at 80 °C under vacuum for 30 min. Elastic polyurethanes were added to the epoxy binder in the range of 10 to 30 parts per hundred resin (phr) to provide a toughening effect to the cured epoxy compositions. The resin composition was poured into a metal mold, followed by heating at 150 °C for 1 h, 170 °C for 1 h, and 190 °C for 1 h to complete the curing.
2.5. Characterization and Analysis
Elemental analysis was performed with an elemental analyzer (EA, Flash 2000, Thermo Fisher, Basingstok, UK). The molecular weight of the synthesized polyol was measured using liquid chromatography–mass spectrometry (LC-MS, G6130, Agilent Technologies, New York, NY, USA), while the structure was analyzed using Fourier-transform nuclear magnetic resonance (FT-NMR, Avance 3300, Bruker, Karlsruhe, Germany). The LC-MS system ionized the sample using electron spray ionization (ESI), and the eluent had a 1:1 ratio of acetonitrile:dried water (formic acid 0.2%) under a 0.7 mL/min flow rate. The molecular weight of the synthesized polyurethane was measured using gel permeation chromatography (GPC, 1260 Series, Agilent Technologies, New York, NY, USA). The glass transition temperature (Tg) of polyurethane was measured using differential scanning calorimetry (DSC, Q2000, TA Instrument, New Castle, DE, USA) from −80 to −20 °C at a heating rate of 10 °C/min. The thermal stability of the polyurethane was examined using thermogravimetric analysis (TGA, Q500, TA Instrument, New Castle, DE, USA) in the range of 25 to 800 °C at a heating rate of 10 °C/min. Then, the tensile strengths of the cured epoxy compositions were measured with a universal testing machine (UTM, INSTRON 5982, Instron, USA) for test specimens processed to sizes of 150 mm × 13 mm × 3 mm based on the ASTM D 638 method. The flexural strengths of the cured compositions processed to sizes of 60 mm × 25 mm × 3 mm were tested by using the ASTM D 790M method. The tensile and flexural tests were repeated five times to obtain average values. The Izod impact strengths were measured using the ASTM D 256 method for cured compositions with sizes of 63.5 mm × 12.7 mm × 3 mm with a pendulum impact tester (HIT-2492, JJ-TEST, Chengde, China). The dynamic mechanical analysis (DMA, Q800, TA Instrument, New Castle, DE, USA) of the epoxy compositions was performed using a Q800 with test specimens measuring 60 mm × 12 mm × 3 mm. The specimens were installed on the dual cantilever probe while the operating temperature increased at a heating rate of 5 °C/min under a 1 Hz frequency to measure both the storage moduli and tan δ values. Furthermore, the thermal stabilities of the cured epoxy compositions were measured using TGA (TGA, Q500, TA Instrument, New Castle DE. USA) in the range of 25 to 800 °C at a heating rate of 10 °C/min.
3. Results and Discussion
3.1. Characterization
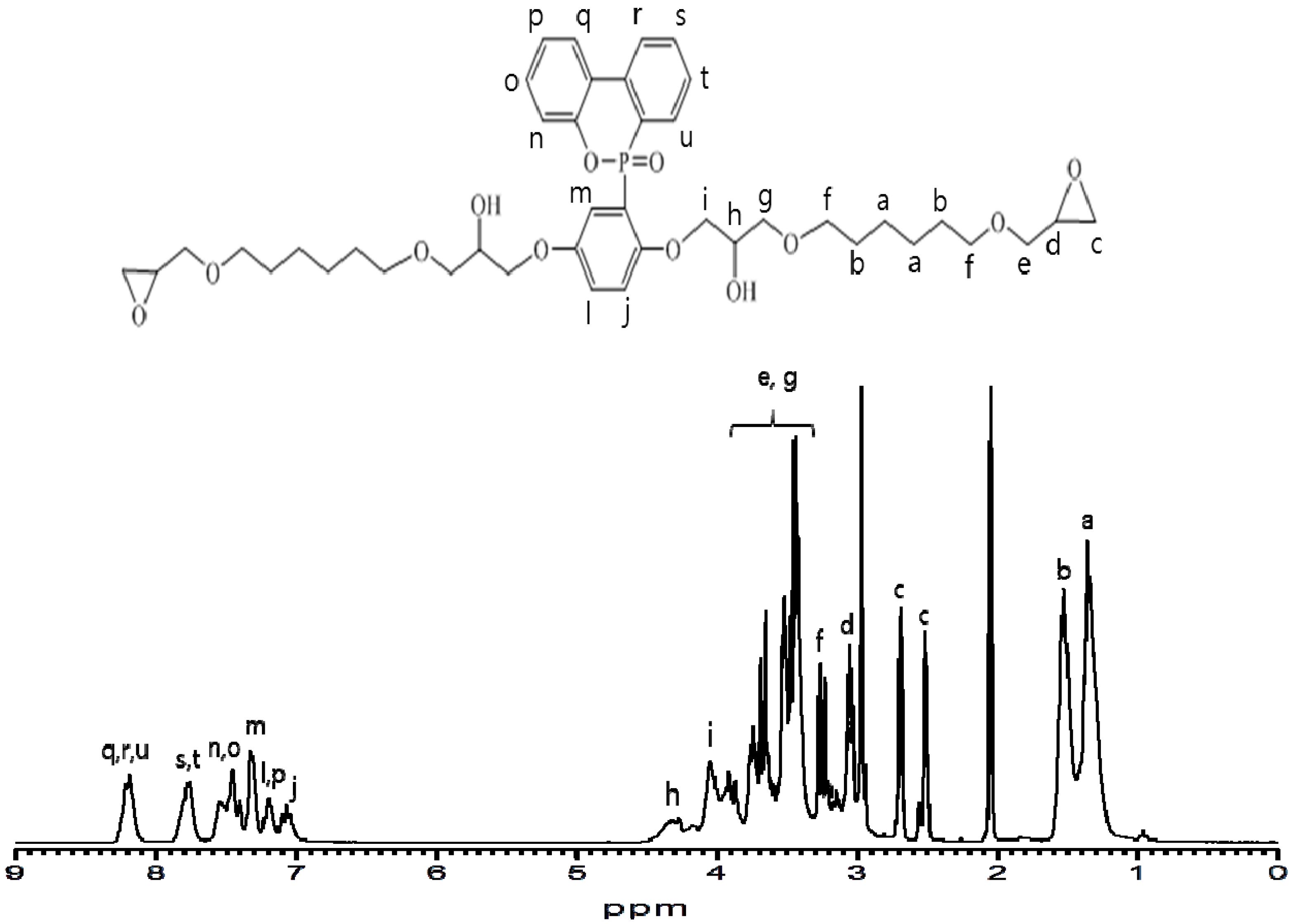
The structures of the prepared polyurethanes were analyzed with
1H-NMR, ESI-MS, and elemental analysis. The proton peaks of secondary carbon (i) and tertiary carbon (h) formed by a ring opening reaction of the epoxy group were observed in the 4.05–4.32 (ppm) range, as shown in
Figure 2.
1H NMR (300 MHz, Acetone): δ (ppm) = 1.36 (8H, m), 1.53 (8H, m), 2.52, 2.69 (4H, d), 3.05 (2H, m), 3.26~3.90 (12H, m), 4.32 (2H, m), 4.05 (4H, d). EA calc’d for C42H57O12P: C, 64.27; H, 7.32; O, 24.46; P, 3.95. found: C, 60.95; H, 5.99; O, 22.86. LC-ESI-MS: found 785.3 (M + H)+, calc’d 784.3.
Polyurethane was prepared by reacting polyol with IPDI followed by capping the terminal-NCO (isocyanate) group with a phenol OH group, as shown in
Figure 3.
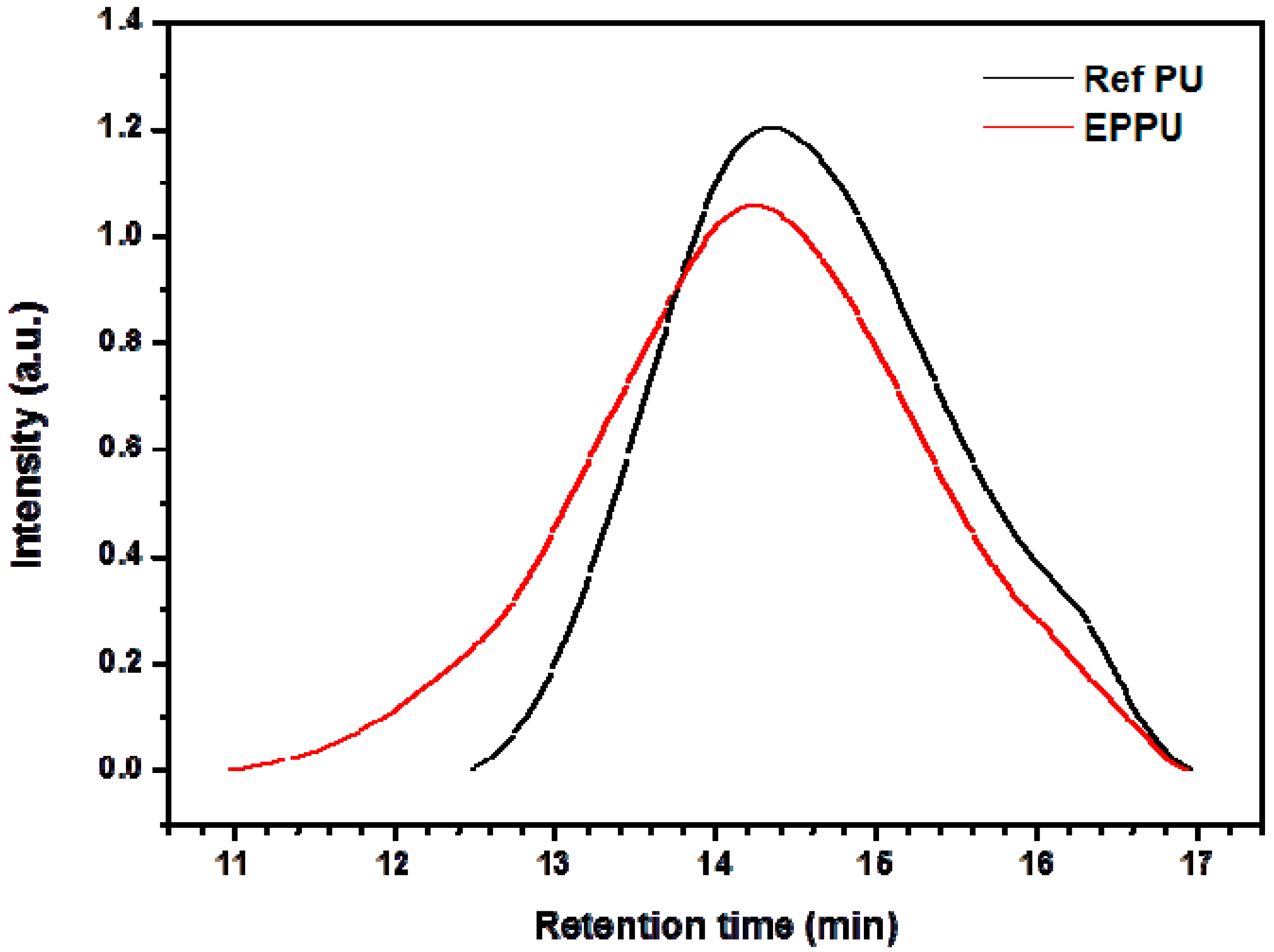
The molecular weights of the prepared polyurethanes were analyzed with GPC (
Figure 4) and the molecular weights and dispersity data are shown in
Table 1.
The prepared polyurethanes were analyzed with GPC to check whether two polyurethanes have a similar range of molecular weight. It turned out that both polyurethanes have a similar range of molecular weight: 9338 g/mol for EPPU and 7556 g/mol for Ref PU. However, EPPU-2 has a small molecular weight because it is synthesized by reacting small molecular weight of EP-DOPO with diisocyanate (
Figure S1).
3.2. Thermal Properties of Polyurethane
3.2.1. Differential Scanning Calorimetry
Polyurethane composed of a hard segment from diisocyanate and soft segment from polyols had two glass transition temperatures due to the individual segments. The glass transition temperature of the soft segment was usually observed at low temperatures, below 0 °C. The DSC data (
Figure 5) shows the
Tg values of Ref PU and EPPU at −69, and −60 °C, respectively. This suggests that EP-DOPO, which is more rigid than PTMG, causes the higher
Tg of the EPPU soft segment. For the same reason,
Tg of EPPU-2 synthesized by reacting EP-DOPO polyol with IPDI was −8.8 ℃ (
Figure S2)
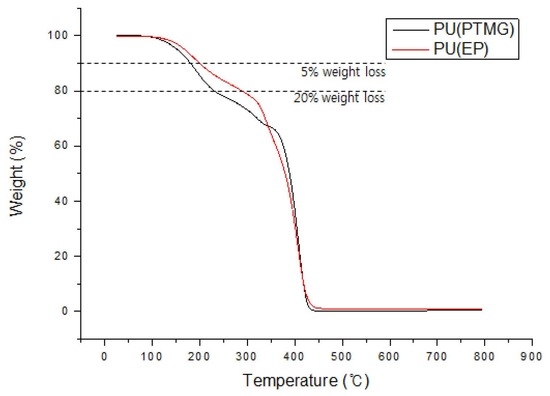
3.2.2. Thermogravimetric Analysis
Van Krevelen suggested that one way to reduce the burning of polymers is to add charcoal-forming additives like phosphor [
16]. Lyon developed a method for measuring flame retardancy from polymers that produce charcoal with TGA [
17]. The phosphor used in this study for flame retardancy is known to react with a combustible material during combustion and form a carbonized layer on the surface of the material. In addition, the phosphorous flame retardant produces phosphoric acid and polyphosphoric acid by pyrolysis, resulting in the formation of a HPO
2 or PO radical that stabilizes the active OH or ∙H radicals [
18,
19]. TGA analysis was carried out to evaluate the thermal stabilities of Ref PU and EPPU by comparing the pyrolysis temperatures at 5% and 20% weight loss ratios. Furthermore, flame retardancy was evaluated by measuring the total residual amount of material remaining after thermal decomposition related to the degree of formation of char. Plots of the weight reduction rate versus the temperature measured under a N
2, or air atmosphere are displayed in
Figure 6 and
Figure 7, and the results are summarized in
Table 2 and
Table 3. In
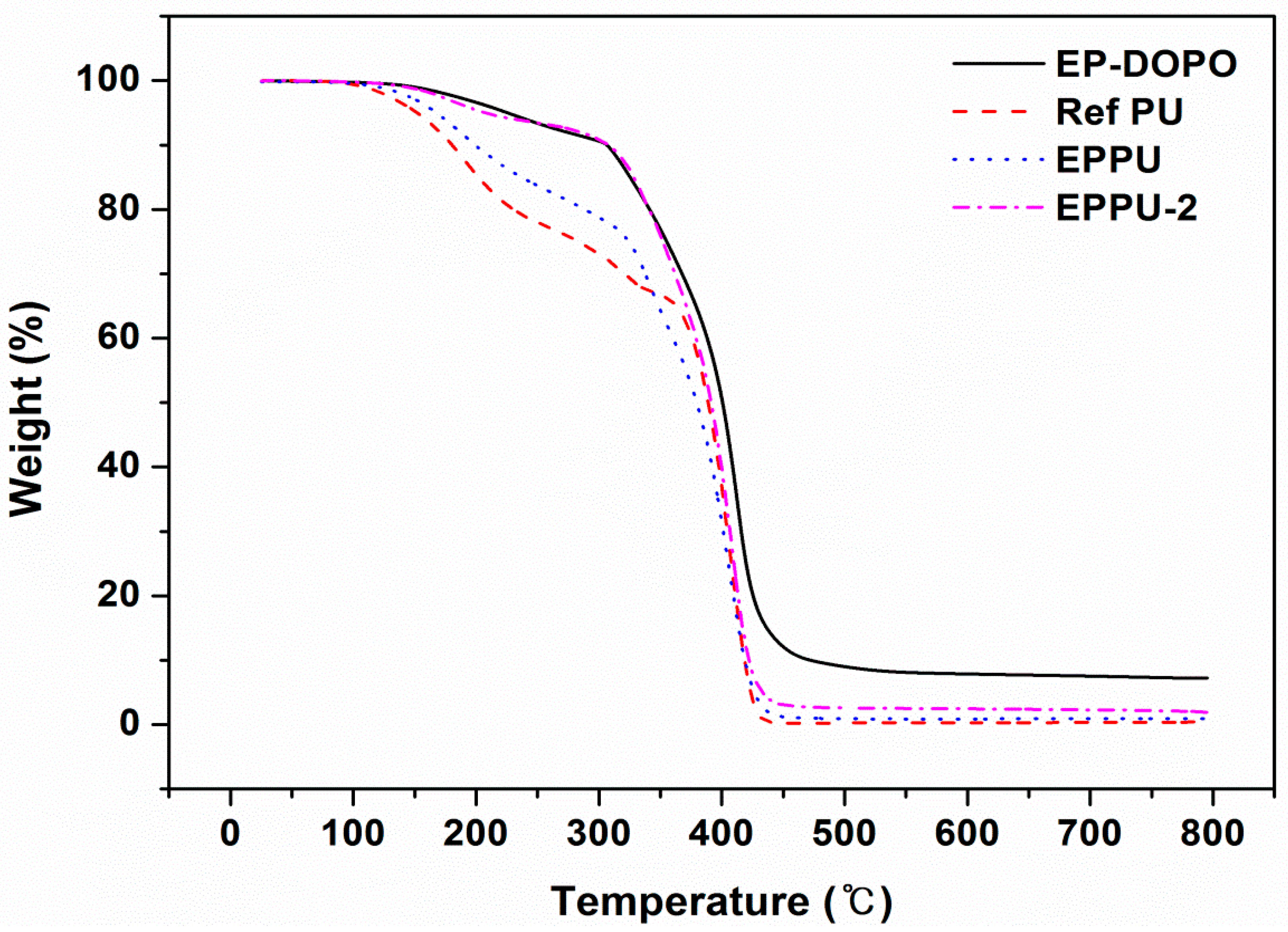
Figure 6, EP-DOPO with a large amount of phosphorus showed a residual amount of 11.9% at over 400 °C under nitrogen gas. Ref PU without phosphorus showed a small residual amount of 0.2%. However, EPPU and EPPU-2 have phosphorus in their structures, but their residual amounts were 1.2% and 3.0%, respectively. The results of TGA analysis under an oxygen atmosphere show that the residual amount of EP-DOPO at temperatures over 400 °C was 28.2% (
Figure 7), which is higher than that measured in the N
2 atmosphere (
Figure 6). In addition, the residual amount of EPPU and EPPU-2, which showed a small residual amount in the N
2 atmosphere, increased to 13.9% and 16.8% in the oxygen atmosphere, respectively. It was confirmed that the flame-retardant effect of phosphorus in the air atmosphere was better than that in the nitrogen atmosphere. This result agrees with a previous report which showed that the residual amount increases as the phosphorus content increases [
20]. The temperatures at which EPPU lost 5% and 20% of its initial weight were as high as 16 and 58 °C compared to those of Ref PU, respectively. Since EPPU is rich in aromatic groups that improve heat resistance, the pyrolysis temperature of EPPU is higher than that of Ref PU. Furthermore, EPPU-2 having higher content of phosphorous than that of EPPU showed better thermal stability than EPPU. The high thermal stability of EPPU is useful in industrial applications, such as cables or wires.
3.3. Characterization of Epoxy Compositions with Polyurethanes
In addition, the properties of the prepared polyurethanes as a toughener were studied by adding them to epoxy compositions composed of an epoxy resin and a curing agent: dicy. The epoxy compositions include various amounts of PU, except for EPPU-2 because it has a high viscosity; it was cured as described previously to prepare the test specimens for the measurements of the mechanical and thermal properties.
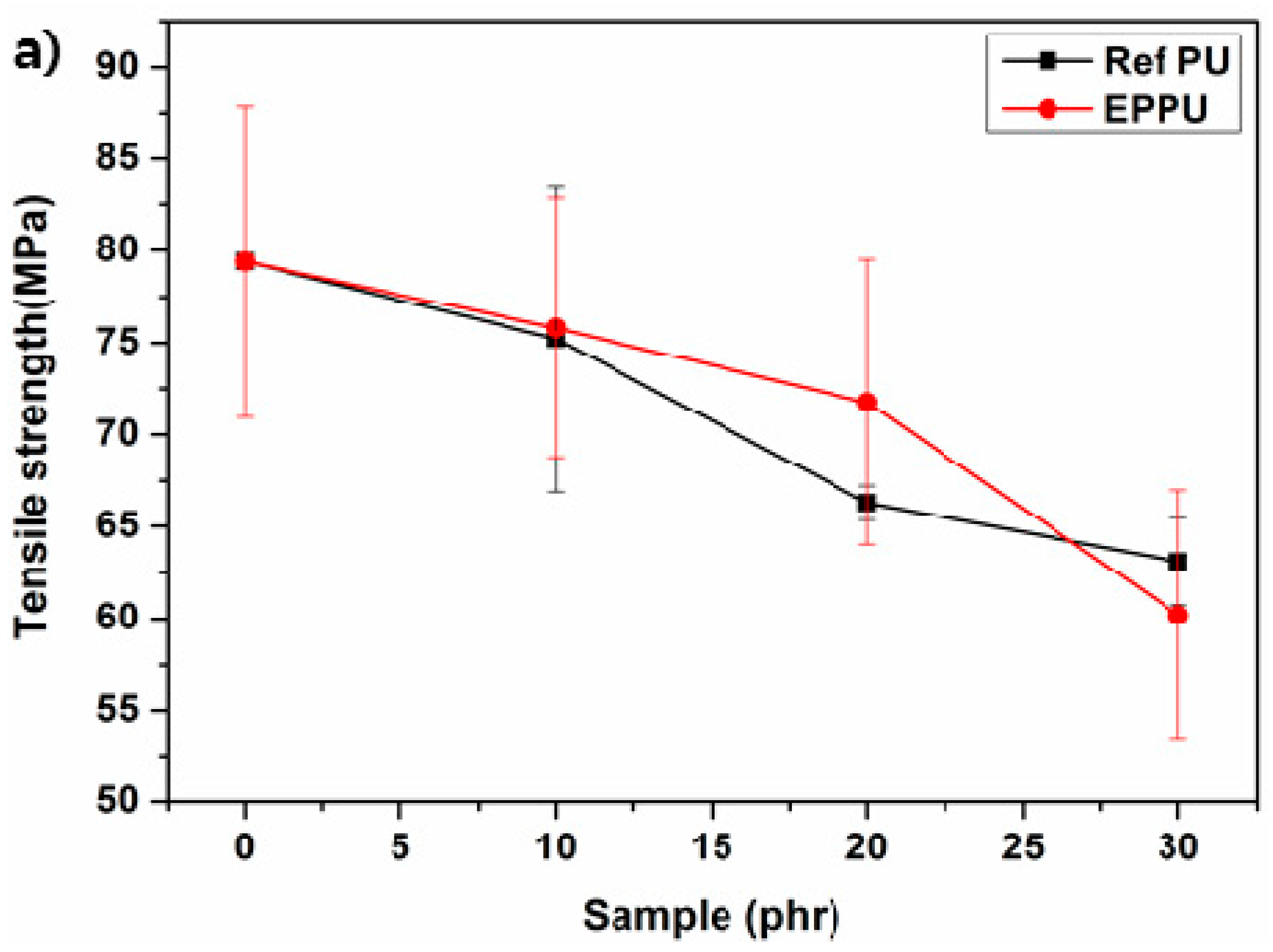
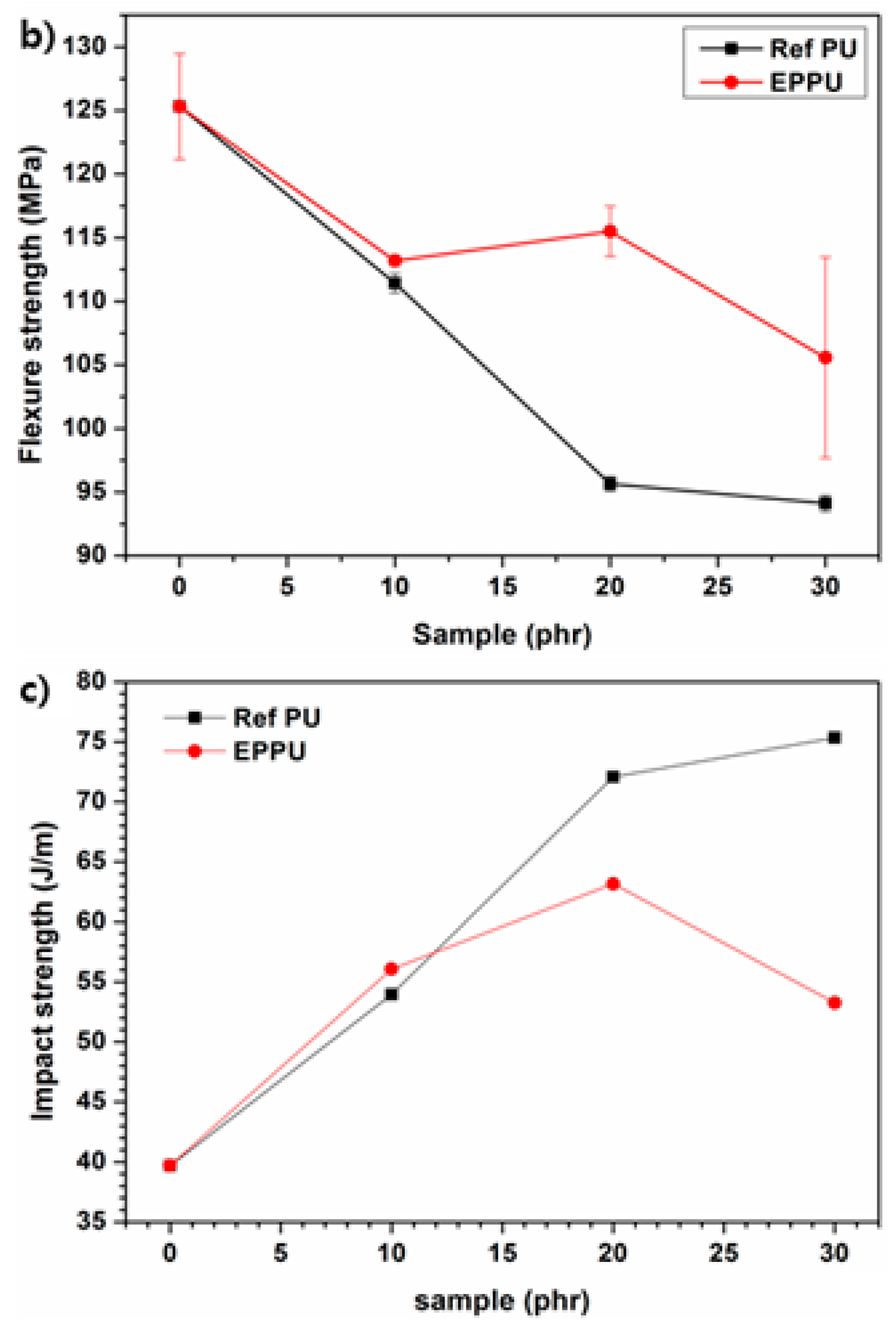
Figure 8a shows that epoxy compositions with synthetic EPPU had higher tensile strengths than those of the epoxy compositions with Ref PU. Furthermore, epoxy compositions containing EPPU have higher flexural strength than epoxy compositions containing Ref PU (
Figure 8b). This indicates that the rigid EPPU in the epoxy compositions compensated for the flexibility of the overall PU and enhanced the mechanical strength. In addition, the impact resistance of the epoxy binder with 10 phr of EPPU increased by ~17% compared to that of neat epoxy (
Figure 8c). However, when more than 10 phr of EPPU was included in the epoxy compositions, the impact resistance became lower than that of the epoxy with Ref PU due to the increased rigidity from aromatic groups in EPPU [
21].
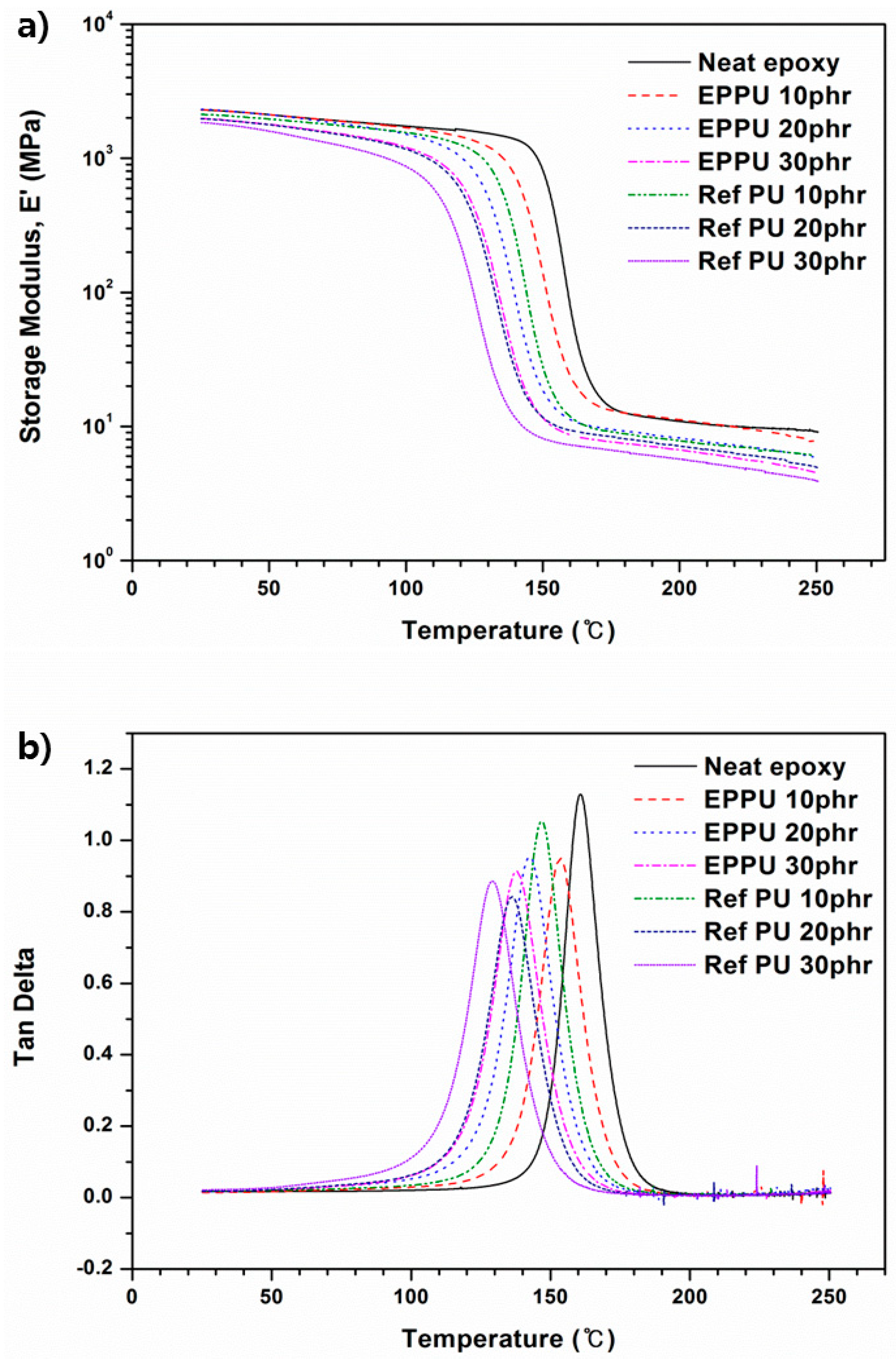
The viscoelastic properties of each epoxy composition were measured using DMA. The storage modulus and tan δ curves are displayed in
Figure 9a,b, and the calculated crosslink density values are shown in
Table 4. The storage modulus (
E′) of neat epoxy exhibited the highest value, while the
E′ of the epoxy compositions decreased as the amount of PU in the epoxy matrix increased. Meanwhile, the storage moduli of the EPPU epoxy compositions were higher than those of the Ref PU epoxy compositions. The high crosslink density of the EPPU epoxy matrix can cause this, because the diglycidyl ethers of EPPU can form a polymer network structure with an amine hardener [
22]. To calculate the crosslink density of each epoxy composition in the rubbery plateau region in the range of 150–250 °C, the following equation was used:
where
Vc is the crosslink density,
E′ is the tensile storage modulus in the rubbery plateau,
T is the temperature in K corresponding to the storage modulus value, and
R is the gas constant. The calculated values for the EPPU epoxy compositions were higher than those of the Ref PU epoxy binder with the same amount of polyurethanes. These results correspond to the storage modulus values listed in
Table 4. However, the
Vc values of the epoxy compositions with EPPU also decreased as the content of polyurethane increased. This was caused by the decreased amount of epoxy resin forming network polymers [
23,
24]. In
Figure 9b, one can see that the curves in the tan δ plot of the epoxy compositions tend to decrease owing to the increase of the flexible segment of the polyurethane, giving rise to chain motion. Specifically, it is obvious that epoxy binders containing EPPU have higher glass transition temperatures than those of the epoxy compositions with Ref PUs because both phenanthrene and the benzene group of EPPU function as hard segments that restrain the chain motion of PU in the polymer matrix. In addition, parameters such as the aromatic density can change the thermal properties of the polymer, increasing
Tg or improving the degradation characteristics [
25].
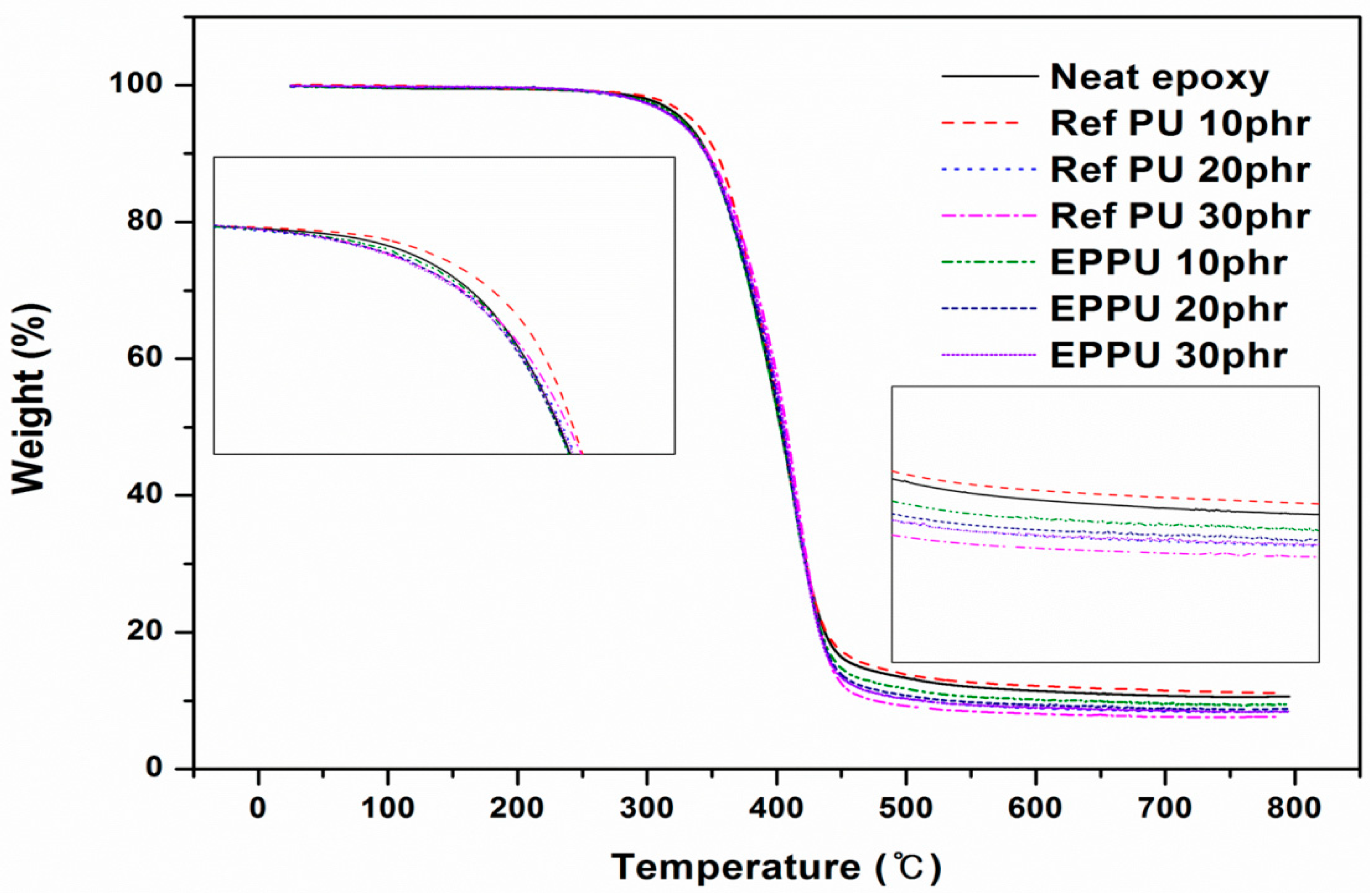
The thermal stabilities of the epoxy compositions containing PUs were measured using TGA and observing the weight loss as a function of temperature (
Figure 10). The thermal data are collected in
Table 5. For a weight loss of 5%, the thermal degradation temperature (
T5%) decreased proportionally to the content of polyurethane in the epoxy binder, indicating reduced thermal stability. Based on the values at
T5%, and
T10%, the epoxy with 10 phr of Ref PU had the highest temperature. This is thought to be related to the morphology of PU in the cured epoxy matrix, as shown in
Figure 11, which shows the scanning electron microscopy (SEM) images of each epoxy sample.
Figure 11b1 shows a large amount of phase-separated PUs in the epoxy binder with 10 phr of Ref PU. The thermal degradation of the volatile segments of the phase-separated PUs was delayed by the surrounding epoxy matrix and resulted in the highest thermal degradation temperature. Neat epoxy and epoxy compositions with PUs exhibited similar degradation temperatures within a ±2% range. This indicates that the content of the flame-retardant phosphor was too low to provide flame retardancy.
Surface images of neat resin (
Figure 11a1,a2) showed mainly smooth surfaces with partial wavy patterns. However, an epoxy composition with 10 phr of Ref PU (
Figure 11b1) or EPPU (
Figure 11cl) showed numerous holes resulting from impact-detached PUs. Other images, including those with >10 phr of polyurethanes, did not show phase-separated polyurethanes but had rough surfaces as the PU content increased. This suggests that the high polyurethane content in the epoxy matrix promoted flexibility, compensating for the embrittlement of the cured epoxy product, but also lowered the tensile and flexural strengths of the epoxy.
4. Conclusions
In this study, a flame-retardant polyol (EP-DOPO) was prepared and characterized with 1H-NMR, electron spray ionization mass spectrometry (ESI-MS), and elemental analysis. In addition, flame retardant polyurethanes (EPPU, and EPPU-2) were prepared by reacting the blend of EP-DOPO and PolyTHF polyol with IPDI. The physical properties of the EPPUs were compared with polyurethanes composed of PolyTHF and IPDI (Ref PU). DSC data showed that EPPU, having a rigid aromatic group, has the disadvantage of having a higher Tg, due to its soft segment, compared to that of Ref PU. However, EPPU exhibited a higher thermal degradation temperature than Ref PU, as revealed by TGA. Therefore, these results suggest that EPPU itself can be useful for industrial applications requiring high thermal stability, such as wires and cables.
Furthermore, the effect of polyurethane in the epoxy binder as a toughener was investigated in terms of mechanical and thermal properties. The tensile strengths of the epoxy compositions with EPPU and Ref-PU were similar, but the EPPU-epoxy compositions had higher flexural strengths than the Ref PU–epoxy compositions, which is beneficial for preparing high-performance epoxy composites. Moreover, EPPU–epoxy compositions exhibited higher impact resistance than neat epoxy, though epoxy compositions containing 20 and 30 phr had lower values than those of epoxy compositions with Ref PU.
Furthermore, the viscoelastic properties of the epoxy compositions with various amounts of EPPU were analyzed using DMA. According to the results, the epoxy polymer with 10 phr of EPPU has higher tan δ value than that of the cured epoxy with 10 phr of Ref PU. Therefore, EPPU is useful for increasing the glass transition temperature (Tg) of the polymer. Moreover, the calculated crosslink density values of the compositions based on the DMA results were also found to increase proportionally to the values of the storage modulus.