SiO2-SnO2:Er3+ Glass-Ceramic Monoliths
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
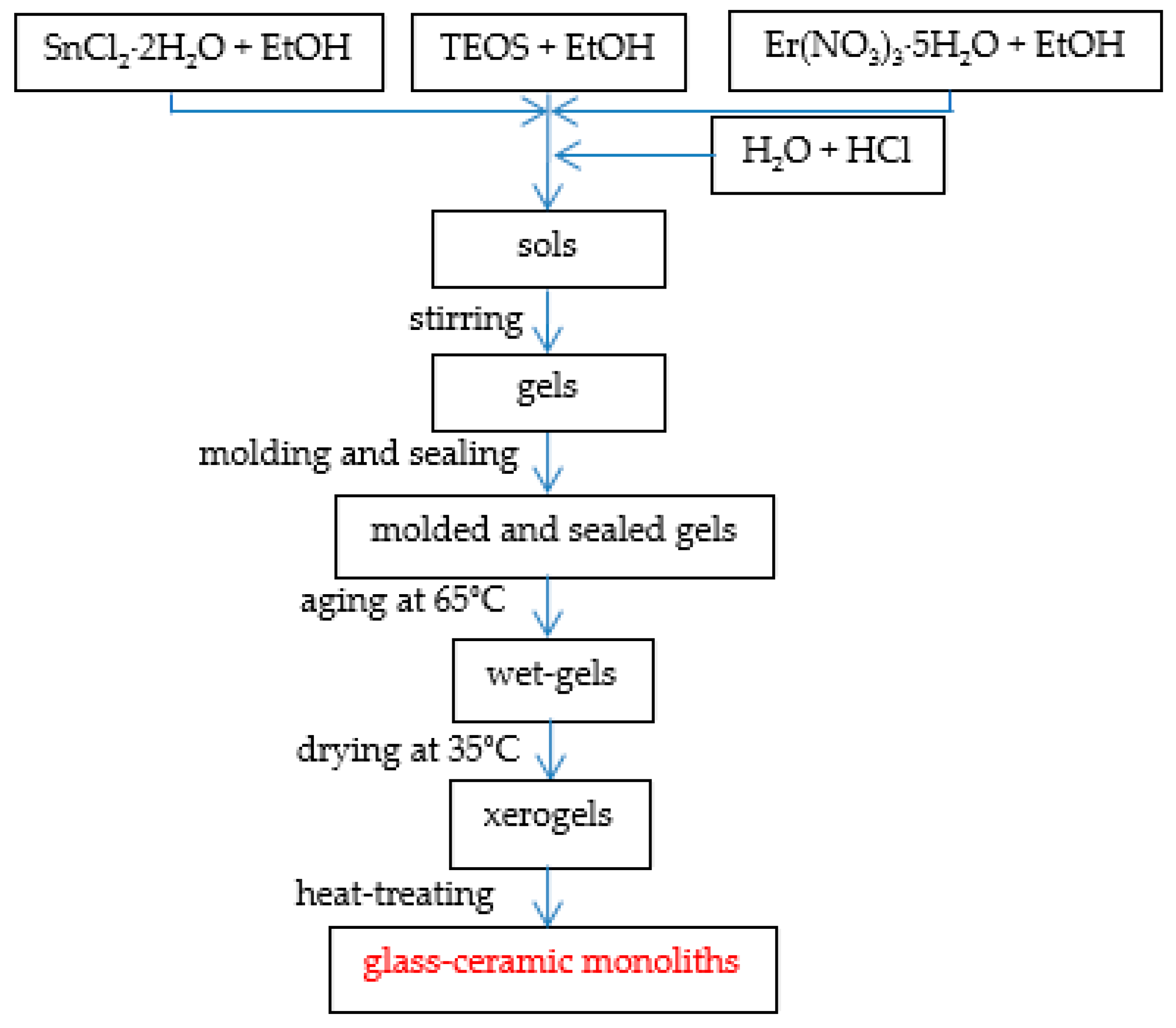
2.1. Sample Preparation: Sol-Gel Derived Route
2.2. Charaterization Methods
3. Results
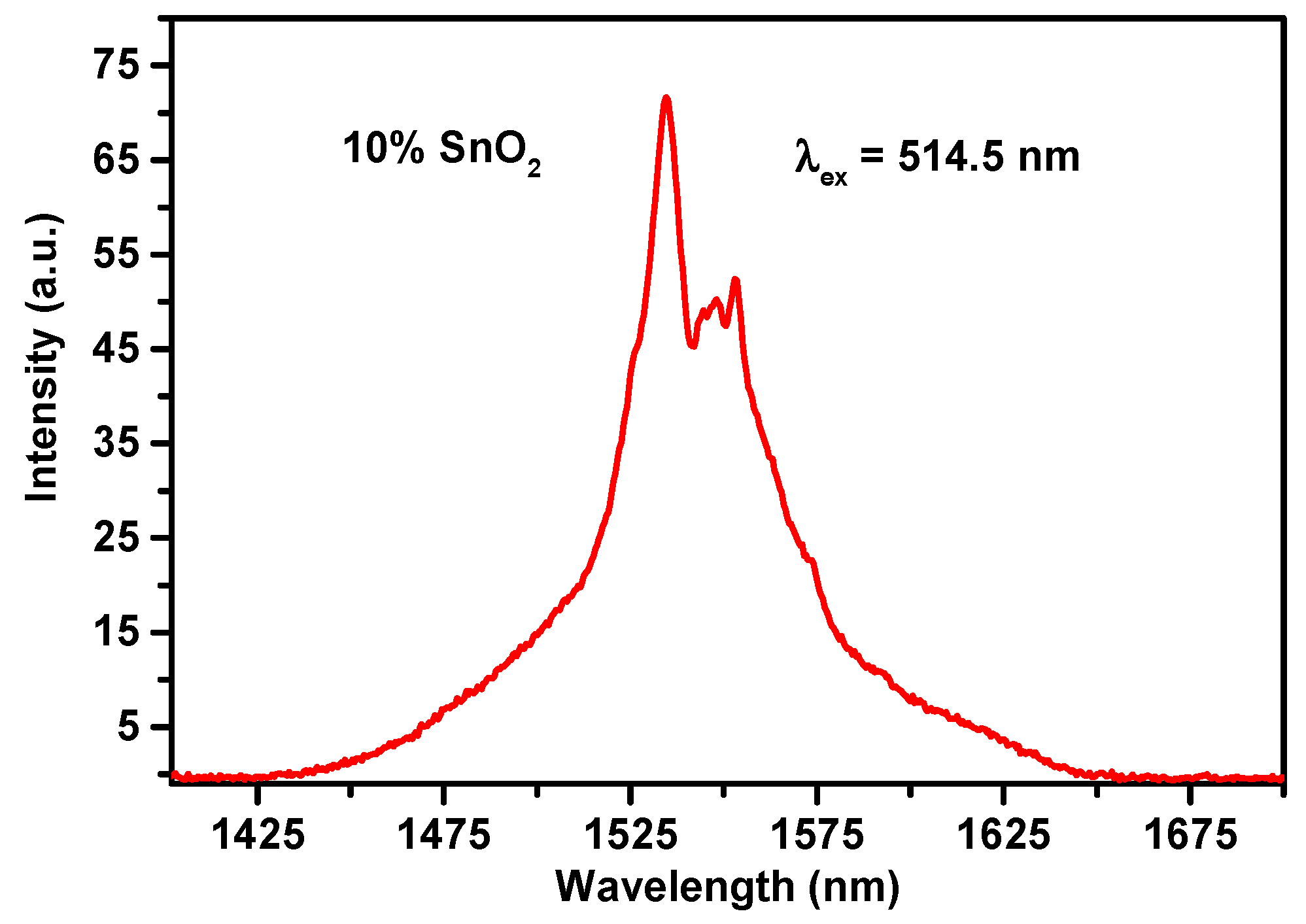
3.1. Emission Spectra
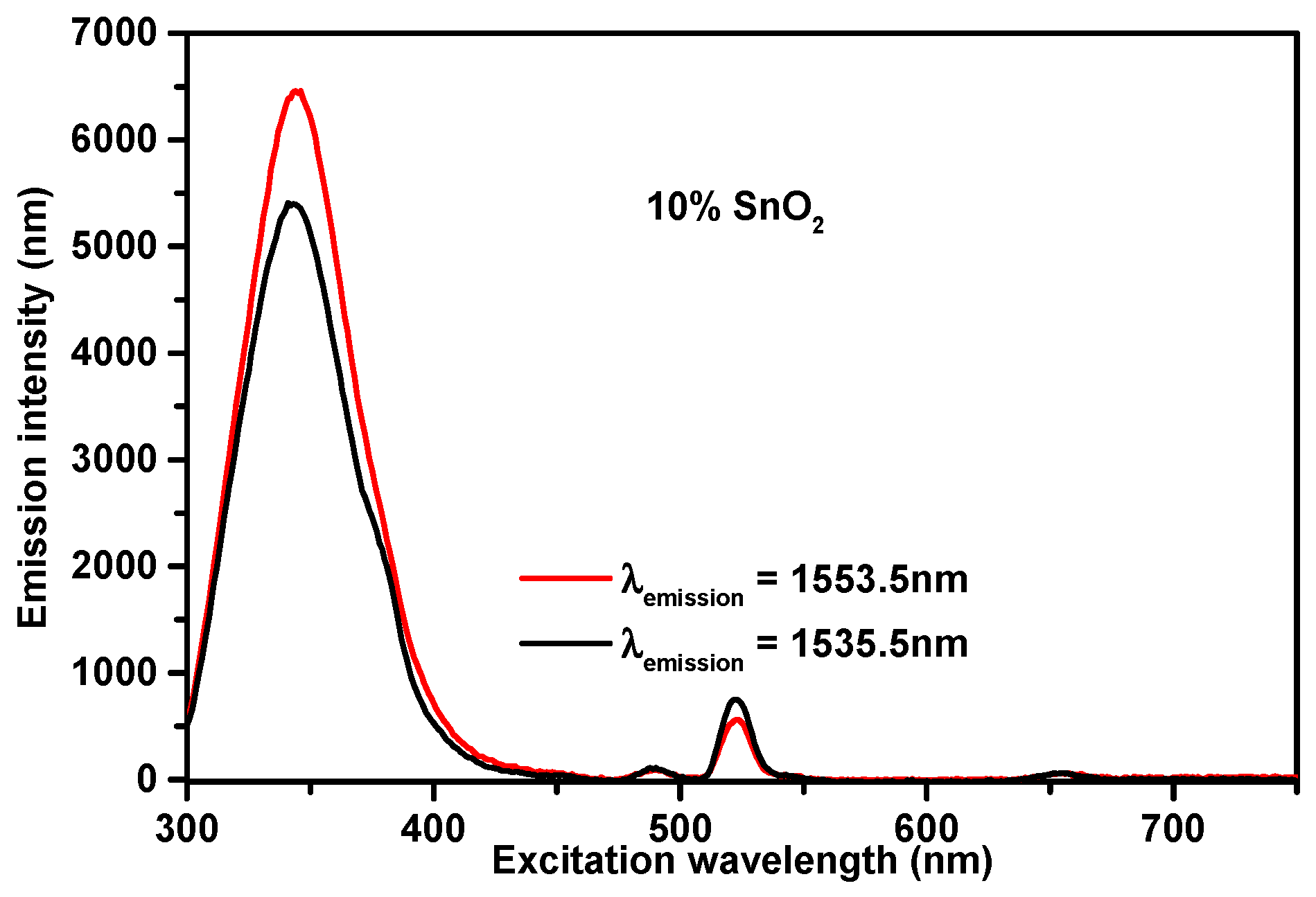
3.2. Excitation Spectra
3.3. Lifetime
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zanotto, E.D. A bright future for glass-ceramics. Am. Ceram. Soc. Bull. 2010, 89, 19–27. [Google Scholar]
- De Pablos-Martin, A.; Ferrari, M.; Pascual, M.J.; Righini, G.C. Glass-ceramics: A class of nanostructured materials for photonics. Riv. Del Nuovo Cimento 2015, 28, 311–369. [Google Scholar]
- Ferrari, M.; Righini, G.C. Glass-Ceramic Materials for Guided-Wave Optics. Int. J. Appl. Glass Sci. 2015, 6, 240–248. [Google Scholar] [CrossRef]
- Zur, L.; Tran, L.T.N.; Meneghetti, M.; Varas, S.; Armellini, C.; Ristic, D.; Chiasera, A.; Scotognella, F.; Pelli, S.; Nunzi Conti, G.; et al. Glass and glass-ceramic photonic systems. In Proceedings of the Integrated Optics: Devices, Materials, and Technologies XXI, San Francisco, CA, USA, 30 January–1 February 2017; pp. 1–12. [Google Scholar]
- Cascales, C.; Balda, R.; Lezama, L.; Fernández, J. Site symmetry and host sensitization-dependence of Eu3+ real time luminescence in tin dioxide nanoparticles. Opt. Express 2018, 26, 16155–16170. [Google Scholar] [CrossRef]
- Zur, L.; Thi, L.; Tran, N.; Meneghetti, M. Sol-gel derived SnO2-based photonic systems. In Handbook of Sol-Gel Science and Technology, 2nd ed.; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer International Publishing AG: Basel, Switzerland, 2017; pp. 1–19. [Google Scholar]
- Gorni, G.; Velázquez, J.J.; Mosa, J.; Balda, R.; Fernández, J.; Durán, A.; Castro, Y. Transparent glass-ceramics produced by Sol-Gel: A suitable alternative for photonic materials. Materials 2018, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- Dymshits, O.; Shepilov, M.; Zhilin, A. Transparent glass-ceramics for optical applications. MRS Bull. 2017, 42, 200–205. [Google Scholar] [CrossRef]
- Gonçalves, M.C.; Santos, L.F.; Almeida, R.M. Rare-earth-doped transparent glass ceramics. Comptes Rendus Chime 2002, 5, 845–854. [Google Scholar] [CrossRef]
- Zur, L.; Tran, L.T.N.; Meneghetti, M.; Tran, V.T.T.; Lukowiak, A.; Chiasera, A.; Zonta, D.; Ferrari, M.; Righini, G.C. Tin-dioxide nanocrystals as Er3+ luminescence sensitizers: Formation of glass-ceramic thin films and their characterization. Opt. Mater. 2017, 63, 95–100. [Google Scholar] [CrossRef]
- Chiodini, N.; Paleari, A.; Brambilla, G.; Taylor, E.R. Erbium doped nanostructured tin-silicate glass-ceramic composites. Appl. Phys. Lett. 2002, 80, 4449–4451. [Google Scholar] [CrossRef]
- Saadeddin, I.; Pecquenard, B.; Manaud, J.P.; Decourt, R.; Labrugère, C.; Buffeteau, T.; Campet, G. Synthesis and characterization of single-and co-doped SnO2 thin films for optoelectronic applications. Appl. Surf. Sci. 2007, 253, 5240–5249. [Google Scholar] [CrossRef]
- Tran, L.T.N.; Zur, L.; Massella, D.; Derkowska-Zielinska, B.; Chiasera, A.; Varas, S.; Armellini, C.; Martucci, A.; Zonta, D.; Tran, T.T.V.; et al. SiO2-SnO2:Er3+ transparent glass-ceramics: fabrication and photonic assessment. In Proceedings of the Fiber Lasers Glass Photonics: Materials through Applications, Strasbourg, France, 22–26 April 2018; pp. 1–10. [Google Scholar]
- Righini, G.C.; Ferrari, M. Photoluminescence of rare-earth-doped glasses. Riv. Del Nuovo Cimento 2005, 28, 1–53. [Google Scholar]
- Duverger, C.; Montagna, M.; Rolli, R.; Ronchin, S.; Zampedri, L.; Fossi, M.; Pelli, S.; Righini, G.G.; Monteil, A.; Armellini, C.; et al. Erbium-activated silica xerogels: Spectroscopic and optical properties. J. Non-Cryst. Solids 2001, 280, 261–268. [Google Scholar] [CrossRef]
- Van, T.T.T.; Turrell, S.; Capoen, B.; Vinh, L.Q.; Cristini-Robbe, O.; Bouazaoui, M.; d’Acapito, F.; Ferrari, M.; Ristic, D.; Lukowiak, A.; et al. Erbium-Doped Tin-Silicate Sol-gel-derived glass-ceramic thin films: Effect of environment segregation on the Er3+ emission. Sci. Adv. Mater. 2015, 7, 301–308. [Google Scholar] [CrossRef]
- Fernández, J.; García-Revilla, S.; Balda, R.; Cascales, C. Rare-earth-doped wide-bandgap tin-oxide nanocrystals: Pumping mechanisms and spectroscopy. In Proceedings of the Optical Components and Materials XV, San Francisco, CA, USA, 30 January–1 February 2018; pp. 1–9. [Google Scholar]
- Tran, T.T.V.; Bui, T.S.; Turrell, S.; Capoen, B.; Roussel, P.; Bouazaoui, M.; Ferrari, M.; Cristini, O.; Kinowski, C. Controlled SnO2 nanocrystal growth in SiO2-SnO2 glass-ceramic monoliths. J. Raman Spectrosc. 2012, 43, 869–875. [Google Scholar] [CrossRef]
- Van, T.T.T.; Turrell, S.; Capoen, B.; Hieu, L.V.; Ferrari, M.; Ristic, D.; Boussekey, L.; Kinowski, C. Environment segregation of Er3+ emission in bulk sol–gel-derived SiO2–SnO2 glass ceramics. J. Mater. Sci. 2014, 49, 8226–8233. [Google Scholar] [CrossRef]
- Zampedri, L.; Ferrari, M.; Armellini, C.; Visintainer, F.; Tosello, C.; Ronchin, S.; Rolli, R.; Montagna, M.; Chiasera, A.; Pelli, S.; et al. Gonçalves, Erbium-activated silica-titania planar waveguides. J. Sol-Gel Sci. Technol. 2003, 26, 1033–1036. [Google Scholar] [CrossRef]
- Bouzidi, C.; Moadhen, A.; Elhouichet, H.; Oueslati, M. Er3+-doped sol-gel SnO2 for optical laser and amplifier applications. Appl. Phys. B 2008, 90, 465–469. [Google Scholar] [CrossRef]
- Chiasera, A.; Alombert-Goget, G.; Ferrari, M.; Berneschi, S.; Pelli, S.; Boulard, B.; Arfuso, C.D. Rare earth–activated glass-ceramic in planar format. Opt. Eng. 2011, 50, 1–10. [Google Scholar] [CrossRef]



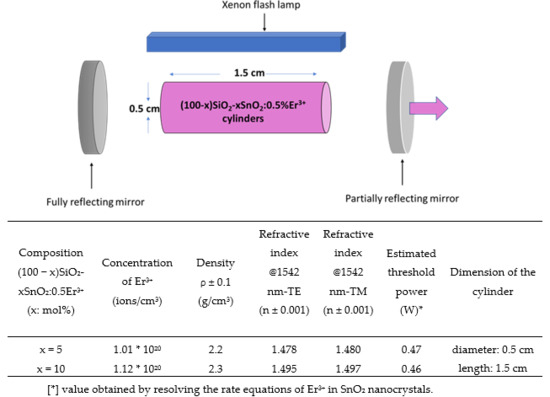
| SnO2 Content x (mol%) | Er3+ Concentration (mol%) | H2O/TEOS | EtOH/TEOS | HCl/TEOS |
|---|---|---|---|---|
| 10 | 0.5 | 10 | 4 | 0.009 |
| A1 | τ1 (ms) | A2 | τ2 (ms) | |
|---|---|---|---|---|
| 0.32 | 4.9 | 1.03 | 0.5 | 75% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, L.T.N.; Massella, D.; Zur, L.; Chiasera, A.; Varas, S.; Armellini, C.; Righini, G.C.; Lukowiak, A.; Zonta, D.; Ferrari, M. SiO2-SnO2:Er3+ Glass-Ceramic Monoliths. Appl. Sci. 2018, 8, 1335. https://doi.org/10.3390/app8081335
Tran LTN, Massella D, Zur L, Chiasera A, Varas S, Armellini C, Righini GC, Lukowiak A, Zonta D, Ferrari M. SiO2-SnO2:Er3+ Glass-Ceramic Monoliths. Applied Sciences. 2018; 8(8):1335. https://doi.org/10.3390/app8081335
Chicago/Turabian StyleTran, Lam Thi Ngoc, Damiano Massella, Lidia Zur, Alessandro Chiasera, Stefano Varas, Cristina Armellini, Giancarlo C. Righini, Anna Lukowiak, Daniele Zonta, and Maurizio Ferrari. 2018. "SiO2-SnO2:Er3+ Glass-Ceramic Monoliths" Applied Sciences 8, no. 8: 1335. https://doi.org/10.3390/app8081335