1. Introduction
In biological testing it is important to use compounds in the form of pure enantiomers, especially for their use in the synthesis of pharmaceuticals. This necessity is evident when a drug has a high enantiomeric excess to avoid undesired side effects by the second enantiomer [
1]. The product of the reduction of α-acetylbutyrolactone
1—α’-1’-hydroxyethyl-γ-butyrolactone
2 possesses two stereogenic centres and may be classified as a β-hydroxyester. This compound is a structural analog of γ-butyrolactone (GLB), which exerts several inhibitory action over the central nervous system (CNS) through the interaction with neuronal high-affinity receptors. Isomers of α’-1’-hydroxyethyl-γ-butyrolactone could be potential ligands of the same receptors [
2].
Microbial reduction of the carbonyl group is a convenient method for obtaining enantiomerically enriched β-hydroxyesters [
3], which encouraged us to use a yeast strain in the biotransformation of α-acetylbutyrolactone. Whole-cell biocatalysts, in comparison to isolated enzymes, exhibit increased stability due to the protective cell matrix and are more profitable due to lower production costs [
1]. Additionally, cofactors of enzymes are already present in the cells and can, therefore, be recycled via the oxidation of the second substrate (i.e., glucose or glycerol) [
4]. Unfortunately, biotransformation can be ineffective when substrates are toxic or have low solubility in water. Overcoming these limitations can be accomplished through the use of a biphasic system, in this case both an organic and aqueous phase. A buffer and a biocatalyst compose the aqueous phase, while the organic phase is water immiscible, which both the substrate and the product seem to strongly prefer. The organic phase acts as substrate reservoir during the reaction and as an in situ extractant for the product [
5]. Deep eutectic solvent (DES) has been recently identified as an innovative solvent because it forms a eutectic mixture of two or more components with a melting point lower than that of both of the individual components [
6,
7,
8]. The charge delocalization that occurs through hydrogen bonding between the salt anion and hydrogen bond donor (HBD) moiety explains their lower melting point [
9]. The most popular DES in biocatalysis today is a combination of choline chloride (ChCl) and glycerol [
10]. Choline chloride is similar to B vitamins and is a biodegradable and non-toxic salt [
8]. In turn, glycerol is a hydrogen bond donor in DES. Glycerol is a conventional solvent used extensively in the food and pharmaceutical industries [
11]. DES based on ChCl and glycerol is hydrophilic but its polarity does not influence the catalytic activity. Viscosity has the ability to change the enzyme activity in the reaction systems by changing the mass-transfer limitations. DES are inexpensive, nontoxic, biodegradable, and do not require further purification [
9]. So far, cholinium-based deep eutectic solvents have been used in asymmetric bioreduction of ketoesters by baker’s yeast [
9,
12,
13] and recombinant
E. coli CCZU-T15 [
14]. Baker’s yeast [
15] and enzymes like
Ralstonia sp. ADH (alcohol dehydrogenase) and horse liver ADH overexpressed in
E. coli [
16] established better selectivity in presence of DES in biotransformation of acetophenones. The aim of the work presented was to obtain pure α’-1’-hydroxyethyl-γ-butyrolactone enantiomers using biotransformation by means of yeast culture. To increase the stereoselectivity and efficiency of biotransformation, different conditions (growing and resting cell cultures, addition of different solvents including DES) were used.
2. Materials and Methods
2.1. Analysis
TLC (thin-layer chromatography) and GC (gas chromatography) analyses were used to monitor the progress of biotransformation processes and to determine the purity of isolated compounds. We used silica gel-coated aluminium plates (DC-Alufolien Kieselgel 60 F254, Merck, Darmstadt, Germany) for TLC analysis. The GC analysis was performed on a CP03380 instrument (Varian, Agilent Technologies, Santa Clara, CA, USA). The temperature program, which was used in GC analysis on DB-1 column ((dimethylpolysiloxane, Agilent), 30 m × 0.25 mm × 0.25 µm), was as follows: injector 250 °C, detector (FID) 300 °C, column temperature: 75 °C (hold 3 min), 75–80 °C (rate 2 °C/min), 80–150 °C (rate 17 °C/min), 150–300 (rate 40 °C/min), 300 °C (hold 1 min) (α-acetylbutyrolactone 1 Rt = 5.9 min; anti stereoisomer of α’-1’-hydroxyethyl-γ-butyrolactone 2 Rt = 6.7 min; syn stereoisomer of 2 Rt = 7.2 min). To determine the enantiomeric excess of the products obtained during biotransformation, GC analysis was performed using chiral column Gamma DEXTM 325 (30 m × 0.25 mm × 0.25 µm, Supelco) under the following conditions: injector 150 °C, detector (FID) 250 °C, column temperature: 80 °C (hold 20 min), 80–107 °C (rate 1 °C/min), 107–200 °C (rate 30 °C/min), 200 °C (hold 1 min) (substrate 1 Rt = 27.9 min; (3S,1’S)-2a Rt = 34.9 min; (3R,1’R)-2b Rt = 35.5 min; (3S,1’R)-2c Rt = 43.8 min; (3R,1’S)-2d Rt = 44.3 min).
Products of biotransformation were purified by column chromatography in silica gel (Kieselgel 60, 230–400 mesh) with eluent: hexane: diethyl ether 1:1. NMR spectra were recorded with a Bruker Avance DRX-500 (Bruker, Billerica, MA, USA) spectrometer in CD3OD solution.
Optical rotation was measured with the P-2000 polarimeter (Jasco, Easton, PA, USA).
2.2. Synthesis of Standards
The chemical reduction of α-acetylbutyrolactone
1 was carried out according to the procedure worked out by Teixeira et al. [
17]. α-Acetylbutyrolactone
1 (1 g, 7.8 mmol) in 30 mL of methanol was stirred 30 min in room temperature in the presence of CaCl
2 (1.73 g, 15.6 mmol). Next, the reaction mixture was cooled to 0 °C and NaBH
4 was slowly added. After the disappearance of substrate
1 crude product was extracted by diethyl ether (3 × 15 mL) and dried over MgSO
4. After evaporation 0.68 g of mixture of diastereomeric α’-1’-hydroxyethyl-γ-butyrolactone
2 was obtained.
1H NMR (500 MHz, CD
3OD,
Figure S2): 1.25 (d, J = 7.0 Hz, 3H, CH
3-2’B), 1.26 (d, J = 6.4 Hz, 3H, CH
3-2’A), 1.22–1.24 (dq, J = 12.8 and 8.5 Hz, 1H, one of CH
2-2A), 2.22–2.30 (m, 1H, one of CH
2-2B), 2.32–2.39 (m, 2H, one of CH
2-2A and one of CH
2-2B), 2.65 (ddd, J = 9.4, 9.4 and 3.3 Hz, 1H, H-3B), 2.74 (ddd, J = 9.4, 8.8 and 4.7 Hz, 1H, H-3A), 4.05 (dq, J = 6.4 and 4.8 Hz, 1H, 1’A), 4.22–4.27 (m, 2H, CH
2-1A), 4.33–4.38 (m, 3H, CH
2-1B and 1’B).
13C NMR (151 MHz, CD
3OD,
Figure S3):
anti δ 18.7 (CH
3), 24.6 (CH
2-2), 45.6 (CH-3), 67.1 (CH
2-1), 67.2 [(CH(OH)], 179.5 (CO);
syn δ 20.5 (CH
3), 21.6 (CH
2-2), 46.5 (CH-3), 65.2 (CH
2-1), 67.2 [(CH(OH)], 178.9 (CO).
The configuration of the individual enantiomers of α’-1’-hydroxyethyl-γ-butyrolactone
2 was determined by comparing it to the biotransformation of
1 by the
Yarrowia lipolytica strain found in literature data [
18] and results of biotransformation
1 by
Rhodotorula marina AM77 described in the
Supplementary Materials. (
Section S1 and Figure S1)
2.3. Microorganisms
The yeast strains used for biotransformation were as follows: Yarrowia lipolytica AM71, Yarrowia lipolytica AM72, Yarrowia lipolytica P26A, Candida viswanathi AM120, Hansenula anomala C2, Saccharomyces cerevisiae K1, and Saccharomyces pombe C1. The microorganisms came from collection of the Department of Chemistry or the Department of Biotechnology and Food Microbiology at Wroclaw University of Environmental and Life Sciences (Poland). These strains were maintained on a gelified medium (glucose 20 g/L, peptone 20 g/L, yeast extract 10 g/L and agar 20 g/L) and were stored at 4 °C.
2.4. Screening Procedure
The microorganisms were cultivated in Erlenmayer flasks (300 mL) containing 50 mL of YPG medium (yeast extract (10 g/L), peptone (20 g/L), glucose (20 g/L), pH = 6. After 4 days of culture growth at 25 °C, 50 µL of substrate (0.060 g, 9.4 mM) was added to shaken cultures (130 rpm). After a maximum 4 days, the medium (20 mL) containing unreacted substrate, product and mycelium was extracted with ethyl acetate (20 mL). The organic fraction was dried over anhydrous magnesium sulphate and the solvent was removed by evaporation in vacuo. The remaining sample was analysed by GC (DB-1 column and chiral column,
Figures S6 and S7).
2.5. Preparation of Resting Cell Suspension
The cultivated cells were harvested by centrifugation (5000 rpm, 3 min) at 4 °C and washed three times with distilled water. Resting cells culture of microorganism was re-suspended in 40 mL of 0.1 M phosphate buffer (KH2PO4 + Na2HPO4, pH = 7.0) to make a resting cell suspension (5.5 g/L). In a separate experiment, the yeast cells were re-suspended in 0.1 M phosphate buffer (KH2PO4 + Na2HPO4, pH = 7.0) containing 1% of glucose to make a resting cell suspension (5.5 g/L). The rest of the procedure was similar to that described above.
2.6. Biotransformation in the Presence of Organic Solvents
Each microorganism culture (20 mL, 11.0 g/L) was centrifuged at 5000 rpm for 3 min. The culture was then re-suspended in 50 mL of sterile medium with the addition of an appropriately chosen organic solvent (ethanol, glycerol, hexane, or isopropanol). The substrate 1 (0.060 g, 9.4 mM) was added. Each suspension was left for 1 day on a rotary shaker (130 rpm, temperature 25 °C), and then the medium (20 mL) containing unreacted substrate, product and mycelium was extracted with ethyl acetate (20 mL). The organic fractions were dried over anhydrous magnesium sulphate, and the solvent was removed by evaporation in vacuo and analysed by GC (chiral column).
2.7. Biotransformation in the Presence of Deep Eutectic Solvents
The deep eutectic solvent was prepared as follows: choline chloride (ChCl, 1 mol) was mixed with glycerol (Gly, 2 mol) at 80 °C for 2 h until a homogeneous liquid was acquired in parallel, the freshly obtained culture of Candida viswanathi AM120, (20 mL, 11.0 g/L) was centrifuged at 5000 rpm for 3 min. Subsequently, cells from the microorganism culture were re-suspended in 40 mL of sterile phosphate buffer (0.1 M, pH = 7) with 10%, 25%, or 50% concentrations of DES respectively. α-Acetylbutyrolactone 1 was added. Incubation of yeast culture with the substrate was continued for 1 day, 2 days, 3 days or 7 days at 25 °C on a rotary shaker (130 rpm). After a suitable period of time, the medium (20 mL) containing unreacted substrate, product and mycelium was extracted with ethyl acetate (20 mL). The organic fractions were dried over anhydrous magnesium sulphate, the solvent was removed by evaporation in vacuo and analysed by GC (chiral column).
3. Results and Discussion
Our investigation started off with the chemical reduction of α-acetylbutyrolactone
1 by NaBH
4. The reaction was carried out according to the procedure worked out by Teixeira et al. [
17] and we derived
anti-to-
syn isomers of α’-1’-hydroxyethyl-γ-butyrolactone
2 in a ratio of 1:7 (
Figure 1), which were used as GC standards for product
2.
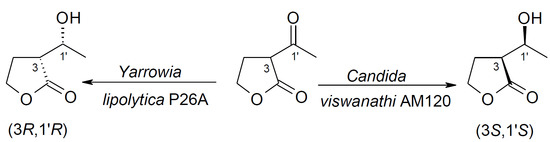
Various yeast strains were examined for their ability to conduct the enantioselective reduction of α-acetylbutyrolactone 1. We commenced with a screening procedure using the following yeast strains: Y. lipolytica P26A, Y. lipolytica AM71, Y. lipolytica AM72, C. viswanathi AM120, H. anomala C2, S. cerevisiae K1, and S. pombe C1.
In our preliminary studies, we used microorganisms grown in medium YPG (glucose (20 g/L), peptone (20 g/L), yeast extract (10 g/L, pH = 6). Biotransformations were carried out for a maximum of 4 days. In the case where compound
1 was transformed completely, the reaction was then interrupted earlier (
Table 1).
Based on the results,
Yarrowia strains prove to be very good biocatalysts for the reduction of α-acetylbutyrolactone
1. However, in all cases, only the formation of the
anti diastereoisomer was observed. It should be emphasized that α-acetylbutyrolactone
1 was transformed completely by
Y. lipolytica P26A after 1 day. The
C. viswanathi AM120 strain bioreduced
1 after 2 days and with less diastereoselectivity. In general, the
Saccharomyces yeast did not transform the substrate at all. This observation is in line with literature data, where Ribeiro et al. [
2] indicated that
S. cerevisiae was not able to transform this substrate. On the other hand, Fantin et al. [
19] and Tala-Tapeha et al. [
20] successfully transformed
1 using this particular microorganism. It should be highlighted that different strains of yeast transform α-acetylbutyrolactone
1 with different stereoselectivity.
The subsequent stage of our research was to investigate the evaluation of the stereoselectivity of biotransformations. Tests were performed using two strains of
Y. lipolytica and one strain of
C. viswanathi, because these strains were capable of bioreducing
1 within 1 or 2 days (
Table 2).
The results presented above indicate that both strains of Y. lipolytica bioreduced 1 to only one isomer, namely 2b, with 100% enantiomeric excess. The strain of C. viswanathi AM120 transformed α-acetylbutyrolactone to a mixture of 2a and 2b isomers, with a large predominance of 2a and trace amounts of optically pure 2d enantiomer.
Next, biotransformation of
1 by
Y. lipolytica P26A was repeated in two flasks (summarily 0.12 g of substrate) and product of biotransformation of
1 was purified by means of column chromatography (61 mg after isolation, yield 51%) and optical rotation was determined. The obtained value of optical rotation (
= −20.4 (c = 0.9; CHCl
3) was compared with literature data to confirm configuration of the biotransformation product [
18].
Stereoselective transformation of α-acetylbutyrolactone
1 can take place either by reducing the carbonyl group or by reducing the double bond in the enol that is readily formed by keto-enol tautomerism. Enol reduction could be catalysed by ene-reductases (flavin-dependent oxidoreductases belonging to the Old Yellow Enzyme family), which require NADPH as a cofactor. If the structure of the enol hydroxyl group is present, it could function as an electron-withdrawing group (EWG) [
21]. In turn, stereoselective reduction of the carbonyl group can be catalysed by dehydrogenases [
22] or ketoreductases [
23,
24].
In the
Y. lipolytica strains, stereoselective dehydrogenases have been identified [
25]. We assumed based on this statement and Ribeiro’s work [
2], that the reduction of
1 would therefore be catalysed by dehydrogenase. In turn, reduction of
1 was carried out by
C. viswanathi AM120 with less stereoselectivity, hence the presence of both enzymes, ene-reductase and dehydrogenase, is possible.
The following phase of our research was to use resting cells in place of growing cultures (
Table 3).
The resting cell suspension, in comparison to the growing cultures, seems to have had a negative effect on bioreduction time. In all three cases, the time needed for transformation was extended by one day. In addition, the formation of an undesired stereoisomer 2a, in biotransformation by means of Yarrowia strains was observed. In contrast, for C. viswanathi AM120, the enantiomeric excess of the expected isomer 2a decreased by about 20%.
Furthermore, we investigated if the addition of sugar would be favourable for biotransformation, as medium containing a high concentration of glucose would facilitate the regeneration of enzyme cofactors. For this purpose, 1% glucose was added to the harvested yeast cells which were re-suspended in phosphate buffer at pH = 7. The results are presented in
Table 4.
Glucose supplementation reduced the time required for complete substrate reduction. Unfortunately, the enantioselectivity of the biotransformation by Y. lipolytica did not improve. Small amounts of undesirable products, namely stereoisomers 2a and 2d, were also identified. In contrast, the C. viswanathi AM120 strain exhibited greater enantioselectivity with respect to the 2a enantiomer, but the diastereoselectivity of reduction was decreased.
In the succeeding experiment, small amounts of organic solvents were added to the medium. Ethanol, glycerol, hexane and isopropanol were used in various proportions. With respect to carbonyl reductase, isopropanol can be used as a co-substrate for the regeneration of NAD
+ or NADP
+ and it increases substrate solubility [
26]. It is also interesting to note that ethanol is a fascinating alternative to glucose as a co-substrate for the regeneration of cofactors. However, it is toxic to the cells at higher concentrations [
27].
The influence of solvent addition was investigated on two strains of yeast,
C. viswanathi AM120 and
Y. lipolytica P26A (
Table 5). Due to the previously obtained results, the time for biotransformation was assumed to be 1 day.
Our results indicate that the addition of organic solvents did not have a positive effect on the biotransformation by C. viswanathi AM120 and Y. lipolytica P26A. The use of 5% ethanol resulted in a significant slowdown of the biotransformation. Increasing the quantity of ethanol to 10% caused complete inhibition of the reaction. Also, the addition of hexane to the medium reduced the substrate’s conversion rate. Supplementation with glycerol or isopropanol had no significant effect on substrate conversion. While noting that, the inclusion of glycerol did increase the amount of 2d product only. Overall, all four solvents used in the experiment resulted in the reduction of the enantiomeric excess of the anti diastereoisomer.
The addition of isopropanol also slightly decreased conversion of substrate by the culture of Y. lipolytica P26A. Moreover, with isopropanol present, formation of the 2d product was observed, which was absent in biotransformation without the presence of solvent. Use of up to 10% glycerol or hexane was ineffective. However, the 20% addition of these solvents led to the formation of trace amounts of stereoisomers 2c and 2d.
Finally, our study concluded with the biotransformation of
1 by
C. viswanathi AM120 in phosphate buffer pH = 7 supplemented with deep eutectic solvent, which is a mixture of choline chloride and glycerol in a ratio of 1:2. The reaction was continued until the substrate was completely reduced, but no longer than 7 days (
Table 6).
If we compare the results obtained from both the biotransformation conducted in just buffer versus in buffer supplemented with DES, the advantage of DES can be noted. The 10% and 25% DES addition reduced the time of transformation to 2 days. With regards to selectivity, DES positively influenced enantio- and stereoselectivity in the process, meaning that it caused an increase. The predominant product was still ster·eoisomer 2a, as the reaction mixture comprised about 80%. Unfortunately, the use of the DES mixture at 50% had adverse effects on the reaction. Despite allowing the biotransformation to progress over 7 days, complete substrate conversion was not achieved and the enantioselectivity of the process was significantly reduced.