Characterizations of Efficient Charge Transfer and Photoelectric Performance in the Cosensitization of Solar Cells
Abstract
:1. Introduction
2. Calculated Methods
3. Results and Discussion
3.1. Structure
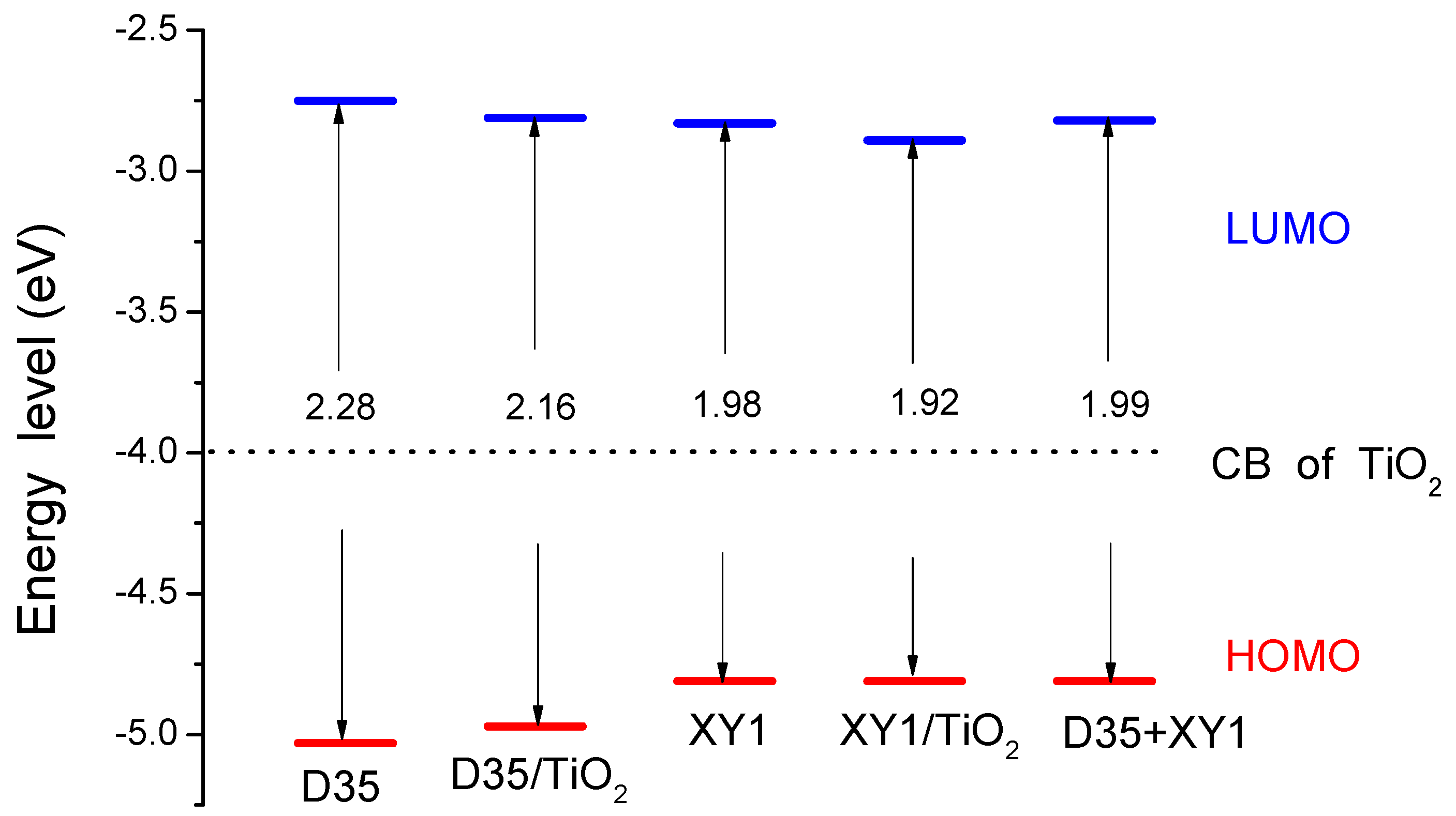
3.2. Energy Levels
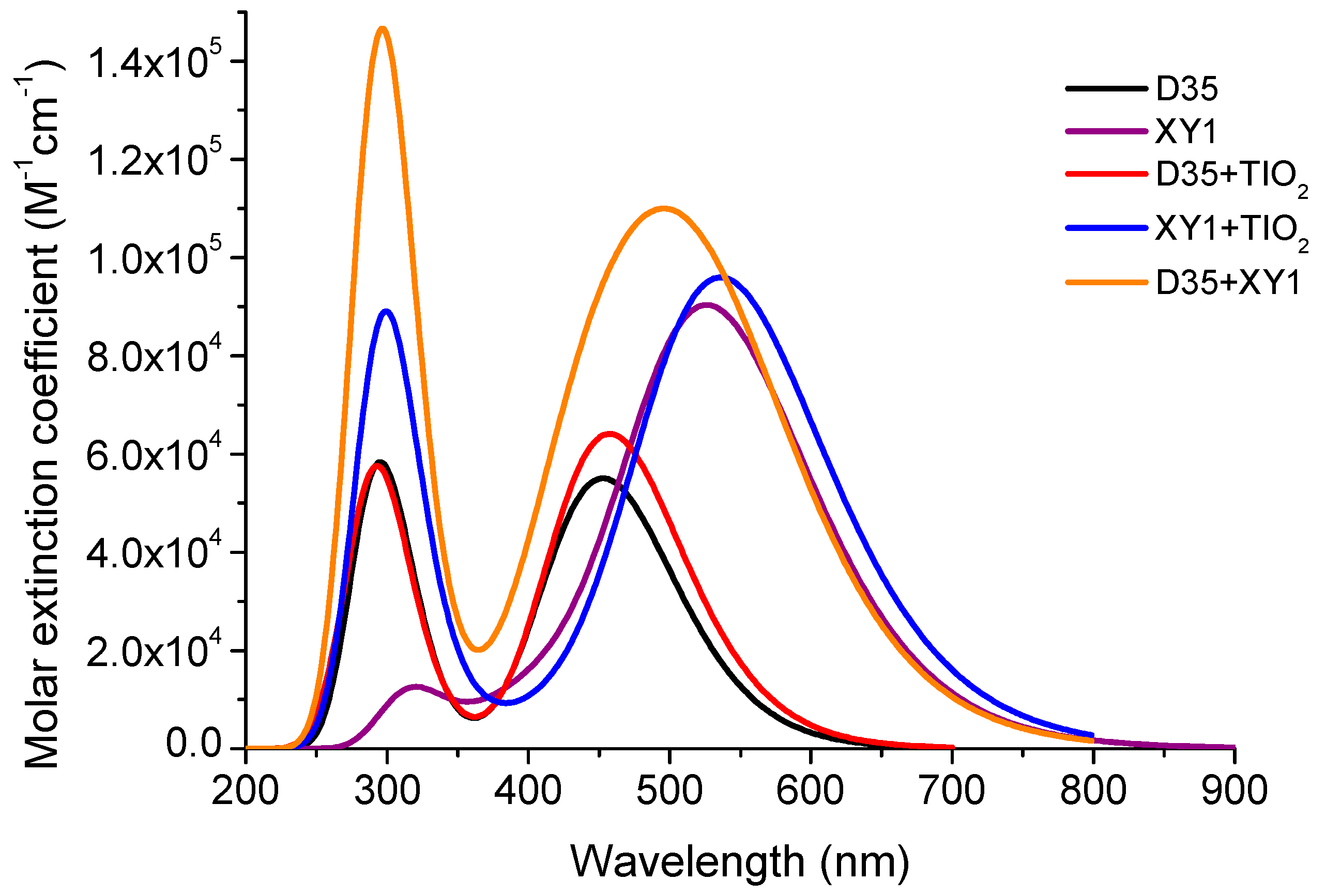
3.3. Absorption Spectra
3.4. Chemical Reactivity Parameters and Reorganization Energy
3.5. Intramolecular and Intermolecular Charge Transfer (CT)
3.6. Factors Influencing JSC and Voc
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Regan, B.O.; Gratzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Cao, Y.M.; Saygili, Y.; Ummadisingu, A.; Teuscher, J.; Luo, J.S.; Pellet, N.; Giordano, F.; Zakeeruddin, S.M.; Moser, J.E.; Freitag, M.; et al. 11% efficiency solid-state dye-sensitized solar cells with copper(II/I) hole transport materials. Nat. Commun. 2017, 8, 15390. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Xu, B.; Liu, P.; Hu, Y.; Yu, Y.; Ding, H.; Kloo, L.; Hua, J.; Sun, L.; Tian, H. High performance solid-state dye-sensitized solar cells based on organic blue-colored dyes. J. Mater. Chem. A 2017, 5, 1242–1247. [Google Scholar] [CrossRef]
- Gong, J.W.; Sumathy, K.; Qiao, Q.; Zhou, Z.P. Review on dye-sensitized solar cells (DSSCs): Advanced techniques and research trends. Renew. Sustain. Energy Rev. 2017, 68, 234–246. [Google Scholar] [CrossRef]
- Li, Y.Z.; Li, H.; Zhao, X.; Chen, M.D. Electronic structure and optical properties of dianionic and dicationic π-Dimers. J. Phys. Chem. A 2010, 114, 6972–6977. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.S.; Tang, Y.Y.; Wu, W.J.; Wang, Y.Q.; Liu, J.C.; Li, X.; Tian, H.; Zhu, W.H. Porphyrin Cosensitization for a Photovoltaic Efficiency of 11.5%: A Record for Non-Ruthenium Solar Cells Based on Iodine Electrolyte. J. Am. Chem. Soc. 2015, 137, 14055–14058. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.S.; Wan, Z.Q.; Jia, C.Y.; Wang, Y.; Wu, X.C.; Yao, X.J. Co-sensitization of Dithiafulvenyl–Phenothiazine Based Organic Dyes with N719 for Efficient Dye-Sensitized Solar Cells. Electrochim. Acta 2016, 211, 364–374. [Google Scholar] [CrossRef]
- Liu, B.; Chai, Q.; Zhang, W.; Wu, W.; Tian, H.; Zhu, W. Cosensitization process effect of DA–π–A featured dyes on photovoltaic Performances. Green Energy Environ. 2016, 1, 84–90. [Google Scholar] [CrossRef]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Kyomen, T.; Hanaya, M. Fabrication of a high-performance dye-sensitized solar cell with 12.8% conversion efficiency using organic silyl-anchor dyes. Chem. Commun. 2015, 51, 6315–6317. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Saygili, Y.; Cong, J.; Eriksson, A.; Yang, W.; Zhang, J.; Polanski, E.; Nonomura, K.; Zakeeruddin, S.M.; Grätzel, M.; et al. Novel Blue Organic Dye for Dye-Sensitized Solar Cells Achieving High Efficiency in Cobalt-Based Electrolytes and by Co-Sensitization. ACS Appl. Mater. Interfaces 2016, 8, 32797–32804. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Chen, B.; Wu, W.J.; Li, X.; Zhu, W.H.; Tian, H.; Xie, Y.S. Efficient Solar Cells Sensitized by Porphyrins with an Extended Conjugation Framework and a Carbazole Donor: From Molecular Design to Cosensitization. Angew. Chem. Int. Ed. 2014, 53, 10779–10783. [Google Scholar] [CrossRef] [PubMed]
- Freitag, M.; Teuscher, J.; Saygili, Y.; Zhang, X.; Giordano, F.; Liska, P.; Hua, J.; Zakeeruddin, S.M.; Moser, J.-E.; Grätzel, M.; et al. Dye-sensitized solar cells for efficient power generation under ambient lighting. Nat. Photonics 2017, 11, 372–378. [Google Scholar] [CrossRef] [Green Version]
- He, L.J.; Wei, W.; Chen, J.; Jia, R.; Wang, J.; Zhang, H.X. The effect of D–[De–π–A]n (n = 1, 2, 3) type dyes on the overall performance of DSSCs: A theoretical investigation. J. Mater. Chem. C 2017, 5, 7510–7520. [Google Scholar] [CrossRef]
- Xu, B.B.; Li, Y.Z.; Song, P.; Ma, F.C.; Sun, M.T. Photoactive layer based on T-shaped benzimidazole dyes used for solar cell: From photoelectric properties to molecular design. Sci. Rep. 2017, 7, 45688. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.N.; Zhou, H.Y.; Sun, P.P.; Chen, S.L.; Li, Z.S. A Promising Candidate with D-A-A-A Architecture as an Efficient Sensitizer for Dye-Sensitized Solar Cells. ChemPhysChem 2015, 16, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ren, P.H.; Wang, X.F.; Li, Y.Z.; Yang, Y.H. Experimental and Theoretical Investigation of Photoelectrical Properties of Tetrabromophenol blue and Bromoxylenol blue based solar cell. J. Nanomater. 2018, 9720595, in press. [Google Scholar]
- Li, Y.Z.; Sun, C.F.; Song, P.; Ma, F.C.; Yang, Y.H. Tuning the electron transport and accepting ability of dyes via introducing different π-conjugated bridges and acceptors for DSSCs. ChemPhysChem 2017, 18, 366–383. [Google Scholar] [CrossRef] [PubMed]
- Bahers, T.L.; Brémond, E.; Ciofini, I.; Adamo, C. The nature of vertical excited states of dyes containing metals for DSSC applications: Insights from TD-DFT and density based indexes. Phys. Chem. Chem. Phys. 2014, 16, 14435–14444. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.A.; Allam, N.K. Propping the optical and electronic properties of potential photo-sensitizers with different π-spacers: TD-DFT insights. Spectrochim. Acta A 2018, 188, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Sharmoukh, W.; Hassan, W.M.I.; Gros, P.C.; Allam, N.K. Design and synthesis of new Ru-complexes as potential photo-sensitizers: Experimental and TD-DFT insights. RSC Adv. 2016, 6, 69647–69657. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Parr, R.G. Density functional theory. Ann. Rev. Phys. Chem. 1983, 34, 631–656. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, R.E.; Scuseria, G.E.; Frisch, M.J. An efficient implementation of time-dependent density-functional theory for the calculation of excitation energies of large molecules. J. Chem. Phys. 1998, 109, 8218–8224. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Yao, B.-Q.; Sun, J.-S.; Tian, Z.-F.; Ren, X.-M.; Gu, D.-W.; Shen, L.-J.; Jiang, X. Ion-pair charge transfer complexes with intense near IR absorption: Syntheses, crystal structures, electronic spectra and DFT calculations. Polyhedron 2008, 27, 2833–2844. [Google Scholar] [CrossRef]
- Marcus, R.A. Electron transfer reactions in chemistry. Theory and experiment. Rev. Mod. Phys. 1993, 65, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Muscat, J.P.; Newns, D.M. Chemisorption on metals. Prog. Surf. Sci. 1978, 9, 1–43. [Google Scholar] [CrossRef]
- Ding, W.L.; Peng, X.L.; Sun, Z.Z.; Li, Z.S. Novel bifunctional aromatic linker utilized in CdSe quantum dots-sensitized solar cells: Boosting the open-circuit voltage and electron injection. J. Mater. Chem. A 2017, 5, 14319–14330. [Google Scholar] [CrossRef]
- Zong, H.; Wang, J.C.; Mu, X.J.; Xu, X.F.; Li, J.; Wang, X.Y.; Long, F.X.; Wang, J.X.; Sun, M.T. Physical mechanism of photoinduced intermolecular charge transfer enhanced by fluorescence resonance energy transfer. Phys. Chem. Chem. Phys. 2018, 20, 13558–13565. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Zhang, Y.J.; Zhang, J.Q.; Wang, Z.Y.; Zhu, L.Y.; Fang, J.F.; Xia, B.Z.; Wang, Z.; Lu, K.; Ma, W.; et al. Fluorination-enabled optimal morphology leads to over 11% efficiency for inverted small-molecule organic solar cells. Nat. Commun. 2016, 7, 13740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cave, R.J.; Newton, M.D. Generalization of the Mulliken–Hush treatment for the calculation of electron transfer matrix elements. Chem. Phys. Lett. 1996, 249, 15–19. [Google Scholar] [CrossRef]
- Obotowo, I.N.; Obot, I.B.; Ekpe, U.J. Organic sensitizers for dye-sensitized solar cell (DSSC): Properties from computation, progress and future perspectives. J. Mol. Struct. 2016, 1122, 80–87. [Google Scholar] [CrossRef]
- Rühle, S.; Greenshtein, M.; Chen, S.G.; Merson, A.; Pizem, H.; Sukenik, C.S.; Cahen, D.; Zaban, A. Molecular Adjustment of the Electronic Properties of Nanoporous Electrodes in Dye-Sensitized Solar Cells. J. Phys. Chem. B 2005, 109, 18907–18913. [Google Scholar] [CrossRef] [PubMed]


| Dye | IP | EA | h | − | + | |
|---|---|---|---|---|---|---|
| D35 | 4.649 | 3.042 | 0.804 | 9.203 | 11.226 | 7.380 |
| XY1 | 4.615 | 3.068 | 0.774 | 9.539 | 11.557 | 7.715 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Lin, X.; Mi, L.; Gao, N.; Song, P.; Ma, F.; Li, Y. Characterizations of Efficient Charge Transfer and Photoelectric Performance in the Cosensitization of Solar Cells. Appl. Sci. 2018, 8, 1122. https://doi.org/10.3390/app8071122
Liu Q, Lin X, Mi L, Gao N, Song P, Ma F, Li Y. Characterizations of Efficient Charge Transfer and Photoelectric Performance in the Cosensitization of Solar Cells. Applied Sciences. 2018; 8(7):1122. https://doi.org/10.3390/app8071122
Chicago/Turabian StyleLiu, Qian, Xiaochen Lin, Lu Mi, Nan Gao, Peng Song, Fengcai Ma, and Yuanzuo Li. 2018. "Characterizations of Efficient Charge Transfer and Photoelectric Performance in the Cosensitization of Solar Cells" Applied Sciences 8, no. 7: 1122. https://doi.org/10.3390/app8071122





