Research Progress of Gas Sensor Based on Graphene and Its Derivatives: A Review
Abstract
:Featured Application
Abstract
1. Introduction
2. Gas Sensor
2.1. Key Parameters of Gas Sensor
2.2. Graphene Gas Sensors with Different Working Principles
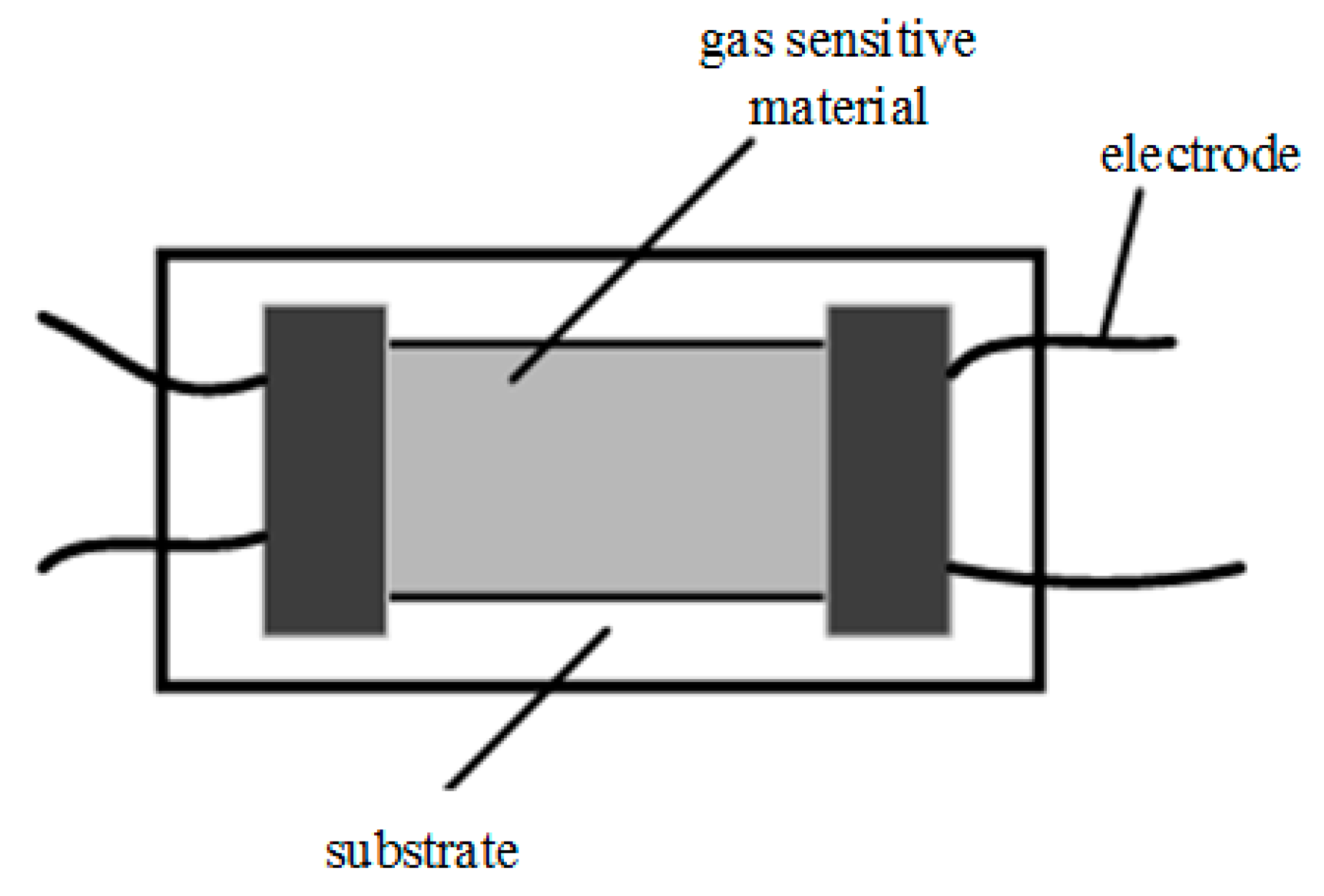
2.2.1. Resistive Gas Sensor
2.2.2. FET Gas Sensor
2.2.3. Quality Sensitive Gas Sensor
2.2.4. MEMS Gas Sensor
3. Preparation and Properties of Graphene
3.1. Preparation of Graphene
3.2. The Property of Graphene
4. Graphene Gas Sensor
4.1. Gas Sensors Based on Pristine Graphene
4.2. Gas Sensors Based on Defective and Functionalized Graphene Materials
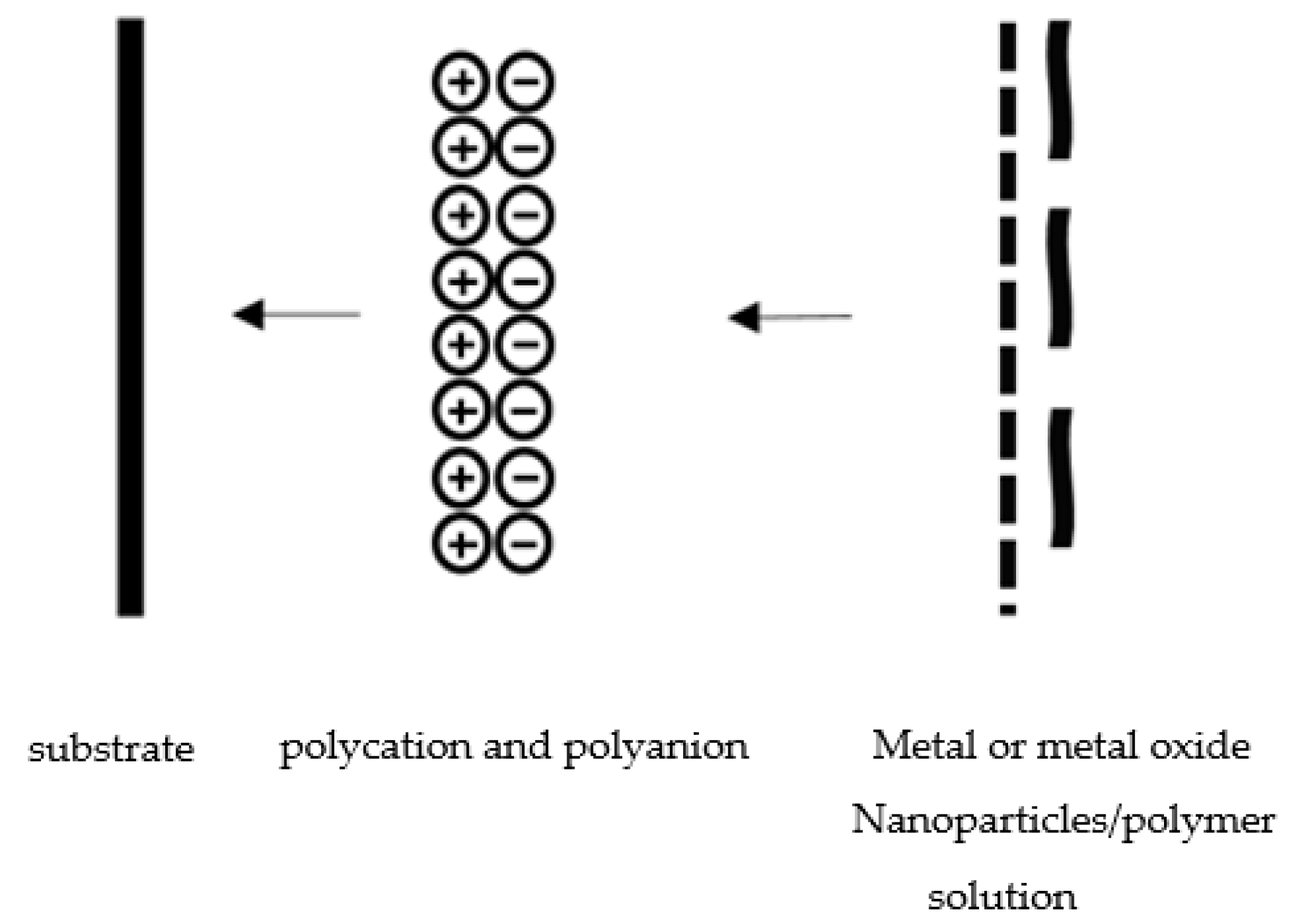
4.3. Gas Sensors Based on Graphene/Polymer Composites
4.4. Gas Sensors Based on Graphene/Metal or Metal Oxide Composites
5. Conclusions
6. Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Basu, S.; Bhattacharyya, P. Recent developments on graphene and graphene oxide based solid state gas sensors. Sens. Actuators B Chem. 2012, 173, 1–21. [Google Scholar] [CrossRef]
- Varghese, S.S.; Varghese, S.H. Two-Dimensional Materials for Sensing: Graphene and Beyond. Electronics 2015, 4, 651–687. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Mubeen, S. Recent progress in carbon nanotube-based gas sensors. Nanotechnology 2008, 19, 332001. [Google Scholar] [CrossRef] [PubMed]
- Govardhan, K.; Grace, A.N. Metal/Metal Oxide Doped Semiconductor Based Metal Oxide Gas Sensors—A Review. Sens. Lett. 2016, 14, 741–750. [Google Scholar] [CrossRef]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Fray, D.J. Development of nanocrystalline TiO2–Er2O3, and TiO2–Ta2O5, thin film gas sensors: Controlling the physical and sensing properties. Sens. Actuators B Chem. 2009, 141, 76–84. [Google Scholar] [CrossRef]
- He, Q.; Wu, S.; Yin, Z.; Zhang, H. Graphene-based electronic sensors. Chem. Sci. 2012, 3, 1764–1772. [Google Scholar] [CrossRef]
- Zhang, S.; Shao, Y.; Liao, H.; Engelhard, M.H.; Yin, G.; Lin, Y. Polyelectrolyte-induced reduction of exfoliated graphite oxide: A facile route to synthesis of soluble graphene nanosheets. ACS Nano 2011, 5, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Falkovsky, L.A. Optical properties of graphene. J. Exp. Theor. Phys. 2008, 115, 012004. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y. Research and Development of Gas Sensors Based on Nanomaterials. Sens. Microsyst. 2013, 32, 1–5. [Google Scholar]
- Wang, T.; Huang, D.; Yang, Z.; Xu, S.; He, G.; Li, X.; Hu, N.; Yin, G.; He, D.; Zhang, L. A Review on Graphene-Based Gas/Vapor Sensors with Unique Properties and Potential Applications. Nano Micro Lett. 2016, 8, 95–119. [Google Scholar] [CrossRef]
- Sriyudthsak, M.; Teeramongkolrasasmee, A. Radial basis neural networks for identification of volatile organic compounds. Sens. Actuators B Chem. 2000, 65, 358–360. [Google Scholar] [CrossRef]
- Wehling, T.O.; Novoselov, K.S. Molecular doping of graphene. Nano Lett. 2008, 8, 173–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearce, R.; Iakimov, T. Epitaxially grown graphene based gas sensors for ultra sensitive NO2, detection. Sens. Actuators B Chem. 2010, 155, 451–455. [Google Scholar] [CrossRef]
- Sun, F.; Xu, S. Application of Graphene Material in Gas-Sensor. J. South China Norm. Univ. 2013, 6, 93–98. [Google Scholar]
- Bouvet, M. Phthalocyanine-based field-effect transistors as gas sensors. Anal. Bioanal. Chem. 2006, 384, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohno, Y.; Maehashi, K. Chemical and biological sensing applications based on graphene field-effect transistors. Biosens. Bioelectron. 2010, 26, 1727–1730. [Google Scholar] [CrossRef] [PubMed]
- Arsat, R.; Breedon, M. Graphene-like nano-sheets for surface acoustic wave gas sensor applications. Chem. Phys. Lett. 2009, 467, 344–347. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J. MEMS-based NO2, gas sensor using ZnO nano-rods for low-power IoT application. J. Korean Phys. Soc. 2017, 70, 924–928. [Google Scholar] [CrossRef]
- Hill, E.W.; Vijayaragahvan, A. Graphene Sensors. IEEE Sens. J. 2011, 11, 3161–3170. [Google Scholar] [CrossRef]
- Katsnelson, M.I. Graphene: Carbon in two dimensions. Mater. Today 2007, 10, 20–27. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, X. A Review of Graphene on NEMS. Recent Pat. Nanotechnol. 2016, 10, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.S.; Kitt, A. Transfer of CVD-Grown Monolayer Graphene onto Arbitrary Substrates. ACS Nano 2011, 5, 6916–6924. [Google Scholar]
- Li, X.; Cai, W. Evolution of graphene growth on Ni and Cu by carbon isotope labeling. Nano Lett. 2009, 9, 4268–4272. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jian, L.; Peng, Z.; Sun, Z.; Zhu, Y.; Li, L.; Xiang, C.; Loic Samuel, E.; Carter, K.; James, M.T. Toward the Synthesis of Wafer-Scale Single-Crystal Graphene on Copper Foils. ACS Nano 2013, 7, 875. [Google Scholar]
- Land, T.A.; Michely, T. STM investigation of single layer graphite structures produced on Pt(111) by hydrocarbon decomposition. Surf. Sci. 1992, 264, 261–270. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X. Large Area, Few-Layer Graphene Films on Arbitrary Substrates by Chemical Vapor Deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Marchini, S.; Günther, S.; Wintterlin, J. Scanning tunneling microscopy of graphene on Ru(0001). Phys. Rev. B 2007, 76, 075429. [Google Scholar] [CrossRef]
- Coraux, J.; N’Diaye, A.T.; Busse, C.; Michely, T. Structural Coherency of Graphene on Ir(111). Nano Lett. 2008, 8, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, S.; Tsuya, D. Coupled Quantum Dots in a Graphene-Based Two-Dimensional Semimetal. Nano Lett. 2009, 9, 2891–2896. [Google Scholar] [CrossRef] [PubMed]
- Somani, P.R.; Somani, S.P. Planer nano-graphenes from camphor by CVD. Chem. Phys. Lett. 2006, 430, 56–59. [Google Scholar] [CrossRef]
- Chen, F.; Quan, Q. Electrochemical Gate-Controlled Charge Transport in Graphene in Ionic Liquid and Aqueous Solution. J. Am. Chem. Soc. 2009, 131, 9908–9909. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, W. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Copel, M.; Reuter, M.C. Surfactants in epitaxial growth. Phys. Phys. Lett. 1989, 63, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Borovikov, V.; Zangwill, A. Step-edge instability during epitaxial growth of graphene from SiC(0001). Phys. Rev. B 2009, 80, 121406. [Google Scholar] [CrossRef]
- Berger, C.; Song, Z. Ultrathin epitaxial graphite: 2D electron gas properties and a route toward graphene-based nanoelectronics. J. Phys. Chem. B 2004, 108, 19912–19916. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 41, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Brodie, B.C. On the Atomic Weight of Graphite. Philos. Trans. R. Soc. Lond. 2009, 149, 249–259. [Google Scholar] [CrossRef]
- Staudenmaier, L. Verfahren zur Darstellung der Graphitsäure. Eur. J. Inorg. Chem. 2010, 32, 1394–1399. [Google Scholar]
- Hirata, M.; Gotou, T. Thin-film particles of graphite oxide 1: High-yield synthesis and flexibility of the particles. Carbon 2004, 42, 2929–2937. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Weitz, R.T.; Yacoby, A. Nanomaterials: Graphene rests easy. Nat. Nanotechnol. 2010, 5, 699–700. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Blake, P. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X. Elastic and frictional properties of graphene. Phys. Status Sol. 2010, 246, 2562–2567. [Google Scholar] [CrossRef]
- Frank, I.W.; Tanenbaum, D.M. Mechanical properties of suspended graphene sheets. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2007, 25, 2558–2561. [Google Scholar] [CrossRef]
- Morozov, S.V.; Novoselov, K.S. Conferences and Symposia: Electron transport in graphene. Phys. Uspekhi 2008, 51, 744–748. [Google Scholar] [CrossRef]
- Sruti, A.N.; Jagannadham, K. Electrical Conductivity of Graphene Composites with In and In-Ga Alloy. J. Electron. Mater. 2010, 39, 1268–1276. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Stoller, M.D.; Park, S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Blake, P. Universal Dynamic Conductivity and Quantized Visible Opacity of Suspended Graphene. arXiv, 2008; arXiv:0803.3718. [Google Scholar]
- Kuila, T.; Bose, S. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Jiang, Y.; Raliya, R. Graphene Oxides in Water: Correlating Morphology and Surface Chemistry with Aggregation Behavior. Environ. Sci. Technol. 2016, 50, 6964–6973. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Raliya, R. Graphene oxides in water: Assessing stability as a function of material and natural organic matter properties. Environ. Sci. Nano 2017, 4, 1484–1493. [Google Scholar] [CrossRef]
- Schedin, F.; Novoselov, K.S. Detection of Individual Gas Molecules by Graphene Sensors. Nat. Mater. 2006, 6, 652–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, S.; Lim, J. Chemical vapor sensing properties of graphene based on geometrical evaluation. Curr. Appl. Phys. 2012, 12, 1017–1022. [Google Scholar] [CrossRef]
- Chen, C.W.; Hung, S.C. Oxygen sensors made by monolayer graphene under room temperature. Appl. Phys. Lett. 2011, 99, 243502. [Google Scholar] [CrossRef]
- Yu, K.; Wang, P. Patterning Vertically Oriented Graphene Sheets for Nanodevice Applications. J. Phys. Chem. Lett. 2011, 2, 537–542. [Google Scholar] [CrossRef]
- Yavari, F.; Castillo, E. High sensitivity detection of NO2 and NH3 in air using chemical vapor deposition grown graphene. Appl. Phys. Lett. 2012, 100, 203120. [Google Scholar] [CrossRef]
- Dutta, D.; Hazra, A. Performance of a CVD grown graphene-based planar device for a hydrogen gas sensor. Meas. Sci. Technol. 2015, 26, 115104. [Google Scholar] [CrossRef]
- Wu, J.; Feng, S. Facile Synthesis of 3D Graphene Flowers for Ultrasensitive and Highly Reversible Gas Sensing. Adv. Funct. Mater. 2016, 26, 7462–7469. [Google Scholar] [CrossRef]
- Yun, J.; Lim, Y. Stretchable patterned graphene gas sensor driven by integrated micro-supercapacitor array. Nano Energy 2015, 19, 401–414. [Google Scholar] [CrossRef]
- Ricciardella, F.; Vollebregt, S. High sensitive gas sensors realized by a transfer-free process of CVD graphene. In Proceedings of the 2016 IEEE Sensors, Orlando, FL, USA, 30 October–3 November 2016. [Google Scholar]
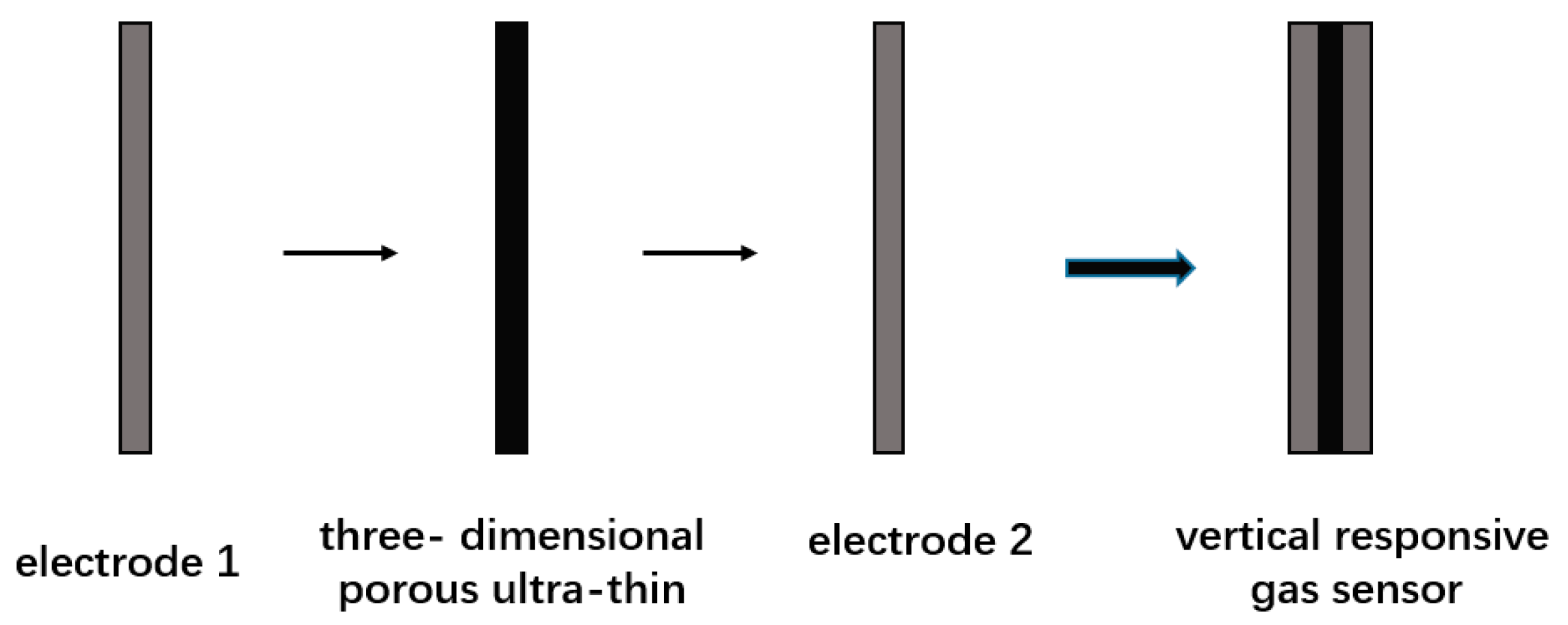
- Wang, Y.; Peng, C. Vertical Response Gas Sensor Based on Three-Dimensional Porous Graphene Ultrathin Film and Its Preparation Method. Patent CN106596654A, 16 April 2017. [Google Scholar]
- Wei, W.; Nong, J. Graphene-Based Long-Period Fiber Grating Surface Plasmon Resonance Sensor for High-Sensitivity Gas Sensing. Sensors 2017, 17, 2. [Google Scholar] [CrossRef] [PubMed]
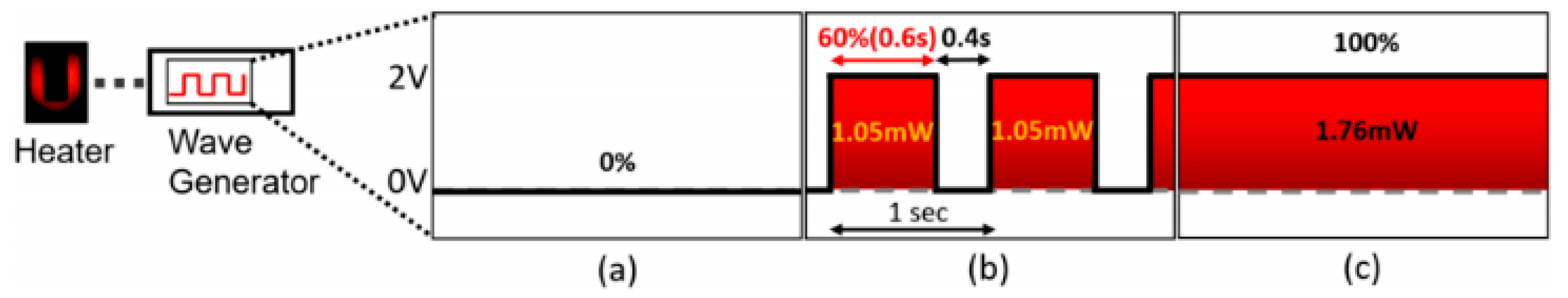
- Kim, J.H.; Zhou, Q. Suspended Graphene-Based Gas Sensor with 1 mW Energy Consumption. Micromachines 2017, 8, 44. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Chen, Y.B. Improving gas sensing properties of graphene by introducing dopants and defects: A first-principles study. Nanotechnology 2009, 20, 185504. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Yuan, J. Gas adsorption on graphene doped with B, N, Al, and S: A theoretical study. Appl. Phys. Lett. 2009, 95, 232105. [Google Scholar] [CrossRef]
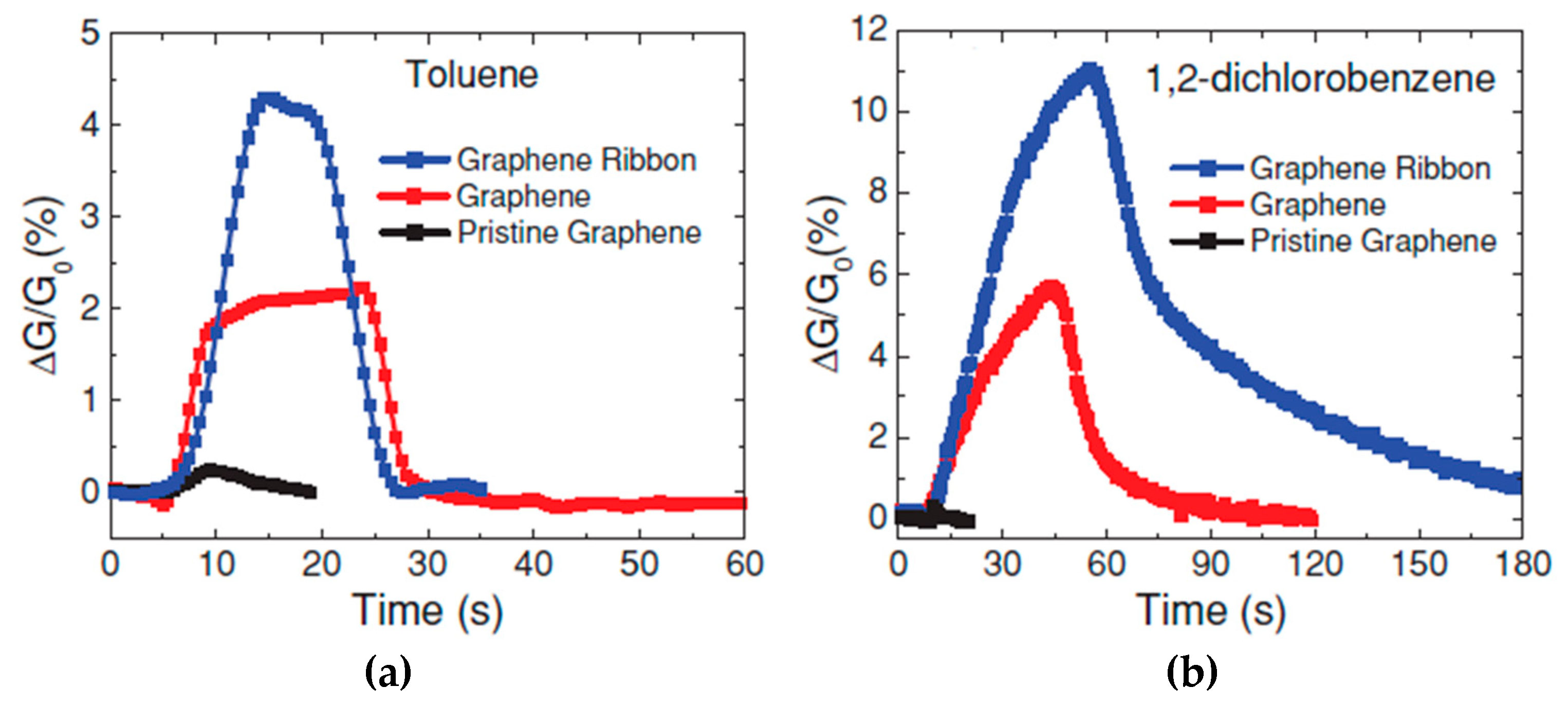
- Salehikhojin, A.; Esreada, D.; Lin, K.P.; Bae, M.H.; Xiong, F.; Pop, E.; Masel, R.I. Polycrystalline graphene ribbons as chemiresistors. Adv. Mater. 2012, 24, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhang, R. A Method of Preparing Graphene Gas Sensor Based on Nanometer Soft Printing Technology. Patent CN102680527A, 23 May 2012. [Google Scholar]
- Sun, J.; Pan, Z. A Gas Sensor for CO Concentration Detection. Patent CN104237356A, 24 December 2014. [Google Scholar]
- Zhang, X.; Yu, L. First-principles study of SF 6, decomposed gas adsorbed on Au-decorated graphene. Appl. Surf. Sci. 2016, 367, 259–269. [Google Scholar] [CrossRef]
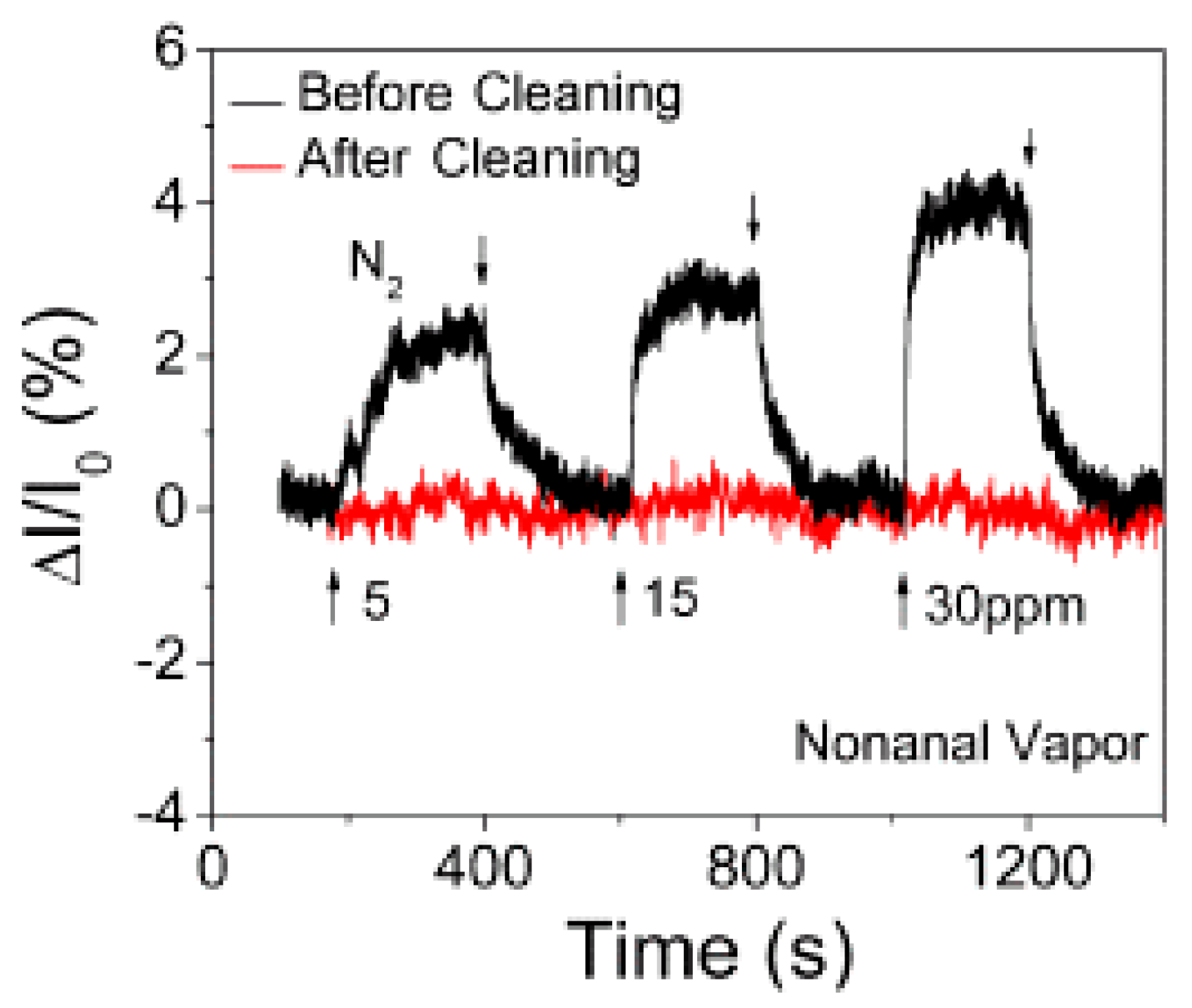
- Zhang, H.; Fan, L. Spectroscopic Investigation of Plasma-Fluorinated Monolayer Graphene and Application for Gas Sensing. ACS Appl. Mater. Interfaces 2016, 8, 8652–8661. [Google Scholar] [CrossRef] [PubMed]
- Ricciardella, F.; Vollebregt, S. Effects of graphene defects on gas sensing properties towards NO2 detection. Nanoscale 2017, 9, 6085–6093. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Dan, Y. Intrinsic Response of Graphene Vapor Sensors. Nano Lett. 2009, 9, 1472–1475. [Google Scholar] [Green Version]
- Zhang, L.; Li, C. Electrosynthesis of graphene oxide/polypyrene composite films and their applications for sensing organic vapors. J. Mater. Chem. 2012, 22, 8438–8443. [Google Scholar] [CrossRef]
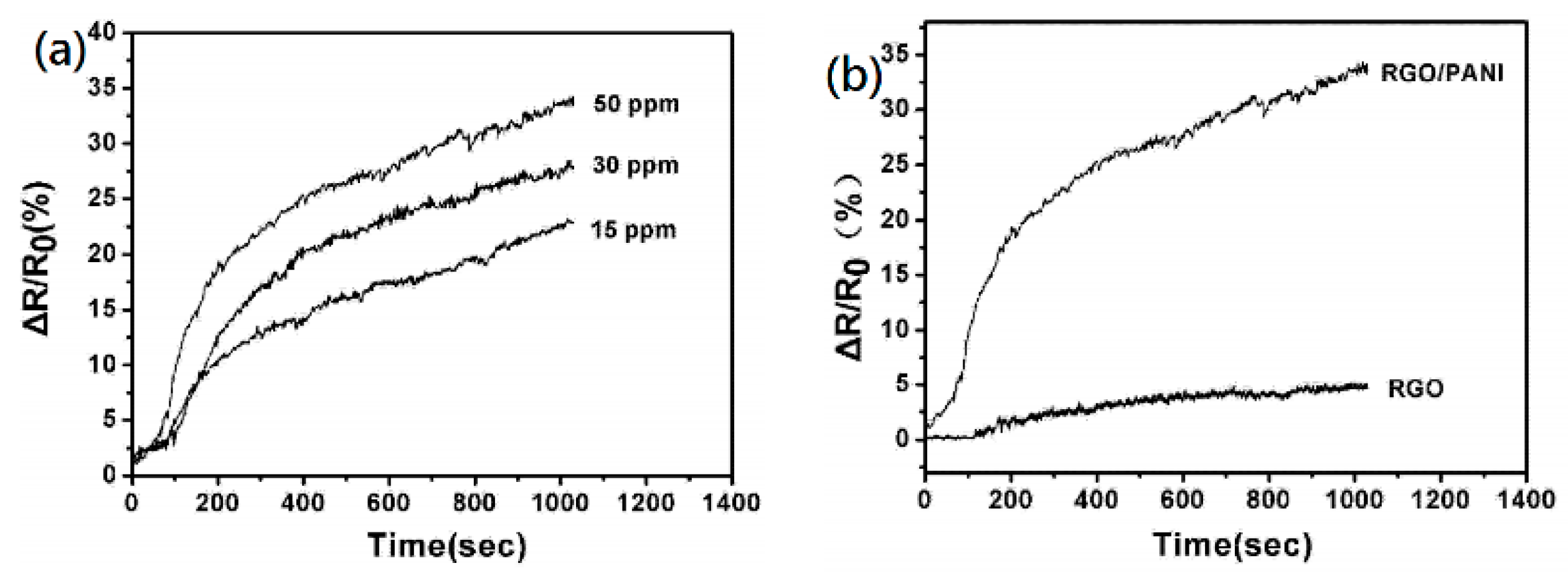
- Huang, X.; Hu, N. Reduced graphene oxide–polyaniline hybrid: Preparation, characterization and its applications for ammonia gas sensing. J. Mater. Chem. 2012, 22, 22488–22495. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, Q. Doping composite of polyaniline and reduced graphene oxide with palladium nanoparticles for room-temperature hydrogen-gas sensing. Int. J. Hydrogen Energy 2016, 41, 5396–5404. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Peng, C. Gas Sensor Based on Graphene/Polyaniline Hybrid Material and Its Preparation Method. Patent CN102879430A, 16 January 2013. [Google Scholar]
- Tai, H.; Ye, Z. Graphene-Based Ternary Composite Film Gas Sensor and Its Preparation Method. Patent CN103926278A, 24 April 2014. [Google Scholar]
- Zhang, Z.; Zou, R. Highly aligned SnO2 nanorods on graphene sheets for gas sensors. J. Mater. Chem. 2011, 21, 17360–17365. [Google Scholar] [CrossRef]
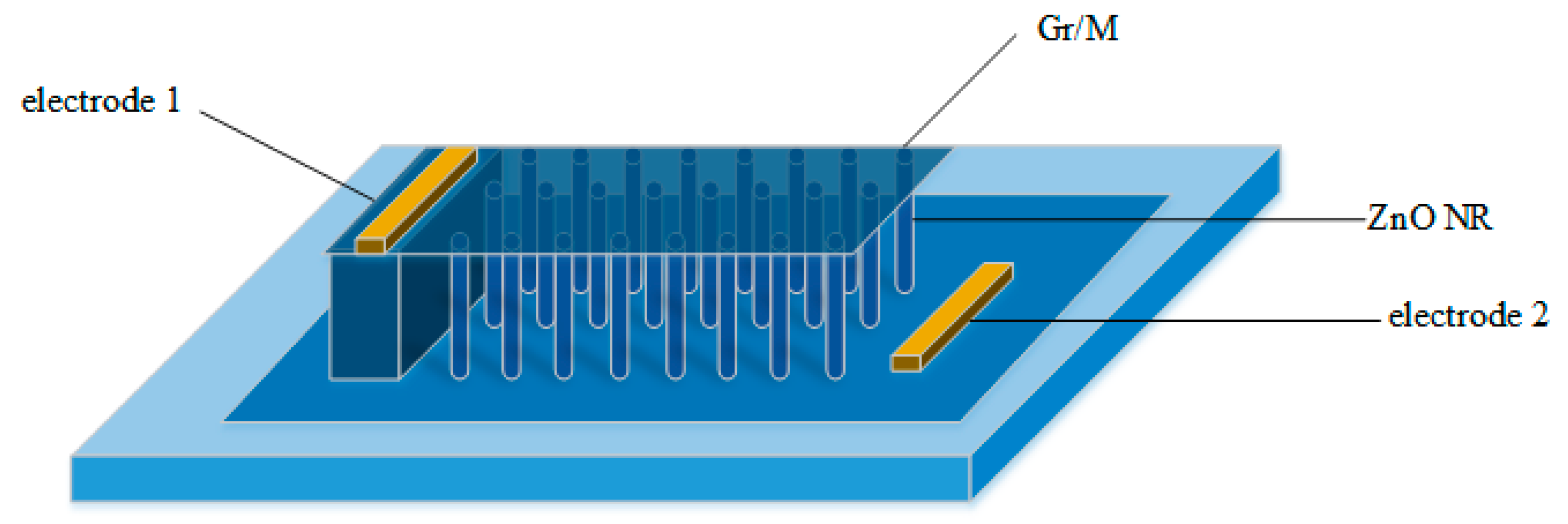
- Yi, J.; Lee, J.M. Vertically aligned ZnO nanorods and graphene hybrid architectures for high-sensitive flexible gas sensors. Sens. Actuators B Chem. 2011, 155, 264–269. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B. Enhancing NO2, gas sensing performances at room temperature based on reduced graphene oxide-ZnO nanoparticles hybrids. Sens. Actuators B Chem. 2014, 202, 272–278. [Google Scholar] [CrossRef]
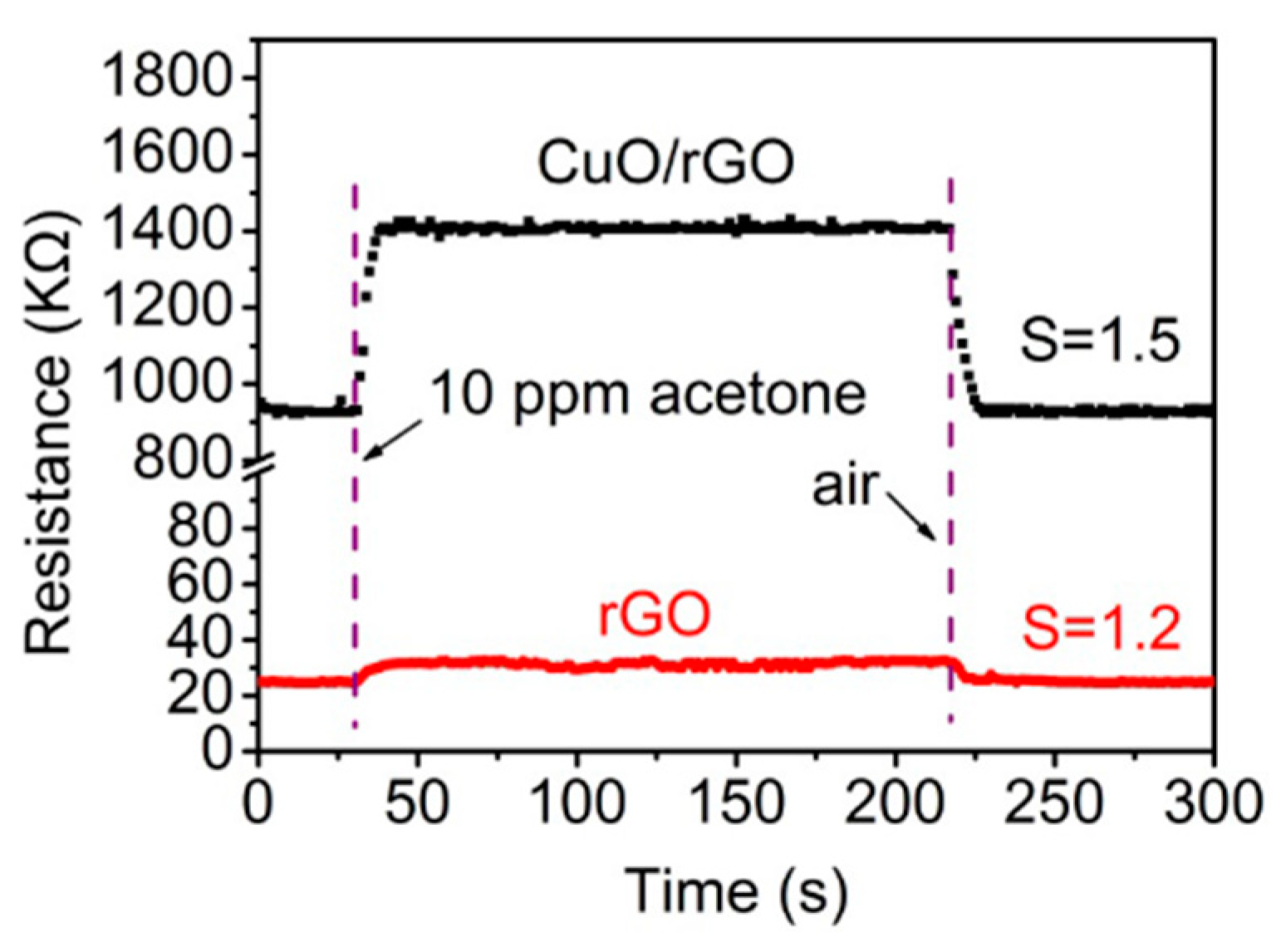
- Wang, C.; Zhu, J. Reduced graphene oxide decorated with CuO-ZnO hetero-junctions: Towards high selective gas-sensing property to acetone. J. Mater. Chem. A 2014, 2, 18635–18643. [Google Scholar] [CrossRef]
- Singkammo, S.; Wisitsoraat, A. Electrolytically-exfoliated Graphene-loaded Flame-made Ni-doped SnO2 Composite Film for Acetone Sensing. ACS Appl. Mater. Interfaces 2015, 7, 3077–3092. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.; Ye, Z. Graphene/Metal Oxide Composite Membrane Gas Sensor and Preparation Method Thereof. Patent CN105092646A, 25 November 2015. [Google Scholar]
- Liu, S.; Wang, Z. A Resistive Gas Sensor Based on Graphene/Tin Dioxide/Zinc Oxide Composite, Its Preparation and Application. Patent CN105891271A, 24 August 2016. [Google Scholar]
- Han, M.; Liu, W. Graphene oxide–SnO2, nanocomposite: Synthesis, characterization, and enhanced gas sensing properties. J. Mater. Sci. Mater. Electron. 2017, 28, 16973–16980. [Google Scholar] [CrossRef]
- Karaduman, I.; Er, E. Room-temperature ammonia gas sensor based on reduced graphene oxide nanocomposites decorated by Ag, Au and Pt nanoparticles. J. Alloys Compd. 2017, 722, 569–578. [Google Scholar] [CrossRef]
- Kim, H.W.; Yong, J.K. Synthesis of zinc oxide semiconductors-graphene nanocomposites by microwave irradiation for application to gas sensors. Sens. Actuators B Chem. 2017, 249, 590–601. [Google Scholar] [CrossRef]
- Wang, T.; Sun, Z. Studies on NH3 gas sensing by zinc oxide nanowire-reduced graphene oxide nanocomposites. Sens. Actuators B Chem. 2017, 252, 284–294. [Google Scholar] [CrossRef]
- Zhou, Y.; Lin, X. Study on gas sensing of reduced graphene oxide/ZnO thin film at room temperature. Sens. Actuators B Chem. 2017, 240, 870–880. [Google Scholar] [CrossRef]
- Bhati, V.S.; Ranwa, S. Improved sensitivity with low limit of detection of hydrogen gas sensor based on rGO loaded Ni doped ZnO nanostructures. ACS Appl. Mater. Interfaces 2018, 10, 11116–11124. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Tai, H. Excellent ammonia sensing performance of gas sensor based on graphene/titanium dioxide hybrid with improved morphology. Appl. Surf. Sci. 2017, 419, 84–90. [Google Scholar] [CrossRef]

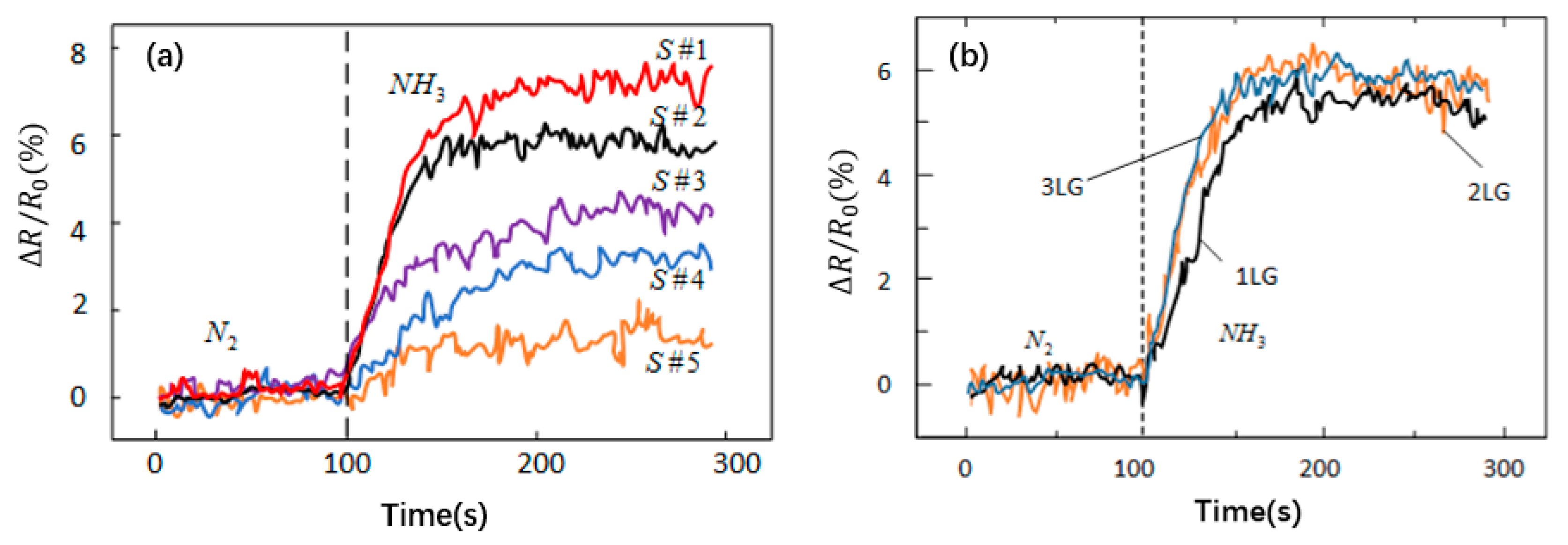



| Method | Carbon Source | Substrate | Temperature (°C) | Reference |
|---|---|---|---|---|
| Micro-mechanical exfoliation | graphite | SiO2/Si | Room temperature | [13] |
| Chemical Vapor Deposition | CH4, C2H2 | Cu, Pt, Ni, Ru, Ir | >1000 | [15,16,17,18,19,20,21,22,23] |
| Epitaxial growth | SiC | SiC | 1200~1600 | [24] |
| The oxidation–reduction method | graphite | -- | <500 | [27] |
| Sample | GO Mass Raction (wt %) | Crystallite Size (nm) | BET (m2·g−1) | Pore Size (nm) | Pore Volume (cm3·g−1) |
|---|---|---|---|---|---|
| Pristine-SnO2 | 0 | 14.1 | 34.87 | 10.41 | 0.0101 |
| 0.3GO-SnO2 | 0.3 | 14.9 | 56.58 | 10.73 | 0.0193 |
| 0.5GO-SnO2 | 0.5 | 14.7 | 59.02 | 10.54 | 0.0177 |
| 1.0GO-SnO2 | 1 | 14.3 | 61.36 | 10.18 | 0.0192 |
| No. | Material | Target Gas | Sensitivity | LOD | Response Time | Year | Reference |
|---|---|---|---|---|---|---|---|
| 1 | CVD Gr | H2 | -- | -- | 11.8 s | 2015 | [60] |
| 2 | MPCVD Gr | NO2 | 1141% | 785 ppt | 2 s | 2016 | [61] |
| 3 | CVD Gr | NO2 | ΔI ≈ 26 nA | 200 ppm | 67 s | 2016 | [62] |
| 4 | CVD Gr | NH3 | 9.3 × 10−5 ppm−1 | 17 ppm | 10 min | 2017 | [63] |
| NO2 | 0.024 ppm−1 | 0.24 ppm | 10 min | ||||
| 5 | r-GO | DMMP | ∆R/R0 > 10% | 50 ppm | 150 s | 2017 | [64] |
| 6 | CVD Gr | methane | 0.344 nm%−1 | 3.5 vol % | 50 s | 2017 | [65] |
| 7 | CVD Gr | NH3 | ∆R/R0 = 4.9% | 18.8 sccm | 70 s | 2017 | [66] |
| 8 | F-Gr | NH3 | ∆R/R0 = 3.8% | 2 ppm | 30 s | 2016 | [73] |
| 9 | CVD-Gr | NO2 | ∆I ≈ 0.0025 A | 0.12 ppm | 4 min | 2017 | [74] |
| LPE-Gr | ∆I ≈ 0.0020 A | 0.45 ppm | |||||
| 11 | Pd-PANI-rGO | H2 | ∆R/R0 = 25% | 1 vol % | 20 s | 2016 | [78] |
| 12 | rGO-ZnO | NO2 | ∆R/R0 = 25.6% | 5 ppm | 165 s | 2014 | [83] |
| 13 | CuO-ZnO/rGO | acetone | Rg/Ra = 1.5 | 10 ppm | 2014 | [84] | |
| 14 | Ni-doped SnO2/GO | acetone | ∆G/G0 = 27.5% | 200 ppm | 5.4 s | 2015 | [85] |
| 15 | GO-SnO2 | ethanol | Ra/Rt = 160 | 200 ppm | -- | 2017 | [88] |
| acetone | Ra/Rt = 200 | ||||||
| formaldehyde | Ra/Rt = 91 | ||||||
| 16 | AgNPs-rGO | NH3 | ∆R/R0 = 6.52% | 1 ppm | 70 s | 2017 | [89] |
| PtNPs-rGO | ∆R/R0 = 2.87% | 80 s | |||||
| AuNPs-rGO | ∆R/R0 = 0.5% | 100 s | |||||
| 17 | ZnO-Gr | NO2 | ∆R/R0 = 12.57% | 1 ppm | 250 s | 2017 | [90] |
| 18 | ZnO NW-rGO | NH3 | ∆R/R0 = 19.2% | 50 ppm | 100 s | 2017 | [91] |
| 19 | ZnO-rGO | chloroform vapor | ∆R/R0 = 1.75% | 20 ppm | 10 s | 2017 | [92] |
| 20 | TiO2-rGO | NH3 | ∆R/R0 = 1.7 | 10 ppm | 114 s | 2017 | [94] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, W.; Liu, X.; Yu, W. Research Progress of Gas Sensor Based on Graphene and Its Derivatives: A Review. Appl. Sci. 2018, 8, 1118. https://doi.org/10.3390/app8071118
Tian W, Liu X, Yu W. Research Progress of Gas Sensor Based on Graphene and Its Derivatives: A Review. Applied Sciences. 2018; 8(7):1118. https://doi.org/10.3390/app8071118
Chicago/Turabian StyleTian, Wenchao, Xiaohan Liu, and Wenbo Yu. 2018. "Research Progress of Gas Sensor Based on Graphene and Its Derivatives: A Review" Applied Sciences 8, no. 7: 1118. https://doi.org/10.3390/app8071118





