Application of Fly Ash as an Adsorbent for Removal of Air and Water Pollutants
Abstract
:1. Introduction
2. Global Overview of Fly Ash
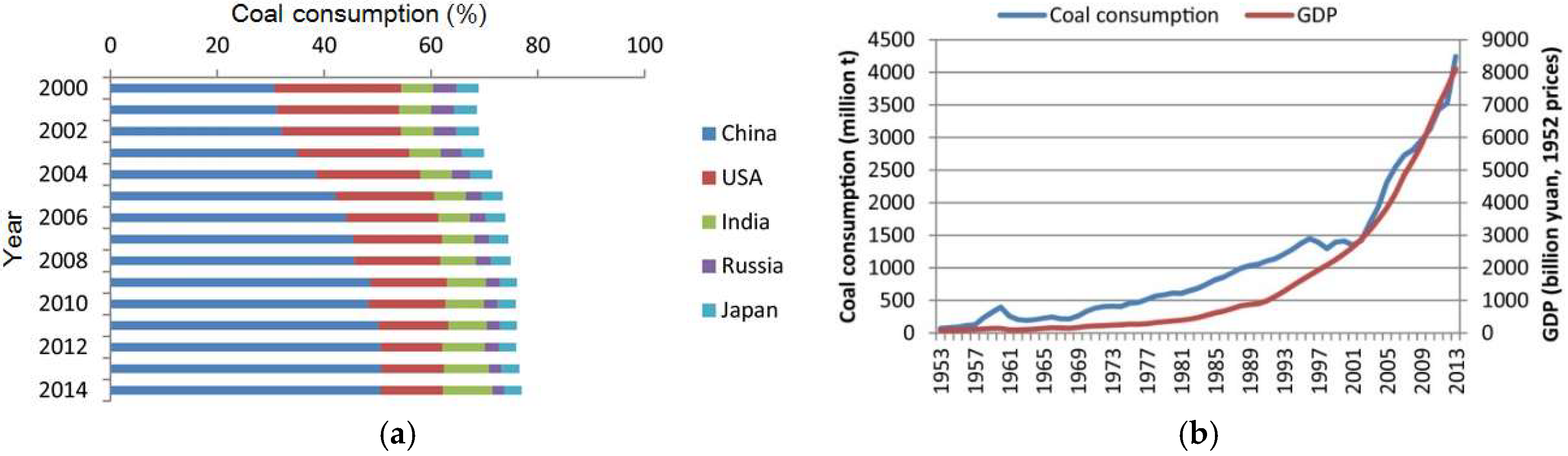
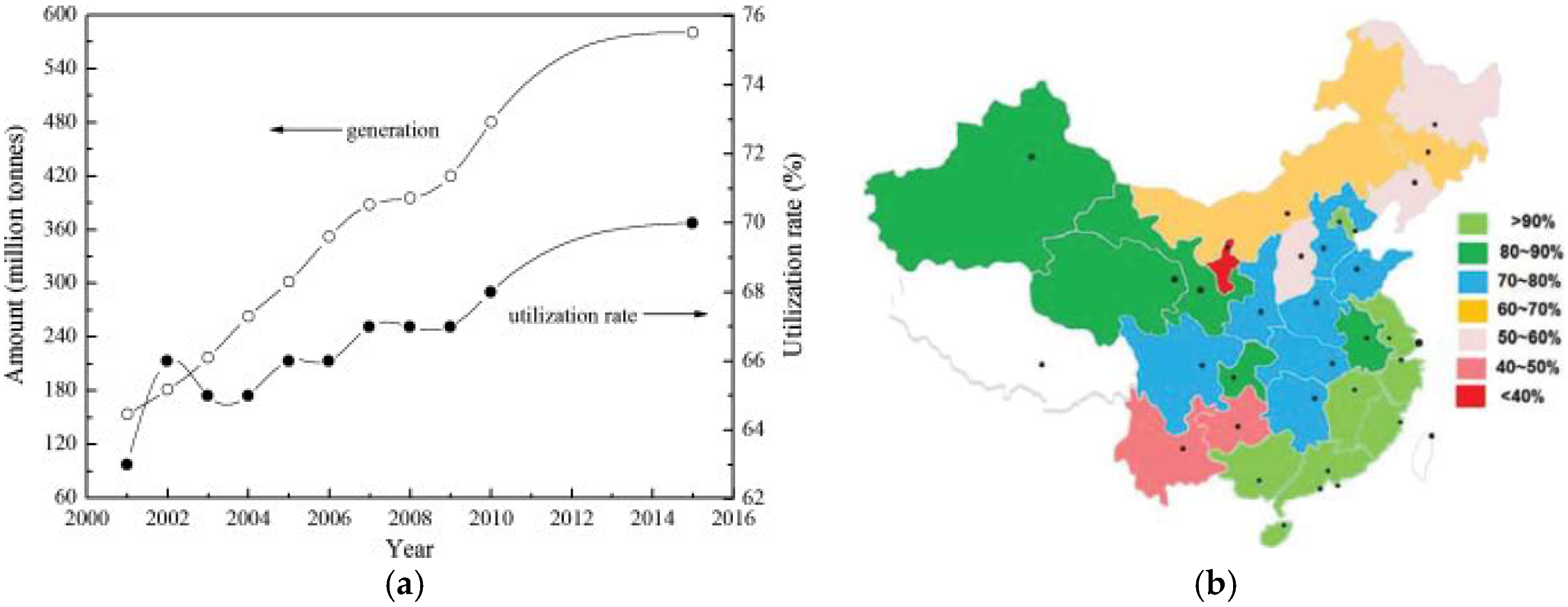
2.1. China
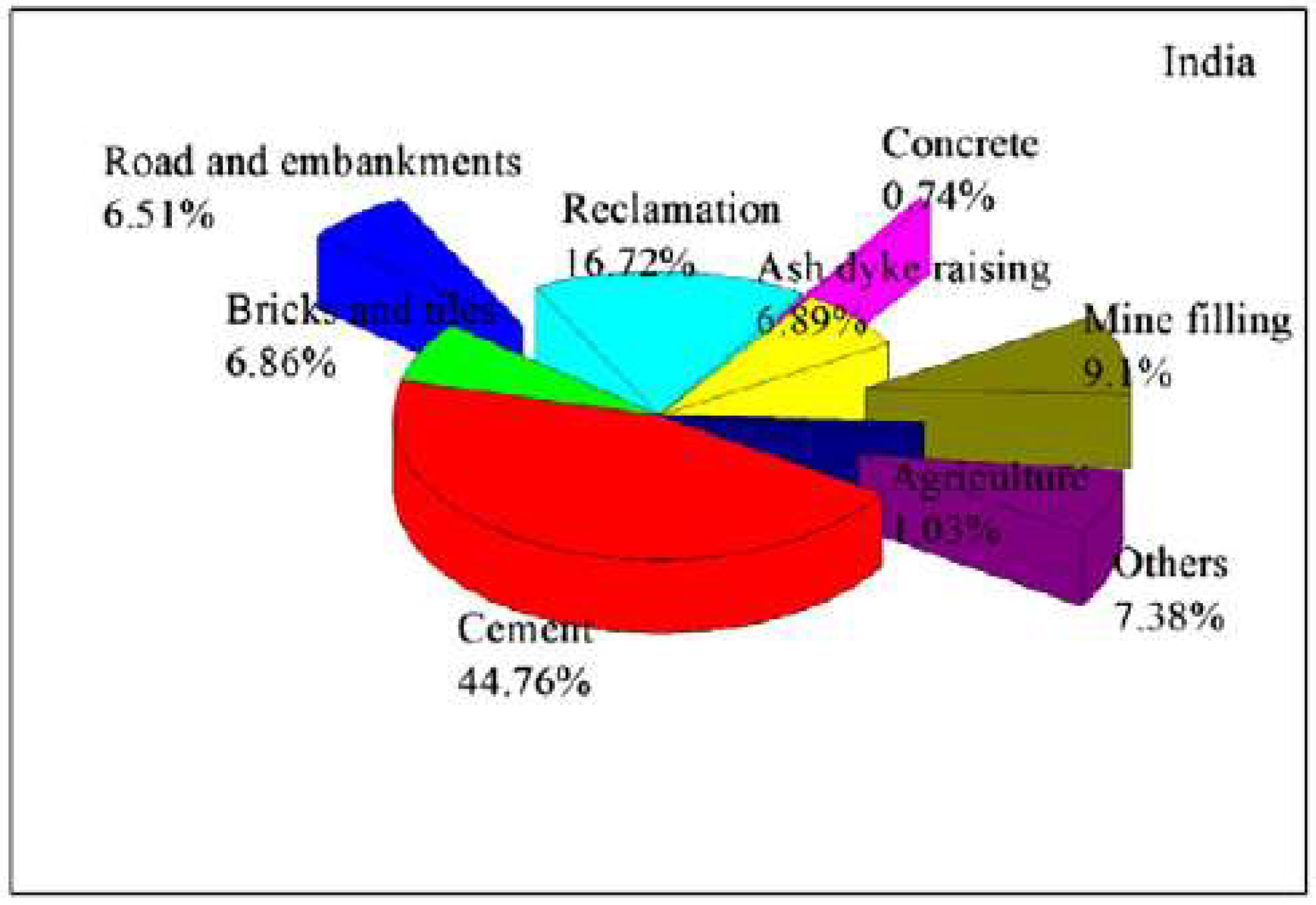
2.2. India
2.3. USA
2.4. Other Countries
3. Physical and Chemical Properties of Fly Ash
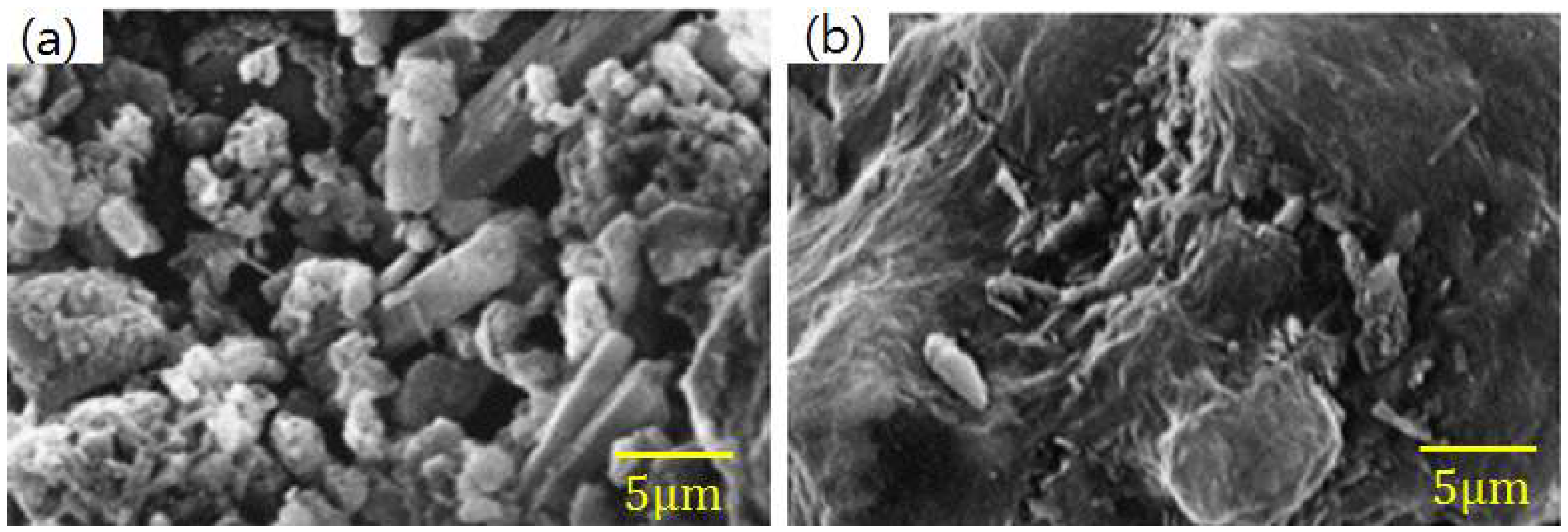
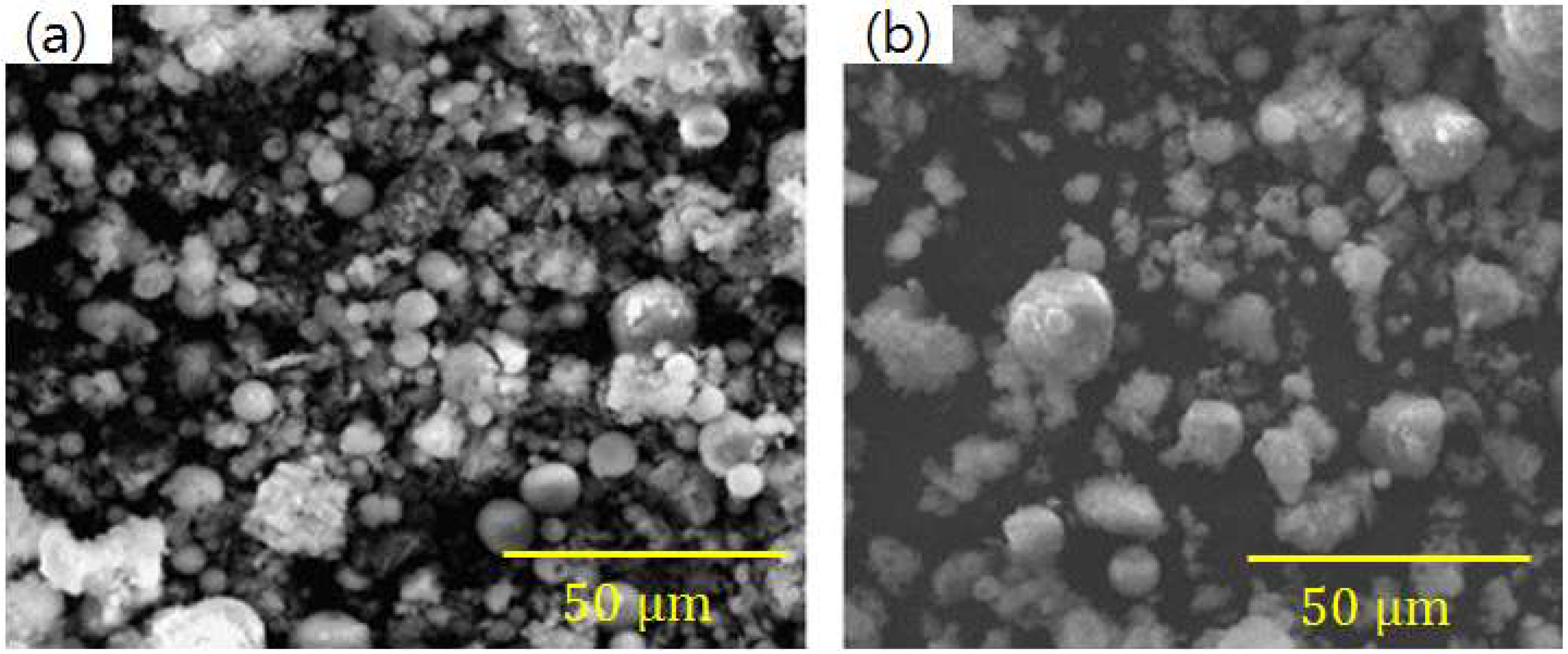
3.1. Morphological Characteristics
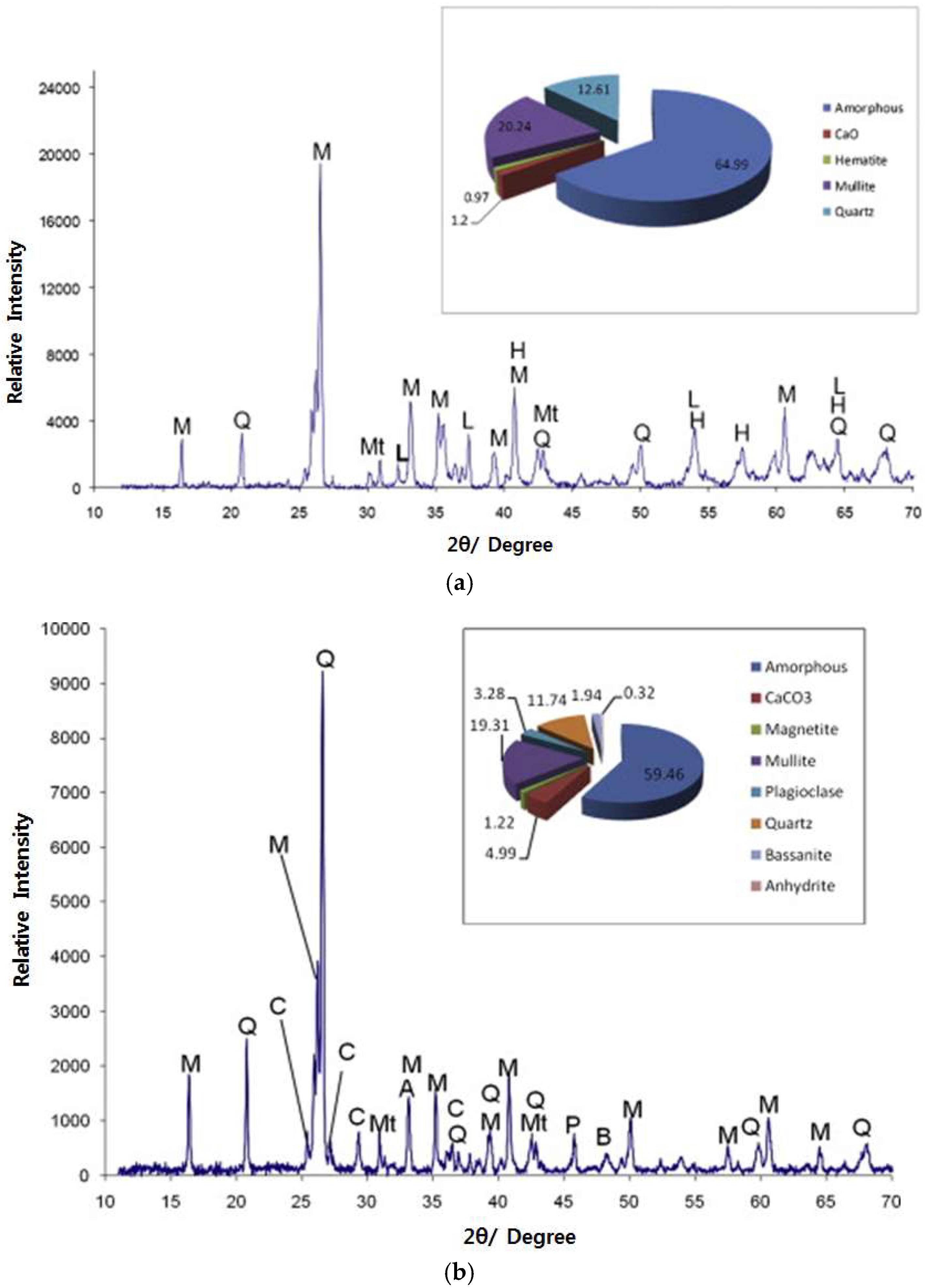
3.2. Chemical and Mineralogical Composition
4. Application of Fly Ash for Treatment of Pollutants
4.1. Removing Air Pollutants
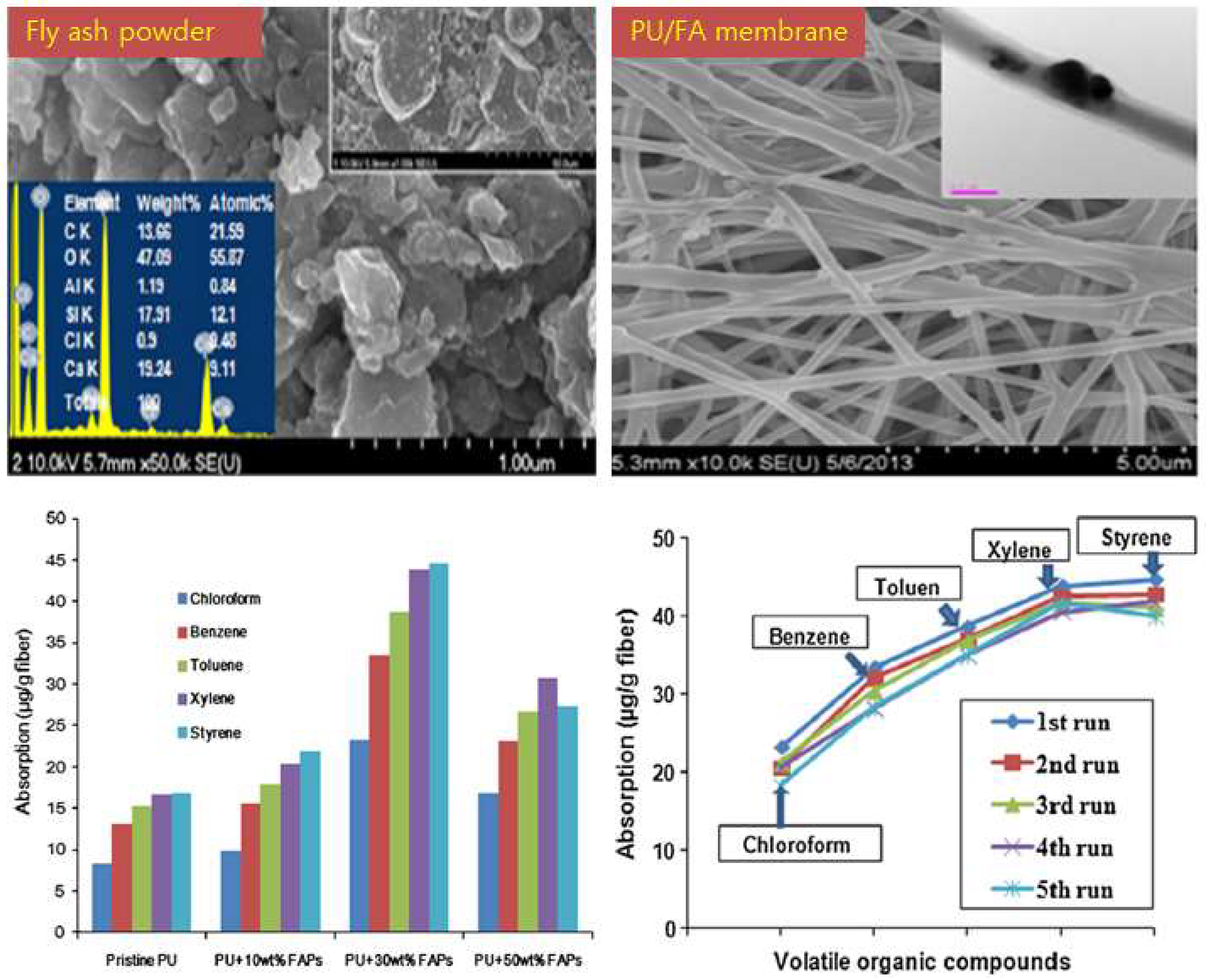
4.1.1. Removal of Volatile Organic Compounds
4.1.2. Removal of Nitrogen Oxides
4.1.3. Removal of Sulfur Dioxide
4.2. Removing Water Pollutants
4.2.1. Removal of Heavy Metals
4.2.2. Removal of Other Organic/Inorganic Components
5. Conclusions
- Fly ash can be used in building materials, building works, roads construction, agriculture, and other fields.
- To improve the adsorption capacity and efficiency of fly ash on environmental pollutants and make full use of fly ash, new technologies for the efficient utilization of fly ash should be developed, such as nanofiber technology.
- Fly ash can be converted to inexpensive and high performance adsorbents by simple modifications due to its unique porous properties. For example, is can be made into various kinds of zeolites.
- Modified fly ash can adsorb VOCs, NOx and SO2 emissions in the air and can also remove some heavy metals and other organic/inorganic pollutants (e.g., phosphorous, fluoride, boron, phenolic compounds, pesticides and dyes) from wastewater, due to its high porosity, high surface area, appropriate pore size, high porosity, alkalinity, negative charge, unburned carbon remaining in the fly ash particles and other unique characteristics.
- In addition, the adsorption effect is not only related to the physicochemical properties of the adsorbent itself but also to the physicochemical properties of the adsorbate, e.g., polar/non-polar, and functional groups.
- Pristine fly ash is a powder, which limits its scope of use. Using nanotechnology, it can be synthesized into low-cost, multi-functional and multi-purpose porous hybrid composites for adsorption of air pollutants and water pollutants. Fly ash porous hybrid composites as an emerging material, which has great potential in the future.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deng, F.; Pan, Y.; Sun, W.; Yang, F.; Zhang, Y.; Huang, Z. An ignition delay time and chemical kinetic study of ethane sensitized by nitrogen dioxide. Fuel 2017, 207, 389–401. [Google Scholar] [CrossRef]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.A.; Alexis, N.; Barnes, C.; Bernstein, I.L.; Nel, A.; Peden, D.; Diaz-Sanchez, D.; Tarlo, S.M.; Williams, P.B. Health effects of air pollution. J. Allergy Clin. Immunol. 2004, 114, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Liu, J.; Wang, Q.; Liang, Z. Health risk assessment of heavy metal exposure to street dust in the zinc smelting district, northeast of China. Sci. Total Environ. 2010, 408, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Zhao, C.; Luo, K. Household consumption of coal and related sulfur, arsenic, fluorine and mercury emissions in China. Energy Policy 2018, 112, 221–232. [Google Scholar] [CrossRef]
- Bartoňová, L. Unburned carbon from coal combustion ash: An overview. Fuel Process. Technol. 2015, 134, 136–158. [Google Scholar] [CrossRef]
- Ding, M.; Han, W.; Li, J.; Ma, E.; Shan, Z. In situ study of the mechanical properties of airborne haze particles. Sci. Chin. Technol. Sci. 2015, 58, 2046–2051. [Google Scholar] [CrossRef]
- Chen, S.; Xu, L.; Zhang, Y.; Chen, B.; Wang, X.; Zhang, X.; Zheng, M.; Chen, J.; Wang, W.; Sun, Y. Direct observations of organic aerosols in common wintertime hazes in north China: Insights into direct emissions from chinese residential stoves. Atmos. Chem. Phys. 2017, 17, 1259–1270. [Google Scholar] [CrossRef]
- Ge, J.C.; Yoon, S.K.; Choi, N.J. Using canola oil biodiesel as an alternative fuel in diesel engines: A review. Appl. Sci. 2017, 7, 881. [Google Scholar] [CrossRef]
- Iyer, R.S.; Scott, J.A. Power station fly ash—A review of value-added utilization outside of the construction industry. Resour. Conserv. Recycl. 2001, 31, 217–228. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Cleaner Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Park, J.H.; Edraki, M.; Mulligan, D.; Jang, H.S. The application of coal combustion by-products in mine site rehabilitation. J. Clean. Prod. 2014, 84, 761–772. [Google Scholar] [CrossRef]
- Lanzerstorfer, C. Fly ash from coal combustion: Dependence of the concentration of various elements on the particle size. Fuel 2018, 228, 263–271. [Google Scholar] [CrossRef]
- Yao, Z.; Ji, X.; Sarker, P.; Tang, J.; Ge, L.; Xia, M.; Xi, Y. A comprehensive review on the applications of coal fly ash. Earth Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Bouzoubaa, N.; Lachemi, M. Self-compacting concrete incorporating high volumes of class F fly ash: Preliminary results. Cem. Concr. Res. 2001, 31, 413–420. [Google Scholar] [CrossRef]
- Abbas, S.; Saleem, M.A.; Kazmi, S.M.; Munir, M.J. Production of sustainable clay bricks using waste fly ash: Mechanical and durability properties. J. Build. Eng. 2017, 14, 7–14. [Google Scholar] [CrossRef]
- Kumar, S. Fly ash–lime–phosphogypsum hollow blocks for walls and partitions. Build. Environ. 2003, 38, 291–295. [Google Scholar] [CrossRef]
- Dilmore, R.M.; Neufeld, R.D. Autoclaved aerated concrete produced with low NOx burner/selective catalytic reduction fly ash. J. Energy Eng. 2001, 127, 37–50. [Google Scholar] [CrossRef]
- Kumar, S. A perspective study on fly ash–lime–gypsum bricks and hollow blocks for low cost housing development. Constr. Build. Mater. 2002, 16, 519–525. [Google Scholar] [CrossRef]
- Andini, S.; Cioffi, R.; Colangelo, F.; Grieco, T.; Montagnaro, F.; Santoro, L. Coal fly ash as raw material for the manufacture of geopolymer-based products. Waste Manag. 2008, 28, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Koukouzas, N.; Ketikidis, C.; Itskos, G.; Spiliotis, X.; Karayannis, V.; Papapolymerou, G. Synthesis of CFB-coal fly ash clay bricks and their characterisation. Waste Biomass Valoriz. 2011, 2, 87–94. [Google Scholar] [CrossRef]
- Jala, S.; Goyal, D. Fly ash as a soil ameliorant for improving crop production—A review. Bioresour. Technol. 2006, 97, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Basu, M.; Pande, M.; Bhadoria, P.; Mahapatra, S. Potential fly-ash utilization in agriculture: A global review. Prog. Nat. Sci. 2009, 19, 1173–1186. [Google Scholar] [CrossRef]
- Ram, L.; Srivastava, N.; Tripathi, R.; Jha, S.; Sinha, A.K.; Singh, G.; Manoharan, V. Management of mine spoil for crop productivity with lignite fly ash and biological amendments. J. Environ. Manag. 2006, 79, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Gandhimathi, R. Removal of heavy metal ions from municipal solid waste leachate using coal fly ash as an adsorbent. J. Hazard. Mater. 2009, 169, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Bayat, B. Comparative study of adsorption properties of turkish fly ashes: I. The case of nickel (II), copper (II) and zinc (II). J. Hazard. Mater. 2002, 95, 251–273. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Kerketta, S.; Kumar, M.S.; Rajanikanth, B. Discharge Plasma Cascaded with Fly Ash for Removal of NOx in Biodiesel Exhaust: A Feasibility Study. Int. J. Plasma Environ. Sci. Technol. 2014, 8, 98–102. [Google Scholar]
- Hower, J.C.; Maroto-Valer, M.M.; Taulbee, D.N.; Sakulpitakphon, T. Mercury capture by distinct fly ash carbon forms. Energy Fuels 2000, 14, 224–226. [Google Scholar] [CrossRef]
- Izquierdo, M.; Rubio, B. Carbon-enriched coal fly ash as a precursor of activated carbons for SO2 removal. J. Hazard. Mater. 2008, 155, 199–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Chen, Y.-L.; Zhang, X.-H.; Tian, F.-M.; Zu, Z.-N. Zeolites developed from mixed alkali modified coal fly ash for adsorption of volatile organic compounds. Mater. Lett. 2014, 119, 140–142. [Google Scholar] [CrossRef]
- Kim, H.J.; Pant, H.R.; Choi, N.J.; Kim, C.S. Fly ash/polyurethane thin film for the adsorption of volatile organic compounds (VOCs) from air. Fibers Polym. 2014, 15, 1393–1398. [Google Scholar] [CrossRef]
- Kim, H.J.; Pant, H.R.; Choi, N.J.; Kim, C.S. Composite electrospun fly ash/polyurethane fibers for absorption of volatile organic compounds from air. Chem. Eng. J. 2013, 230, 244–250. [Google Scholar] [CrossRef]
- Joo Kim, H.; Raj Pant, H.; Hee Kim, J.; Jung Choi, N.; Sang Kim, C. Fabrication of multifunctional TiO2–fly ash/polyurethane nanocomposite membrane via electrospinning. Ceram. Int. 2014, 40, 3023–3029. [Google Scholar] [CrossRef]
- Pant, H.R.; Kim, H.J.; Joshi, M.K.; Pant, B.; Park, C.H.; Kim, J.I.; Hui, K.; Kim, C.S. One-step fabrication of multifunctional composite polyurethane spider-web-like nanofibrous membrane for water purification. J. Hazard. Mater. 2014, 264, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhou, M.; Li, H. The drag effect of coal consumption on economic growth in China during 1953–2013. Resour. Conserv. Recycl. 2018, 129, 326–332. [Google Scholar] [CrossRef]
- He, Y.; Luo, Q.; Hu, H. Situation analysis and countermeasures of China’s fly ash pollution prevention and control. Procedia Environ. Sci. 2012, 16, 690–696. [Google Scholar] [CrossRef]
- Dong, P.; Wang, S.Y. Risks and countermeasures of the shale gas development in China. Adv. Mater. Res. 2013, 734, 1253–1256. [Google Scholar] [CrossRef]
- Hu, D.; Xu, S. Opportunity, challenges and policy choices for China on the development of shale gas. Energy Policy 2013, 60, 21–26. [Google Scholar] [CrossRef]
- Pi, G.; Dong, X.; Dong, C.; Guo, J.; Ma, Z. The status, obstacles and policy recommendations of shale gas development in China. Sustainability 2015, 7, 2353–2372. [Google Scholar] [CrossRef]
- Yao, Z.; Xia, M.; Sarker, P.K.; Chen, T. A review of the alumina recovery from coal fly ash, with a focus in China. Fuel 2014, 120, 74–85. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Wang, L.; Sun, X. Study on adsorption of Cr(VI) using singlephase zeolites synthesized from fly ash. Chin. J. Environ. Eng. 2008, 2, 1121–1126. [Google Scholar]
- Ram, L.; Masto, R. Fly ash for soil amelioration: A review on the influence of ash blending with inorganic and organic amendments. Earth Sci. Rev. 2014, 128, 52–74. [Google Scholar] [CrossRef]
- Singh, J.; Mantha, S.S.; Phalle, V.M. Characterizing domestic electricity consumption in the indian urban household sector. Energy Build. 2018, 170, 74–82. [Google Scholar] [CrossRef]
- Singh, R.; Gupta, N. Value added utilization of fly ash-prospective and sustainable solutions. Int. J. Appl. Sci. Eng. Res. 2014, 3, 1–16. [Google Scholar]
- Surabhi. Fly ash in India: Generation vis-à-vis Utilization and Global perspective. Int. J. Appl. Chem. 2017, 13, 29–52. [Google Scholar]
- Parab, N.; Mishra, S.; Bhonde, S. Prospects of bulk utilization of fly ash in agriculture for integrated nutrient management. Bull. Nat. Inst. Ecol. 2012, 23, 31–46. [Google Scholar]
- Kalra, N.; Harit, R.; Sharma, S. Effect of flyash incorporation on soil properties of texturally variant soils. Bioresour. Technol. 2000, 75, 91–93. [Google Scholar] [CrossRef]
- Yeledhalli, N.; Prakash, S.; Gurumurthy, S.; Ravi, M. Coal fly ash as modifier of physico-chemical and biological properties of soil. Karnataka J. Agric. Sci. 2010, 20, 531–534. [Google Scholar]
- Garg, R.N.; Pathak, H.; Das, D.; Tomar, R. Use of flyash and biogas slurry for improving wheat yield and physical properties of soil. Environ. Monit. Assess. 2005, 107, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dhadse, S.; Kumari, P.; Bhagia, L. Fly ash characterization, utilization and government initiatives in India —A review. J. Sci. Ind. Res. 2008, 67, 11–18. [Google Scholar]
- Dermatas, D.; Meng, X. Utilization of fly ash for stabilization/solidification of heavy metal contaminated soils. Eng. Geol. 2003, 70, 377–394. [Google Scholar] [CrossRef]
- Production and Use of Coal Combustion Products in the U.S. Market Forecast through 2033. Prepared by: American road & Transportation builders association. Prepared for: American coal ash association. June 2015. Available online: https://www.acaa-usa.org/Portals/9/Files/PDFs/ReferenceLibrary/ARTBA-final-historical.compressed.pdf (accessed on 3 June 2015).
- Kishor, P.; Ghosh, A.; Kumar, D. Use of flyash in agriculture: A way to improve soil fertility and its productivity. Asian J. Agric. Res. 2010, 4, 1–14. [Google Scholar] [CrossRef]
- Kolbe, J.L.; Lee, L.S.; Jafvert, C.T.; Murarka, I.P. Proceedings of the Use of Alkaline Coal Ash for Reclamation of a Former Strip Mine, World of Coal Ash (WOCA) Conference, Denver, CO, USA, 9–12 May 2011; pp. 1–15.
- Grubb, D.G.; Guimaraes, M.A.S.; Valencia, R. Phosphate immobilization using an acidic type F fly ash. J. Hazard. Mater. 2000, 76, 217–236. [Google Scholar] [CrossRef]
- Nyambura, M.G.; Mugera, G.W.; Felicia, P.L.; Gathura, N.P. Carbonation of brine impacted fractionated coal fly ash: Implications for CO2 sequestration. J. Environ. Manag. 2011, 92, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Kalaw, M.E.; Culaba, A.; Hinode, H.; Kurniawan, W.; Gallardo, S.; Promentilla, M.A. Optimizing and characterizing geopolymers from ternary blend of philippine coal fly ash, coal bottom ash and rice hull ash. Materials 2016, 9, 580. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, T.; Kim, J.; Hardcastle, S.; Rohatgi, P. Crystallinity and selected properties of fly ash particles. Mater. Sci. Eng. A 2002, 325, 333–343. [Google Scholar] [CrossRef]
- Safiuddin, M.; Jumaat, M.Z.; Salam, M.; Islam, M.; Hashim, R. Utilization of solid wastes in construction materials. Int. J. Phys. Sci. 2010, 5, 1952–1963. [Google Scholar]
- Cao, J.; Dong, X.; Li, L.; Dong, Y.; Hampshire, S. Recycling of waste fly ash for production of porous mullite ceramic membrane supports with increased porosity. J. Eur. Ceram. Soc. 2014, 34, 3181–3194. [Google Scholar] [CrossRef]
- Yang, T.; Ji, H.; Yoon, S.; Kim, B.; Park, H. Porous mullite composite with controlled pore structure processed using a freeze casting of TBA-based coal fly ash slurries. Resour. Conserv. Recycl. 2010, 54, 816–820. [Google Scholar] [CrossRef]
- Kutchko, B.G.; Kim, A.G. Fly ash characterization by SEM–EDS. Fuel 2006, 85, 2537–2544. [Google Scholar] [CrossRef]
- Külaots, I.; Hurt, R.H.; Suuberg, E.M. Size distribution of unburned carbon in coal fly ash and its implications. Fuel 2004, 83, 223–230. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, X.; Dai, S.; Huang, B.; He, Q. Fractional characteristics of coal fly ash for beneficial use. J. Mater. Civ. Eng. 2012, 25, 63–69. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Menendez, R.; Alvarez, D.; Diaz-Somoano, M.; Martinez-Tarazona, M.R. Phase-mineral and chemical composition of coal fly ashes as a basis for their multicomponent utilization. 1. Characterization of feed coals and fly ashes☆. Fuel 2003, 82, 1793–1811. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Menendez, R. Phase-mineral and chemical composition of coal fly ashes as a basis for their multicomponent utilization. 4. Characterization of heavy concentrates and improved fly ash residues. Fuel 2005, 84, 973–991. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. Methods for characterization of composition of fly ashes from coal-fired power stations: A critical overview. Energy Fuels 2005, 19, 1084–1098. [Google Scholar] [CrossRef]
- Sarode, D.B.; Jadhav, R.N.; Khatik, V.A.; Ingle, S.T.; Attarde, S.B. Extraction and leaching of heavy metals from thermal power plant fly ash and its admixtures. Polish J. Environ. Stud. 2010, 6, 1325–1330. [Google Scholar]
- Polat, H.; Vengosh, A.; Pankratov, I.; Polat, M. A new methodology for removal of boron from water by coal and fly ash. Desalination 2004, 164, 173–188. [Google Scholar] [CrossRef] [Green Version]
- Akar, G.; Polat, M.; Galecki, G.; Ipekoglu, U. Leaching behavior of selected trace elements in coal fly ash samples from Yenikoy coal-fired power plants. Fuel Process. Technol. 2012, 104, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Sushil, S.; Batra, V.S. Analysis of fly ash heavy metal content and disposal in three thermal power plants in india. Fuel 2006, 85, 2676–2679. [Google Scholar] [CrossRef]
- Fan, M.; Brown, R.C. Comparison of the loss-on-ignition and thermogravimetric analysis techniques in measuring unburned carbon in coal fly ash. Energy Fuels 2001, 15, 1414–1417. [Google Scholar] [CrossRef]
- Querol, X.; Umaña, J.C.; Plana, F.; Alastuey, A.; Lopez-Soler, A.; Medinaceli, A.; Valero, A.; Domingo, M.J.; Garcia-Rojo, E. Synthesis of zeolites from fly ash at pilot plant scale. Examples of potential applications. Fuel 2001, 80, 857–865. [Google Scholar] [CrossRef]
- Inada, M.; Tsujimoto, H.; Eguchi, Y.; Enomoto, N.; Hojo, J. Microwave-assisted zeolite synthesis from coal fly ash in hydrothermal process. Fuel 2005, 84, 1482–1486. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Mid-infrared spectroscopic studies of alkali-activated fly ash structure. Microporous Mesoporous Mater. 2005, 86, 207–214. [Google Scholar] [CrossRef]
- Wang, S.; Boyjoo, Y.; Choueib, A.; Zhu, Z. Removal of dyes from aqueous solution using fly ash and red mud. Water Res. 2005, 39, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Montes-Hernandez, G.; Perez-Lopez, R.; Renard, F.; Nieto, J.; Charlet, L. Mineral sequestration of CO2 by aqueous carbonation of coal combustion fly-ash. J. Hazard. Mater. 2009, 161, 1347–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belviso, C. State-of-the-art applications of fly ash from coal and biomass: A focus on zeolite synthesis processes and issues. Prog. Energy Combust. Sci. 2018, 65, 109–135. [Google Scholar] [CrossRef]
- Wang, S.; Ma, Q.; Zhu, Z.H. Characteristics of coal fly ash and adsorption application. Fuel 2008, 87, 3469–3473. [Google Scholar] [CrossRef]
- Ge, J.C.; Kim, H.Y.; Yoon, S.K.; Choi, N.J. Reducing volatile organic compound emissions from diesel engines using canola oil biodiesel fuel and blends. Fuel 2018, 218, 266–274. [Google Scholar] [CrossRef]
- Yilmaz, N.; Davis, S.M. Polycyclic aromatic hydrocarbon (PAH) formation in a diesel engine fueled with diesel, biodiesel and biodiesel/n-butanol blends. Fuel 2016, 181, 729–740. [Google Scholar] [CrossRef]
- Li, G.; Wei, W.; Shao, X.; Nie, L.; Wang, H.; Yan, X.; Zhang, R. A comprehensive classification method for VOC emission sources to tackle air pollution based on VOC species reactivity and emission amounts. J. Environ. Sci. 2018, 67, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Malakar, S.; Saha, P.D.; Baskaran, D.; Rajamanickam, R. Comparative study of biofiltration process for treatment of VOCs emission from petroleum refinery wastewater—A review. Environ. Technol. Innov. 2017, 8, 441–461. [Google Scholar] [CrossRef]
- Lee, S.; Lam, S.; Fai, H.K. Characterization of VOCs, ozone, and PM10 emissions from office equipment in an environmental chamber. Build. Environ. 2001, 36, 837–842. [Google Scholar] [CrossRef]
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Sakai, N.; Yamamoto, S.; Matsui, Y.; Khan, M.F.; Latif, M.T.; Mohd, M.A.; Yoneda, M. Characterization and source profiling of volatile organic compounds in indoor air of private residences in Selangor state, Malaysia. Sci. Total Environ. 2017, 586, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.C.; Choi, N.J. Fabrication of functional polyurethane/rare earth nanocomposite membranes by electrospinning and its VOCs absorption capacity from air. Nanomaterials 2017, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Mendell, M.J. Indoor residential chemical emissions as risk factors for respiratory and allergic effects in children: A review. Indoor Air 2007, 17, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Yoon, J.-W.; Choi, K.-I.; Jang, H.W.; Umar, A.; Lee, J.-H. Ultraselective and sensitive detection of xylene and toluene for monitoring indoor air pollution using Cr-doped NiO hierarchical nanostructures. Nanoscale 2013, 5, 7066–7073. [Google Scholar] [CrossRef] [PubMed]
- Rayalu, S.; Meshram, S.; Biniwale, R.B.; Srivasatava, A.; Jadhav, P.; Devotta, S. Volatile organic carbon monitoring in indoor environment using a versatile hydrophobic flyash-based zeolite as adsorbent. Curr. Sci. 2006, 497–503. [Google Scholar]
- Guo, H.; Lee, S.; Li, W.; Cao, J. Source characterization of btex in indoor microenvironments in Hong Kong. Atmos. Environ. 2003, 37, 73–82. [Google Scholar] [CrossRef]
- Barna, M.; Lamb, B.; Westberg, H. Modeling the effects of VOC/NOx emissions on ozone synthesis in the cascadia airshed of the pacific northwest. J. Air Waste Manag. Assoc. 2001, 51, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Park, R.J.; Kim, J.-J. Urban air quality modeling with full O3–NOx–VOC chemistry: Implications for O3 and PM air quality in a street canyon. Atmos. Environ. 2012, 47, 330–340. [Google Scholar] [CrossRef]
- Shao, M.; Zhang, Y.; Zeng, L.; Tang, X.; Zhang, J.; Zhong, L.; Wang, B. Ground-level ozone in the pearl river delta and the roles of VOC and NOx in its production. J. Environ. Manag. 2009, 90, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Cheng, S.; Li, G.; Wang, G.; Wang, H. Characteristics of ozone and ozone precursors (VOCs and NOx) around a petroleum refinery in Beijing, China. J. Environ. Sci. 2014, 26, 332–342. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Kato, S.; Ataka, Y.; Chino, S. Performance test for evaluating the reduction of VOCs in rooms and evaluating the lifetime of sorptive building materials. Build. Environ. 2009, 44, 207–215. [Google Scholar] [CrossRef]
- Chmielewski, A.; Ostapczuk, A.; Zimek, Z.; Licki, J.; Kubica, K. Reduction of VOCs in flue gas from coal combustion by electron beam treatment. Radiat. Phys. Chem. 2002, 63, 653–655. [Google Scholar] [CrossRef]
- Kim, S. The reduction of formaldehyde and VOCs emission from wood-based flooring by green adhesive using cashew nut shell liquid (CNSL). J. Hazard. Mater. 2010, 182, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Vijayalakshmi, G.; Rajmohan, B.; Subbaramaiah, V. Adsorption of benzene vapor onto activated biomass from cashew nut shell: Batch and column study. Recent Patents Chem. Eng. 2012, 5, 116–133. [Google Scholar] [CrossRef]
- Tseng, T.K.; Lin, Y.S.; Chen, Y.J.; Chu, H. A review of photocatalysts prepared by sol-gel method for VOCs removal. Int. J. Mol. Sci. 2010, 11, 2336–2361. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhu, Q.; Jiang, Z.; Zhang, Y.; Wang, Y. Preparation and formation mechanism of mesoporous CuO–CeO2 mixed oxides with excellent catalytic performance for removal of VOCs. Microporous Mesoporous Mater. 2008, 113, 427–434. [Google Scholar] [CrossRef]
- Belkouch, J.; Ould-Dris, A.; Taouk, B. Removal of hazardous chlorinated VOCs over Mn–Cu mixed oxide based catalyst. J. Hazard. Mater. 2009, 169, 758–765. [Google Scholar]
- Kim, S.C.; Shim, W.G. Catalytic combustion of VOCs over a series of manganese oxide catalysts. Appl. Catal. B 2010, 98, 180–185. [Google Scholar] [CrossRef]
- Scire, S.; Minico, S.; Crisafulli, C.; Satriano, C.; Pistone, A. Catalytic combustion of volatile organic compounds on gold/cerium oxide catalysts. Appl. Catal. B 2003, 40, 43–49. [Google Scholar] [CrossRef]
- Liotta, L. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Chuang, C.; Chiang, P.; Chang, E. Modeling VOCs adsorption onto activated carbon. Chemosphere 2003, 53, 17–27. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Chiang, P.-C.; Huang, C.-P. Effects of pore structure and temperature on VOC adsorption on activated carbon. Carbon 2001, 39, 523–534. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Liu, J. Surface modification of coconut shell based activated carbon for the improvement of hydrophobic VOC removal. J. Hazard. Mater. 2011, 192, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Gaur, V.; Verma, N. Removal of volatile organic compound by activated carbon fiber. Carbon 2004, 42, 2949–2962. [Google Scholar] [CrossRef]
- Tsai, J.-H.; Chiang, H.-M.; Huang, G.-Y.; Chiang, H.-L. Adsorption characteristics of acetone, chloroform and acetonitrile on sludge-derived adsorbent, commercial granular activated carbon and activated carbon fibers. J. Hazard. Mater. 2008, 154, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.-Y.; Ju, Y.-W.; Jung, H.-R.; Lee, W.-J. Preparation of the novel manganese-embedded pan-based activated carbon nanofibers by electrospinning and their toluene adsorption. J. Anal. Appl. Pyrolysis 2008, 81, 211–217. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, Z.-H.; Kang, F. Synthesis of reduced graphene oxide/phenolic resin-based carbon composite ultrafine fibers and their adsorption performance for volatile organic compounds and water. J. Mater. Chem. A 2013, 1, 9536–9543. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, C.; Jiao, T.; Song, J.; Zhang, X.; Xing, R.; Zhou, J.; Zhang, L.; Peng, Q. Self-assembled AgNP-containing nanocomposites constructed by electrospinning as efficient dye photocatalyst materials for wastewater treatment. Nanomaterials 2018, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Gupta, V. Application of coal fly ash in air quality management. Ind. Eng. Chem. Res. 2012, 51, 15299–15314. [Google Scholar] [CrossRef]
- Qian, Q.; Gong, C.; Zhang, Z.; Yuan, G. Removal of VOCs by activated carbon microspheres derived from polymer: A comparative study. Adsorption 2015, 21, 333–341. [Google Scholar] [CrossRef]
- Huang, Z.-H.; Kang, F.; Zheng, Y.-P.; Yang, J.-B.; Liang, K.-M. Adsorption of trace polar methy-ethyl-ketone and non-polar benzene vapors on viscose rayon-based activated carbon fibers. Carbon 2002, 40, 1363–1367. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Z.; Liu, F.; Wang, S. Study of NO/NOx removal from flue gas contained fly ash and water vapor by pulsed corona discharge. J. Electrostat. 2005, 63, 155–164. [Google Scholar] [CrossRef]
- Rokni, E.; Panahi, A.; Ren, X.; Levendis, Y.A. Curtailing the generation of sulfur dioxide and nitrogen oxide emissions by blending and oxy-combustion of coals. Fuel 2016, 181, 772–784. [Google Scholar] [CrossRef]
- Rubio, B.; Izquierdo, M.T.; Mayoral, M.C.; Bona, M.T.; Andres, J.M. Unburnt carbon from coal fly ashes as a precursor of activated carbon for nitric oxide removal. J. Hazard. Mater. 2007, 143, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Rubel, A.; Andrews, R.; Gonzalez, R.; Groppo, J.; Robl, T. Adsorption of Hg and NOx on coal by-products. Fuel 2005, 84, 911–916. [Google Scholar] [CrossRef]
- Hwang, J.; Sun, X.; Li, Z. Unburned carbon from fly ash for mercury adsorption: I. Separation and characterization of unburned carbon. J. Miner. Mater. Charact. Eng. 2002, 1, 39. [Google Scholar] [CrossRef]
- Mehmood, S.; Reddy, B.V.; Rosen, M.A. Energy analysis of a biomass co-firing based pulverized coal power generation system. Sustainability 2012, 4, 462–490. [Google Scholar] [CrossRef]
- Bukhari, S.S.; Behin, J.; Kazemian, H.; Rohani, S. Conversion of coal fly ash to zeolite utilizing microwave and ultrasound energies: A review. Fuel 2015, 140, 250–266. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, B.; Bi, J. More efforts, more benefits: Air pollutant control of coal-fired power plants in China. Energy 2015, 80, 1–9. [Google Scholar] [CrossRef]
- Long, X.-L.; Xin, Z.-L.; Wang, H.-X.; Xiao, W.-D.; Yuan, W.-K. Simultaneous removal of no and SO2 with hexamminecobalt (II) solution coupled with the hexamminecobalt (II) regeneration catalyzed by activated carbon. Appl. Catal. B 2004, 54, 25–32. [Google Scholar] [CrossRef]
- Liu, Y.x.; Zhang, J. Photochemical oxidation removal of NO and SO2 from simulated flue gas of coal-fired power plants by wet scrubbing using UV/H2O2 advanced oxidation process. Ind. Eng. Chem. Res. 2011, 50, 3836–3841. [Google Scholar] [CrossRef]
- Srivastava, R.; Miller, C.; Erickson, C.; Jambhekar, R. Emissions of sulfur trioxide from coal-fired power plants. J. Air Waste Manag. Assoc. 2004, 54, 750–762. [Google Scholar] [CrossRef]
- Ishizuka, T.; Tsuchiai, H.; Murayama, T.; Tanaka, T.; Hattori, H. Preparation of active absorbent for dry-type flue gas desulfurization from calcium oxide, coal fly ash, and gypsum. Ind. Eng. Chem. Res. 2000, 39, 1390–1396. [Google Scholar] [CrossRef]
- Renedo, M.; Fernandez, J. Preparation, characterization, and calcium utilization of fly ash/Ca(OH)2 sorbents for dry desulfurization at low temperature. Ind. Eng. Chem. Res. 2002, 41, 2412–2417. [Google Scholar] [CrossRef]
- Lin, R.-B.; Shih, S.-M.; Liu, C.-F. Characteristics and reactivities of Ca(OH)2/silica fume sorbents for low-temperature flue gas desulfurization. Chem. Eng. Sci. 2003, 58, 3659–3668. [Google Scholar] [CrossRef]
- Lee, K.; Mohamed, A.; Bhatia, S.; Chu, K. Removal of sulfur dioxide by fly ash/CaO/CaSO4 sorbents. Chem. Eng. J. 2005, 114, 171–177. [Google Scholar] [CrossRef]
- Lee, K.T.; Bhatia, S.; Mohamed, A.R. Preparation and characterization of sorbents prepared from ash (waste material) for sulfur dioxide (SO2) removal. J. Mater. Cycles Waste Manag. 2005, 7, 16–23. [Google Scholar] [CrossRef]
- Lin, R.-B.; Shih, S.-M.; Liu, C.-F. Structural properties and reactivities of Ca(OH)2/fly ash sorbents for flue gas desulfurization. Ind. Eng. Chem. Res. 2003, 42, 1350–1356. [Google Scholar] [CrossRef]
- Duruibe, J.; Ogwuegbu, M.; Egwurugwu, J. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Wang, S.-L.; Xu, X.-R.; Sun, Y.-X.; Liu, J.-L.; Li, H.-B. Heavy metal pollution in coastal areas of south China: A review. Mar. Pollut. Bull. 2013, 76, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Rios, C.A.; Williams, C.D.; Roberts, C.L. Removal of heavy metals from acid mine drainage (AMD) using coal fly ash, natural clinker and synthetic zeolites. J. Hazard. Mater. 2008, 156, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Huisman, J.L.; Schouten, G.; Schultz, C. Biologically produced sulphide for purification of process streams, effluent treatment and recovery of metals in the metal and mining industry. Hydrometallurgy 2006, 83, 106–113. [Google Scholar] [CrossRef]
- Amuda, O.; Giwa, A.; Bello, I. Removal of heavy metal from industrial wastewater using modified activated coconut shell carbon. BioChem. Eng. J. 2007, 36, 174–181. [Google Scholar] [CrossRef]
- Edelstein, M.; Ben-Hur, M. Heavy metals and metalloids: Sources, risks and strategies to reduce their accumulation in horticultural crops. Sci. Hortic. 2018, 234, 431–444. [Google Scholar] [CrossRef]
- Cho, H.; Oh, D.; Kim, K. A study on removal characteristics of heavy metals from aqueous solution by fly ash. J. Hazard. Mater. 2005, 127, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.; Chao, C.Y.H.; Kot, S. Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash. J. Hazard. Mater. 2005, 127, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Itskos, G.; Koukouzas, N.; Vasilatos, C.; Megremi, I.; Moutsatsou, A. Comparative uptake study of toxic elements from aqueous media by the different particle-size-fractions of fly ash. J. Hazard. Mater. 2010, 183, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Weng, C.-H.; Huang, C. Adsorption characteristics of Zn(II) from dilute aqueous solution by fly ash. Colloids Surf. A 2004, 247, 137–143. [Google Scholar] [CrossRef]
- Salam, O.E.A.; Reiad, N.A.; ElShafei, M.M. A study of the removal characteristics of heavy metals from wastewater by low-cost adsorbents. J. Adv. Res. 2011, 2, 297–303. [Google Scholar] [CrossRef]
- Nascimento, M.; Soares, P.S.M.; de Souza, V.P. Adsorption of heavy metal cations using coal fly ash modified by hydrothermal method. Fuel 2009, 88, 1714–1719. [Google Scholar] [CrossRef]
- Kandel, S.; Vogel, J.; Penn, C.; Brown, G. Phosphorus retention by fly ash amended filter media in aged bioretention cells. Water 2017, 9, 746. [Google Scholar] [CrossRef]
- Yildiz, E. Phosphate removal from water by fly ash using crossflow microfiltration. Sep. Purif. Technol. 2004, 35, 241–252. [Google Scholar] [CrossRef]
- Agyei, N.M.; Strydom, C.; Potgieter, J. The removal of phosphate ions from aqueous solution by fly ash, slag, ordinary portland cement and related blends. Cem. Concr. Res. 2002, 32, 1889–1897. [Google Scholar] [CrossRef]
- Chen, J.; Kong, H.; Wu, D.; Chen, X.; Zhang, D.; Sun, Z. Phosphate immobilization from aqueous solution by fly ashes in relation to their composition. J. Hazard. Mater. 2007, 139, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Oguz, E. Sorption of phosphate from solid/liquid interface by fly ash. Colloids Surf. A 2005, 262, 113–117. [Google Scholar] [CrossRef]
- Xu, X.; Li, Q.; Cui, H.; Pang, J.; Sun, L.; An, H.; Zhai, J. Adsorption of fluoride from aqueous solution on magnesia-loaded fly ash cenospheres. Desalination 2011, 272, 233–239. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Adsorption of phenolic compounds on low-cost adsorbents: A review. Adv. Colloid Interface Sci. 2008, 143, 48–67. [Google Scholar] [CrossRef] [PubMed]
- Kao, P.-C.; Tzeng, J.-H.; Huang, T.-L. Removal of chlorophenols from aqueous solution by fly ash. J. Hazard. Mater. 2000, 76, 237–249. [Google Scholar] [CrossRef]
- Matheswaran, M.; Karunanithi, T. Adsorption of chrysoidine R by using fly ash in batch process. J. Hazard. Mater. 2007, 145, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Singh, K.P.; Singh, G.; Kumar, K. Removal of dyes from wastewater using flyash, a low-cost adsorbent. Ind. Eng. Chem. Res. 2002, 41, 3688–3695. [Google Scholar] [CrossRef]







| Item | Specific Utilization |
|---|---|
| Building materials | Cement, fly ash bricks, fly ash ceramics, fly ash blocks, concrete, mortar |
| Road construction | Embankments, pavement bases and pavement |
| Backfill | Structure backfill, construction backfill, filling in low-lying areas and wastelands, filling mines, filling coal mining subsidence areas, building materials for pits, tidal marshes |
| Agriculture | Soil improvement, fertilizer production, land reclamation |
| Recycling useful raw materials | Hollow microspheres, Al2O3, Fe2O3, SiO2, carbon granules |
| Component (wt.%) | Bituminous | Sub-Bituminous | Lignite |
|---|---|---|---|
| SiO2 | 20–60 | 40–60 | 15–45 |
| Al2O3 | 5–35 | 20–30 | 10–25 |
| Fe2O3 | 10–40 | 4–10 | 4–15 |
| CaO | 1–12 | 5–30 | 15–40 |
| MgO | 0–5 | 1–6 | 3–10 |
| SO3 | 0–4 | 0–2 | 0–10 |
| Na2O | 0–4 | 0–2 | 0–6 |
| K2O | 0–3 | 0–4 | 0–4 |
| LOI a | 0–15 | 0–3 | 0–5 |
| Heavy Metals | Unit | Fly ash 1 | Fly ash 2 | Fly ash 3 | Fly ash 4 | Fly ash 5 | Fly ash 6 | Fly ash 7 |
|---|---|---|---|---|---|---|---|---|
| Fe | mg/kg | 4.2 | 3.2 | 4 | 2.8 | 4 | 4.6 | 3.4 |
| Ca | mg/kg | 67,040 | 55,360 | 58,400 | 48,320 | 78,720 | 85,120 | 74,880 |
| Zn | mg/kg | 2 | 1.6 | 1.8 | 1.6 | 2 | 2 | 2 |
| Cd | mg/kg | 2.8 | 2.2 | 2.4 | 2 | 2.8 | 2.8 | 3 |
| Co | mg/kg | 3.2 | 2.8 | 2.2 | 2 | 2 | 2.6 | 2.4 |
| Ni | mg/kg | 4 | 3.6 | 4.8 | 4 | 3.4 | 3.4 | 5.6 |
| Cu | mg/kg | 2.8 | 2.6 | 2.6 | 2.6 | 3.2 | 3.2 | 3.4 |
| Pb | mg/kg | 11 | 10 | 11 | 10.4 | 14.2 | 15 | 14.4 |
| Mn | mg/kg | 1.8 | 1.6 | 1.6 | 1.4 | 1.6 | 1.6 | 1.8 |
| Volatile Organic Compounds | Dangerous Concentration | Unit | Sources | Health Effects |
|---|---|---|---|---|
| Benzene | 500 | ppm | Petroleum products; Incomplete combustion of liquid fuels; Adhesives; Lacquers | Carcinogen; Damage the ozone layer; Produce photochemical smog & pose mutagenic hazards |
| Toluene | 500 | ppm | ||
| Ethylbenzene | 800 | ppm | ||
| Carbon tetrachloride | 200 | ppm | Chemical extractants; Paints; Adhesives; Polymer syntheses; Water purification systems | Strong bioaccumulation potential; Acute toxicity; Destruction of the ozone; Cause greenhouse gas effects |
| Chlorobenzene | 1000 | ppm | ||
| 1,1,2-Trichloroethane | 100 | ppm | ||
| 1,1,2,2-Tetrachloroethane | 100 | ppm | ||
| Trichloroethylene | 1000 | ppm | ||
| Tetrachloroethylene | 150 | ppm | ||
| Acetone | 2500 | ppm | Varnishes; Window cleaners; Paint thinners; Adhesives | Irritation of eyes, nose, & throat; Central nervous system depression; Headache and nausea |
| Ethyl butyl ketone | 1000 | ppm | ||
| Formaldehyde | 20 | ppm | Decorative & construction; Materials; Cosmetics & plastic adhesives; Fabrics & bio-waste decomposition; Biomass burning; Degradation of VOCs in multiple steps oxidations | Irritation of the throat, eyes, & skin; Nasal tumors; Predecessor of ozone |
| Acetaldehyde | 2000 | ppm | ||
| Methanol | 6000 | ppm | Antiseptics; Preservative; Cosmetics & personal care products | Throat irritation & shortness of breath; Eye irritation; Central nervous system depression |
| Ethyl alcohol | 3300 | ppm | ||
| Isopropyl alcohol | 2000 | ppm |
| Metal | Target Organs | Primary Sources | Clinical Effects |
|---|---|---|---|
| As | Pulmonary nervous system, skin | Industrial dusts, medicinal uses of polluted water | Perforation of nasal septum, respiratory cancer, peripheral neuropathy: dermatomes, skin cancer |
| Cd | Renal, skeletal, pulmonary | Industrial dust and fumes and polluted water and food | Proteinuria, glucosuria, osteomalacia, aminoaciduria, emphysema |
| Cr | Pulmonary | Industrial dust and fumes and polluted food | Ulcer, perforation of nasal septum, respiratory cancer |
| Mn | Nervous system | Industrial dust and fumes | Central and peripheral neuropathies |
| Pb | Nervous system, hematopoietic system, renal | Industrial dust and fumes and polluted food | Encephalopathy, peripheral neuropathy, central nervous disorders, anemia |
| Ni | Pulmonary, skin | Industrial dust, aerosols | Cancer, dramatis |
| Sn | Nervous system, pulmonary | Medicinal uses, industrial dusts | Central nervous system disorders, visual defects and EEG changes, pneumoconiosis |
| Hg | Nervous system, renal | Industrial dust and fumes and polluted water and food | Proteinuria |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, J.C.; Yoon, S.K.; Choi, N.J. Application of Fly Ash as an Adsorbent for Removal of Air and Water Pollutants. Appl. Sci. 2018, 8, 1116. https://doi.org/10.3390/app8071116
Ge JC, Yoon SK, Choi NJ. Application of Fly Ash as an Adsorbent for Removal of Air and Water Pollutants. Applied Sciences. 2018; 8(7):1116. https://doi.org/10.3390/app8071116
Chicago/Turabian StyleGe, Jun Cong, Sam Ki Yoon, and Nag Jung Choi. 2018. "Application of Fly Ash as an Adsorbent for Removal of Air and Water Pollutants" Applied Sciences 8, no. 7: 1116. https://doi.org/10.3390/app8071116





