Study on Cleaning the Surface of Stainless Steel 316 Using Plasma Electrolysis Technology
Abstract
:Featured Application
Abstract
1. Introduction
2. Experimental Method
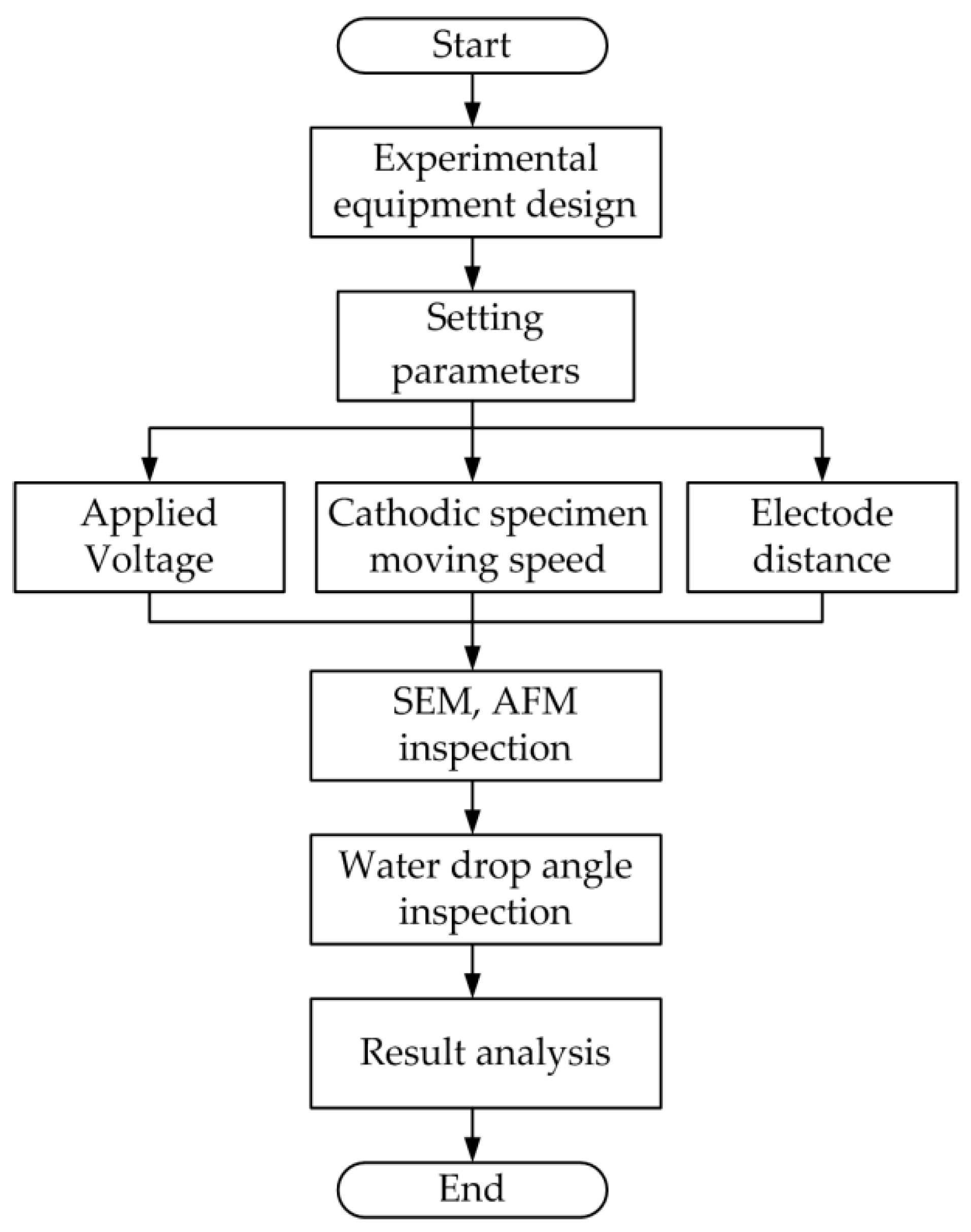
2.1. Experimental Procedure
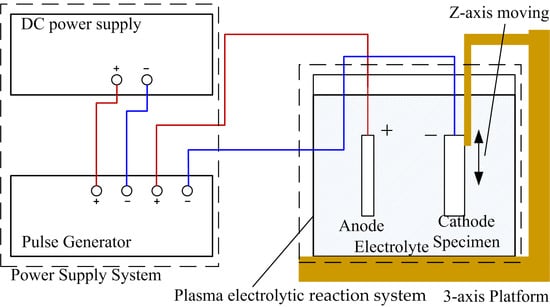
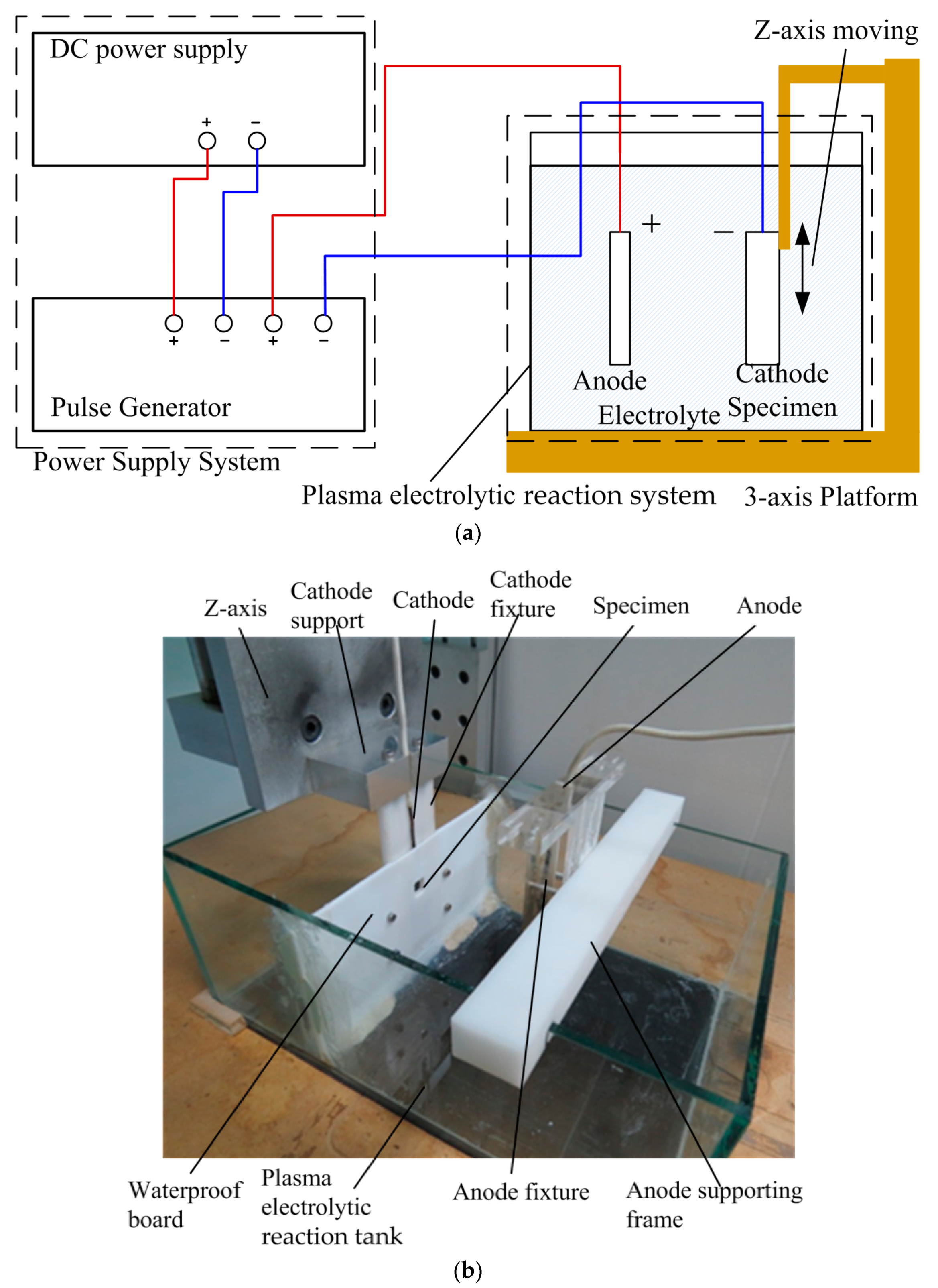
2.2. Experimental Equipment
2.3. Setting the Parameters
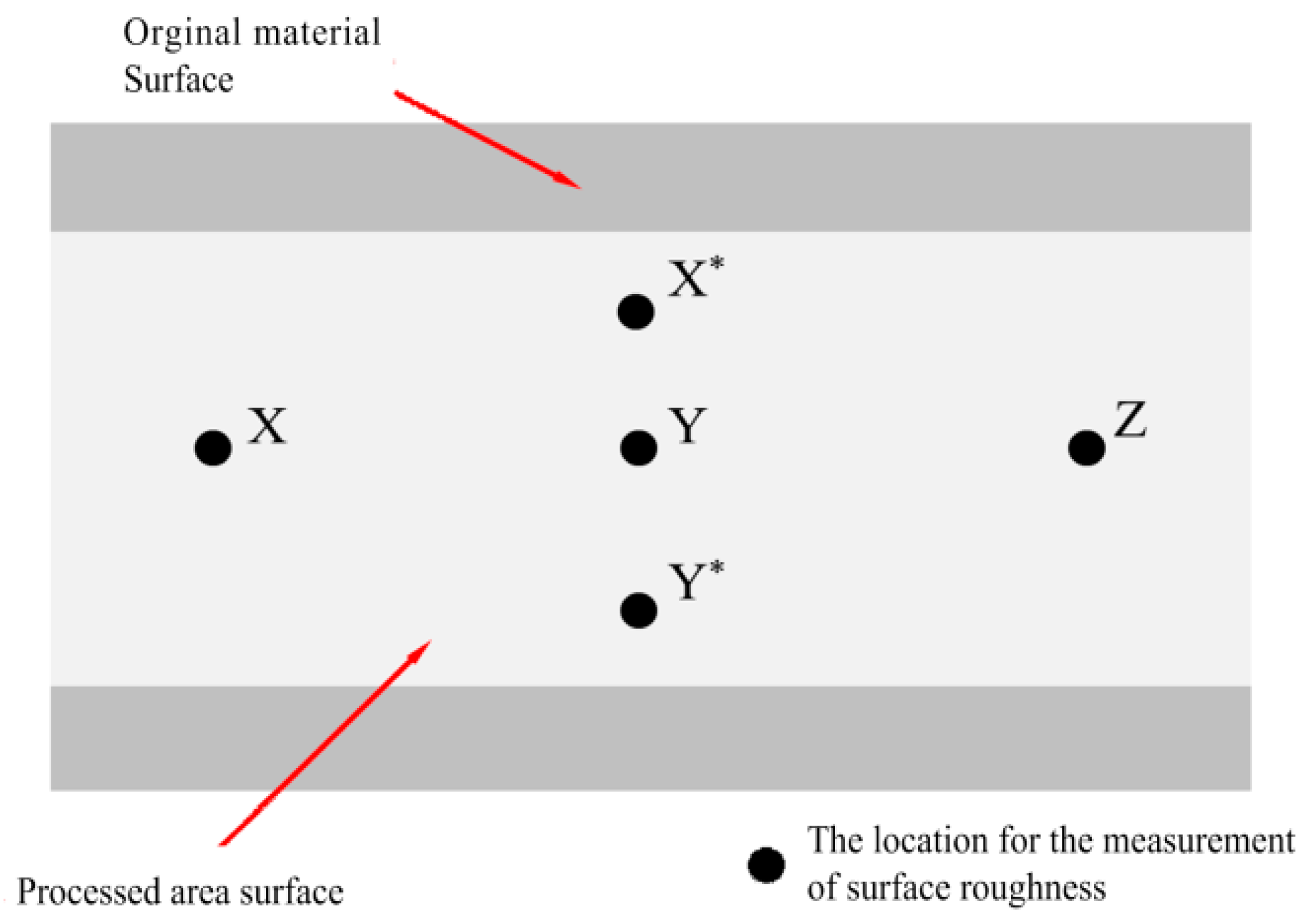
2.4. Post-Processing
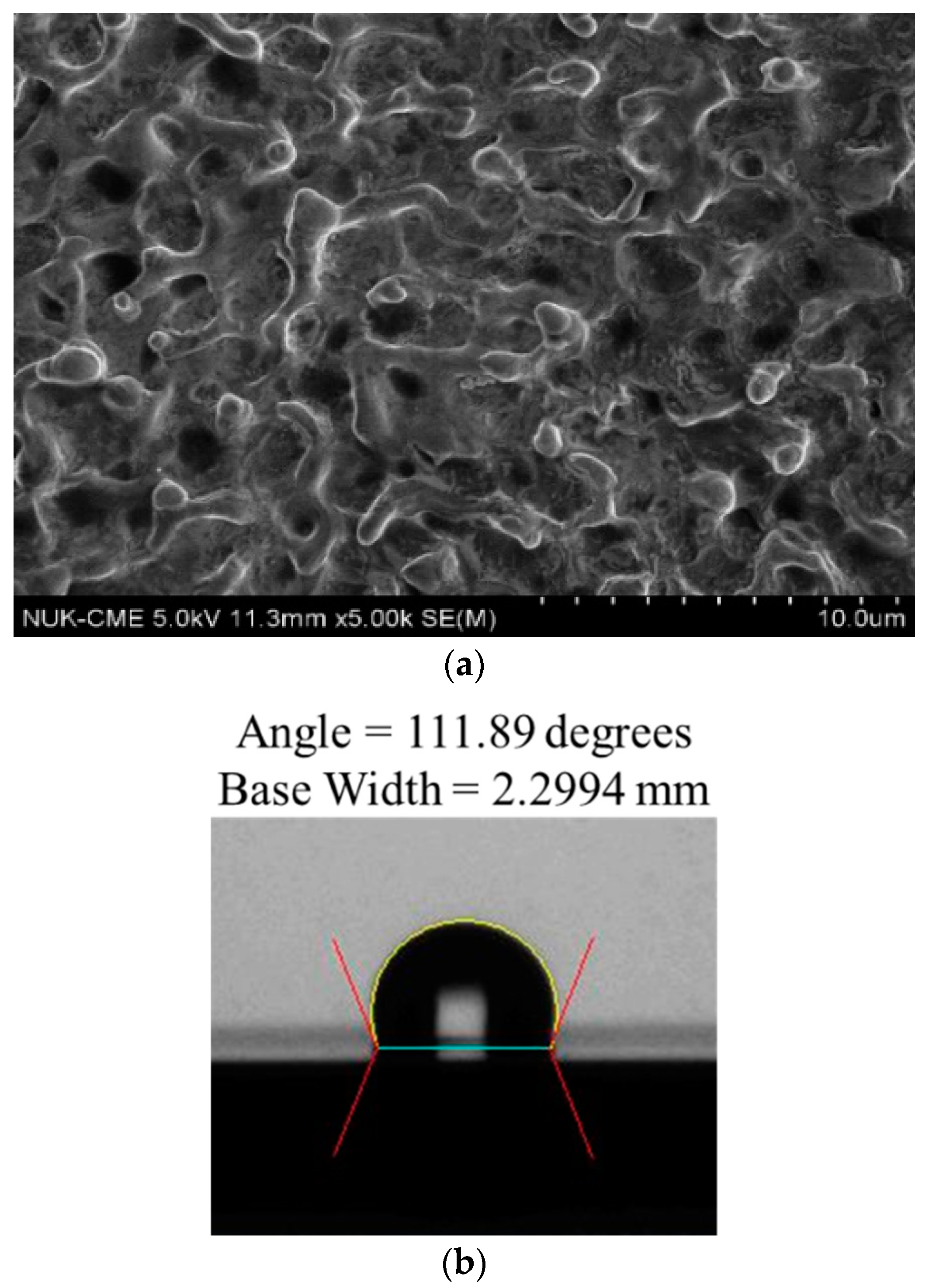
3. Results and Discussion
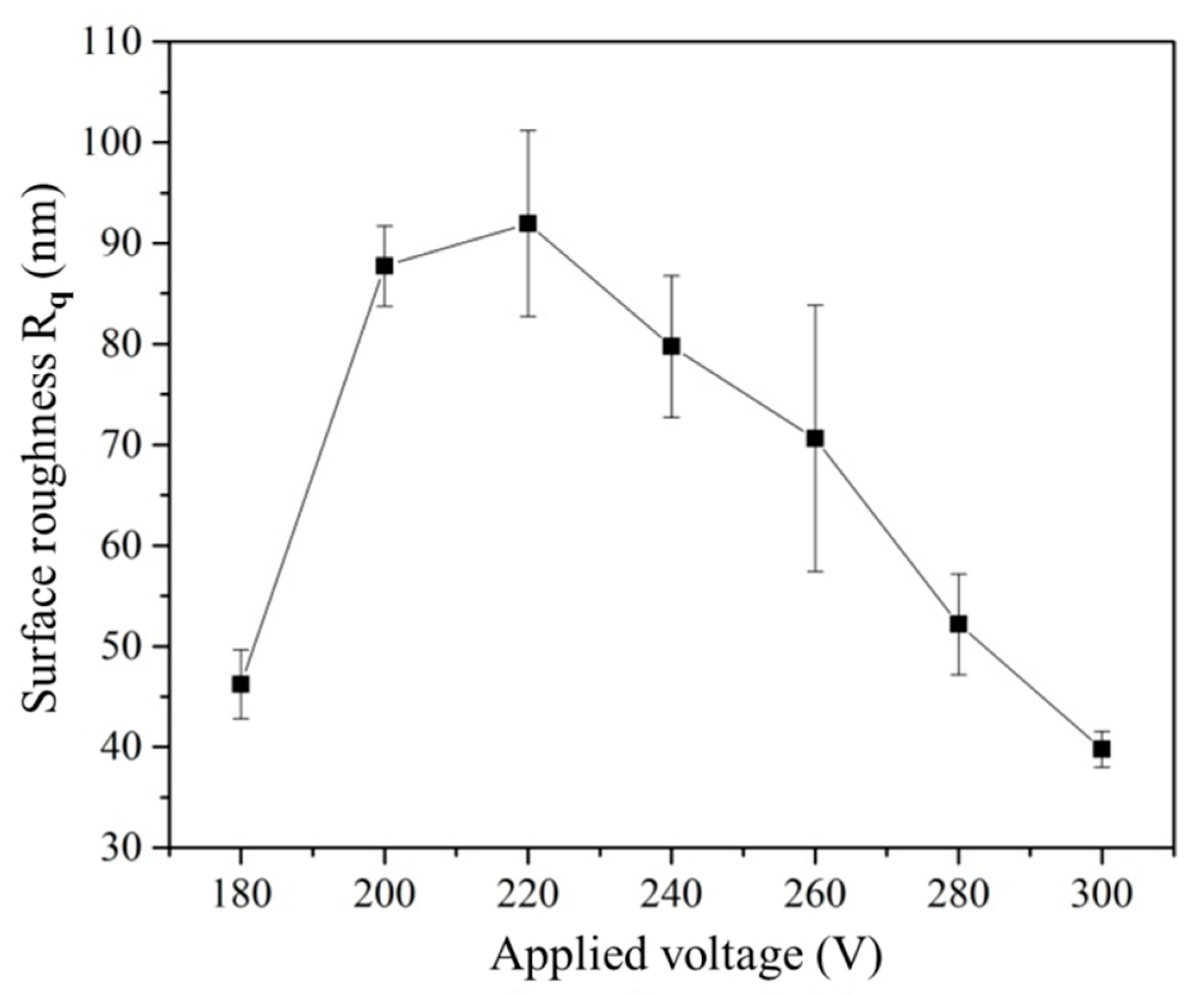
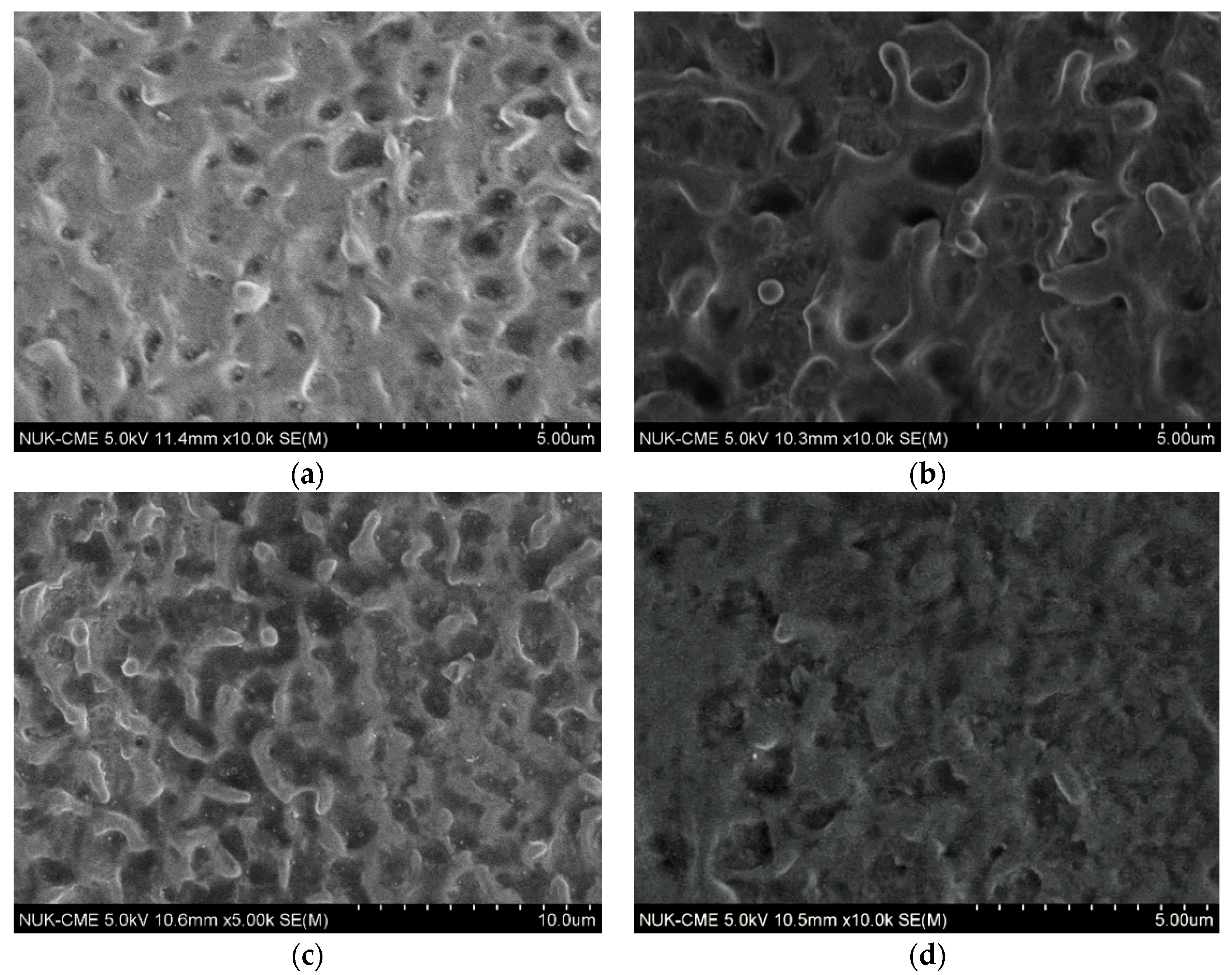
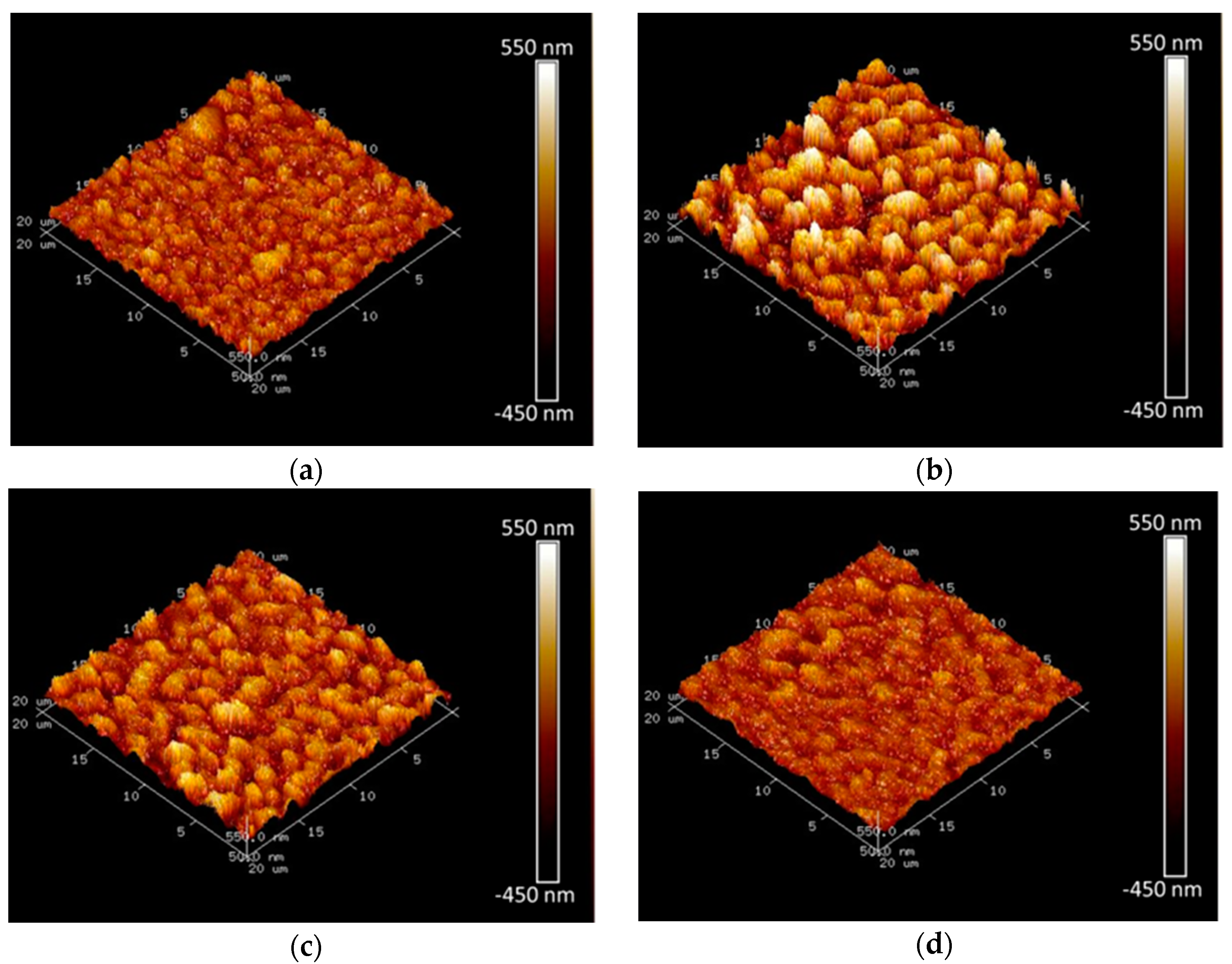
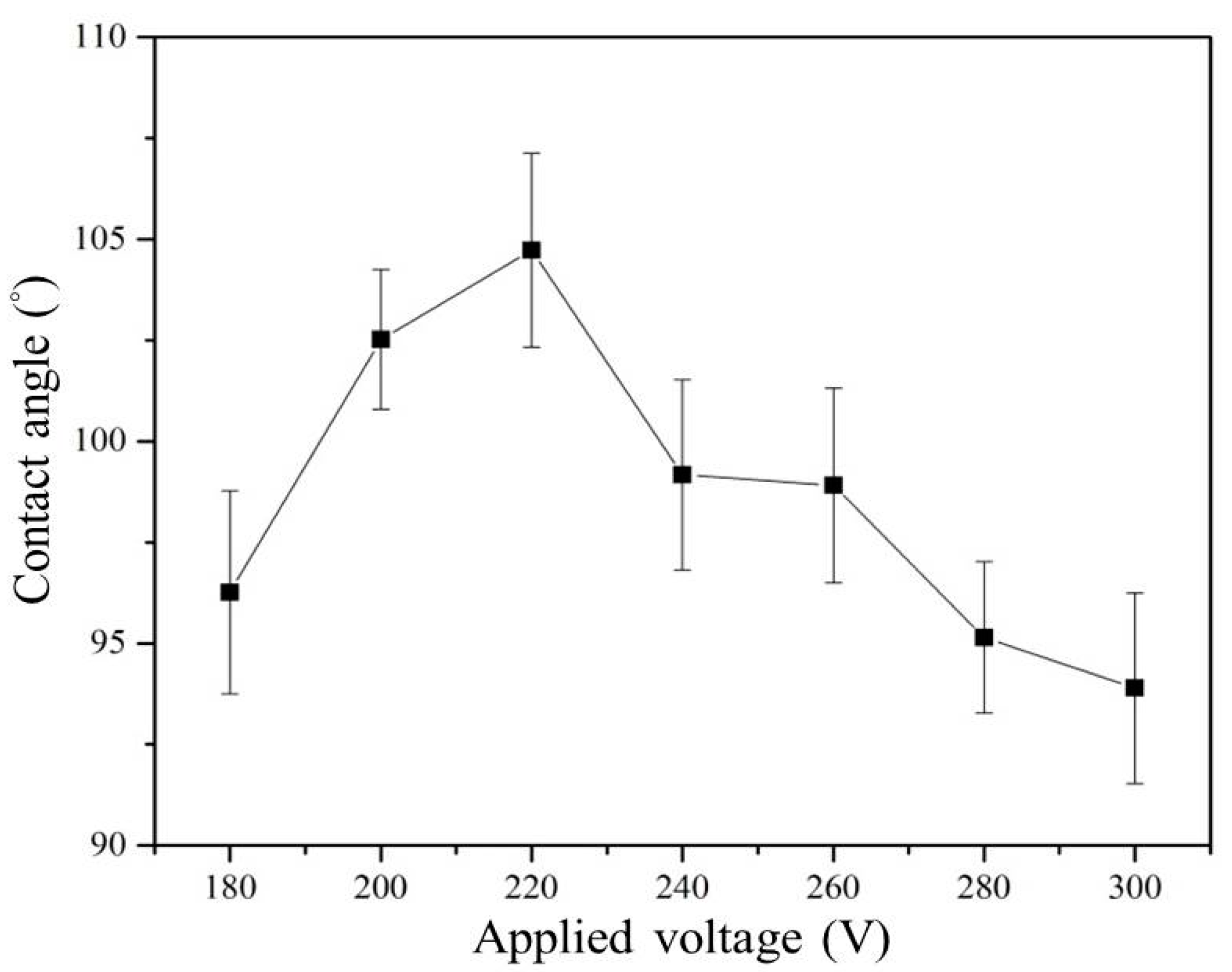
3.1. Effect of Working Voltage on Plasma Electrolysis Reaction
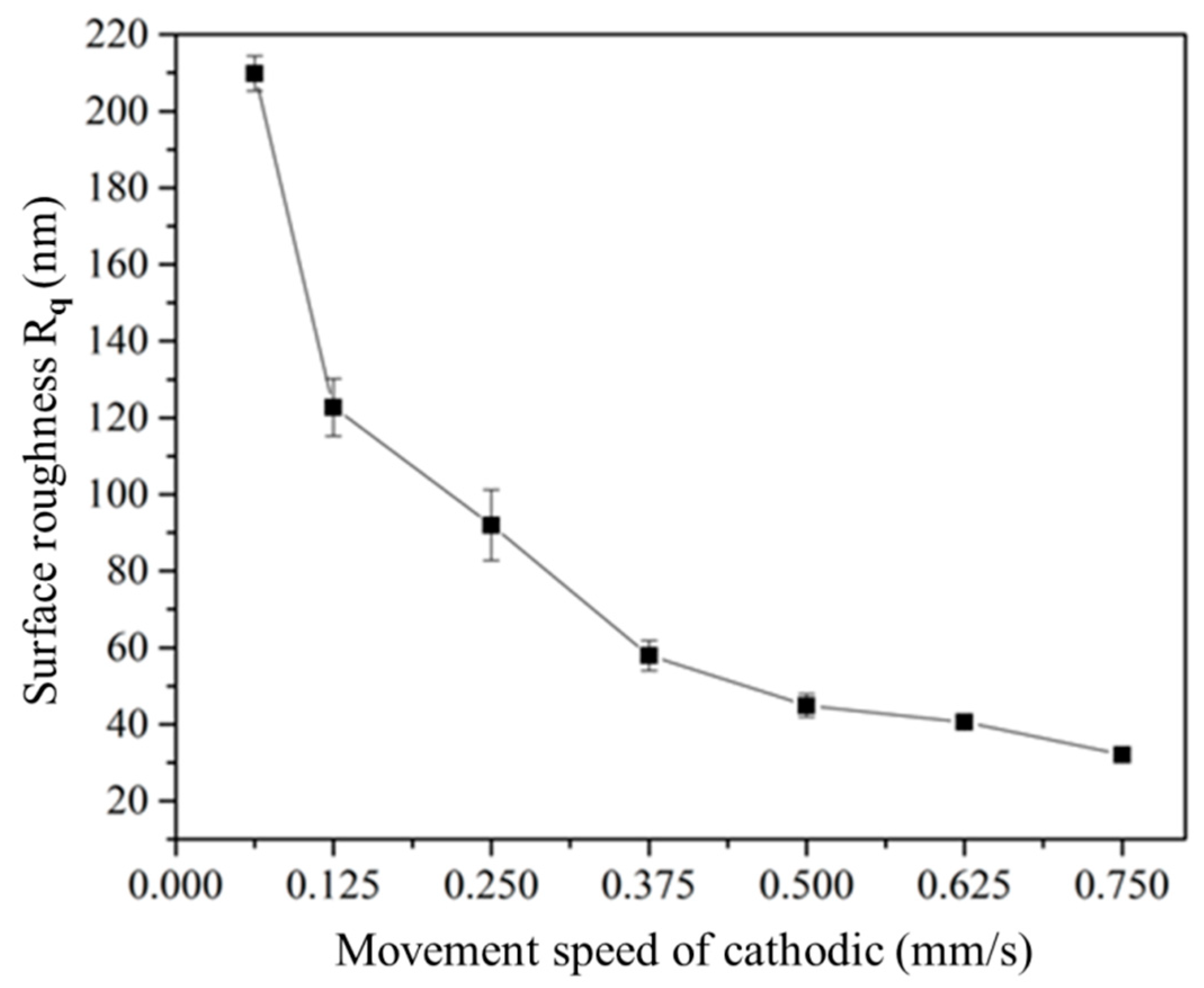
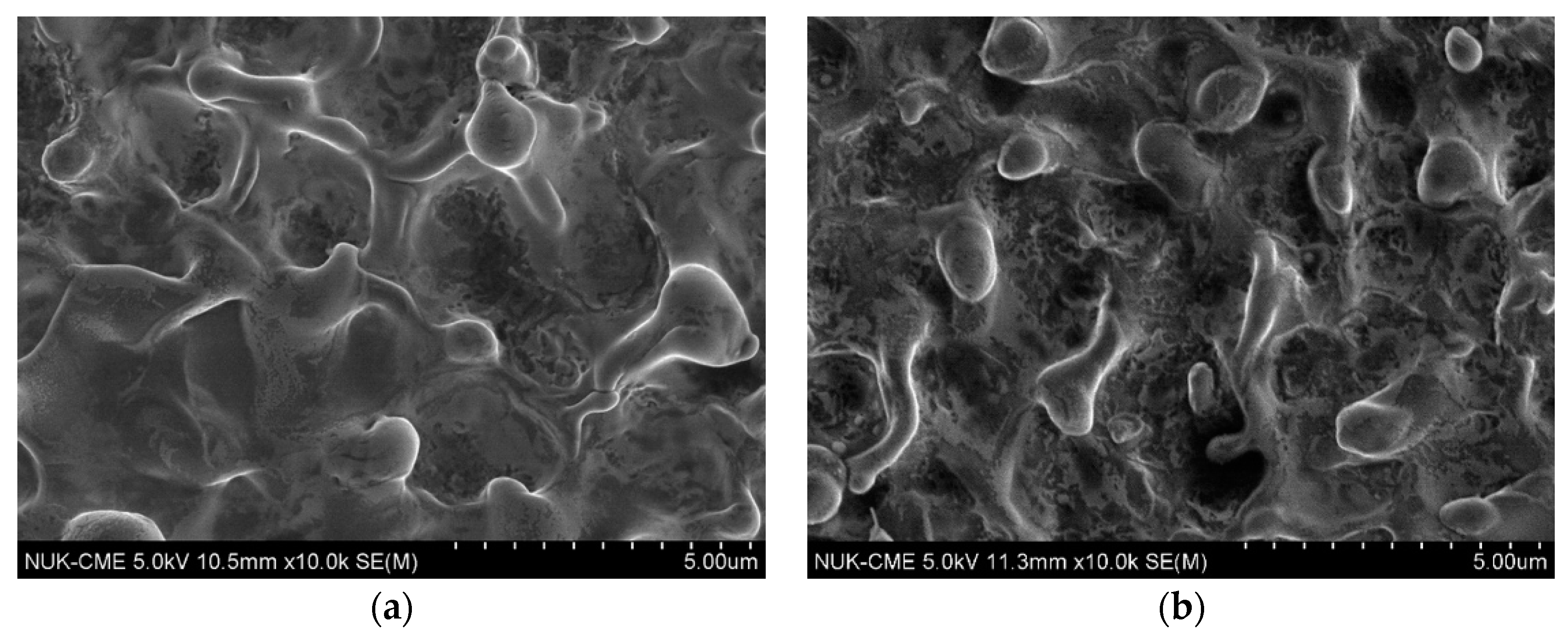
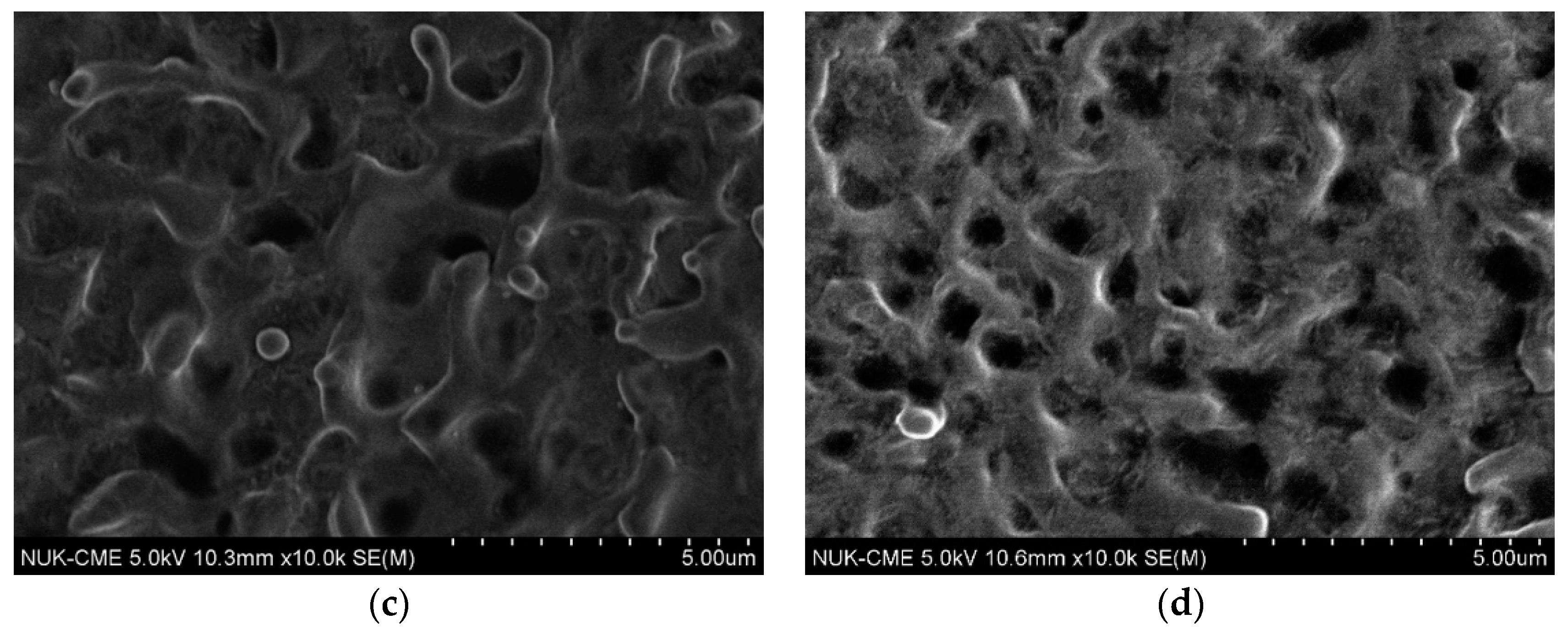
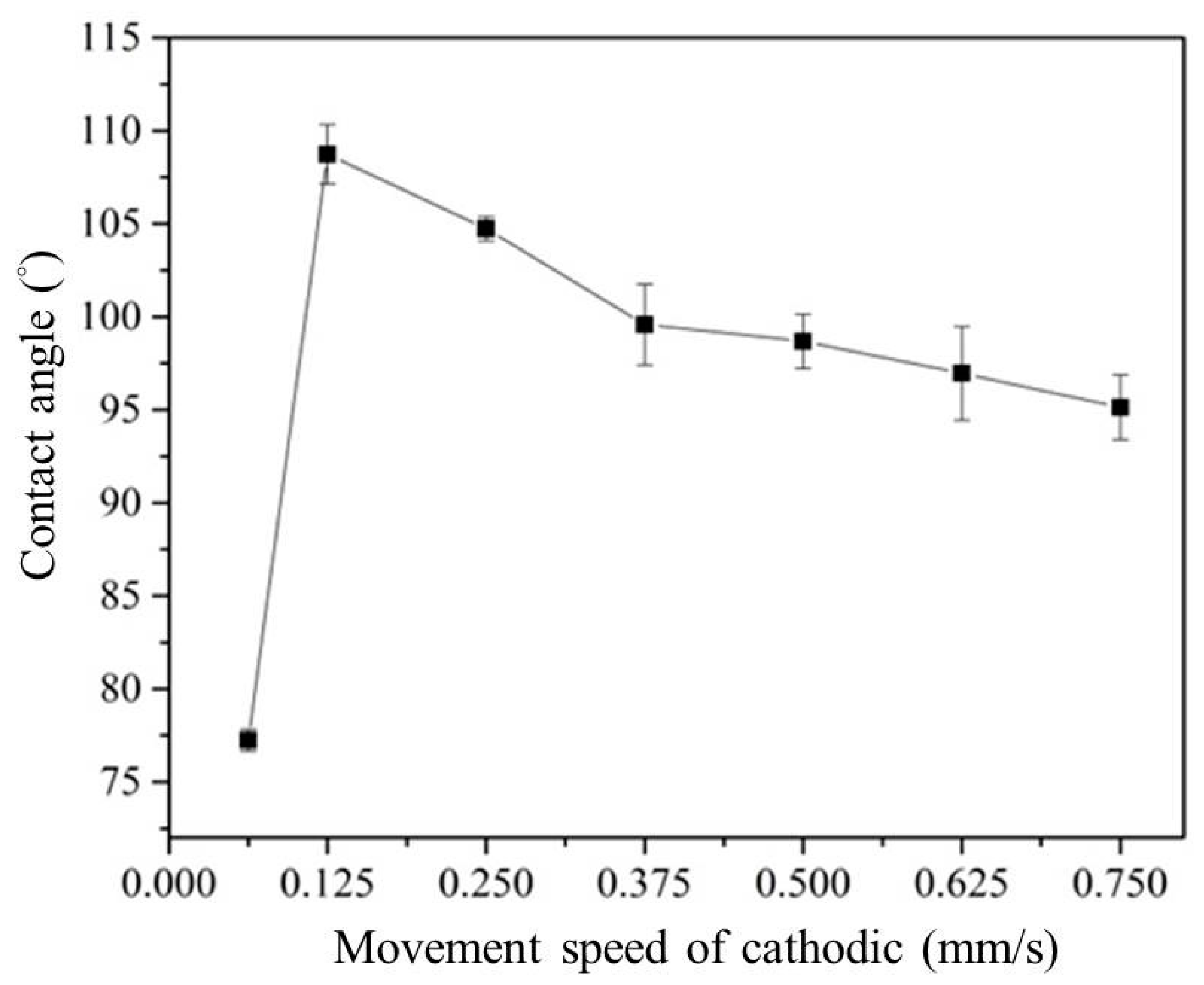
3.2. Effect of the Movement Speed of the Cathodic Specimen on Plasma Electrolysis
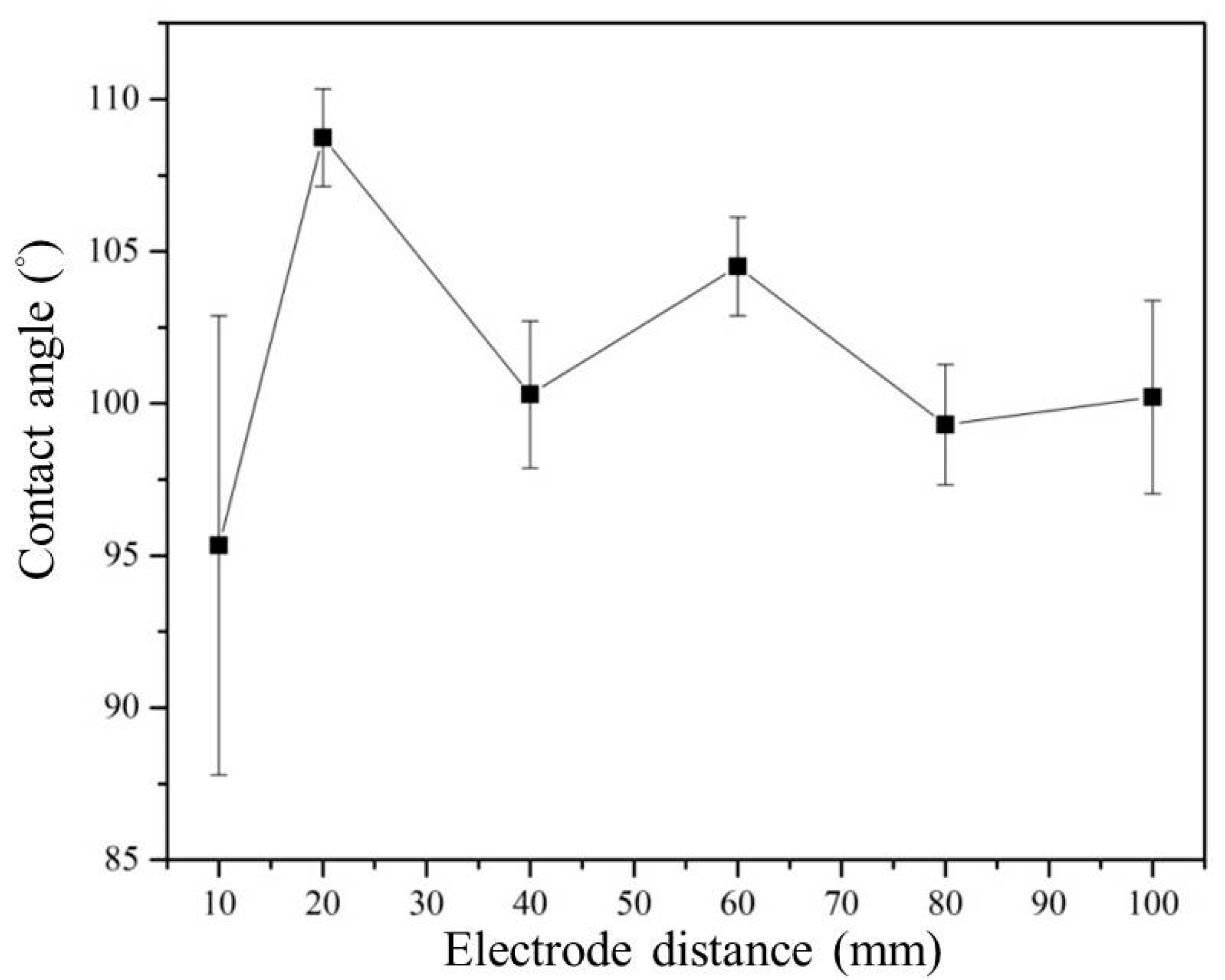
3.3. Effect of Electrode Distance on the Plasma Electrolytic Reaction
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Aliofkhazraei, M.; Rouhaghdam, A.S. Fabrication of Nanostructures by Plasma Electrolysis; Wiley-VCH Verlag GmbH & Go. KGaA: Weinheim, Germany, 2010. [Google Scholar] [CrossRef]
- Parfenov, E.V.; Yerokhin, A.; Nevyantseva, R.R.; Gorbatkov, M.V.; Liang, C.-J.; Matthews, A. Towards smart electrolytic plasma technologies: An overview of methodological approaches to process modeling. Surf. Coat. Technol. 2015, 269, 2–22. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A. Characterization of oxide films produced by plasma electrolytic oxidation of a Ti–6Al–4V alloy. Surf. Coat. Technol. 2000, 130, 195–206. [Google Scholar] [CrossRef]
- Snizhkoa, L.O.; Yerokhin, A.L.; Pilkington, A.; Gurevina, N.L.; Misnyankin, D.O.; Leyland, A.; Matthews, A. Anodic processes in plasma electrolytic oxidation of aluminum in alkaline solutions. Electrochim. Acta 2004, 49, 2085–2095. [Google Scholar] [CrossRef]
- Gu, W.; Shen, D.; Wang, Y.; Chen, G.; Feng, W.; Zhang, G.; Fan, S.; Liu, C.; Yang, S. Deposition of duplex Al2O3/aluminum coatings on steel using a combined technique of arc spraying and plasma electrolytic oxidation. Appl. Surf. Sci. 2006, 252, 2927–2932. [Google Scholar] [CrossRef]
- Ma, Y.; Nie, X.; Northwood, D.O.; Hu, H. Systematic study of the electrolytic plasma oxidation process on a Mg alloy for corrosion protection. Thin Solid Films 2006, 494, 296–301. [Google Scholar] [CrossRef]
- Liang, J.; Hu, L.; Hao, J. Improvement of corrosion properties of microarc oxidation coating on magnesium alloy by optimizing current density parameters. Appl. Surf. Sci. 2007, 253, 6939–6945. [Google Scholar] [CrossRef]
- Mu, W.; Han, Y. Study on Micro-Arc Oxidized Coatings on Magnesium in Three Different Electrolytes. Rare Met. Mater. Eng. 2010, 39, 1129–1134. [Google Scholar]
- Moon, S. Corrosion behavior of PEO treated AZ31 Mg alloy in chloride solution. J. Solid State Electrochem. 2014, 18, 341–346. [Google Scholar] [CrossRef]
- Cui, X.-J.; Lin, X.-Z.; Liu, C.-H.; Yang, R.-S.; Zheng, X.-W.; Gong, M. Fabrication and corrosion resistance of a hydrophobic micro-arc oxidation coating on AZ31 Mg alloy. Corros. Sci. 2015, 90, 402–412. [Google Scholar] [CrossRef]
- Krishna, L.R.; Poshal, G.; Jyothirmayi, A.; Sundararajan, G. Relative hardness and corrosion behavior of micro arc oxidation coatings deposited on binary and ternary magnesium alloys. Mater. Des. 2015, 77, 6–14. [Google Scholar] [CrossRef]
- Gupta, P.; Tenhundfeld, G.; Daigle, E.O.; Ryabkov, D. Electrolytic plasma technology: Science and engineering—An overview. Surf. Coat. Technol. 2007, 201, 8746–8760. [Google Scholar] [CrossRef]
- Schilling, P.J.; Herrington, P.D.; Daigle, E.O.; Meletis, E. Surface characteristics of structural steel processed using electro-plasma techniques. J. Mater. Eng. Perform. 2002, 11, 26–31. [Google Scholar] [CrossRef]
- Meletis, E.I.; Nie, X.; Wang, F.L.; Jiang, J.C. Electrolytic plasma processing for cleaning and metal-coating of steel surfaces. Surf. Coat. Technol. 2002, 150, 246–256. [Google Scholar] [CrossRef]
- Kumruoglu, L.C.; Özel, A. Electrolytic Plasma Surface Cleaning of Industrial Metallic Components. Acta Phys. Pol. A 2014, 125, 379–381. [Google Scholar] [CrossRef]
- Cheng, M.-H.; Liang, T.-J.; Chen, S.-M. A Phase-Shift Resonant Power System with Novel Plasma Impedance Measurement Methodology for Plasma Cleaning Applications. In Proceedings of the 2017 IEEE 3rd International Future Energy Electronics Conference and ECCE Asia (IFEEC 2017—ECCE Asia), Kaohsiung, Taiwan, 3–7 June 2017. [Google Scholar] [CrossRef]
- Lin, A.-D.; Kung, C.-L.; Cao, Y.-Q.; Hsu, C.-M.; Chen, C.-Y. Stainless Steel Surface Coating with Nanocrystalline Ag Film by Plasma Electrolysis Technology. Coatings 2018, 8, 222. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, A.-D.; Kung, C.-L.; Hsieh, W.-C.; Hsu, C.-M.; Chen, C.-Y. Study on Cleaning the Surface of Stainless Steel 316 Using Plasma Electrolysis Technology. Appl. Sci. 2018, 8, 1060. https://doi.org/10.3390/app8071060
Lin A-D, Kung C-L, Hsieh W-C, Hsu C-M, Chen C-Y. Study on Cleaning the Surface of Stainless Steel 316 Using Plasma Electrolysis Technology. Applied Sciences. 2018; 8(7):1060. https://doi.org/10.3390/app8071060
Chicago/Turabian StyleLin, Ah-Der, Chi-Liang Kung, Wei-Chen Hsieh, Chao-Ming Hsu, and Cheng-Yi Chen. 2018. "Study on Cleaning the Surface of Stainless Steel 316 Using Plasma Electrolysis Technology" Applied Sciences 8, no. 7: 1060. https://doi.org/10.3390/app8071060