1. Introduction
Clinical advances have identified the podocyte as the main cell involved in kidney disease, because structural alteration of podocytes such as hypertrophy, dedifferentiation, detachment, and apoptosis contribute to the development of idiopathic nephrotic syndrome (INS), minimal change disease, focal segmental glomerulosclerosis, membranous glomerulopathy, and lupus nephritis [
1,
2,
3].
Podocytes alterations are associated with loss of podocalyxin, a protein involved in structural maintenance pedicels (podocyte foot processes) which anchor the cell to the glomerular basal membrane [
2,
4,
5].
Currently, cell lines are used for the study of kidney disease; however, the process of immortalization affects the expression of anchor and structural proteins, hindering the understanding of the physiopathologic mechanisms [
6,
7]. Primary cultures of cells extracted from patients have been used to elucidate the origin of kidney-associated diseases, however, the viability of primary podocytes in vitro is around 2 or 3 weeks at most, with loss of morphology and drastic decrease of viability [
8]. Thus, it is necessary to improve culture conditions and develop new techniques that prolong the half-life of primary podocytes while keeping the production of their specific proteins.
Scaffolds are support structures that simulate the physiological conditions for the recovery and repair of damaged organs and tissues, [
9,
10,
11] and are an option for primary cell cultures [
12].
Scaffolds are made of bio-compatible polymers that are either present in the physiological environment of the cell or that provide requirements for a specific cell type. One of the polymers used for scaffolds is chitosan, a nitrogenous polysaccharide obtained from the alkaline deacetylation of chitin derived from the exoskeleton of marine animals such as crabs, shrimps, lobsters, and krill [
13,
14]. Several studies show that chitosan stimulates cell adhesion, migration and proliferation. Chitosan interacts with other molecules through its amine groups, improving their mechanical and biological properties. Polyvinyl alcohol (PVA) a nontoxic synthetic polymer, is one of such molecules. Blends of chitosan-PVA have been studied as base for hydrogels and scaffolds with biological applications [
15,
16].
We found no reports of chitosan-PVA scaffolds for podocyte primary culture; furthermore, for their maintenance, podocytes require other substances such as integrin α
3β
1, necessary for attachment to the basal membrane [
17] and IV collagen, a main component of the basal membrane.
The aim of this work is to study the performance of a chitosan/PVA/IV collagen/integrin α3β1 scaffold for primary podocytes culture, evaluating their half-life, cell adhesion, and specific protein expression, as an alternative in vitro model for the study of physio-pathological mechanisms involved in renal disease.
2. Materials and Methods
2.1. Reagents
Chitosan medium molecular weight, poly (vinyl alcohol), collagen from human placenta type IV, Roswell Park Memorial Institute medium (RPMI 1640) (Sigma Aldrich, St. Louis, MO, USA), recombinant human integrin α3β1 (R&D Systems, Minneapolis, MN, USA). MAbs-IgG1 mouse anti-podocalyxin antibody, anti-podocin antibody, anti-CD80 (B7.1) antibody, anti-IgG Rabbit antibody (FITC), anti-IgG1 mouse antibody (TR), anti-IgG goat antibody (FITC) (Santa Cruz Biotechnology, Dallas, TX, USA). Anti-IgG1 mouse antibody (Rat anti-mouse IgG1 MicroBeads), MiniMACS separator (Miltenyi Biotec, Bergisch Gladbach, Germany), fetal bovine serum (Gibco, Thermo Fisher Scientific, Waltham, MA, USA). Kit Quick-Prep™ Urinalysis, L-Lactate Assay Kit (Cayman Chemical Company, Ann Arbor, MI, USA), 24-well tissue culture polystyrene plates (Corning Costar, Sigma-Aldrich, Saint Louis, MO, USA).
2.2. Chitosan, PVA, Type IV Collagen, and Integrin α3β1 Scaffold Preparation
PVA hydrogels were prepared by fully dissolving 1.0 g of polymer powder in 100 mL of Milli-Q water, under magnetic stirring at 90 °C (solution A). Chitosan hydrogels were prepared by fully dissolving 2 g of polymer powder in 100 mL of 0.2 M CH3COOH under magnetic stirring for 24 h (solution B). Subsequently, solution A was added to solution B to obtain Chitosan/PVA with a 7:1 (v/v) mass ratio. Finally, type IV collagen and recombinant human integrin α3β1, at 7% w/v or 0.03% w/v ratio were added. The solution was poured into plastic molds and dried in an oven for 48 h at 30 °C.
2.3. Characterization for Fourier Transform Infrared Spectroscopy (FTIR)
The study of the contribution of each component to the interaction was carried by means of attenuated total reflection (ATR) used in conjunction with Fourier Transform Infrared Spectroscopy (FTIR) technique. The spectra of CTS, PVA, CTS/PVA, CTS/PVA/type IV collagen and CTS/PVA/type IV collagen/integrin α3β1 samples were recorded by a Universal ATR Sampling Accessory Perkin Elmer by the accumulation of 16 scan at 4 cm−1 resolution in the wavenumber range 4500–500 cm−1. Specimens were placed onto the diamond ATR crystal using a top-plate and pressure arm. Data were analyzed with the software PERKIN ELMER spectrum.
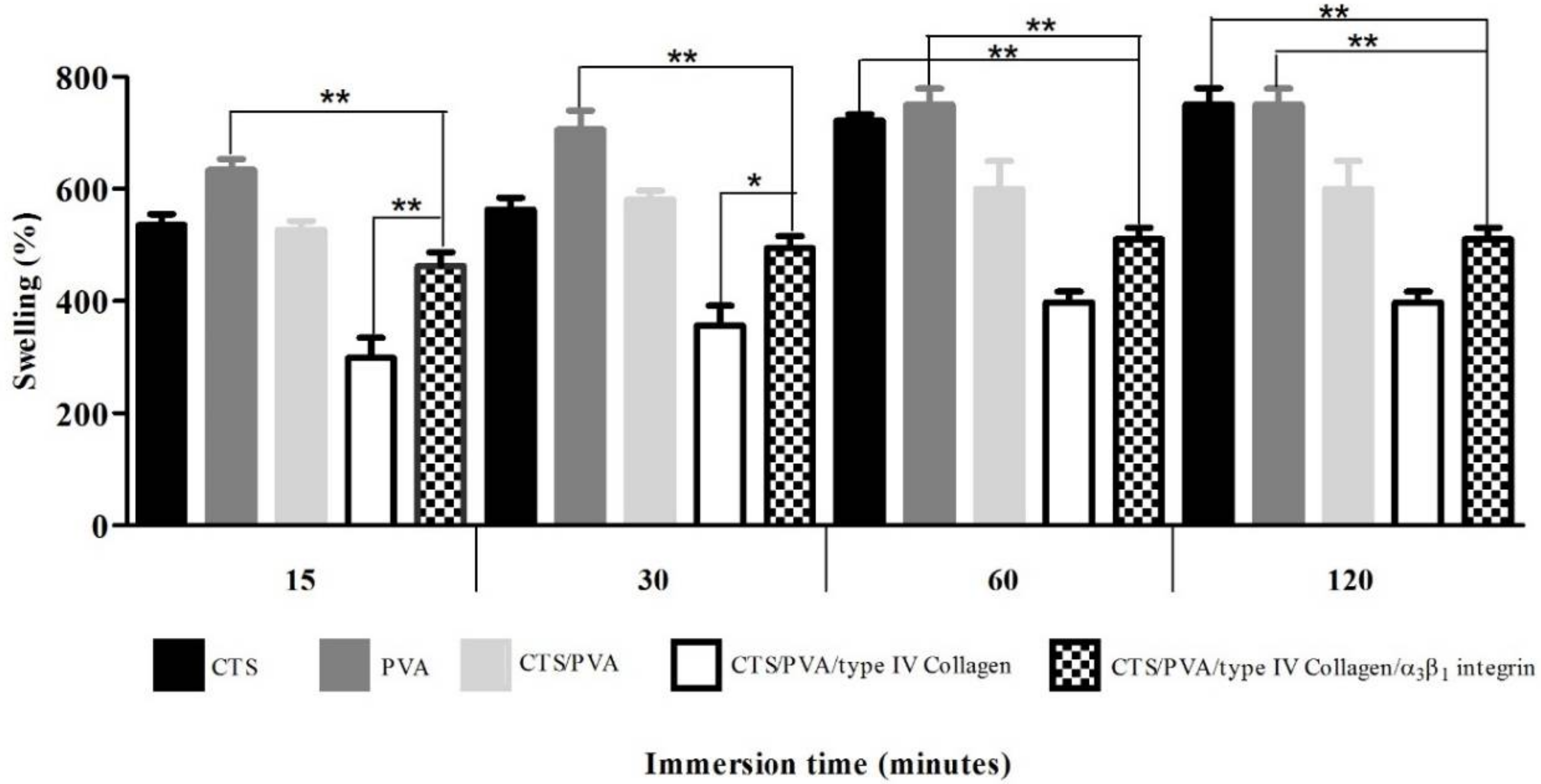
2.4. Swelling and Degradation Tests under Physiological Mimicking Conditions
The CTS, PVA, CTS/PVA, CTS/PVA/type IV collagen and CTS/PVA/type IV collagen/integrin α3β1 scaffolds were cut into 2 × 2 cm2 pieces and soaked in RPMI 1640 medium for the swelling and degradation assay. At the end of each soaking period, the solution excess on the samples was wiped with a lint-free tissue paper.
2.4.1. Swelling Test
The swelling test indicates the nutrient uptake of our scaffold from the culture medium and their delivery to the cell. A rigid structure is produced by the polymer interaction, which reduces the flexibility and number of hydrophobic groups in the scaffold, affecting the swelling velocity.
For fluid uptake measurements, CTS, PVA, CTS/PVA, CTS/PVA/type IV collagen and CTS/PVA/type IV collagen/integrin α
3β
1 scaffold samples were weighed before being immersed in RPMI 1640 medium at 37 °C. At determinate time intervals (3, 7, 15, and 30 min), the samples were removed quickly and carefully, dried lightly to remove excess medium, and then were weighed. The degree of welling was determinate by the following Equation (1): medium absorption (
S), wet weight (
Wf) and dry weight (
W0).
Each medium absorption experiment was made by triplicate and the average value is reported.
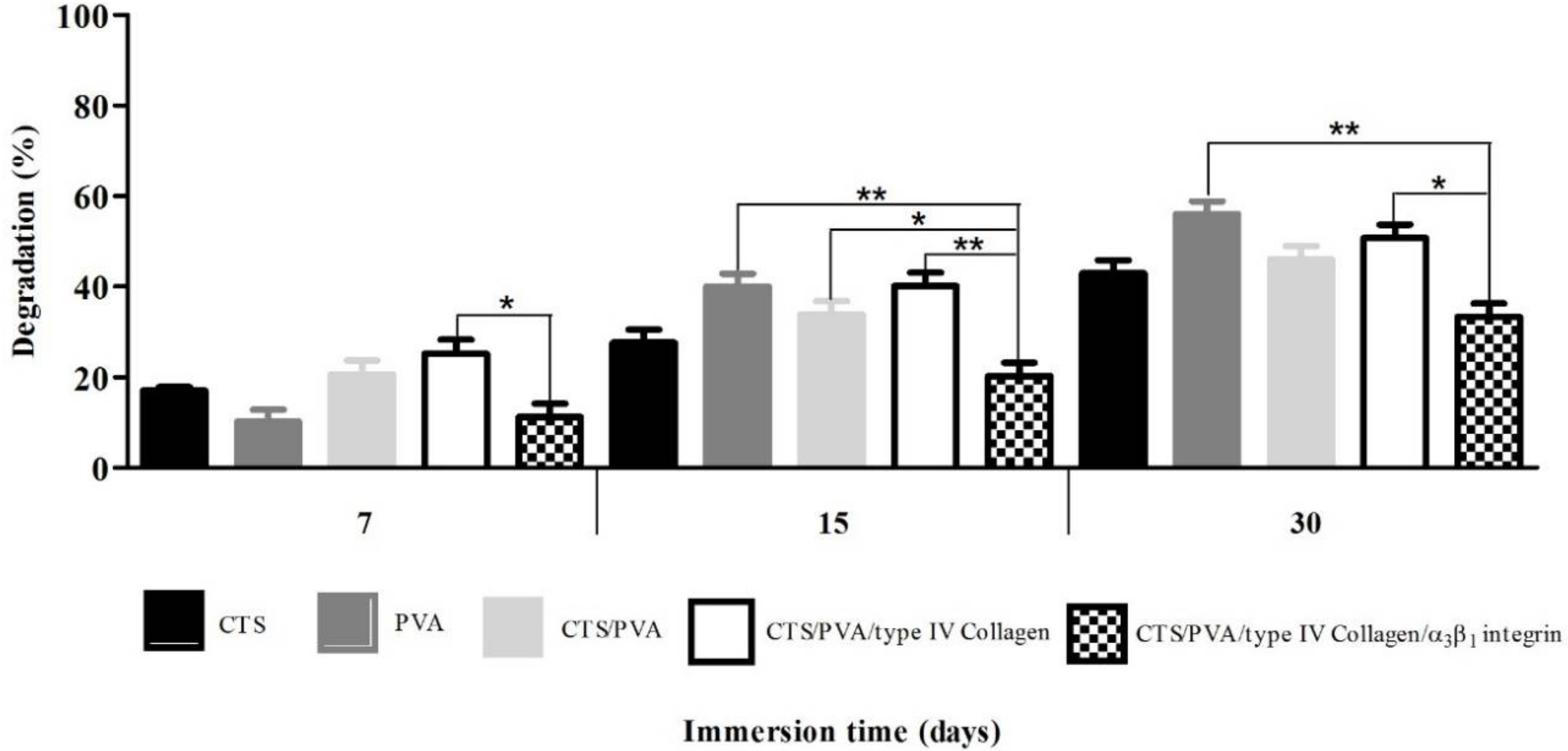
2.4.2. Degradation Test
The degradation study determines time-dependent alterations in the physicochemical characteristics of the scaffolds, this includes polymer mass loss through solvation and depolymerization. To find out the degradation index (
Di), samples were incubated in RPMI 1640 medium at 37 °C. At determinate time intervals (3, 7, 15, and 30 days), the samples were removed quickly and carefully, dried lightly to remove excess medium, and then were weighed. The degradation index was calculated based on the mass loss using the Equation (2): wet weight (
Wt) and dry weight (
W0).
Each degradation experiment was made by triplicate and the average value is reported.
2.5. Biological Samples
Urine samples were obtained from twenty-two children: twelve patients with INS, 7 boys and 5 girls with an average age of 4.8 years, and ten healthy children 6 boys and 4 girls, with an average age of 7.8 years (
Table 1). All patients were diagnosed according to the criteria of the International Study of Kidney Disease in Children. Informed consent was obtained from the parents of each child as necessary. This study was approved by the Ethics Committee of the Zacatecas General Hospital, Mexico.
2.6. Podocyte Isolation
Podocytes were isolated from urine and mice kidneys. The freshly urine were processed in less than 30 min and were centrifuged for 5 min at 1500 rpm. The Balb/c mice kidneys were trypsinized for 10 min at 37 °C and then centrifuged for 5 min at 1500 rpm. After decanting the supernatant, both pellets were labeled with mAbs anti-Podocalyxin antibody (Podocalyxin-like 1 (3D3) mAbs-IgG
1 mouse) for a 1 h. Subsequently, the cells were magnetically labeled with the anti-IgG
1 mouse antibody (Rat anti-mouse IgG
1). Then, the cell suspension was loaded onto a MACS Column, which was placed in the magnetic field of a MiniMACS separator. The viability and number of podocytes obtained by positive selection was determined using the Quick-Prep TM Urinalysis kit, using the Equation (3):
2.7. Podocyte Culture
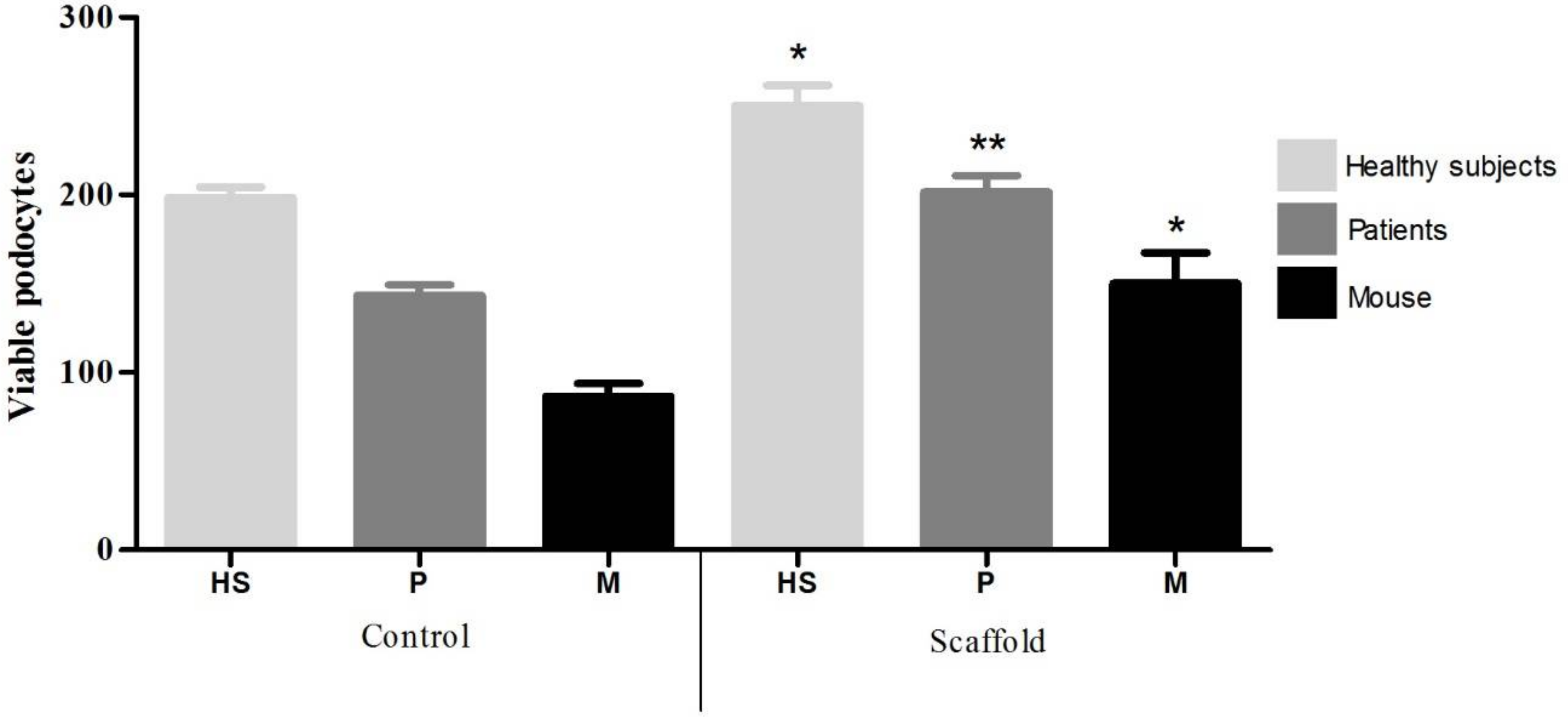
The CTS/PVA/type IV collagen/integrin α3β1 scaffold was placed in a 24-well tissue culture polystyrene plate (Corning Costar, Sigma-Aldrich, Saint Louis, MO, USA). Then, 1 mL cell suspension at a density of 1 × 104 cell/mL in RPMI 1640 medium was placed on each well and maintained in a humidified atmosphere with 5% CO2 at 37 °C. As a culture control, we used a polystyrene plate (Corning Costar, Sigma-Aldrich, Saint Louis, MO, USA), where 1 mL cell suspension at a density of 1 × 104 cell/mL in RPMI 1640 medium were added. After 30 days of culture, podocytes were stained with 4′,6-diamidino-2-phenylindole (DAPI) to be counted and determine the number of viable cells.
2.8. Co-Localization of Podocin, Podocalyxin, and CD80
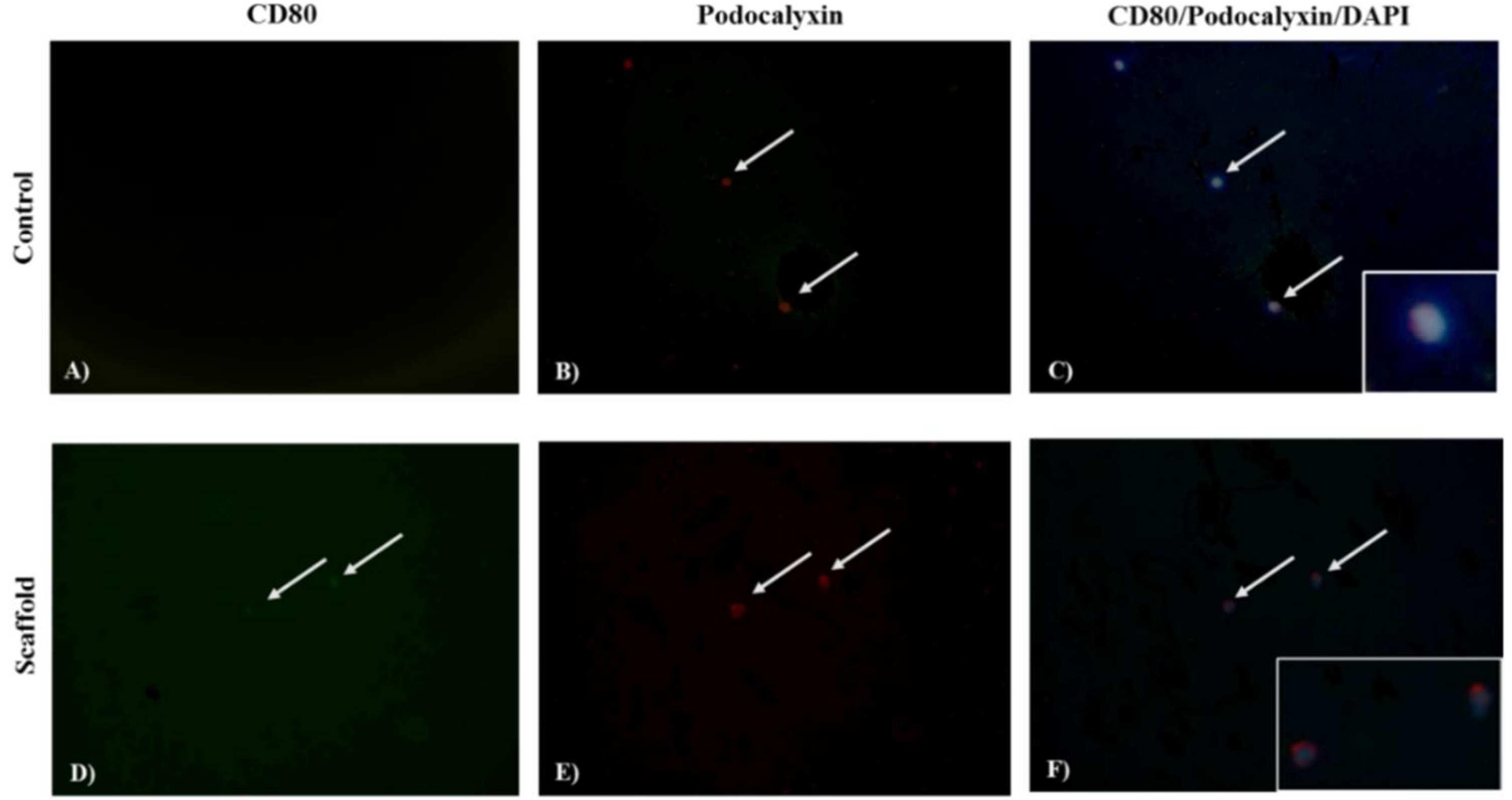
Expression of Podocin, Podocalyxin, and CD80 was identified by indirect immunofluorescent assay (IF). Podocytes were washed with PBS and fixed with 4% paraformaldehyde at 4 °C for 5 min. Then, were permeabilized with Triton 1X (Triton 0.1% with 1% sodium citrate in PBS) for 2 min, and washes three times with PBS. After, the nonspecific binding sites were blocked with 20% fetal bovine serum in PBS for 30 min and incubated for 1 h with anti-Podocin antibody (rabbit anti-human polyclonal IgG, Podocalyxin-like 1 (3D3) mAbs-IgG1 mouse or anti-CD80 (B7.1) antibody Goat anti-human polyclonal IgG diluted 1:100 in PBS. After washes with PBS, the presence of bound antibodies was detected by Goat anti-rabbit IgG FITC, Goat anti-mouse IgG1 TR, and Donkey anti-goat IgG FITC, staining. Podocytes were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) for 30 min, after washing with methanol and methanol-PBS were mounted in PBS/glycerol (3/7) for be photographed at 40X in a microscope (Olympus BX-40). The images were analyzed using Image-Pro Plus software, version 7.0. (Media Cybernetics, Rockville, MD, USA) and the intensity of signal was expressed in pixels. Podocin and CD80 were identified by emitting fluorescence in green, and Podocalixin red color, with the contrast of the DAPI was determined the cellular localization. The analysis was conducted independently by two researchers.
2.9. Cell Viability Assay
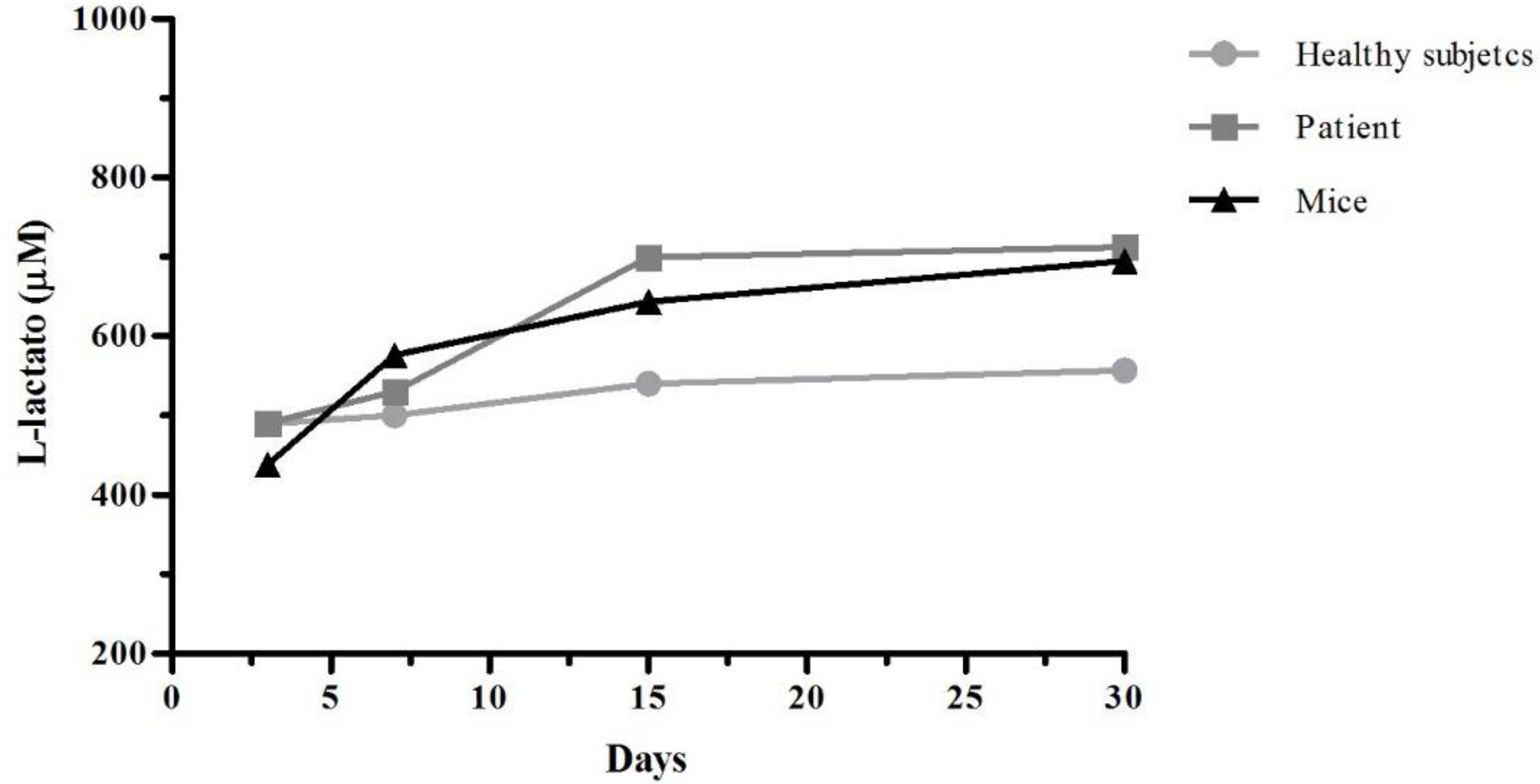
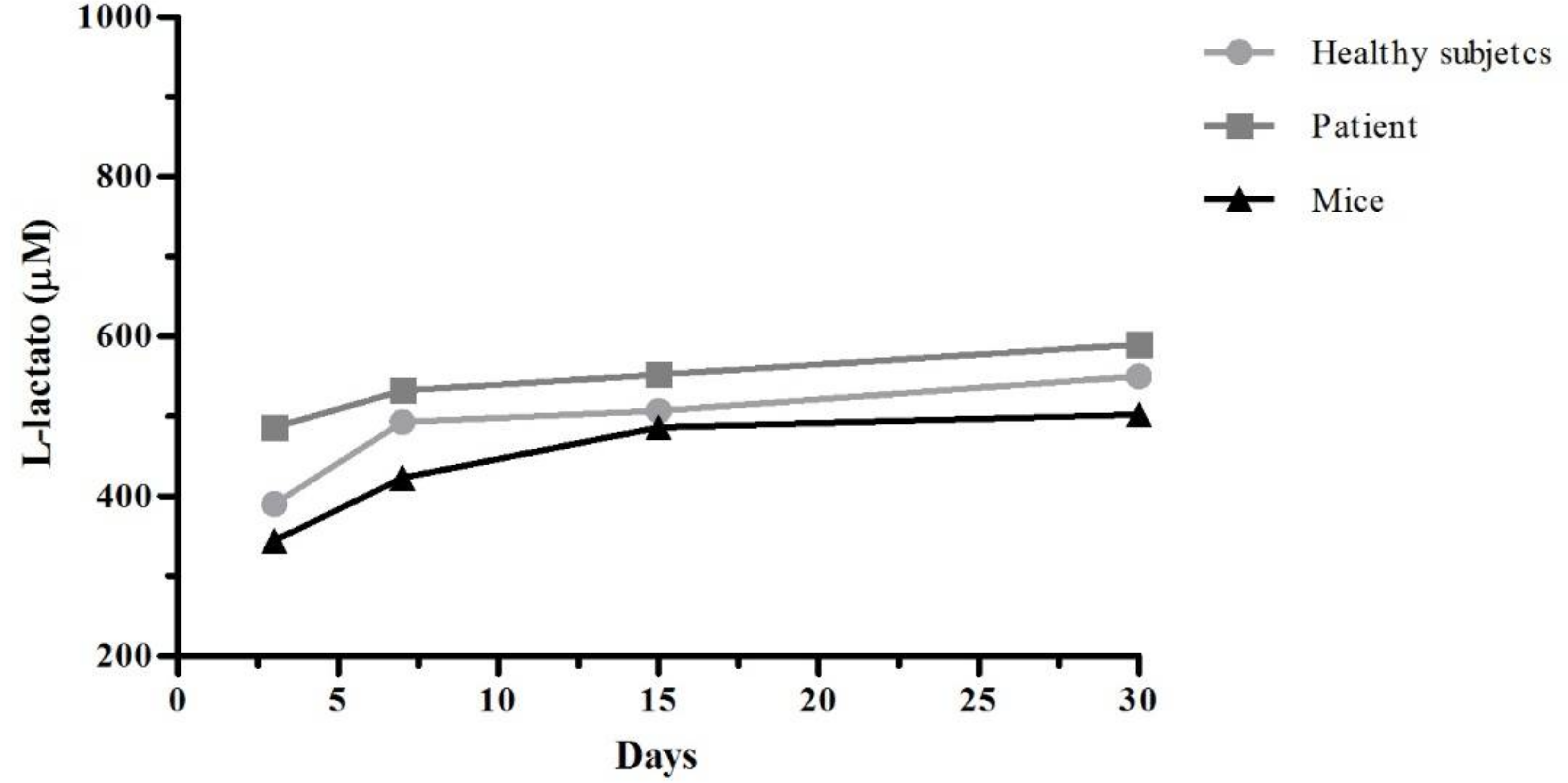
Lactate dehydrogenase (LDH) is a cytoplasmic enzyme that is present in the cells. When the cytoplasmic membranes suffer damage, the release of LDH is produce. The quantification of this enzyme in cell culture supernatants it can be used as an indicator of cell death. After 3, 7, 15, and 30 days of culture, the supernatants were recovered and was work according with specifications of L-Lactate Assay Kit (Cayman Chemical Company, Ann Arbor, MI, USA). In the assay, lactate dehydrogenase catalyzes the oxidation of lactate to pyruvate, along with the concomitant reduction of NAD+ to NADH. The measure of formation of NADH was done at 585 nm.
2.10. Statistical Analysis
Statistical analyses were performed using GraphPad Prism 6 program. The results are presented as the mean ± SD. Comparisons were made using one-way ANOVA followed by a Tukey test contrast with a confidence interval of 95%. * p < 0.05, ** p < 0.01 and *** p < 0.001.
4. Discussion
Keeping ex vivo podocytes alive will help to clarify the pathophysiological mechanisms involved in glomerular diseases to design new strategies for diagnosis and treatment. Because of this, the development of biocompatible materials that simulate the environmental conditions of specific cells could be useful in future studies.
Our results indicate that swelling percentages of scaffolds CTS and PVA are of the order of 600%, decreasing with the increasing crosslinking type IV collagen and integrin α
3β
1. These results coincide with those of Caruapoma and Santiago [
18] who report that scaffolds of CTS, PVA, and CTS/PVA present swelling percentages of the order of 600%, although they used 10 kilograys (kGy) of gamma radiation to obtain the scaffolds.
Costa et al. [
13] reported a decrease in the degradation percentage of scaffold CTS/PVA by increasing of glutaraldehyde (GA) as a crosslinker agent. These results and ours indicate that cross-linking of the polymer chains maintains the stability of the supports and reduce degradation; this is confirmed by the results obtained from FTIR, indicating polymeric crosslinking of type IV collagen and integrin α
3β
1 through the group O=C–N of Chitosan. These findings coincide with the results reported by Costa et al., where GA has higher affinity for amino groups (NH
2) of Chitosan in comparison to hydroxyl groups (OH) of PVA.
In patients with certain glomerular diseases, podocytes in urine have been detected, correlating with the loss of kidney function. Therefore, we obtained a higher number of podocytes in ISN patients (6000 cells) than HS (2000 cells). These results are consistent with those published by Petermann et al. [
19], reporting the presence of viable podocytes in a urine a model of passive Heymann nephritis induced in rats (2500 cells), in contrast to controls where no presence of viable podocytes was observed. Our results are also consistent with those of Nakamura et al. [
20] that detected viable podocytes (0.8–5.3 cells/mL) in the urine of 50 diabetic patients and an absence of viable podocytes in the 10 HS.
Evaluating the scaffold in culture, it was observed by IF that a higher number of podocytes adhered to the scaffolds (HS = 2, ISN = 3.5 and mouse = 2), in contrast to the control culture (HS = 1.75, ISN = 2.5 and 1.5 = mouse). These results agree with those of Chuang et al. [
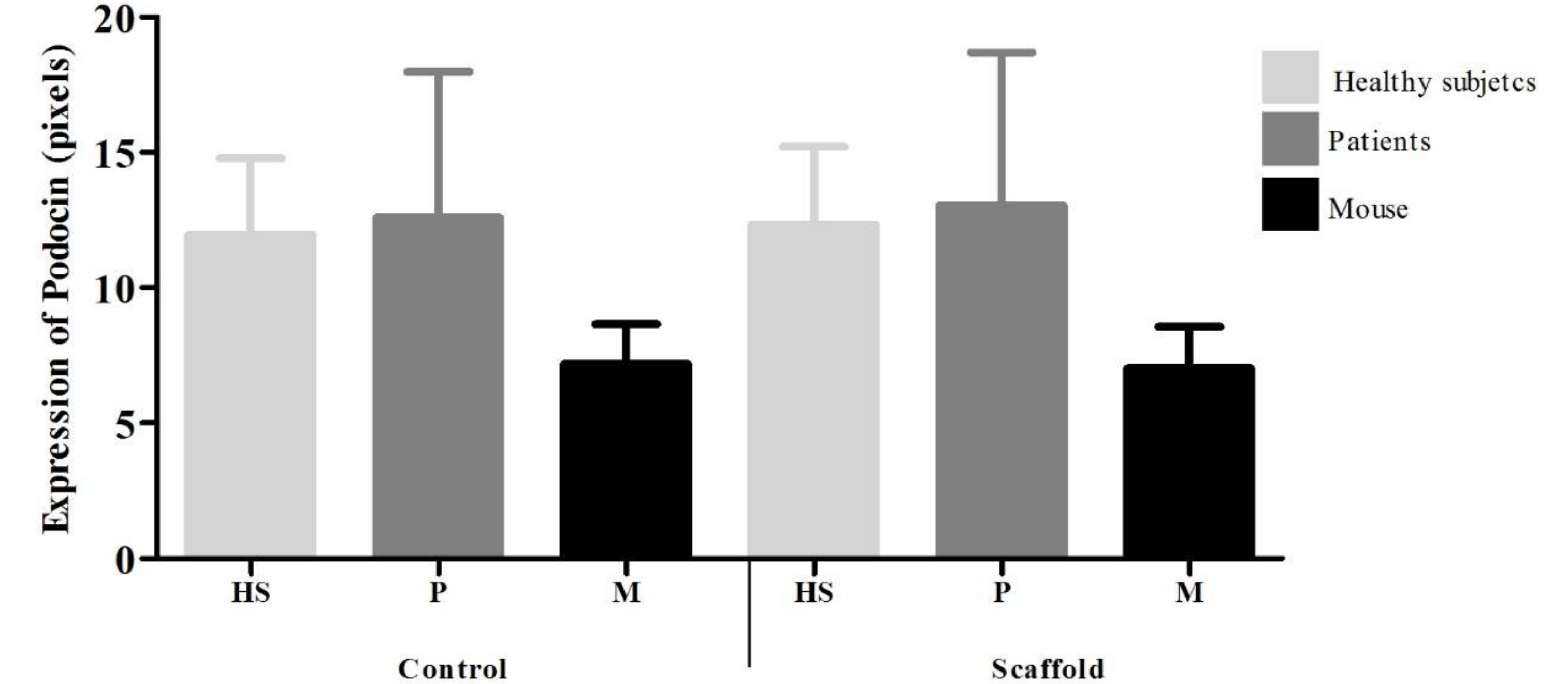
12] who reported greater adherence of fibroblasts taken from skin (5 cells per field) on a support of CTS/PVA and presence of microvilli, in contrast to a support of PVA where fewer cells adhered (3 cells per field) and without presence of microvilli. Expression of podocalyxin in podocytes is observed homogeneously both on the scaffold (HS = 10.3, INS = 10.3 and mouse = 11.4) and control cultures (HS = 9.4, INS = 9.4 and mouse = 9.4) however the expression of CD80 is increased in podocytes grown on the scaffold (HS = 7, INS = 7.3 and mouse = 7.4) in contrast to the control culture (HS = 3.9, INS = 3.9 and mouse = 4.5). There are reports that have observed that biomaterials promote increase in the expression of costimulatory molecules (e.g., Ex. CD80, CD86, CD40) of primary culture cells [
21]. Recent data also indicate that podocyte cells, under certain circumstances, can be induced to express CD80 (B7.1) [
22]. So, we believe that CTS provides an active signaling pathway that leads to the expression of this receptor in podocytes. Cytotoxicity tests using cell cultures are the first step to determine the safety of biomaterials and identifying active compounds. Our results of cell viability show a greater LDH release in culture control in contrast with scaffold culture podocytes. These results agree those of Chuang et al. [
12] where CTS/PVA scaffold cultured fibroblast viability was of 70–80%, compared to 30% viability on PVA alone; however, they determined cell viability by Thiazolyl Blue Tetrazolium Bromide MTT technique; and they measured cell viability on day 4 of culture.