Compound K Inhibits the Lipopolysaccharide-Induced Inflammatory Responses in Raw 264.7 Cell Line and Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Cytotoxicity Assay
2.4. Nitric Oxide Determination
2.5. Enzyme-Linked Immunosorbent Assays
2.6. RNA Isolation and Reverse-Transcription Polymerase Chain Reaction
2.7. Western Blot Analysis
2.8. Zebrafish Care and Experimental Protocol
2.9. Nitric Oxide Determination in Zebrafish
2.10. Statistical Analysis
3. Results
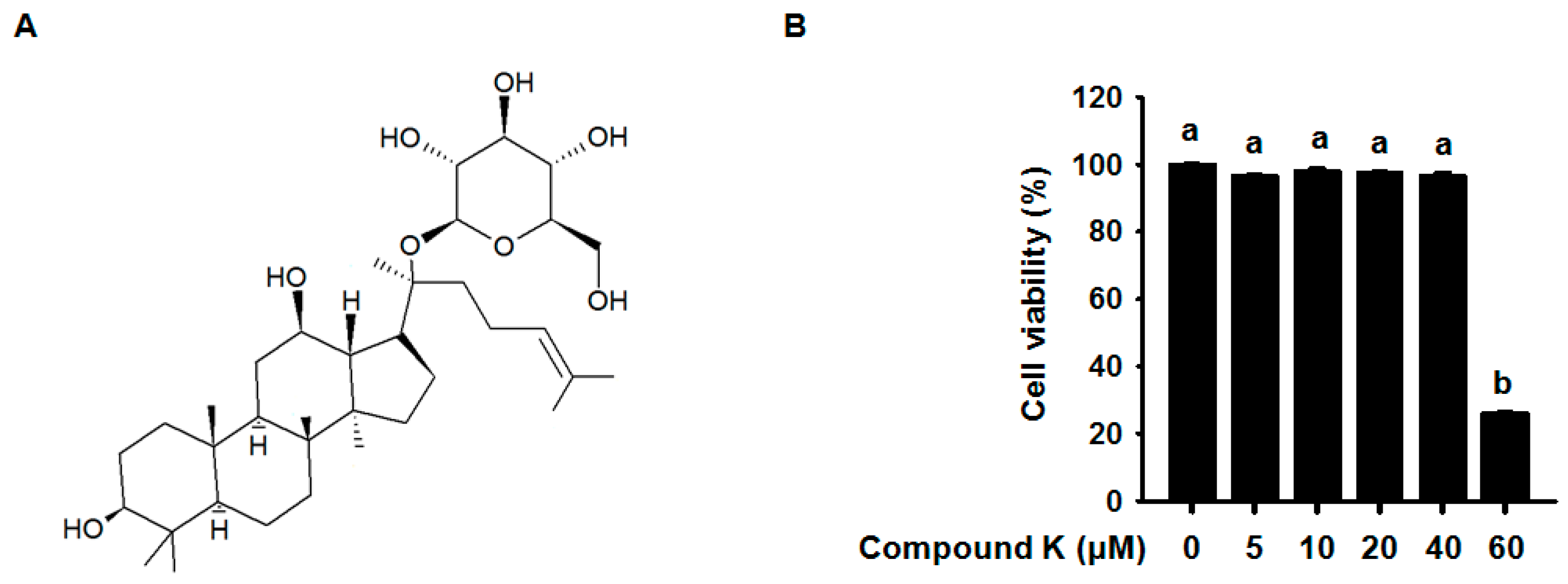
3.1. Effect of Compound K on RAW264.7 Cell Viability
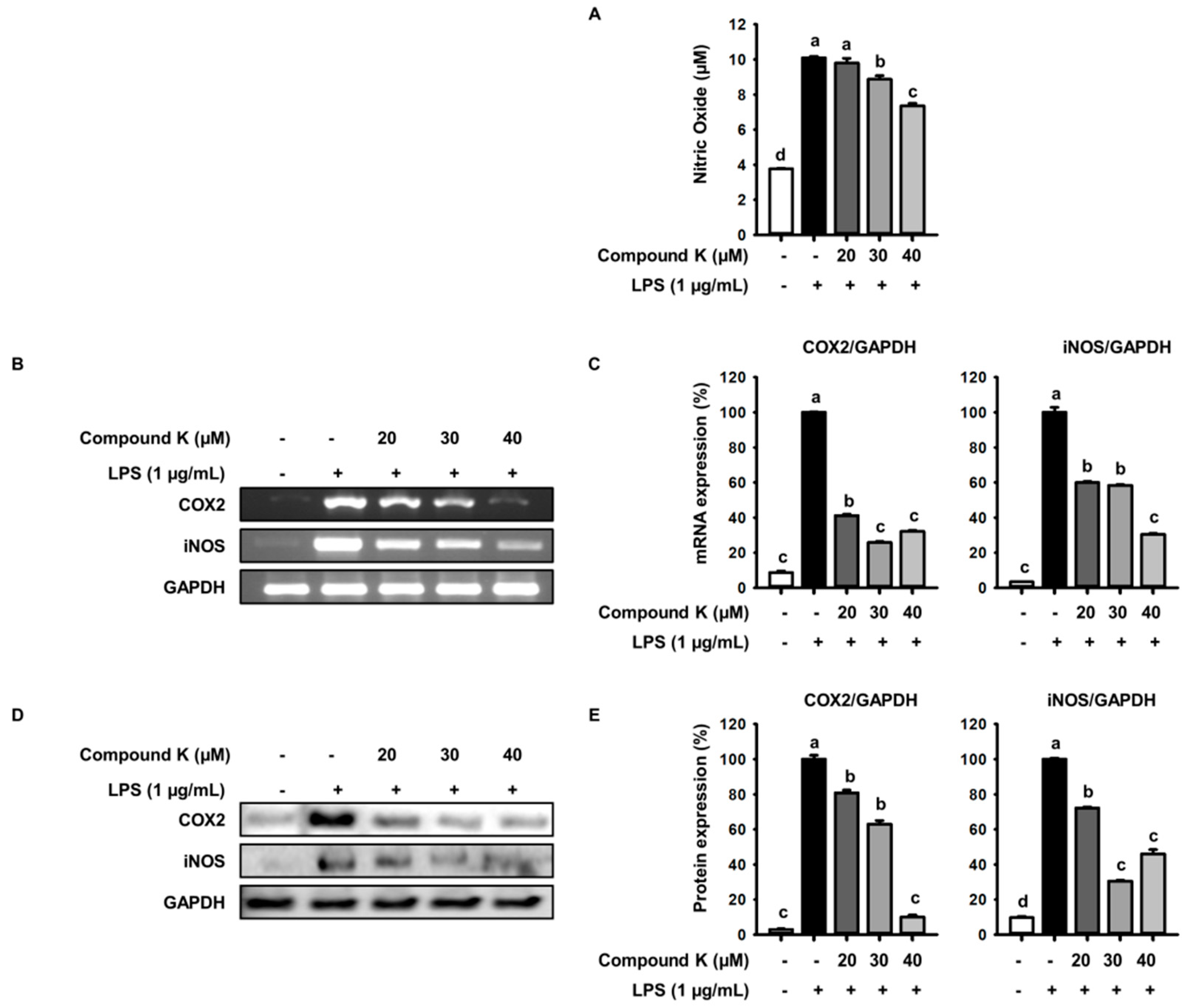
3.2. Inhibitory Effect of Compound K on LPS-Induced NO Production and iNOS and COX-2 Expression Levels
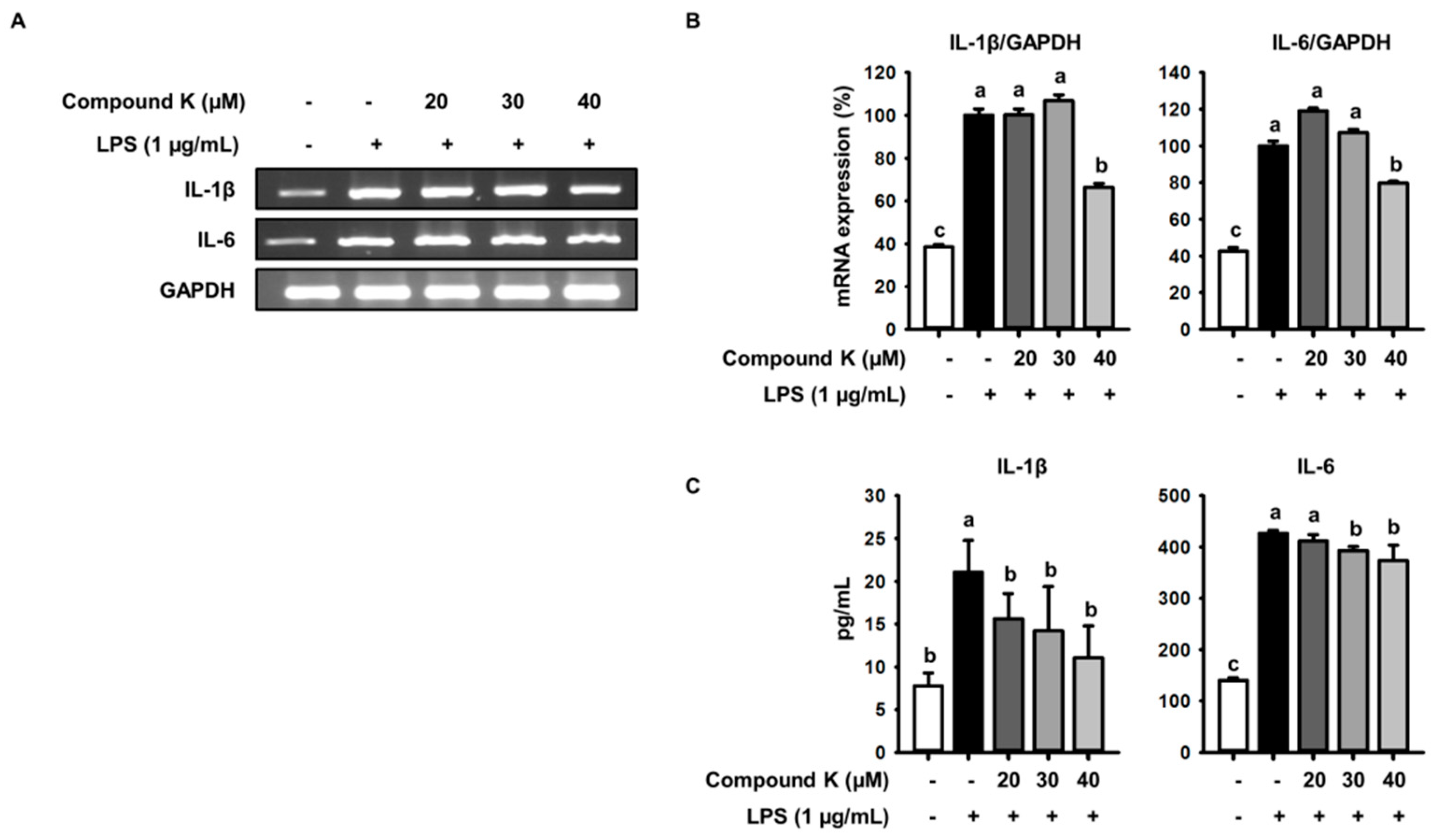
3.3. Inhibitory Effect of Compound K on LPS-Induced IL-1β and IL-6 Release and Their Expressions
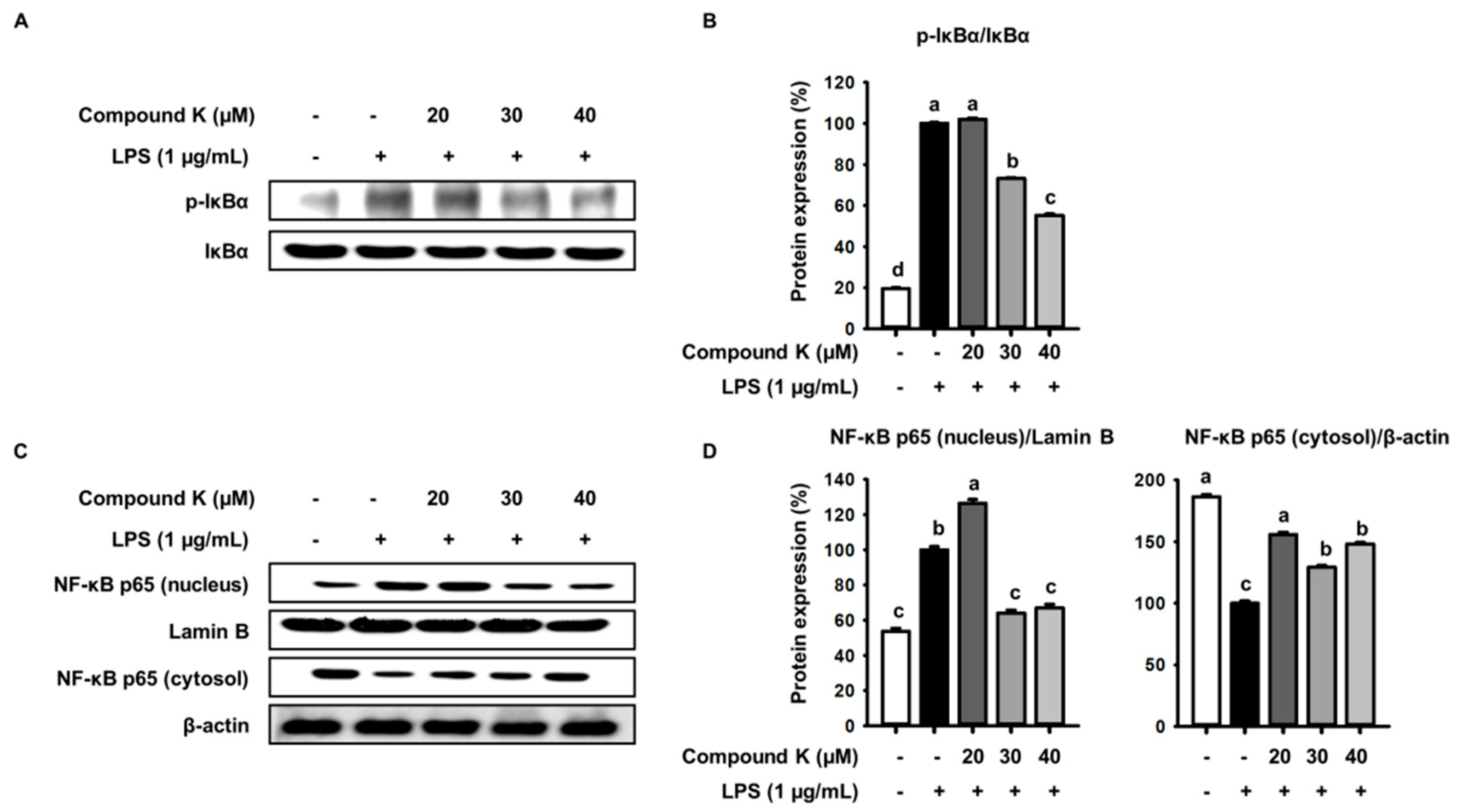
3.4. Inhibitory Effect of Compound K on LPS-Induced IκB Phosphorylation and NF-κB Translocation
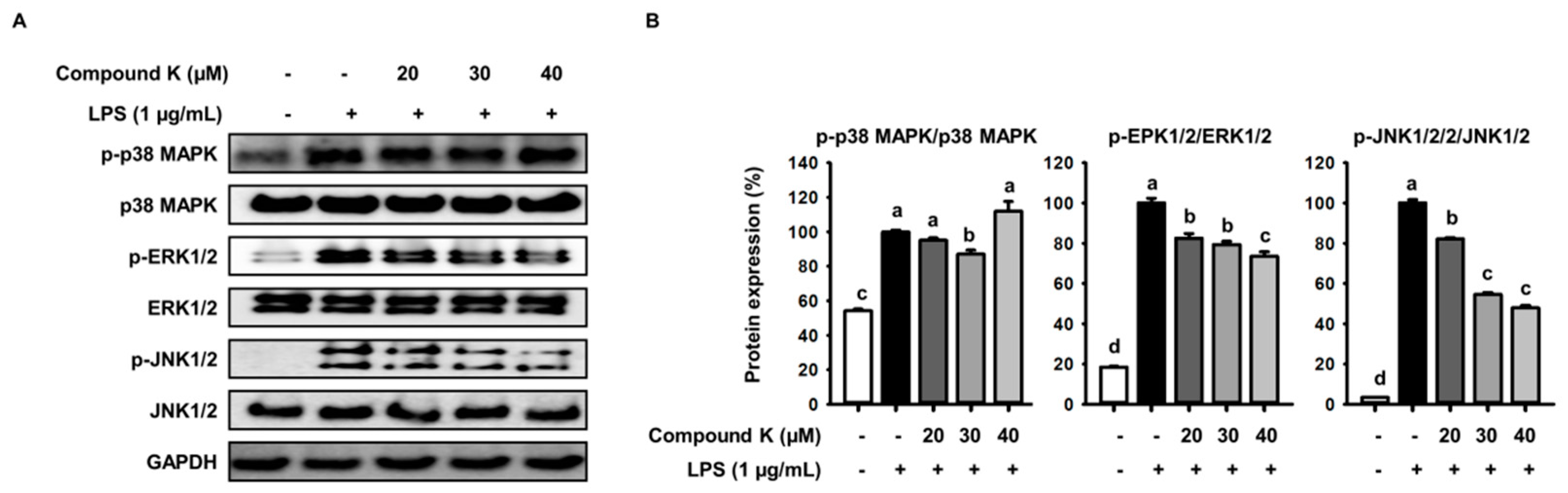
3.5. Inhibitory Effect of Compound K on LPS-Induced MAPKs Activation
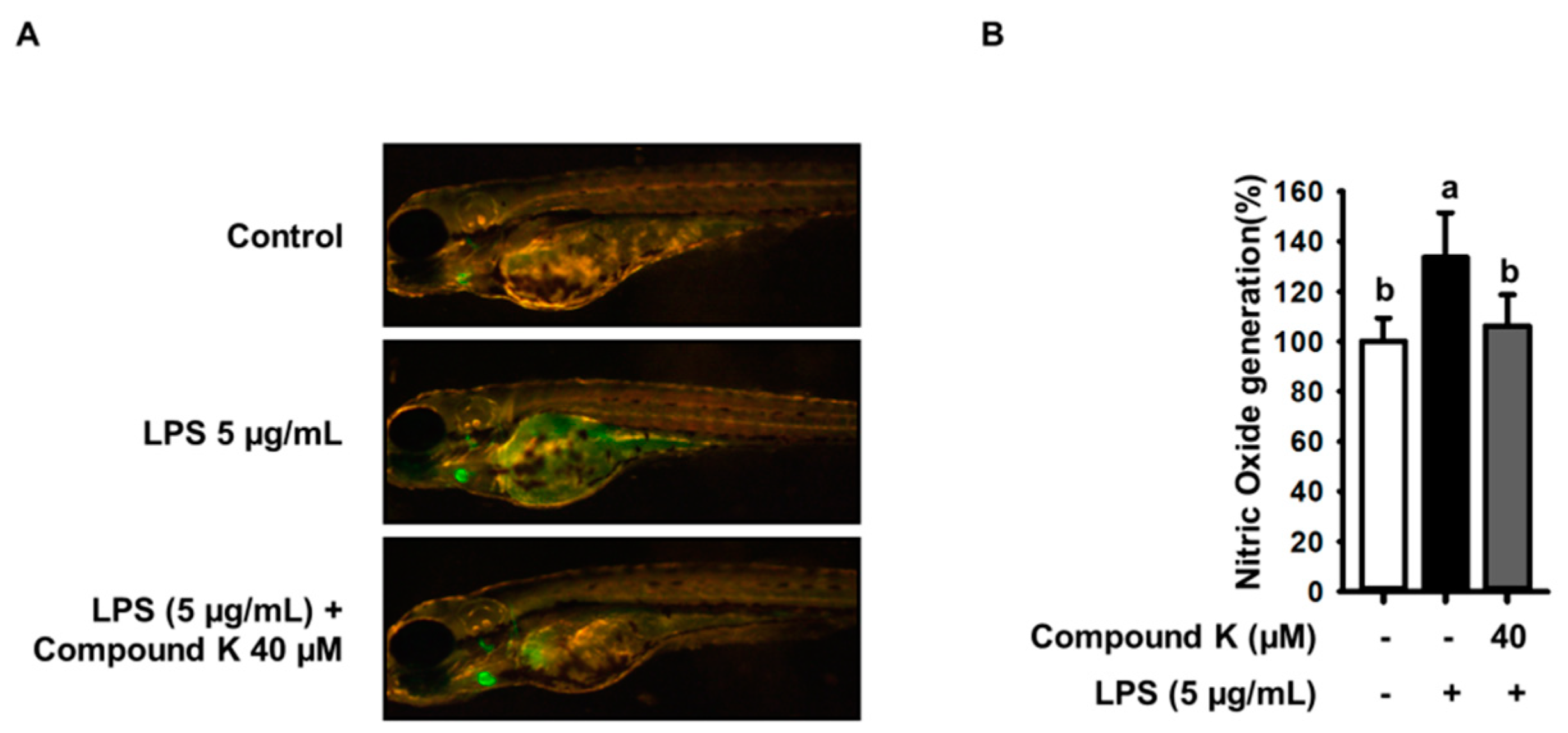
3.6. Inhibitory Effect of Compound K on LPS-Induced NO Productions in Experimental Zebrafish Model
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Bautista, L.E. Inflammation, endothelial dysfunction, and the risk of high blood pressure: Epidemiologic and biological evidence. J. Hum. Hypertens. 2003, 17, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.L.; Cosseau, C.; Gardy, J.L.; Hancock, R.E. Complexities of targeting innate immunity to treat infection. Trends Immunol. 2007, 28, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Keating, S.E.; Maloney, G.M.; Moran, E.M.; Bowie, A.G. Irak-2 participates in multiple toll-like receptor signaling pathways to nfκb via activation of traf6 ubiquitination. J. Biol. Chem. 2007, 282, 33435–33443. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A. Tlrs: Professor mechnikov, sit on your hat. Trends Immunol. 2004, 25, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Vanden Berghe, W.; Plaisance, S.; Boone, E.; De Bosscher, K.; Schmitz, M.L.; Fiers, W.; Haegeman, G. P38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-kappab p65 transactivation mediated by tumor necrosis factor. J. Biol. Chem. 1998, 273, 3285–3290. [Google Scholar] [CrossRef] [PubMed]
- Von Knethen, A.; Callsen, D.; Brüne, B. Nf-κb and ap-1 activation by nitric oxide attenuated apoptotic cell death in raw 264.7 macrophages. Mol. Biol. Cell 1999, 10, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Driever, W.; Solnica-Krezel, L.; Schier, A.; Neuhauss, S.; Malicki, J.; Stemple, D.; Stainier, D.; Zwartkruis, F.; Abdelilah, S.; Rangini, Z. A genetic screen for mutations affecting embryogenesis in zebrafish. Development 1996, 123, 37–46. [Google Scholar] [PubMed]
- Kimmel, C.B. Genetics and early development of zebrafish. Trends Genet. 1989, 5, 283–288. [Google Scholar] [CrossRef]
- Trede, N.S.; Zapata, A.; Zon, L.I. Fishing for lymphoid genes. Trends Immunol. 2001, 22, 302–307. [Google Scholar] [CrossRef]
- Correa, R.G.; Tergaonkar, V.; Ng, J.K.; Dubova, I.; Izpisua-Belmonte, J.C.; Verma, I.M. Characterization of nf-kappa b/i kappa b proteins in zebra fish and their involvement in notochord development. Mol. Cell. Biol. 2004, 24, 5257–5268. [Google Scholar] [CrossRef] [PubMed]
- Hermann, A.C.; Kim, C.H. Effects of arsenic on zebrafish innate immune system. Mar. Biotechnol. (N. Y.) 2005, 7, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Rojo, I.; de Ilarduya, O.M.; Estonba, A.; Pardo, M.A. Innate immune gene expression in individual zebrafish after listonella anguillarum inoculation. Fish Shellfish Immunol. 2007, 23, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Fujita, M.; Itokawa, H.; Tanaka, O.; Ishii, T. Studies on the constituents of japanese and chinese crude drugs. Xi. Panaxadiol, a sapogenin of ginseng roots. Chem. Pharm. Bull. 1963, 11, 759–761. [Google Scholar] [CrossRef] [PubMed]
- Park, E.H.; Yum, J.; Ku, K.B.; Kim, H.M.; Kang, Y.M.; Kim, J.C.; Kim, J.A.; Kang, Y.K.; Seo, S.H. Red ginseng-containing diet helps to protect mice and ferrets from the lethal infection by highly pathogenic h5n1 influenza virus. J. Ginseng Res. 2014, 38, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Kim, M.; Ryu, J.; Choi, C. Ginsenosides compound k and rh(2) inhibit tumor necrosis factor-alpha-induced activation of the nf-kappab and jnk pathways in human astroglial cells. Neurosci. Lett. 2007, 421, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kim, J.-H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J. Ginseng Res. 2014, 38, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Akao, T.; Kida, H.; Kanaoka, M.; Hattori, M.; Kobashi, K. Intestinal bacterial hydrolysis is required for the appearance of compound k in rat plasma after oral administration of ginsenoside rb1 from panax ginseng. J. Pharm. Pharmacol. 1998, 50, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Han, G.C.; Ko, S.K.; Sung, J.H.; Chung, S.H. Compound k enhances insulin secretion with beneficial metabolic effects in db/db mice. J. Agric. Food Chem. 2007, 55, 10641–10648. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, C.; Murakami, K.; Hasegawa, H.; Murata, J.; Saiki, I. An intestinal bacterial metabolite of ginseng protopanaxadiol saponins has the ability to induce apoptosis in tumor cells. Biochem. Biophys. Res. Commun. 1998, 246, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, E.; Kim, D.; Lee, J.; Yoo, J.; Koh, B. Studies on absorption, distribution and metabolism of ginseng in humans after oral administration. J. Ethnopharmacol. 2009, 122, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Akao, T.; Kanaoka, M.; Kobashi, K. Appearance of compound k, a major metabolite of ginsenoside rb1 by intestinal bacteria, in rat plasma after oral administration--measurement of compound k by enzyme immunoassay. Biol. Pharm. Bull. 1998, 21, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.J.; Choi, H.S.; Yoon, K.Y.; Lee, O.H.; Kim, K.J.; Lee, B.Y. Oleuropein suppresses lps-induced inflammatory responses in raw 264.7 cell and zebrafish. J. Agric. Food Chem. 2015, 63, 2098–2105. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Ko, J.-Y.; Kim, E.-A.; Hwang, E.-K.; Park, C.S.; Lee, J.-S.; Kim, C.-Y.; Lee, H.-S.; Kang, H.-K.; Cha, S.-H. Identification and large isolation of an anti-inflammatory compound from an edible brown seaweed, undariopsis peterseniana, and evaluation on its anti-inflammatory effect in in vitro and in vivo zebrafish. J. Appl. Phycol. 2017, 29, 1587–1596. [Google Scholar] [CrossRef]
- Lee, S.-H.; Ko, C.-I.; Jee, Y.; Jeong, Y.; Kim, M.; Kim, J.-S.; Jeon, Y.-J. Anti-inflammatory effect of fucoidan extracted from ecklonia cava in zebrafish model. Carbohydr. Polym. 2013, 92, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Yang, H.-W.; Ding, Y.; Wang, Y.; Jeon, Y.-J.; Moon, S.-H.; Jeon, B.-T.; Sung, S.-H. Anti-inflammatory effects of enzymatic hydrolysates of velvet antler in raw 264.7 cells in vitro and zebrafish model. EXCLI J. 2015, 14, 1122–1132. [Google Scholar] [PubMed]
- Arroyo, P.L.; Hatch-Pigott, V.; Mower, H.; Cooney, R. Mutagenicity of nitric oxide and its inhibition by antioxidants. Mutat. Res. Lett. 1992, 281, 193–202. [Google Scholar] [CrossRef]
- Miwa, M.; Stuehr, D.J.; Marletta, M.A.; Wishnok, J.S.; Tannenbaum, S.R. Nitromation of amines by stimulated macrophages. Carcinogenesis 1987, 8, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Wink, D.A.; Kasprzak, K.S.; Maragos, C.M.; Elespuru, R.K.; Misra, M.; Dunams, T.M.; Cebula, T.A.; Koch, W.H.; Andrews, A.; Allen, J.S. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science 1991, 1001–1003. [Google Scholar] [CrossRef]
- Hernández, G.L.; Volpert, O.V.; Íñiguez, M.A.; Lorenzo, E.; Martínez-Martínez, S.; Grau, R.; Fresno, M.; Redondo, J.M. Selective inhibition of vascular endothelial growth factor–mediated angiogenesis by cyclosporin a: Roles of the nuclear factor of activated t cells and cyclooxygenase 2. J. Exp. Med. 2001, 193, 607–620. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007, 7, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Molloy, R.; Mannick, J.; Rodrick, M. Cytokines, sepsis and immunomodulation. Br. J. Surg. 1993, 80, 289–297. [Google Scholar] [CrossRef] [PubMed]
- West, M.A.; Seatter, S.C.; Bellingham, J.; Clair, L. Mechanisms of reprogrammed macrophage endotoxin signal transduction after lipopolysaccharide pretreatment. Surgery 1995, 118, 220–228. [Google Scholar] [CrossRef]
- Van Snick, J. Interleukin-6: An overview. Annu. Rev. Immunol. 1990, 8, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Dixit, V.; Mak, T.W. Nf-κb signaling: Many roads lead to madrid. Cell 2002, 111, 615–619. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. Nf-kappab: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Eisen, J.S. Zebrafish make a big splash. Cell 1996, 87, 969–977. [Google Scholar] [CrossRef]
- Fishman, M.C. Zebrafish genetics: The enigma of arrival. Proc. Natl. Acad. Sci. USA 1999, 96, 10554–10556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Den Hertog, J. Chemical genetics: Drug screens in zebrafish. Biosci. Rep. 2005, 25, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Pichler, F.B.; Laurenson, S.; Williams, L.C.; Dodd, A.; Copp, B.R.; Love, D.R. Chemical discovery and global gene expression analysis in zebrafish. Nat. Biotechnol. 2003, 21, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Lepiller, S.; Laurens, V.; Bouchot, A.; Herbomel, P.; Solary, E.; Chluba, J. Imaging of nitric oxide in a living vertebrate using a diaminofluorescein probe. Free Radic. Biol. Med. 2007, 43, 619–627. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, S.-J.; Choi, J.; Lee, J.-S.; Choi, H.-S.; Yoon, K.-Y.; Hwang, J.-H.; Kim, K.J.; Lee, B.-Y. Compound K Inhibits the Lipopolysaccharide-Induced Inflammatory Responses in Raw 264.7 Cell Line and Zebrafish. Appl. Sci. 2018, 8, 924. https://doi.org/10.3390/app8060924
Ryu S-J, Choi J, Lee J-S, Choi H-S, Yoon K-Y, Hwang J-H, Kim KJ, Lee B-Y. Compound K Inhibits the Lipopolysaccharide-Induced Inflammatory Responses in Raw 264.7 Cell Line and Zebrafish. Applied Sciences. 2018; 8(6):924. https://doi.org/10.3390/app8060924
Chicago/Turabian StyleRyu, Su-Jung, Jia Choi, Jong-Seok Lee, Hyeon-Son Choi, Kye-Yoon Yoon, Ji-Hyun Hwang, Kui Jin Kim, and Boo-Yong Lee. 2018. "Compound K Inhibits the Lipopolysaccharide-Induced Inflammatory Responses in Raw 264.7 Cell Line and Zebrafish" Applied Sciences 8, no. 6: 924. https://doi.org/10.3390/app8060924



