A State-of-the-Art Review of Nanoparticles Application in Petroleum with a Focus on Enhanced Oil Recovery
Abstract
:1. Introduction
2. Applications of Nanotechnology in the Petroleum Industry
2.1. Exploration and Reservoir Characterization
2.2. Drilling and Completion
2.2.1. Mud Additives
2.2.2. Cement Additives
2.2.3. Drilling Tools
2.3. Production and Stimulation
2.3.1. Production Problem Solution
2.3.2. Stimulation Process Improvement
2.4. Refining
3. Nanoparticles in EOR
3.1. Silica-Based
3.2. Aluminum Oxides
3.3. Iron Oxide
3.4. Nickel Oxide
3.5. Titanium Oxide
3.6. Zinc Oxide
3.7. Zirconium Oxide
3.8. Polymer and Polymer-Coated
3.9. CNT
4. Parameters Affecting Nano-EOR
4.1. NP Size
4.2. NP Concentration
4.3. Salinity
4.4. Reservoir Temperature
4.5. Rock Wettability
5. Mechanism
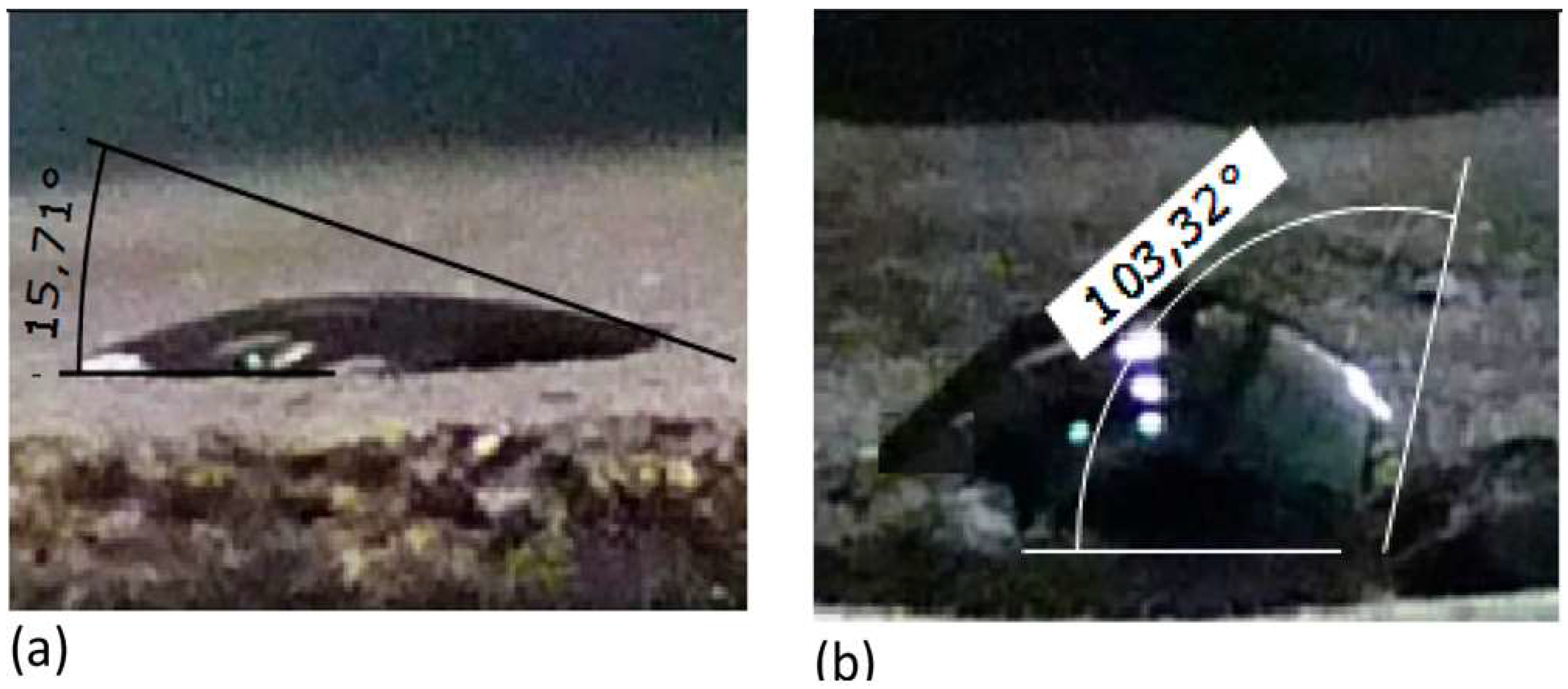
5.1. Wettability Alteration
5.2. Interfacial Tension Reduction
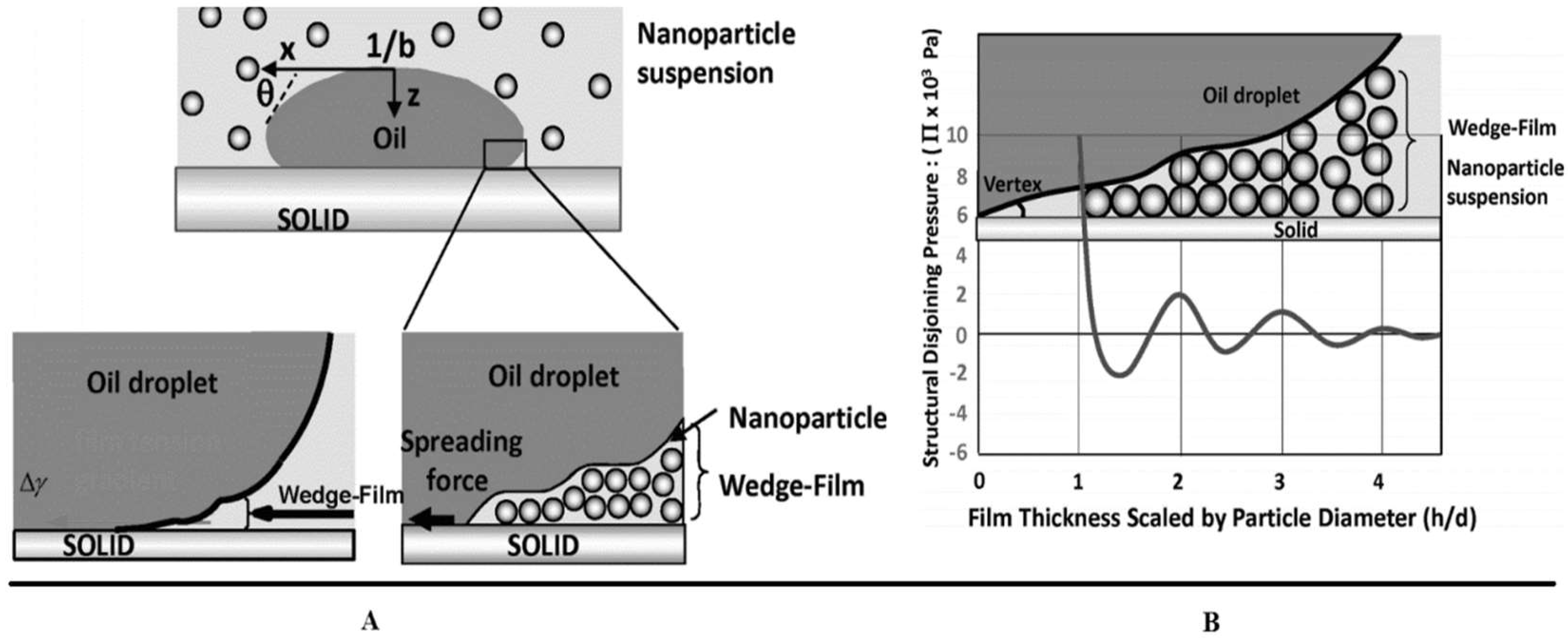
5.3. Disjoining Pressure
5.4. Viscosity Control
6. Challenges and Future Research
- Stable nanofluids for larger-scale application are not difficult. As NPs tend to agglomerate under reservoir conditions (high temperature, pressure, and salinity), the preparation of homogenous NP suspensions is still a challenge.
- Several mechanisms for improving recovery in nano-EOR have been proposed. However, these mechanisms and the influencing parameters are not thoroughly understood. The interactions between NPs and rock surfaces need to be studied further.
- A fundamental understanding of nano-EOR is limited. The lack of theoretical and numerical investigation into nano-EOR seems to be one of the reasons for this. Most numerical studies refer to the colloidal particle model, which does not perfectly describe NP behavior. The models are also limited to the physical interactions of NPs and have not yet considered the chemical interactions.
- Due to the fast advancement of nanotechnology, health and safety studies are lagging behind. One of the important challenges for NP development is the lack of knowledge on the effects of various types of nanoparticles on the human body. Due to nano-scale size, NPs can be easily inhaled by humans and potentially deposited inside the lungs.
- In light of these challenges, future research on nano-EOR should focus on the following aspects:
- Research on the preparation of stable nanofluids in large quantities needs to be performed in consideration of economic aspects.
- Several NPs have proved to exhibit different characteristics and mechanisms for EOR. However, no research has yet been proposed on the application of mixtures of nanofluids. New applications and better performance may be possible by enabling two or more nanofluids to function together.
- Pilot projects of nano-EOR need to be carried out. These projects will improve the understanding of the nano-EOR processes in real-world conditions. Optimization studies on nano-EOR parameters are also recommended to improve recovery and cost efficiency.
- Experimental work needs to be done to confirm the adsorption and desorption behavior during NP flow. Reversible and irreversible adsorption need be studied further, both experimentally and theoretically, because they will affect the deliverability of NPs to the target zone.
- Integrated research on the health and safety of NPs must be done to prevent the risk to humans and the environment.
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kong, X.; Ohadi, M. Applications of micro and nano technologies in the oil and gas industry-an overview of the recent progress. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, UAE, 1–4 Nomverber 2010; Society of Petroleum Engineering: Richardson, TX, USA, 2010. [Google Scholar]
- Hendraningrat, L.; Torsæter, O. Metal oxide-based nanoparticles: Revealing their potential to enhance oil recovery in different wettability systems. Appl. Nanosci. 2015, 5, 181–199. [Google Scholar] [CrossRef]
- Ju, B.; Fan, T. Enhanced oil recovery by flooding with hydrophilic nanoparticles. China Particuol. 2006, 4, 41–46. [Google Scholar] [CrossRef]
- Li, S. An experimental Investigation of Enhanced Oil Recovery Mechanisms in Nanofluid Injection Process. PhD Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2016. [Google Scholar]
- Fletcher, A.; Davis, J. How EOR can be transformed by nanotechnology. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010; Society of Petroleum Engineering: Richardson, TX, USA, 2010. [Google Scholar]
- Guo, K.; Li, H.; Yu, Z. In-situ heavy and extra-heavy oil recovery: A review. Fuel 2016, 185, 886–902. [Google Scholar] [CrossRef]
- Zhang, T.; Roberts, M.; Bryant, S.L.; Huh, C. Foams and emulsions stabilized with nanoparticles for potential conformance control applications. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 20–22 April 2009; Society of Petroleum Engineers: Richardson, TX, USA, 2010. [Google Scholar]
- Espinoza, D.A.; Caldelas, F.M.; Johnston, K.P.; Bryant, S.L.; Huh, C. Nanoparticle-stabilized supercritical CO2 foams for potential mobility control applications. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA,, 24–28 April 2010; Society of Petroleum Engineers: Richardson, TX, USA, 2010. [Google Scholar]
- Ju, B.; Dai, S.; Luan, Z.; Zhu, T.; Su, X.; Qiu, X. A study of wettability and permeability change caused by adsorption of nanometer structured polysilicon on the surface of porous media. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Melbourne, Australia, 8–10 October 2002; Society of Petroleum Engineers: Richardson, TX, USA, 2002. [Google Scholar]
- Guo, K.; Li, H.; Yu, Z. Metallic nanoparticles for enhanced heavy oil recovery: Promises and challenges. Energy Procedia 2015, 75, 2068–2073. [Google Scholar] [CrossRef]
- Hendraningrat, L.; Engeset, B.; Suwarno, S.; Torsæter, O. Improved oil recovery by nanofluids flooding: An experimental study. In Proceedings of the SPE Kuwait International Petroleum Conference and Exhibition, Kuwait City, Kuwait, 10–12 December 2012; Society of Petroleum Engineers: Richardson, TX, USA, 2012. [Google Scholar]
- Onyekonwu, M.O.; Ogolo, N.A. Investigting the use of nanoparticles in enhancing oil recovery. In Proceedings of the Nigeria Annual International Conference and Exhibition, Tinapa-Calabar, Nigeria, 31 July–7 August 2010; Society of Petroleum Engineers: Richardson, TX, USA, 2010. [Google Scholar]
- Sun, X.; Zhang, Y.; Chen, G.; Gai, Z. Application of nanoparticles in enhanced oil recovery: A critical review of recent progress. Energies 2017, 10, 345. [Google Scholar] [CrossRef]
- Cheraghian, G.; Hendraningrat, L. A review on applications of nanotechnology in the enhanced oil recovery part A: Effects of nanoparticles on interfacial tension. Int. Nano Lett. 2016, 6, 129–138. [Google Scholar] [CrossRef]
- Cheraghian, G.; Hendraningrat, L. A review on applications of nanotechnology in the enhanced oil recovery part B: Effects of nanoparticles on flooding. Int. Nano Lett. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Guo, K.; Zhang, Y.H.; Shi, Q.; Yu, Z.X. The effect of carbon-supported nickel nanoparticles in the reduction of carboxylic acids for in situ upgrading of heavy crude oil. Energy Fuels 2017, 31, 6045–6055. [Google Scholar] [CrossRef]
- Guo, K.; Gu, M.F.; Yu, Z.X. Carbon nanocatalysts for aquathermolysis of heavy crude oil: Insights into thiophene hydrodesulfurization. Energy Technol. 2017, 5, 1228–1234. [Google Scholar] [CrossRef]
- Guo, K.; Hansen, V.F.; Li, H.L.; Yu, Z.X. Monodispersed nickel and cobalt nanoparticles in desulfurization of thiophene for in-situ upgrading of heavy crude oil. Fuel 2018, 211, 697–703. [Google Scholar] [CrossRef]
- Zhang, T. Modeling of Nanoparticle Transport in Porous Media. PhD Thesis, University of Texas Austin, Austin, TX, USA, 2012. [Google Scholar]
- Song, Y.; Marcus, C. Hyperpolarized silicon nanoparticles: Reinventing oil exploration. Presented at the Schlumberger Seminar, Schlumberger, College Station, TX, USA, 29–31 January 2007. [Google Scholar]
- Jahagirdar, S.R. Oil-microbe detection tool using nano optical fibers. In Proceedings of the SPE Western Regional and Pacific Section AAPG Joint Meeting, Bakersfield, CA, USA, 29 March–4 April 2008; Society of Petroleum Engineers: Richardson, TX, USA, 2008. [Google Scholar]
- Pratyush, S.; Sumit, B. Nano-Robots System and Methods for Well Logging and Borehole Measurements. U.S. Patent 20100242585A1, 12 November 2010. [Google Scholar]
- Li, J.; Meyyappan, M. Real Time Oil Reservoir Evaluation Using Nanotechnology. U.S. Patent 7875455B1, 25 January 2011. [Google Scholar]
- Al-Shehri, A.A.; Ellis, E.S.; Servin, J.M.F.; Kosynkin, D.V.; Kanj, M.Y.; Schmidt, H.K. Illuminating the reservoir: Magnetic nanomappers. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 10–13 March 2013; Society of Petroleum Engineers: Richardson, TX, USA, 2013. [Google Scholar]
- Rahmani, A.R.; Bryant, S.; Huh, C.; Athey, A.E.; Ahmadian, M.; Chen, J.; Wilt, M. Crosswell magnetic sensing of superparamagnetic nanoparticles for subsurface applications. SPE J. 2015, 20, 1067–1082. [Google Scholar] [CrossRef]
- Sharma, M.M.; Zhang, R.; Chenevert, M.E.; Ji, L.; Guo, Q.; Friedheim, J. A new family of nanoparticle based drilling fluids. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 8–10 October 2012; Society of Petroleum Engineers: Richardson, TX, USA, 2012. [Google Scholar]
- Cai, J.; Chenevert, M.E.; Sharma, M.M.; Friedheim, J.E. Decreasing water invasion into atoka shale using nonmodified silica nanoparticles. SPE Drill. Complet. 2012, 27, 103–112. [Google Scholar] [CrossRef]
- Chakraborty, S.; Agrawal, G.; DiGiovanni, A.; Scott, D.E. The trick is the surface-Functionalized nanodiamond PDC technology. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, Noordwijk, The Netherlands, 12–14 June 2012; Society of Petroleum Engineers: Richardson, TX, USA, 2012. [Google Scholar]
- Santra, A.K.; Boul, P.; Pang, X. Influence of nanomaterials in oilwell cement hydration and mechanical properties. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, Noordwijk, The Netherlands, 12–14 June 2012; Society of Petroleum Engineers: Richardson, TX, USA, 2012. [Google Scholar]
- Gurluk, M.R.; Wang, G.; Nasr-El-Din, H.A.; Crews, J.B. The effect of different brine solutions on the viscosity of VES micelles. In Proceedings of the SPE European Formation Damage Conference & Exhibition, Noordwijk, The Netherlands, 5–7 June 2013; Society of Petroleum Engineers: Richardson, TX, USA, 2013. [Google Scholar]
- Murtaza, M.; Rahman, M.; Al-Majed, A. Mechanical and microstructural studies of nanoclay based oil well cement mix under high pressure and temperature application. In Proceedings of the International Petroleum Technology Conference, Bangkok, Thailand, 14–16 November 2016; International Petroleum Technology Conference: Richardson, TX, USA, 2016. [Google Scholar]
- Khan, W.; Rahman, M.; Mahmoud, M.; Sarmah, P. MWCNT for enhancing mechanical properties of oil well cement for HPHT applications. In Proceedings of the SPE/IADC Middle East Drilling Technology Conference and Exhibition, Abu Dhabi, UAE, 26–28 January 2016; Society of Petroleum Engineers: Richardson, TX, USA, 2016. [Google Scholar]
- Sun, X.; Wu, Q.; Zhang, J.; Qing, Y.; Wu, Y.; Lee, S. Rheology, Curing temperature and mechanical performance of oil well cement: Combined effect of cellulose nanofibers and graphene nano-platelets. Mater. Des. 2017, 114, 92–101. [Google Scholar] [CrossRef]
- Crews, J.B.; Huang, T.; Wood, W.R. The future of fracturing-fluid technology and rates of hydrocarbon recovery. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 21–24 September 2008; Society of Petroleum Engineers: Richardson, TX, USA, 2008. [Google Scholar]
- Bhatia, K.H.; Chacko, L.P. Ni-Fe nanoparticle: An innovative approach for recovery of hydrates. In Proceedings of the Brasil Offshore, Macaé, Brazil, 14–17 June 2011; Society of Petroleum Engineers: Richardson, TX, USA, 2011. [Google Scholar]
- Crews, J.B.; Gomaa, A.M. Nanoparticle associated surfactant micellar fluids: An alternative to crosslinked polymer systems. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, Noordwijk, The Netherlands, 12–14 June 2012; Society of Petroleum Engineers: Richardson, TX, USA, 2012. [Google Scholar]
- Li, L.; Al-Muntasheri, G.A.; Liang, F. Nanomaterials-enhanced high-temperature fracturing fluids prepared with untreated seawater. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dubai, UAE, 26–28 September 2016; Society of Petroleum Engineers: Richardson, TX, USA, 2016. [Google Scholar]
- Suleimanov, B.; Ismailov, F.; Veliyev, E. Nanofluid for enhanced oil recovery. J. Petrol. Sci. Eng. 2011, 78, 431–437. [Google Scholar] [CrossRef]
- Mohajeri, A.; Rashidi, A.; Jozani, K.J.; Khorami, P.; Amini, B.; Parviz, D.; Kalbasi, M. Hydrodesulphurization Nanocatalyst, Its Use and A Process for Its Production. U.S. Patent 20100167915A1, 22 January 2010. [Google Scholar]
- Hansen, L.P.; Ramasse, Q.M.; Kisielowski, C.; Brorson, M.; Johnson, E.; Topsøe, H.; Helveg, S. Atomic-scale edge structures on industrial-style MoS2 nanocatalysts. Angew. Chem. Int. Ed. 2011, 50, 10153–10156. [Google Scholar] [CrossRef] [PubMed]
- Sotto, A.; Boromand, A.; Balta, S.; Kim, J.; Van der Bruggen, B. Doping of polyethersulfone nanofiltration membranes: Antifouling effect observed at ultralow concentrations of TiO2 nanoparticles. J. Mater. Chem. 2011, 21, 10311–10320. [Google Scholar] [CrossRef]
- Mohammadi, M.; Akbari, M.; Fakhroueian, Z.; Bahramian, A.; Azin, R.; Arya, S. Inhibition of asphaltene precipitation by TiO2, SiO2, and ZrO2 nanofluids. Energy Fuels 2011, 25, 3150–3156. [Google Scholar] [CrossRef]
- Ko, S.; Prigiobbe, V.; Huh, C.; Bryant, S.L.; Bennetzen, M.V.; Mogensen, K. Accelerated oil droplet separation from produced water using magnetic nanoparticles. In Proceedings of the SPE Annual Technical Conference and Exhibition, Amsterdam, The Netherlands, 27–29 October 2014; Society of Petroleum Engineers: Richardson, TX, USA, 2014. [Google Scholar]
- Patiño, J.E.; Cortés, F.B. Nanocatalysts for Hydrocracking and Methods of Their Use. U.S. Patent 9339796B2, 17 May 2016. [Google Scholar]
- Bera, A.; Belhaj, H. Application of nanotechnology by means of nanoparticles and nanodispersions in oil recovery-a comprehensive review. J. Nat. Gas Sci. Eng. 2016, 34, 1284–1309. [Google Scholar] [CrossRef]
- Kapusta, S.D.; Balzano, L.; te Riele, P. Nanotechnology applications in oil and gas exploration and production. In Proceedings of the International Petroleum Technology Conference, Bangkok, Thailand, 15–17 November 2011; International Petroleum Technology Conference: Richardson, TX, USA, 2011. [Google Scholar]
- Bennetzen, M.V.; Mogensen, K. Novel applications of nanoparticles for future enhanced oil recovery. In Proceedings of the International Petroleum Technology Conference, Kuala Lumpur, Malaysia, 10–12 December 2014; International Petroleum Technology Conference: Richardson, TX, USA, 2014. [Google Scholar]
- Schröder, L.; Lowery, T.J.; Hilty, C.; Wemmer, D.E.; Pines, A. Molecular imaging using a targeted magnetic resonance hyperpolarized biosensor. Science 2006, 314, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, M.; Chan, H.; Ross, B.; Bhattacharya, P.; Marcus, C.M. In vivo magnetic resonance imaging of hyperpolarized silicon particles. Nat. Nanotechnol. 2013, 8, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jin, X.; Ding, B. Application of nanotechnology in petroleum exploration and development. Petrol. Explor. Dev. 2016, 43, 1107–1115. [Google Scholar] [CrossRef]
- Amanullah, M.; Al-Tahini, A. Nano-technology-its significance in smart fluid development for oil and gas field application. In Proceedings of the SPE Saudi Arabia Section Technical Symposium, Al-Khobar, Saudi Arabia, 9–11 May 2009; Society of Petroleum Engineers: Richardson, TX, USA, 2009. [Google Scholar]
- Apaleke, A.S.; Al-Majed, A.A.; Hossain, M.E. Drilling fluid: State of the art and future trend. In Proceedings of the North Africa Technical Conference and Exhibition, Cairo, Egypt, 20–22 February 2012; Society of Petroleum Engineers: Richardson, TX, USA, 2012. [Google Scholar]
- Amanullah, M.; AlArfaj, M.K.; Al-abdullatif, Z.A. Preliminary test results of nano-based drilling fluids for oil and gas field application. In Proceedings of the SPE/IADC Drilling Conference and Exhibition, Amsterdam, The Netherlands, 1–3 March 2011; Society of Petroleum Engineers: Richardson, TX, USA, 2011. [Google Scholar]
- Hoelscher, K.P.; De Stefano, G.; Riley, M.; Young, S. Application of nanotechnology in drilling fluids. In Proceedings of the SPE international oilfield nanotechnology conference and exhibition, Noordwijk, The Netherlands, 12–14 June 2012; Society of Petroleum Engineers: Richardson, TX, USA, 2012. [Google Scholar]
- Srivatsa, J.T.; Ziaja, M.B. An experimental investigation on use of nanoparticles as fluid loss additives in a surfactant-polymer based drilling fluids. In Proceedings of the International Petroleum Technology Conference, Bangkok, Thailand, 15–17 November 2011. [Google Scholar]
- Aderibigbe, A.A.; Cheng, K.; Heidari, Z.; Killough, J.E.; Fuss, T.; Stephens, W. Detection of propping agents in fractures using magnetic susceptibility measurements enhanced by magnetic nanoparticles. In Proceedings of the SPE Annual Technical Conference and Exhibition, Amsterdam, The Netherlands, 27–29 October 2014; Society of Petroleum Engineers: Richardson, TX, USA, 2014. [Google Scholar]
- Contreras, O.; Hareland, G.; Husein, M.; Nygaard, R.; Al-Saba, M. Application of in-house prepared nanoparticles as filtration control additive to reduce formation damage. In Proceedings of the SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 26–28 February 2014; Society of Petroleum Engineers: Richardson, TX, USA, 2014. [Google Scholar]
- Ho, C.Y.; Yusup, S.; Soon, C.V.; Arpin, M.T. Rheological behaviour of graphene nano-sheets in hydrogenated oil-based drilling fluid. Procedia Eng. 2016, 148, 49–56. [Google Scholar] [CrossRef]
- Quercia, G.; Brouwers, H.; Garnier, A.; Luke, K. Influence of olivine nano-silica on hydration and performance of oil-well cement slurries. Mater. Des. 2016, 96, 162–170. [Google Scholar] [CrossRef]
- Jafariesfad, N.; Gong, Y.; Geiker, M.R.; Skalle, P. Nano-sized mgo with engineered expansive property for oil well cement systems. In Proceedings of the SPE Bergen One Day Seminar, Grieghallen, Bergen, Norway, 20 April 2016; Society of Petroleum Engineers: Richardson, TX, USA, 2016. [Google Scholar]
- De la Roij, R.; Egyed, C.; Lips, J.-P. Nano-engineered oil well cement improves flexibility and increases compressive strength: A laboratory study. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, Noordwijk, The Netherlands, 12–14 June 2012; Society of Petroleum Engineers: Richardson, TX, USA, 2012. [Google Scholar]
- Sengupta, S.; Kumar, A. Nano-ceramic coatings-a means of enhancing bit life and reducing drill string trips. In Proceedings of the IPTC, Beijing, China, 26–28 March 2013; International Petroleum Technology Conference: Richardson, TX, USA, 2013. [Google Scholar]
- Mueller, H.; Herold, C.-P.; Bongardt, F.; Herzog, N.; von Tapavicza, S. Lubricants for Drilling Fluids. U.S. Patent 6806235B1, 19 October 2004. [Google Scholar]
- Moh’d Husein, M.; Hareland, G. Drilling Fluids with Nano and Granular Particles and Their Use for Wellbore Strengthening. U.S. Patent 20150126415A1, 20 March 2018. [Google Scholar]
- Roddy, C.W.; Covington, R.L. Cement Compositions and Methods Utilizing Nano-Clay. U.S. Patent 9512346B2, 6 December 2016. [Google Scholar]
- Roddy, C.W. Cement Compositions and Methods Utilizing Nano-Hydraulic Cement. U.S. Patent 8603952B2, 10 December 2013. [Google Scholar]
- Kuhn, K.-D. Bone Cement Mixture and X-ray Contrast Medium as Well as Method for Their Preparation. U.S. Patent 20040029996A1, 15 August 2006. [Google Scholar]
- Wu, J.; Lei, Q.; Xiong, C.; Cao, G.; Zhang, J.; Li, J.; Fang, J.; Tan, J.; Ai, T.; Li, N.; et al. A nano-particle foam unloading agent applied in unloading liquid of deep gas well. Pet. Explor. Dev. 2016, 43, 695–700. [Google Scholar] [CrossRef]
- Sun, Z.; Jing, G.; Tu, Z. Effect of modified nano-silica/EVA on flow behavior and wax crystallization of model oils with different wax content. J. Dispers. Sci. Technol. 2018, 39, 71–76. [Google Scholar] [CrossRef]
- Davidson, A.; Huh, C.; Bryant, S.L. Focused magnetic heating utilizing superparamagnetic nanoparticles for improved oil production applications. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, Noordwijk, The Netherlands, 12–14 June 2012; Society of Petroleum Engineers: Richardson, TX, USA, 2012. [Google Scholar]
- Gavrielatos, E.; Mohan, R.; Shoham, O. Effect of intermediate wettability nanoparticles on oil-water emulsion stability. J. Petrol. Sci. Eng. 2017, 152, 664–674. [Google Scholar] [CrossRef]
- Singh, R.; Panthi, K.; Mohanty, K.K. Microencapsulation of acids by nanoparticles for acid treatment of shales. Energy Fuels 2017, 31, 11755–11764. [Google Scholar] [CrossRef]
- Huang, T.; Crews, J.B. Nanotechnology applications in viscoelastic surfactant stimulation fluids. SPE Prod. Oper. 2008, 23, 512–517. [Google Scholar] [CrossRef]
- Barati, R.; Johnson, S.J.; McCool, S.; Green, D.W.; Willhite, G.P.; Liang, J.T. Fracturing fluid cleanup by controlled release of enzymes from polyelectrolyte complex nanoparticles. J. Appl. Polym. Sci. 2011, 121, 1292–1298. [Google Scholar] [CrossRef]
- Ghahfarokhi, R.B. Nano-Proppants for Fracture Conductivity. U.S. Patent 20160355727A1, 8 December 2016. [Google Scholar]
- Al-Muntasheri, G.; Liang, F.; Ow, H.; Cox, J.; Poitzsch, M.E. High Temperature Fracturing Fluids with Nano-Crosslinkers. U.S. Patent 9862878B2, 9 January 2018. [Google Scholar]
- Ying, J.Y.; Sun, T. Research needs assessment on nanostructured catalysts. J. Electroceram. 1997, 1, 219–238. [Google Scholar] [CrossRef]
- Kisielowski, C.; Ramasse, Q.M.; Hansen, L.P.; Brorson, M.; Carlsson, A.; Molenbroek, A.M.; Topsøe, H.; Helveg, S. Imaging MoS2 nanocatalysts with single-atom sensitivity. Angew. Chem. Int. Ed. 2010, 49, 2708–2710. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.R.; Lara, L.S.D.; Tonetto, B.C. Stability and mobility of functionalized silica nanoparticles for enhanced oil recovery applications. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, Noordwijk, The Netherlands, 12–14 June 2012; Society of Petroleum Engineers: Richardson, TX, USA, 2012. [Google Scholar]
- Nazari Moghaddam, R.; Bahramian, A.; Fakhroueian, Z.; Karimi, A.; Arya, S. Comparative study of using nanoparticles for enhanced oil recovery: Wettability alteration of carbonate rocks. Energy Fuels 2015, 29, 2111–2119. [Google Scholar] [CrossRef]
- Haroun, M.R.; Alhassan, S.; Ansari, A.A.; Al Kindy, N.A.M.; Abou Sayed, N.; Kareem, A.; Ali, B.; Sarma, H.K. Smart nano-eor process for abu dhabi carbonate reservoirs. In Proceedings of the Abu Dhabi International Petroleum Conference and Exhibition, Abu Dhabi, UAE, 11–14 November 2012; Society of Petroleum Engineers: Richardson, TX, USA, 2012. [Google Scholar]
- Shahrabadi, A.; Bagherzadeh, H.; Roostaie, A.; Golghanddashti, H. Experimental investigation of hlp nanofluid potential to enhance oil recovery: A mechanistic approach. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, Noordwijk, The Netherlands, 12–14 June 2012; Society of Petroleum Engineers: Richardson, TX, USA, 2012. [Google Scholar]
- Maghzi, A.; Kharrat, R.; Mohebbi, A.; Ghazanfari, M.H. The impact of silica nanoparticles on the performance of polymer solution in presence of salts in polymer flooding for heavy oil recovery. Fuel 2014, 123, 123–132. [Google Scholar] [CrossRef]
- Hendraningrat, L.; Li, S.; Torsaeter, O. Enhancing oil recovery of low-permeability berea sandstone through optimised nanofluids concentration. In Proceedings of the SPE Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 2–4 July 2013; Society of Petroleum Engineers: Richardson, TX, USA, 2013. [Google Scholar]
- Ehtesabi, H.; Ahadian, M.M.; Taghikhani, V. Enhanced heavy oil recovery using TiO2 nanoparticles: Investigation of deposition during transport in core plug. Energy Fuels 2014, 29, 1–8. [Google Scholar] [CrossRef]
- Joonaki, E.; Ghanaatian, S. The application of nanofluids for enhanced oil recovery: Effects on interfacial tension and coreflooding process. Petrol. Sci. Technol. 2014, 32, 2599–2607. [Google Scholar] [CrossRef]
- Hendraningrat, L.; Torsaeter, O. Unlocking the potential of metal oxides nanoparticles to enhance the oil recovery. In Proceedings of the Offshore Technology Conference-Asia, Kuala Lumpur, Malaysia, 25–28 March 2014. [Google Scholar]
- Sharma, T.; Kumar, G.S.; Sangwai, J.S. Comparative effectiveness of production performance of pickering emulsion stabilized by nanoparticle-surfactant-polymer over surfactant–polymer (sp) flooding for enhanced oil recoveryfor brownfield reservoir. J. Petrol. Sci. Eng. 2015, 129, 221–232. [Google Scholar] [CrossRef]
- El-diasty, A.I.; Aly, A.M. Understanding the mechanism of nanoparticles applications in enhanced oil recovery. In Proceedings of the SPE North Africa Technical Conference and Exhibition, Cairo, Egypt, 14–16 September 2015; Society of Petroleum Engineers: Richardson, TX, USA, 2015. [Google Scholar]
- Cheraghian, G. Effects of nanoparticles on wettability: A review on applications of nanotechnology in the enhanced oil recovery. Int. J. Nano Dimens. 2015, 6, 443–452. [Google Scholar]
- Cheraghian, G. Effects of titanium dioxide nanoparticles on the efficiency of surfactant flooding of heavy oil in a glass micromodel. Petrol. Sci. Technol. 2016, 34, 260–267. [Google Scholar] [CrossRef]
- Alnarabiji, M.S.; Yahya, N.; Shafie, A.; Solemani, H.; Chandran, K.; Hamid, S.B.A.; Azizi, K. The influence of hydrophobic multiwall carbon nanotubes concentration on enhanced oil recovery. Procedia Eng. 2016, 148, 1137–1140. [Google Scholar] [CrossRef]
- Jafarnezhad, M.; Giri, M.S.; Alizadeh, M. Impact of SnO2 nanoparticles on enhanced oil recovery from carbonate media. Energy Source Part A 2017, 39, 121–128. [Google Scholar] [CrossRef]
- Towler, B.F.; Lehr, H.L.; Austin, S.W.; Bowthorpe, B.; Feldman, J.H.; Forbis, S.K.; Germack, D.; Firouzi, M. Spontaneous imbibition experiments of enhanced oil recovery with surfactants and complex nano-fluids. J. Surfactants Deterg. 2017, 20, 367–377. [Google Scholar] [CrossRef]
- Zhang, T.; Davidson, D.; Bryant, S.L.; Huh, C. Nanoparticle-stabilized emulsions for applications in enhanced oil recovery. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010; Society of Petroleum Engineers: Tulsa, OK, USA, 2010. [Google Scholar]
- Binks, B.P.; Philip, J.; Rodrigues, J.A. Inversion of silica-stabilized emulsions induced by particle concentration. Langmuir 2005, 21, 3296–3302. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.P.; Rodrigues, J.A. Inversion of emulsions stabilized solely by ionizable nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Dickson, J.L.; Binks, B.P.; Johnston, K.P. Stabilization of carbon dioxide-in-water emulsions with silica nanoparticles. Langmuir 2004, 20, 7976–7983. [Google Scholar] [CrossRef] [PubMed]
- Adkins, S.S.; Gohil, D.; Dickson, J.L.; Webber, S.E.; Johnston, K.P. Water-in-carbon dioxide emulsions stabilized with hydrophobic silica particles. Phys. Chem. Chem. Phys. 2007, 9, 6333–6343. [Google Scholar] [CrossRef] [PubMed]
- Son, H.; Kim, H.; Lee, G.; Kim, J.; Sung, W. Enhanced oil recovery using nanoparticle-stabilized oil/water emulsions. Korean J. Chem. Eng. 2013, 31, 338–342. [Google Scholar] [CrossRef]
- Maurya, N.K.; Mandal, A. Investigation of synergistic effect of nanoparticle and surfactant in macro emulsion based eor application in oil reservoirs. Chem. Eng. Res. Des. 2018, 132, 370–384. [Google Scholar] [CrossRef]
- Sharma, T.; Suresh Kumar, G.; Sangwai, J.S. Enhanced oil recovery using oil-in-water (o/w) emulsion stabilized by nanoparticle, surfactant and polymer in the presence of nacl. Geosyst. Eng. 2014, 17, 195–205. [Google Scholar] [CrossRef]
- Guo, F.; Aryana, S. An experimental investigation of nanoparticle-stabilized CO2 foam used in enhanced oil recovery. Fuel 2016, 186, 430–442. [Google Scholar] [CrossRef]
- Yang, W.P.; Wang, T.F.; Fan, Z.X. Highly stable foam stabilized by alumina nanoparticles for eor: Effects of sodium cumenesulfonate and electrolyte concentrations. Energy Fuels 2017, 31, 9016–9025. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, N.; Li, Z.M.; Wang, Y.H. Nanoparticle-stabilized foam for mobility control in enhanced oil recovery. Energy Technol. 2016, 4, 1084–1096. [Google Scholar] [CrossRef]
- Yang, W.P.; Wang, T.F.; Fan, Z.X.; Miao, Q.; Deng, Z.Y.; Zhu, Y.Y. Foams stabilized by in situ-modified nanoparticles and anionic surfactants for enhanced oil recovery. Energy Fuels 2017, 31, 4721–4730. [Google Scholar] [CrossRef]
- Yu, J.; Khalil, M.; Liu, N.; Lee, R. Effect of particle hydrophobicity on CO2 foam generation and foam flow behavior in porous media. Fuel 2014, 126, 104–108. [Google Scholar] [CrossRef]
- Wang, P.; You, Q.; Han, L.; Deng, W.B.; Liu, Y.F.; Fang, J.C.; Gao, M.W.; Dai, C.L. Experimental study on the stabilization mechanisms of CO2 foams by hydrophilic silica nanoparticles. Energy Fuels 2018, 32, 3709–3715. [Google Scholar] [CrossRef]
- Hendraningrat, L. Unlocking the Potential of Hydrophilic Nanoparticles as Novel Enhanced Oil Recovery Method: An Experimental Investigation. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2015. [Google Scholar]
- Metin, C.O.; Baran, J.R.; Nguyen, Q.P. Adsorption of surface functionalized silica nanoparticles onto mineral surfaces and decane/water interface. J. Nanopart. Res. 2012, 14, 1246. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Z.; Yang, H.; Yang, G. The study of thermal stability of the SiO2 powders with high specific surface area. Mater. Chem. Phys. 1999, 57, 260–263. [Google Scholar] [CrossRef]
- Maghzi, A.; Mohebbi, A.; Kharrat, R.; Ghazanfari, M.H. Pore-scale monitoring of wettability alteration by silica nanoparticles during polymer flooding to heavy oil in a five-spot glass micromodel. Transp. Porous Media 2011, 87, 653–664. [Google Scholar] [CrossRef]
- Sharma, T.; Sangwai, J.S. Silica nanofluids in polyacrylamide with and without surfactant: Viscosity, surface tension, and interfacial tension with liquid paraffin. J. Petrol. Sci. Eng. 2017, 152, 575–585. [Google Scholar] [CrossRef]
- Sharma, T.; Iglauer, S.; Sangwai, J.S. Silica nanofluids in an oilfield polymer polyacrylamide: Interfacial properties, wettability alteration, and applications for chemical enhanced oil recovery. Ind. Eng. Chem. Res. 2016, 55, 12387–12397. [Google Scholar] [CrossRef]
- Johnston, K.P.; da Rocha, S.R. Colloids in supercritical fluids over the last 20 years and future directions. J. Supercrit. Fluids 2009, 47, 523–530. [Google Scholar] [CrossRef]
- Enick, R.M.; Olsen, D.K.; Ammer, J.R.; Schuller, W. Mobility and conformance control for CO2 EOR via thickeners, foams, and gels–a literature review of 40 years of research and pilot tests. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012; Society of Petroleum Engineers: Richardson, TX, USA, 2012. [Google Scholar]
- AttarHamed, F.; Zoveidavianpoor, M.; Jalilavi, M. The incorporation of silica nanoparticle and alpha olefin sulphonate in aqueous CO2 foam: Investigation of foaming behavior and synergistic effect. Petrol. Sci. Technol. 2014, 32, 2549–2558. [Google Scholar] [CrossRef]
- Kim, I.; Worthen, A.J.; Johnston, K.P.; DiCarlo, D.A.; Huh, C. Size-dependent properties of silica nanoparticles for pickering stabilization of emulsions and foams. J. Nanopart. Res. 2016, 18, 1–12. [Google Scholar] [CrossRef]
- Hafshejani, L.D.; Tangsir, S.; Koponen, H.; Riikonen, J.; Karhunen, T.; Tapper, U.; Lehto, V.-P.; Moazed, H.; Naseri, A.A.; Hooshmand, A. Synthesis and characterization of Al2O3 nanoparticles by flame spray pyrolysis (FSP)-role of fe ions in the precursor. Powder Technol. 2016, 298, 42–49. [Google Scholar] [CrossRef]
- Chang, H.; Chang, Y.C. Fabrication of Al2O3 nanofluid by a plasma arc nanoparticles synthesis system. J. Mater. Process. Technol. 2008, 207, 193–199. [Google Scholar] [CrossRef]
- Tsuzuki, T.; McCormick, P.G. Mechanochemical synthesis of nanoparticles. J. Mater. Sci. 2004, 39, 5143–5146. [Google Scholar] [CrossRef]
- Ogolo, N.; Olafuyi, O.; Onyekonwu, M. Enhanced oil recovery using nanoparticles. In Proceedings of the SPE Saudi Arabia Section Technical Symposium and Exhibition, Al-Khobar, Al-Khobar, Saudi Arabia, 8–11 April 2012; Society of Petroleum Engineers: Richardson, TX, USA, 2012. [Google Scholar]
- Giraldo, J.; Benjumea, P.; Lopera, S.; Cortés, F.B.; Ruiz, M.A. Wettability alteration of sandstone cores by alumina-based nanofluids. Energy Fuels 2013, 27, 3659–3665. [Google Scholar] [CrossRef]
- Zaid, H.M.; Yahya, N.; Latiff, N.R.A. The effect of nanoparticles crystallite size on the recovery efficiency in dielectric nanofluid flooding. J. Nano Res. 2013, 21, 103–108. [Google Scholar] [CrossRef]
- Alomair, O.A.; Matar, K.M.; Alsaeed, Y.H. Experimental study of enhanced-heavy-oil recovery in berea sandstone cores by use of nanofluids applications. SPE Reserv. Eval. Eng. 2015, 18, 387–399. [Google Scholar] [CrossRef]
- Negin, C.; Ali, S.; Xie, Q. Application of nanotechnology for enhancing oil recovery–A review. Petroleum 2016, 2, 324–333. [Google Scholar] [CrossRef]
- Shekhawat, D.S.; Aggarwal, A.; Agarwal, S.; Imtiaz, M. Magnetic recovery-injecting newly designed magnetic fracturing fluid with applied magnetic field for EOR. In Proceedings of the SPE Asia Pacific Hydraulic Fracturing Conference, Beijing, China, 24–26 August 2016; Society of Petroleum Engineers: Richardson, TX, USA, 2016. [Google Scholar]
- Nassar, N.N.; Al-Jabari, M.E.; Husein, M.M. Removal of asphaltenes from heavy oil by nickel nano and micro particle adsorbents. In Proceedings of the IASTED International Conference, Crete, Greece, 29 September–1 October 2008; ACTA Press: Calgary, AB, Canada, 2008. [Google Scholar]
- Nwidee, L.; Al-Anssari, S.; Barifcani, A.; Sarmadivaleh, M.; Iglauer, S. Nanofluids for enhanced oil recovery processes: Wettability alteration using zirconium oxide. In Proceedings of the Offshore Technology Conference Asia, Kuala Lumpur, Malaysia, 22–25 March 2016; Society of Petroleum Engineers: Richardson, TX, USA, 2016. [Google Scholar]
- Ehtesabi, H.; Ahadian, M.M.; Taghikhani, V.; Ghazanfari, M.H. Enhanced heavy oil recovery in sandstone cores using TiO2 nanofluids. Energy Fuels 2013, 28, 423–430. [Google Scholar] [CrossRef]
- Cheraghian, G. Effect of nano titanium dioxide on heavy oil recovery during polymer flooding. Petrol. Sci. Technol. 2016, 34, 633–641. [Google Scholar] [CrossRef]
- Sedaghat, M.; Mohammadi, H.; Razmi, R. Application of SiO2 and TiO2 nano particles to enhance the efficiency of polymer-surfactant floods. Energ. Sources Part A 2016, 38, 22–28. [Google Scholar] [CrossRef]
- Tajmiri, M.; Mousavi, S.M.; Ehsani, M.R.; Roayaei, E.; Emadi, A. Wettability alteration of sandstone and carbonate rocks by using zno nanoparticles in heavy oil reservoirs. Iran. J. Oil Gas Sci. Technol. 2016, 4, 50–66. [Google Scholar]
- Latiff, N.R.A.; Yahya, N.; Zaid, H.M.; Demiral, B. Novel enhanced oil recovery method using dielectric zinc oxide nanoparticles activated by electromagnetic waves. In Proceedings of the National Postgraduate Conference (NPC), Kuala Lumpur, Malaysia, 19–20 September 2011. [Google Scholar]
- Adil, M.; Zaid, H.M.; Chuan, L.K.; Latiff, N.R.A. Effect of dispersion stability on electrorheology of water-based zno nanofluids. Energy Fuels 2016, 30, 6169–6177. [Google Scholar] [CrossRef]
- Karimi, A.; Fakhroueian, Z.; Bahramian, A.; Pour Khiabani, N.; Darabad, J.B.; Azin, R.; Arya, S. Wettability alteration in carbonates using zirconium oxide nanofluids: EOR implications. Energy Fuels 2012, 26, 1028–1036. [Google Scholar] [CrossRef]
- Moslan, M.S.; Sulaiman, W.; Rosli, W.; Ismail, A.R.; Jaafar, M.Z.; Ismail, I. Wettability alteration of dolomite rock using nanofluids for enhanced oil recovery. Mater. Sci. Forum 2016, 864, 194–198. [Google Scholar] [CrossRef]
- Esmaeilzadeh, P.; Hosseinpour, N.; Bahramian, A.; Fakhroueian, Z.; Arya, S. Effect of ZrO2 nanoparticles on the interfacial behavior of surfactant solutions at air-water and n-heptane-water interfaces. Fluid Phase Equilib. 2014, 361, 289–295. [Google Scholar] [CrossRef]
- Fakirov, S.; Bhattacharyya, D.; Panamoottil, S.M. Converting of bulk polymers into nanosized materials with controlled nanomorphology. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 777–793. [Google Scholar] [CrossRef]
- Rao, J.P.; Geckeler, K.E. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- ShamsiJazeyi, H.; Miller, C.A.; Wong, M.S.; Tour, J.M.; Verduzco, R. Polymer-coated nanoparticles for enhanced oil recovery. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Li, G.; Zhang, J.; Ding, B. Preparation of microgel nanospheres and their application in EOR. In Proceedings of the International Oil and Gas Conference and Exhibition in China, Beijing, China, 8–10 June 2010; Society of Petroleum Engineers: Richardson, TX, USA, 2010. [Google Scholar]
- Horikoshi, S.; Serpone, N. Introduction to nanoparticles. In Microwaves in Nanoparticle Synthesis: Fundamentals and Applications; Horikoshi, S., Serpone, N., Eds.; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Shen, M.; Resasco, D.E. Emulsions stabilized by carbon nanotube—Silica nanohybrids. Langmuir 2009, 25, 10843–10851. [Google Scholar] [CrossRef] [PubMed]
- Villamizar, L.C.; Lohateeraparp, P.; Harwell, J.H.; Resasco, D.E.; Shiau, B.J.B. Interfacially active swnt/silica nanohybrid used in enhanced oil recovery. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010; Society of Petroleum Engineers: Richardson, TX, USA, 2010. [Google Scholar]
- Kadhum, M.J.; Swatske, D.P.; Chen, C.; Resasco, D.E.; Harwell, J.H.; Shiau, B. Propagation of carbon nanotube hybrids through porous media for advancing oilfield technology. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 13–15 April 2015; Society of Petroleum Engineers: Richardson, TX, USA, 2015. [Google Scholar]
- AfzaliTabar, M.; Alaei, M.; Khojasteh, R.R.; Motiee, F.; Rashidi, A. Preference of multi-walled carbon nanotube (MWCNT) to single-walled carbon nanotube (SWCNT) and activated carbon for preparing silica nanohybrid pickering emulsion for chemical enhanced oil recovery (C-EOR). J. Solid State Chem. 2017, 245, 164–173. [Google Scholar] [CrossRef]
- Kondiparty, K.; Nikolov, A.; Wu, S.; Wasan, D. Wetting and spreading of nanofluids on solid surfaces driven by the structural disjoining pressure: Statics analysis and experiments. Langmuir 2011, 27, 3324–3335. [Google Scholar] [CrossRef] [PubMed]
- Mcelfresh, P.; Holcomb, D.; Ector, D. Application of nanofluid technology to improve recovery in oil and gas. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, Noordwijk, The Netherlands, 12–14 June 2012; Society of Petroleum Engineers: Richardson, TX, USA, 2012. [Google Scholar]
- Hendraningrat, L.; Li, S.; Torsæter, O. Effect of some parameters influencing enhanced oil recovery process using silica nanoparticles: An experimental investigation. In Proceedings of the SPE Reservoir Characterization and Simulation Conference and Exhibition, Abu Dhabi, UAE, 16–18 September 2013; Society of Petroleum Engineers: Richardson, TX, USA, 2013. [Google Scholar]
- Chengara, A.; Nikolov, A.D.; Wasan, D.T.; Trokhymchuk, A.; Henderson, D. Spreading of nanofluids driven by the structural disjoining pressure gradient. J. Colloid Interface Sci. 2004, 280, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Hendraningrat, L.; Li, S.; Torsæter, O. A coreflood investigation of nanofluid enhanced oil recovery. J. Petrol. Sci. Eng. 2013, 111, 128–138. [Google Scholar] [CrossRef]
- Somasundaran, P.; Agar, G. The zero point of charge of calcite. J. Colloid Interface Sci. 1967, 24, 433–440. [Google Scholar] [CrossRef]
- Worthen, A.J.; Tran, V.; Cornell, K.A.; Truskett, T.M.; Johnston, K.P. Steric stabilization of nanoparticles with grafted low molecular weight ligands in highly concentrated brines including divalent ions. Soft Matter 2016, 12, 2025–2039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Murphy, M.J.; Yu, H.; Bagaria, H.G.; Yoon, K.Y.; Nielson, B.M.; Bielawski, C.W.; Johnston, K.P.; Huh, C.; Bryant, S.L. Investigation of nanoparticle adsorption during transport in porous media. SPE J. 2014, 20, 667–677. [Google Scholar] [CrossRef]
- Kanj, M.Y.; Funk, J.J.; Al-Yousif, Z. Nanofluid coreflood experiments in the ARAB-D. In Proceedings of the SPE Saudi Arabia Section Technical Symposium, Al-Khobar, Saudi Arabia, 9–11 May 2009; Society of Petroleum Engineers: Richardson, TX, USA, 2009. [Google Scholar]
- Caldelas, F.M.; Murphy, M.; Huh, C.; Bryant, S.L. Factors governing distance of nanoparticle propagation in porous media. In Proceedings of the SPE Production and Operations Symposium, Oklahoma City, OK, USA, 27–29 March 2011; Society of Petroleum Engineers: Richardson, TX, USA, 2011. [Google Scholar]
- Owens, W.; Archer, D. The effect of rock wettability on oil-water relative permeability relationships. J. Petrol. Technol. 1971, 23, 873–878. [Google Scholar] [CrossRef]
- Morrow, N.R. Wettability and its effect on oil recovery. J. Petrol. Technol. 1990, 42, 476–484. [Google Scholar] [CrossRef]
- Lorenz, P.B.; Donaldson, E.C.; Thomas, R.D. Use of Centrifugal Measurements of Wettability to Predict Oil Recovery; US Department of the Interior, Report of Investigations; US Department of the Interior: Washington, DC, USA, 1974. [Google Scholar]
- Morrow, N.R. A review of the effects of initial saturation, pore structure and wettability on oil recovery by waterflooding. In Proceedings of the North Sea Oil and Gas Reservoirs, Trondheim, Norway, 2–4 December 1985; Graham and Trotman, Ltd.: London, UK, 1987. [Google Scholar]
- Khilar, K.C.; Fogler, H.S. Migrations of Fines in Porous Media; Fogler, H.S., Ed.; Springer Science & Business Media: Berlin, Germany, 1998; p. 12. [Google Scholar]
- Wang, Y.; Xu, H.; Yu, W.; Bai, B.; Song, X.; Zhang, J. Surfactant induced reservoir wettability alteration: Recent theoretical and experimental advances in enhanced oil recovery. Petrol. Sci. 2011, 8, 463–476. [Google Scholar] [CrossRef]
- Maerker, J.; Gale, W. Surfactant flood process design for loudon. SPE Reserv. Eng. 1992, 7, 36–44. [Google Scholar] [CrossRef]
- Hammond, P.S.; Unsal, E. Spontaneous imbibition of surfactant solution into an oil-wet capillary: Wettability restoration by surfactant-contaminant complexation. Langmuir 2011, 27, 4412–4429. [Google Scholar] [CrossRef] [PubMed]
- Al-Anssari, S.; Barifcani, A.; Wang, S.; Iglauer, S. Wettability alteration of oil-wet carbonate by silica nanofluid. J. Colloid Interface Sci. 2016, 461, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Hendraningrat, L.; Torsaeter, O. Understanding fluid-fluid and fluid-rock interactions in the presence of hydrophilic nanoparticles at various conditions. In Proceedings of the SPE Asia Pacific Oil & Gas Conference and Exhibition, Adelaide, Australia, 14–16 October 2014; Society of Petroleum Engineers: Richardson, TX, USA, 2014. [Google Scholar]
- Roustaei, A.; Bagherzadeh, H. Experimental investigation of SiO2 nanoparticles on enhanced oil recovery of carbonate reservoirs. J. Petrol. Explor. Prod. Technol. 2015, 5, 27–33. [Google Scholar] [CrossRef]
- Chatzis, I.; Morrow, N.R. Correlation of capillary number relationships for sandstone. SPE J. 1984, 24, 555–562. [Google Scholar] [CrossRef]
- Melrose, J. Role of capillary forces in detennining microscopic displacement efficiency for oil recovery by waterflooding. J. Can. Petrol. Technol. 1974, 13. [Google Scholar] [CrossRef]
- Munshi, A.; Singh, V.; Kumar, M.; Singh, J. Effect of nanoparticle size on sessile droplet contact angle. J. Appl. Phys. 2008, 103, 084315. [Google Scholar] [CrossRef]
- Roustaei, A.; Moghadasi, J.; Bagherzadeh, H.; Shahrabadi, A. An experimental investigation of polysilicon nanoparticles’ recovery efficiencies through changes in interfacial tension and wettability alteration. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, Noordwijk, The Netherlands, 12–14 June 2012; Society of Petroleum Engineers: Richardson, TX, USA, 2012. [Google Scholar]
- Moghadam, T.F.; Azizian, S. Effect of ZnO nanoparticles on the interfacial behavior of anionic surfactant at liquid/liquid interfaces. Colloids Surf. A 2014, 457, 333–339. [Google Scholar] [CrossRef]
- Bergeron, V. Forces and structure in thin liquid soap films. J. Phys. Condens. Matter 1999, 11, R215. [Google Scholar] [CrossRef]
- Derjaguin, B.; Churaev, N. Structural component of disjoining pressure. J. Colloid Interface Sci. 1974, 49, 249–255. [Google Scholar] [CrossRef]
- Basu, S.; Sharma, M.M. Measurement of critical disjoining pressure for dewetting of solid surfaces. J. Colloid Interface Sci. 1996, 181, 443–455. [Google Scholar] [CrossRef]
- Wasan, D.T.; Nikolov, A.D. Spreading of nanofluids on solids. Nature 2003, 423, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Wasan, D.; Nikolov, A.; Kondiparty, K. The wetting and spreading of nanofluids on solids: Role of the structural disjoining pressure. Curr. Opin. Colloid Interface Sci. 2011, 16, 344–349. [Google Scholar] [CrossRef]
- Piech, M.; Walz, J.Y. Direct measurement of depletion and structural forces in polydisperse, charged systems. J. Colloid. Interface Sci. 2002, 253, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dong, M. Optimum effective viscosity of polymer solution for improving heavy oil recovery. J. Petrol. Sci. Eng. 2009, 67, 155–158. [Google Scholar] [CrossRef]
- Sheng, J. Modern Chemical Enhanced Oil Recovery: Theory and Practice; Sheng, J., Ed.; Gulf Professional Publishing: Houston, TX, USA, 2010. [Google Scholar]
- Ramsden, D.; McKay, K. Degradation of polyacrylamide in aqueous solution induced by chemically generated hydroxyl radicals: Part i-Fenton’s reagent. Polym. Degrad. Stab. 1986, 14, 217–229. [Google Scholar] [CrossRef]
- Ramsden, D.; McKay, K. The degradation of polyacrylamide in aqueous solution induced by chemically generated hydroxyl radicals: Part ii-autoxidation of Fe2+. Polym. Degrad. Stab. 1986, 15, 15–31. [Google Scholar] [CrossRef]
- Zeyghami, M.; Kharrat, R.; Ghazanfari, M. Investigation of the applicability of nano silica particles as a thickening additive for polymer solutions applied in eor processes. Energy Sources Part A 2014, 36, 1315–1324. [Google Scholar] [CrossRef]
- Cheraghian, G.; Hemmati, M.; Bazgir, S.; Singh, M.R. Application of TiO2 and fumed silica nanoparticles and improve the performance of drilling fluids. AIP Conf. Proc. 2014, 1590, 266–270. [Google Scholar]
- Yousefvand, H.; Jafari, A. Enhanced oil recovery using polymer/nanosilica. Procedia Mater. Sci. 2015, 11, 565–570. [Google Scholar] [CrossRef]
- Cheraghian, G.; Khalilinezhad, S. Effect of nanoclay on heavy oil recovery during polymer flooding. Petrol. Sci. Technol. 2015, 33, 999–1007. [Google Scholar] [CrossRef]
- Cheraghian, G. Thermal resistance and application of nanoclay on polymer flooding in heavy oil recovery. Petrol. Sci. Technol. 2015, 33, 1580–1586. [Google Scholar] [CrossRef]
- Tarek, M.; El-Banbi, A.H. Comprehensive investigation of effects of nano-fluid mixtures to enhance oil recovery. In Proceedings of the SPE North Africa Technical Conference and Exhibition, Cairo, Egypt, 14–16 September 2015; Society of Petroleum Engineers: Richardson, TX, USA, 2015. [Google Scholar]




| Area | Nano Type | Purpose | References |
|---|---|---|---|
| Exploration | Hyperpolarized silicon NPs | Imaging sensors for oil in a hydrocarbon reservoir | [20] |
| Nano-optical fiber | Detect oil-microbe, estimate reservoir pressure and temperature | [21] | |
| Nano-robots | Well logging and borehole measurement (patent) | [22] | |
| Coated carbon-nano structure | Real-time oil reservoir evaluation with two-dimensional detection technology | [23] | |
| Magnetic NP | Detect flood front, fluid contact, hydrocarbon bypass and fracture | [24] | |
| Superparamagnetic NP | Cross well magnetic sensor for tracking flood front | [25] | |
| Drilling & Completion | Silica NPs | Reduce or stop water invasion in shale by plugging shale pore | [26,27] |
| Nanodiamond | Improve drilling process in harsh and demanding environment | [28] | |
| Silica and alumina NPs | Cement accelerator | [29] | |
| MgO and ZnO NPs | Improve thermal stability of drilling fluid | [30] | |
| Nanoclay | Reduce permeability and porosity of cement and enhanced compressive strength | [31] | |
| Carbon nanotubes | Improve compressive strength in HPHT | [32] | |
| Cellulose nanofibers & graphene nano-platelets | Increase yield stresses, degree of hydration, flexural and compressive strengths | [33] | |
| Stimulation & Production | Pyroelectric NP | Additive for fracturing + viscoelastic surfactant to increase efficiency in fracturing | [34] |
| Ni-Fe NPs | Hydrate mitigation in the well | [35] | |
| ZnO NPs | Increase low shear rate viscosity on Threadlike micelle fluids and more stable | [36] | |
| Metal oxides based | Improve fracturing fluid stability and viscosity at high temperature (300 F). | [37] | |
| Non-ferrous NPS | Combine with surfactant for IFT reduction | [38] | |
| Refinery | Nano-supported HDS | Patent on nano-supported HDS catalyst | [39] |
| MoS2 nano-catalyst | Observe atomic-scale edge structures of MoS2 | [40] | |
| TiO2 NPs | Improve water treatment by reducing fouling effect | [41] | |
| TiO2, ZrO2, and SiO2 NPs | Additive for stabilizing asphaltene in oil under acidic conditions | [42] | |
| Magnetic NPs | Accelerate oil removal in water-oil emulsion | [43] | |
| Nickel oxides and Al2O3 NPs | Patent on nanocatalyst for hydrocracking | [44] |
| NP Type | Concentration (wt %) | Additional RF (%) | Fluids | Porous Media | References |
|---|---|---|---|---|---|
| SiO2 | 0.02–0.03 | - | Water | sandstone core | [3] |
| FeO, CuO, NiO | 5 | 14 | Water | carbonate core | [81] |
| Al2O3, Ni2O3, MgO, Fe2O3, ZnO, ZrO2, SnO, SiO2 | 0.3 | 12 | ethanol brine & water | sandstone core | [12] |
| HLP & LHP SiO2 | 0.1–0.4 | 19.31 | ethanol | sandstone core | [82] |
| SiO2 | 0.1 | 10 | polyacrylamide | glass bed | [83] |
| LHP SiO2 | 0.01–0.1 | 10 | brine (NaCl 3 wt %) | Berea sandstone | [84] |
| TiO2 | 0.01 and 1 | 51 | brine (NaCl 0.5 wt %) | sandstone core | [85] |
| Fe2O3, Al2O3, silane | 0.05–0.3 | 22.5 | propanol | sand pack | [86] |
| Al2O3, TiO2, SiO2 | 0.05 | 7–11 | brine (NaCl 3 wt %) | sandstone core | [87] |
| SiO2 | 1 | 21 | surfactant and polymer | Berea sandstone | [88] |
| SiO2 | 0.01–3 | 29 | Water | sandstone core | [89] |
| Nanoclay | 0.9 | 5.8 | Water | sandstone core | [90] |
| ZrO2, TiO2, MgO, Al2O3, CeO2, CNT, CaCO3, SiO2 | 5 | 8–9 | brine (3–12 wt %) | sandstone core | [80] |
| TiO2 | 1.9–2.5 | 4 | polymer and water | sandstone core | [91] |
| MWCNT | 0.01–0.10 | 31.8 | MWCNT fluid | glass bed | [92] |
| SnO2 | 0.1 | 22 | Brine | carbonate core | [93] |
| Complex nanofluid | 1 | 16–21 | water, brine, surfactant | Tensleep core | [94] |
| NP Type | Surface Coating | Concentration (wt %) | Influencing Factor | Emulsion/Foam Type | References |
|---|---|---|---|---|---|
| SiO2 | Hydrophilic | 0.05–5 | NP wettability | decane-in-water | [95] |
| Hydrophobic | water-in-decane | ||||
| Fumed SiO2 | Neutral-wet | 0.1–5 | Concentration | W/O or O/W | [96] |
| Polystyrene | Carboxy-coated | 2 | Brine salinity | W/O or O/W | [97] |
| SiO2 | Different SiOH | 1 | SiOH coverage | CO2-in-water | [98] |
| SiO2 | Silylating agent | 0.9–2.4 | CO2 density | Water-in-CO2 | [99] |
| SiO2 | Hexadecyltrimethoxysilane and polyvinyl alcohol | 3 | - | O/W | [100] |
| SiO2 | sodium dodecyl sulfate and cetyl trimethyl ammonium bromide | 0.1–0.5 | Concentration, surfactant | O/W | [101] |
| SiO2 | Hydrophilic | 1 | NP type | O/W | [102] |
| Clay | Partially hydrophobic | ||||
| SiO2, clay | Alpha-olefin sulfonate, sodium dodecyl sulfate and betaine | 500 ppm | Surfactant, NP type | CO2-in-water | [103] |
| AlOOH | Sodium cumenesulfonate (SC) | 1 | SC concentration | CO2-in-water | [104] |
| SiO2 | Partially hydrophobic | 0–2 | Surfactant | Gas-in-water | [105] |
| AlOOH | Sodium dodecyl sulfate | 1 | Surfactant | N2-in-water | [106] |
| SiO2 | Three wetting states | 5000 ppm | NP wetting state | CO2-in-water | [107] |
| SiO2 | Hydrophilic | 0.5 | Four surfactants | CO2-in-water | [108] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agista, M.N.; Guo, K.; Yu, Z. A State-of-the-Art Review of Nanoparticles Application in Petroleum with a Focus on Enhanced Oil Recovery. Appl. Sci. 2018, 8, 871. https://doi.org/10.3390/app8060871
Agista MN, Guo K, Yu Z. A State-of-the-Art Review of Nanoparticles Application in Petroleum with a Focus on Enhanced Oil Recovery. Applied Sciences. 2018; 8(6):871. https://doi.org/10.3390/app8060871
Chicago/Turabian StyleAgista, Madhan Nur, Kun Guo, and Zhixin Yu. 2018. "A State-of-the-Art Review of Nanoparticles Application in Petroleum with a Focus on Enhanced Oil Recovery" Applied Sciences 8, no. 6: 871. https://doi.org/10.3390/app8060871





