Prophylactic Effects of Polymethoxyflavone-Rich Orange Peel Oil on Nω-Nitro-L-Arginine-Induced Hypertensive Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples, Standards and Solvents
2.2. HPLC Analysis of the Polymethoxyflavones in OPO
2.3. GC-MS Analysis of D-Limonene in OPO
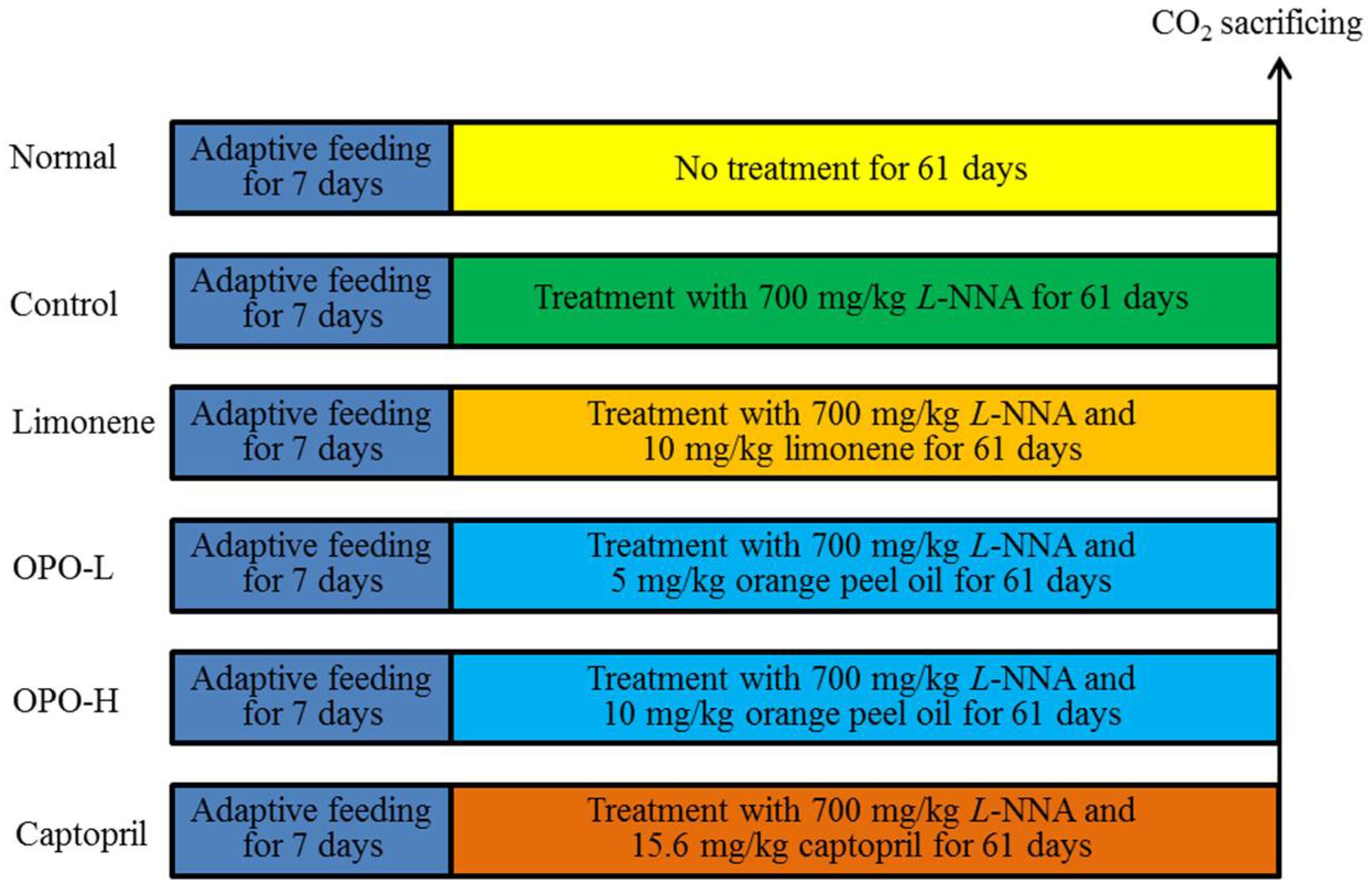
2.4. Animal Experiments
2.5. Serum Level Assay
2.6. Tissue Level Assay
2.7. Real-Time Quantitative PCR Assay
2.8. Western Blot Assay
2.9. Statistical Analysis
3. Results
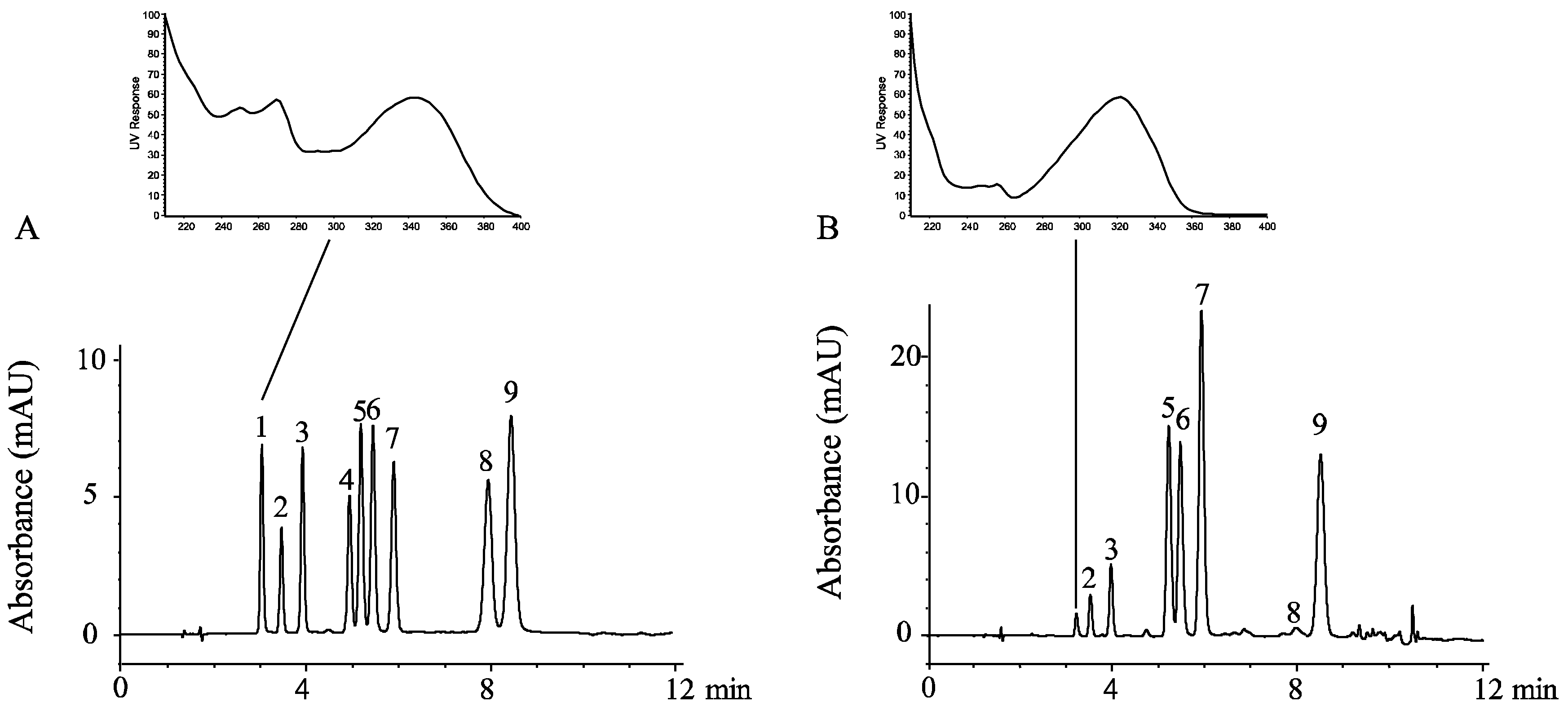
3.1. Polymethoxyflavones and D-Limonene Contents in Orange Peel Oil
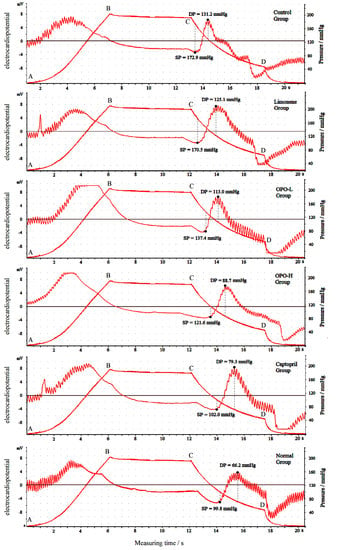
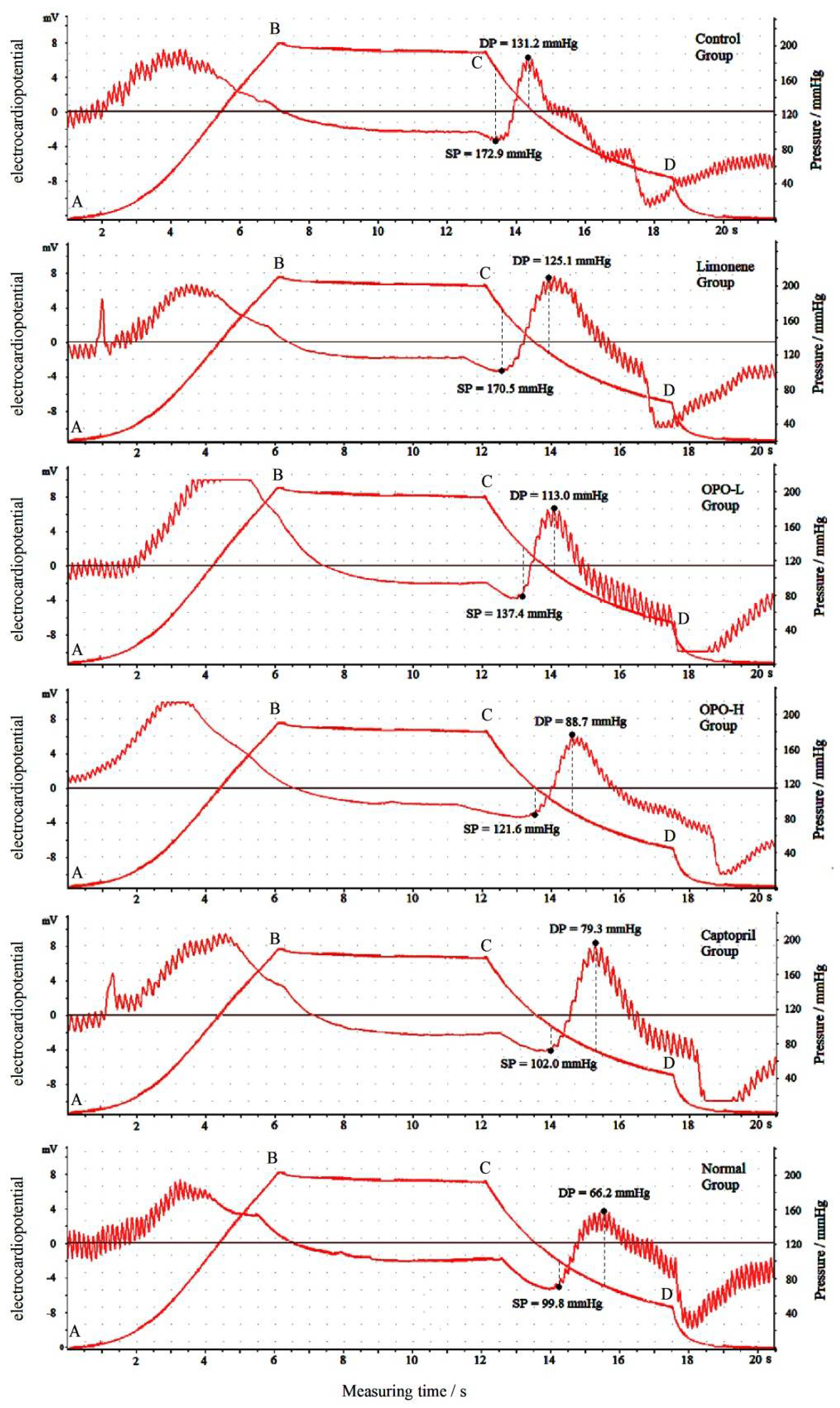
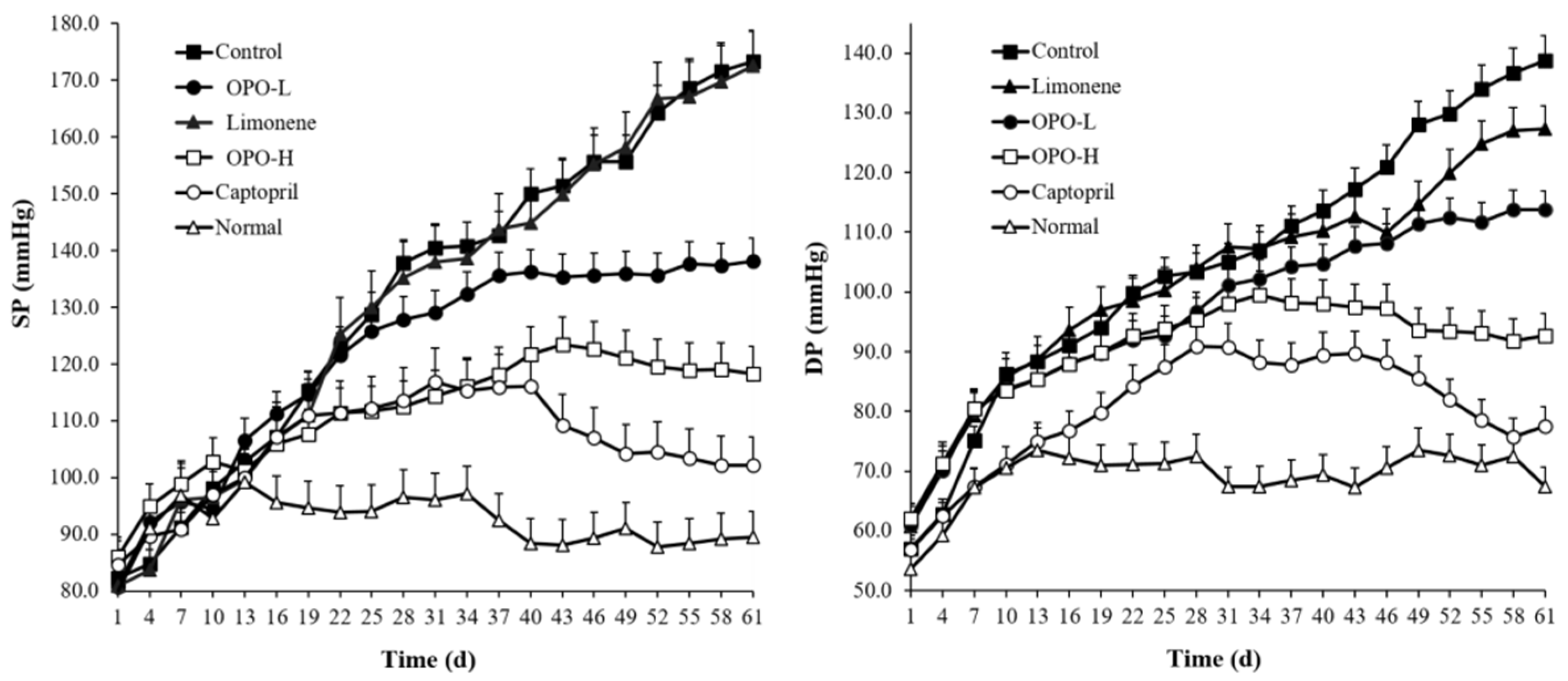
3.2. Effects of Orange Peel Oil on Blood Pressure
3.3. Nitric Oxide Content
3.4. Malondialdehyde Content
3.5. Serum Levels of ET-1, CGRP, VEGF and E-Selectin
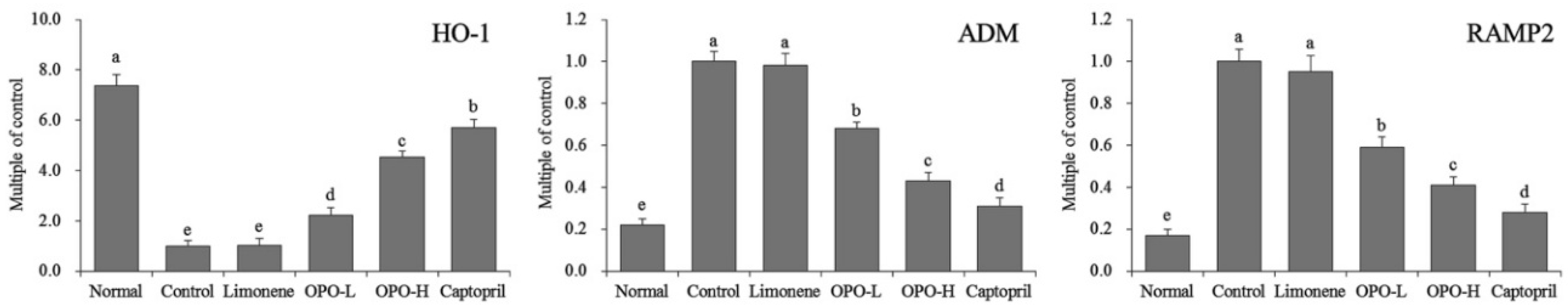
3.6. mRNA Expression of Myocardial Tissue
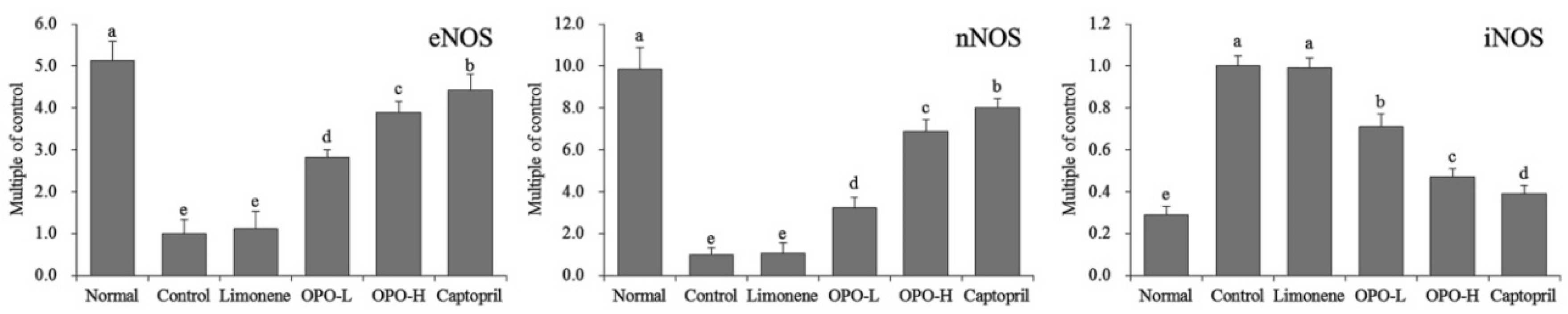
3.7. Protein Expression in Myocardial Tissue
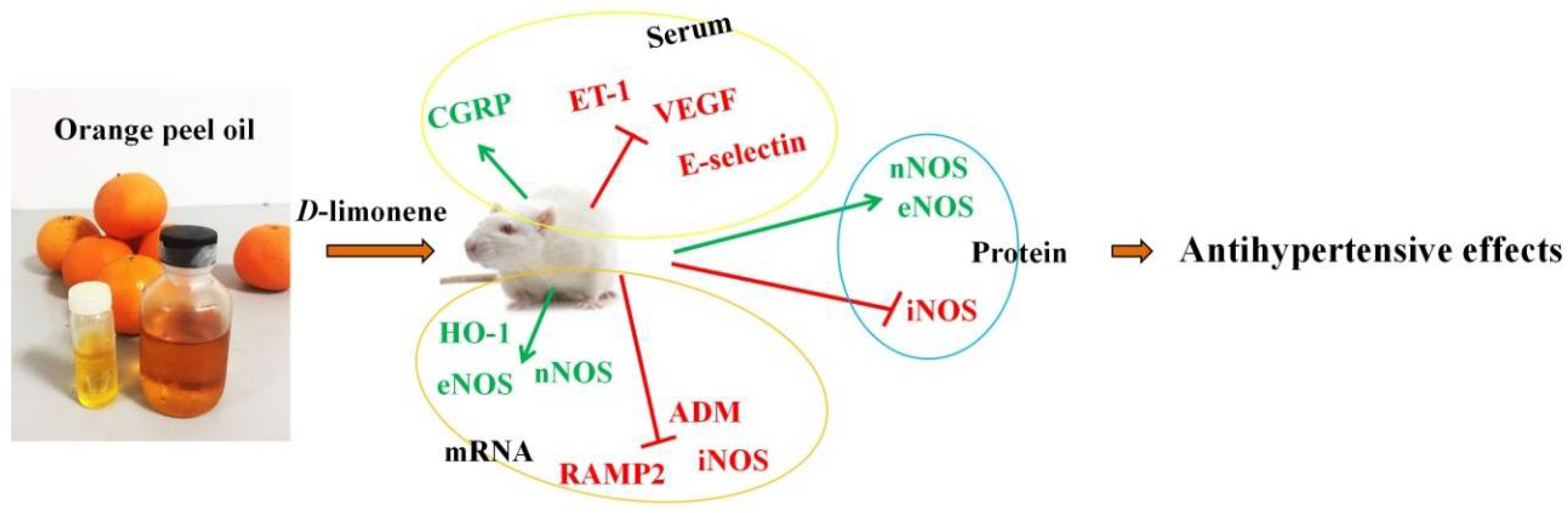
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Koes, R.E.; Quattrocchio, F.; Mol, J.N.M. The flavonoid biosynthetic pathway in plants: Function and evolution. Bioessays 1994, 1, 123–132. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Fang, L.; Zheng, Z.; Zhi, D.; Wang, S.; Li, S.; Ho, C.T.; Zhao, H. Anticancer activities of citrus peel polymethoxyflavones related to angiogenesis and others. Biomed. Res. Int. 2014, 2014, 453972. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Nakamura, Y.; Torikai, K.; Tanaka, T.; Koshiba, T.; Koshimizu, K.; Kuwahara, S.; Takahashi, Y.; Ogawa, K.; Yano, M.; et al. Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion in rats. Cancer Res. 2000, 60, 5059–5066. [Google Scholar] [PubMed]
- Ou, M.C.; Liu, Y.H.; Sun, Y.W.; Chan, C.F. The composition, antioxidant and antibacterial activities of cold-pressed and distilled essential oils of Citrus paradisi and Citrus grandis (L.) osbeck. Evid. Based Complement. Alternat. Med. 2015, 2015, 804091. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Kato, T.; Azuma, T.; Kikuzaki, H.; Abe, K. Anti-allergenic activity of polymethoxyflavones from Kaempferia parviflora. J. Funct. Food. 2015, 13, 100–107. [Google Scholar] [CrossRef]
- Montanari, A.; Chen, J.; Widmer, W. Citrus flavonoids: Citrus flavonoids: A review of past biological activity against disease. Discovery of new flavonoids from Dancy tangerine cold pressed peel oil solids and leaves. Adv. Exp. Med. Biol. 1998, 439, 103–116. [Google Scholar] [PubMed]
- Manthey, J.A.; Guthrie, N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J. Agric. Food Chem. 2002, 50, 5837–5843. [Google Scholar] [CrossRef] [PubMed]
- Morley, K.L.; Ferguson, P.J.; Koropatnick, J. Tangeretin and nobiletin induce G1 cell cycle arrest but not apoptosis in human breast and colon cancer cells. Cancer Lett. 2007, 251, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, Y.; Tao, L.; Tao, X.; Su, X.; Wei, D. Tyrosinase inhibitory effects and inhibition mechanisms of nobiletin and hesperidin from citrus peel crude extracts. J. Enzyme Inhibit. Med. Chem. 2007, 22, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Kharya, M.D.; Gajbhiye, A.; Sara, U.V.S.; Sharma, V.K. Flavonoids as nutraceuticals: Review. Herba Polonica 2010, 7, 1089–1099. [Google Scholar]
- Li, S.; Wang, Y.; Dushenkov, S.; Ho, C.T. Bioavailability of polymethoxyflavones. Acs Symp. 2008, 987, 233–245. [Google Scholar]
- Ohta, F.; Takagi, T.; Sato, H.; Ignarro, L.J. Low-dose L-arginine administration increases microperfusion of hindlimb muscle without affecting blood pressure in rats. Proc. Natl. Acad. Sci. USA 2007, 104, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, K.; Rubin, I.; Lauritzen, M. NO- and non-NO-, non-prostanoid-dependent vasodilatation in rat sciatic nerve during maturation and developing experimental diabetic neuropathy. J. Physiol. 2002, 543, 977–993. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.J.; Detterich, J.A.; Connes, P. Nitric oxide, vasodilation and the red blood cell. Biorheology 2014, 51, 121–134. [Google Scholar] [PubMed]
- Gangula, P.; Ravella, K.; Chukkapalli, S.; Rivera, M.; Srinivasan, S.; Hale, A.; Channon, K.; Southerland, J.; Kesavalu, L. Polybacterial periodontal pathogens alter vascular and gut BH4/nNOS/NRF2-phase II enzyme expression. PLoS ONE 2015, 10, e0129885. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, A.; Hirawa, N.; Fujita, M.; Kobayashi, Y.; Okuyama, Y.; Yatsu, K.; Katsumata, M.; Yamamoto, Y.; Ichihara, N.; Saka, S.; et al. Impaired nitric oxide production and increased blood pressure in systemic heterozygous ATP2B1 null rats. J. Hypertens 2014, 32, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Mahinur, B.; Yi, Y.; Chen, L.D.; Aisa, H.A.; Wang, M.H. Alkaloids of Nitraria sibirica Pall. decrease hypertension and albuminuria in angiotensin II-salt. Chin. J. Nat. Med. 2014, 12, 266–272. [Google Scholar]
- Li, L.; Yang, H.G.; Yuan, T.Y.; Zhao, Y.; Du, G.H. Rho kinase inhibition activity of pinocembrin in rat aortic rings contracted by angiotensin II. Chin. J. Nat. Med. 2013, 11, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhu, K.; Wang, C.; Liu, W.W.; Peng, D.G.; Zhao, X. Prophylactic effects of alkaloids from Ba lotus seeds on L-NNA-induced hypertension in rats. Chin. J. Nat. Med. 2016, 14, 835–843. [Google Scholar] [PubMed]
- Xue, B.J.; Zhang, Z.M.; Johnson, R.F.; Johnson, A.K. Sensitization of slow pressor angiotensin II (ANGII) initiated hypertension: Induction of sensitization by prior ANGII treatment. Hypertension 2012, 59, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.L.; Shi, Z.X.; Yu, Y.X.; Wu, Y.W.; Lu, B.W.; Hui, K.K. Distribution characteristics of syndrome types in essentialhypertension. Zhong Xi Yi Jie He Xue Bao 2010, 8, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.Y.; Wang, Y.Q. Investigation and application for the therapy of hypertension with traditional Chinese medicine. China Prac. Med. 2008, 3, 189–192. [Google Scholar]
- Shen, C.Z.; Peng, M.C.; Kuang, H.R.; Cheng, Z.Q. The effect of Chinese food therapy on the quality of life in patients with hypertension. Chin. J. Nurs. 2009, 44, 510–513. [Google Scholar]
- Silva, I.; Teixeira, A.; Oliveira, J.; Almeida, I.; Almeida, R.; Vasconcelos, C. Predictive value of vascular disease biomarkers for digital ulcers in systemic sclerosis patients. Clin. Exp. Rheumatol. 2015, 33, 127–130. [Google Scholar]
- Calò, L.A.; Dal Maso, L.; Pagnin, E.; Ravarotto, V.; Facco, M.; Boscaro, E.; Maiolino, G.; Pessina, A.C.; Rossi, G.P. Effect of olmesartan medoxomil on number and survival of circulating endothelial progenitor cells and calcitonin gene related peptide in hypertensive patients. J. Hypertens. 2014, 32, 193–199. [Google Scholar]
- Wang, Z.G.; Liu, J.L.; Liu, Y.; Wen, S.J.; Wen, J.; Liu, Y.P.; Chen, X.J.; Wu, Z.S. Increased plasma vasoactive substances and antioxidant enzymes levels in prehypertensive patients. Zhonghua Xin Xue Guan Bing Za Zhi 2007, 35, 719–722. [Google Scholar] [PubMed]
- Serban, D.; Leng, J.; Cheresh, D. H-ras regulates angiogenesis and vascular permeability by activation of distinct downstream effectors. Circ. Res. 2008, 102, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Muti, L.A.; Pârvu, A.E.; Crăciun, A.M.; Miron, N.; Acalovschi, M. Nitro-oxidative stress, VEGF and MMP-9 in patients with cirrhotic and non-cirrhotic portal hypertension. Clujul. Med. 2015, 88, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Qin, Y.W.; Zheng, X.; Qiu, J.L.; Zhang, L.Z.; Zhang, J.R.; Bian, J.L. Relevance of serum soluble E-selectin level and blood pressure in hypertensive patients. Shanghai Med. J. 2004, 27, 7–9. [Google Scholar]
- Chen, J.; Li, J. Clinical curative effect and influence on E-selectin, iNOS, eNOS in patient of essential hypertension with massotherapy. China J. Tradit. Chin. Med. Pharm. 2010, 25, 1708–1710. [Google Scholar]
- Zhou, Y.; Zhao, L.; Zhang, Z.; Lu, X. Protective effect of enalapril against methionine-enriched diet-induced hypertension: Role of endoplasmic reticulum and oxidative stress. Biomed. Res. Int. 2015, 2015, 724876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, T.; Xu, X.; Wang, M.; Zhong, L.; Yang, Y.; Zhai, Z.; Xiao, F.; Wang, C. Oxidative stress and nitric oxide signaling related biomarkers in patients with pulmonary hypertension: A case control study. BMC Pulm. Med. 2015, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.M.; Chuang, M.J.; Liu, J.H.; Liu, X.Q.; Ho, L.K.; Pan, W.H.; Zhang, X.M.; Liu, C.M.; Tsai, S.K.; Kong, C.W.; et al. Baicalein protects against retinal ischemia by antioxidation, antiapoptosis, down-regulation of HIF-1α, VEGF, and MMP-9 and upregulation of HO-1. J. Ocul. Pharmacol. Ther. 2013, 29, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.; Man, R.Y.; Vanhoutte, P.M. Upregulation of heme oxygenase-1 potentiates EDH-type relaxations in the mesenteric artery of the spontaneously hypertensive rat. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Kuroi, N.; Nakayama, T.; Sato, N.; Izumi, Y.; Soma, M.; Kokubun, S. Association study of calcitonin-receptor-like receptor gene in essential hypertension. Am. J. Hypertens. 2005, 18, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.S.; Fan, X.F.; Wu, X.M.; Hu, L.G.; Tang, C.S.; Pang, Y.Z.; Qi, Y.F. Changes of intermedin/adrenomedullin 2 and its receptors in the right ventricle of rats with chronic hypoxic pulmonary hypertension. Sheng Li Xue Bao 2007, 59, 210–214. [Google Scholar] [PubMed]
- Basralı, F.; Nasırcılar Ülker, S.; Koçer, G.; Ülker Karadamar, P.; Özyurt, D.; Cengiz, M.; Şentürk, Ü.K. Effect of magnesium on vascular reactivity in NOS inhibition-induced hypertension. Magnes. Res. 2015, 28, 64–74. [Google Scholar] [PubMed]
- Nieto, C.I.; Cabildo, M.P.; Cornago, M.P.; Sanz, D.; Claramunt, R.M.; Torralba, M.C.; Torres, M.R.; Elguero, J.; García, J.A.; López, A.; Acuña-Castroviejo, D. Fluorination effects on NOS inhibitory activity of pyrazoles related to curcumin. Molecules 2015, 20, 15643–15665. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Wang, S.S.; Chang, C.C.; Lee, F.Y.; Lin, H.C.; Hou, M.C.; Teng, T.H.; Chen, Y.C.; Lee, S.D. Evolution of portal-systemic collateral vasopressin response in endotoxemic portal hypertensive rats. Shock 2009, 32, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Pulgar, V.M.; Yamaleyeva, L.M.; Varagic, J.; McGee, C.; Bader, M.; Dechend, R.; Brosnihan, K.B. Functional changes in the uterine artery precede the hypertensive phenotype in a transgenic model of hypertensive pregnancy. Am. J. Physiol. Endocrinol. Metab. 2015, 309, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sun, P.; Zhao, X.; Yi, R.; Qian, J.; Shi, Y.; Wang, R. Gardenia jasminoides has therapeutic effects on L-NNA-induced hypertension in vivo. Mol. Med. Rep. 2017, 15, 4360–4373. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Suzuki, A.; Hase, T. Short-term effects of glucosyl hesperidin and hesperetin on blood pressure and vascular endothelial function in spontaneously hypertensive rats. J. Nutr. Sci. Vitaminol. 2008, 54, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, K.; Abe, A.H.; Kondo, M.; Uemura, K.; Iwasaki, Y.; Kondo, Y. Effects of long-term administration of hesperidin and glucosyl hesperidin to spontaneously hypertensive rats. J. Nutr. Sci. Vitaminol. 2002, 48, 420. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Ademosun, A.O. Phenolic extracts from grapefruit peels (Citrus paradisi) inhibit key enzymes linked with type 2 diabetes and hypertension. J. Food Biochem. 2011, 35, 1703–1709. [Google Scholar] [CrossRef]
- Díazjuárez, J.A.; Tenoriolópez, F.A.; Zarcoolvera, G.; Vallemondragón, L.D.; Torresnarváez, J.C.; Pastelínhernández, G. Effect of citrus paradisi extract and juice on arterial pressure both in vitro and in vivo. Phytother. Res. 2009, 23, 948. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


| Gene Name | Sequence |
|---|---|
| HO-1 | Forward: 5′-GGAACTTTCAGAAGGGCCAG-3′ |
| Reverse: 5′-GTCCTTGGTGTCATGGGTCA-3′ | |
| ADM | Forward: 5′-GCTGGTTTCCGTCGCCCTGATGT-3′ |
| Reverse: 5′-CGTTGTCCTTGTCCTTATCTGTG-3′ | |
| RAMP2 | Forward: 5′-GGACGGTGAAGAACTATGAG-3′ |
| Reverse: 5′-ATCATGGCCAGGAGTACATC-3′ | |
| nNOS | Forward: 5′-GAATACCAGCCTGATCCATGGAA-3′ |
| Reverse: 5′-TCCTCCAGGAGGGTGTCCACCGCATG-3′ | |
| eNOS | Forward: 5′-GGAGAGGCTGCATGACATTG-3′ |
| Reverse: 5′-GGTAGAGCCATAGTGGAATGAC-3′ | |
| iNOS | Forward: 5′-AGAGAGATCGGGTTCACA-3′ |
| Reverse: 5′-CACAGAACTGAGGGTACA-3′ | |
| GAPDH | Forward: 5′-TGCACCACCAACTGCTTAG-3′ |
| Reverse: 5′-GATGCAGGGATGATGTTC-3′ |
| Compounds | Quant. Method | Peak Area % | Calibration Equation * | Concentration (mg/L) |
|---|---|---|---|---|
| isosinensetin | HPLC-UV | N.D. | Y = 2.59X − 2.15 | N.D. |
| sinensetin | 2.2 ± 0.1 | Y = 3.33X + 0.23 | 5.10 ± 0.77 | |
| hexamethoxyflavone | 4.3 ± 0.2 | Y = 1.57X + 1.84 | 20.23 ± 1.44 | |
| tetramethyl-O-isoscutellarein | N.D. | Y = 2.87X + 0.52 | N.D. | |
| nobiletin | 16.7 ± 0.2 | Y = 2.64X + 4.41 | 47.72 ± 1.69 | |
| tetramethyl-O-scutellarein | 16.0 ± 0.2 | Y = 5.19X + 3.49 | 23.30 ± 1.25 | |
| heptamethoxyflavone | 28.6 ± 0.3 | Y = 5.29X + 0.30 | 42.02 ± 1.78 | |
| 5-demethylnobiletin | 0.9 ± 0.0 | Y = 2.41X + 5.73 | 0.44 ± 0.10 | |
| tangeretin | 22.7 ± 0.3 | Y = 6.88X + 1.31 | 25.55 ± 1.13 | |
| total PMFs | 91.4 ± 0.3 | - | 164.18 ± 3.14 | |
| D-limonene | GC-MS | 95.3 ± 0.5 | Y = 388,293X + 507,631 | 809.10 ± 7.31 (g/L) |
| Group | Serum (μmol/L) | Heart (µmol/gprot) | Liver (µmol/gprot) | Kidney (µmol/gprot) |
|---|---|---|---|---|
| Normal | 105.31 ± 4.92 a | 15.29 ± 1.35 a | 17.11 ± 0.46 a | 13.51 ± 0.57 a |
| Control | 58.91 ± 3.12 e | 7.35 ± 0.97 e | 5.83 ± 0.34 e | 6.65 ± 0.72 c |
| Limonene | 59.03 ± 2.70 e | 7.95 ± 0.40 e | 5.97 ± 1.00 e | 6.44 ± 1.85 c |
| OPO-L | 70.99 ± 2.54 d | 9.48 ± 1.13 d | 9.29 ± 0.82 d | 8.16 ± 0.28 b |
| OPO-H | 86.35 ± 3.48 c | 12.11 ± 1.76 c | 11.75 ± 0.76 c | 12.03 ± 1.34 a |
| Captopril | 97.37 ± 3.06 b | 13.54 ± 2.02 b | 14.63 ± 2.38 b | 13.21 ± 2.29 a |
| Group | Serum (μmol/L) | Heart (nmol/gprot) | Liver (nmol/gprot) | Kidney (nmol/gprot) |
|---|---|---|---|---|
| Normal | 2.23 ± 0.49 d | 2.12 ± 0.21 d | 0.50 ± 0.06 d | 1.35 ± 0.15 c |
| Control | 5.89 ± 0.31 a | 4.36 ± 0.23 a | 1.13 ± 0.08 a | 2.66 ± 0.17 a |
| Limonene | 5.69 ± 0.27 a | 4.33 ± 0.12 a | 1.14 ± 0.05 a | 2.40 ± 0.18 a |
| OPO-L | 4.09 ± 0.25 b | 3.75 ± 0.14 b | 0.92 ± 0.08 b | 1.81 ± 0.20 b |
| OPO-H | 3.68 ± 0.34 c | 2.98 ± 0.27 c | 0.72 ± 0.07 c | 1.62 ± 0.13 bc |
| Captopril | 2.97 ± 0.30 d | 2.70 ± 0.16 c | 0.58 ± 0.05 d | 1.52 ± 0.22 c |
| Group | ET-1 (pg/mL) | CGRP (pg/mL) | VEGF (pg/mL) | E-selectin (ng/mL) |
|---|---|---|---|---|
| Normal | 62.47 ± 4.78 e | 125.72 ± 7.92 a | 82.36 ± 5.20 e | 231.28 ± 22.17 e |
| Control | 197.36 ± 12.79 a | 41.82 ± 3.37 e | 155.73 ± 11.38 a | 533.03 ± 38.29 a |
| Limonene | 195.71 ± 17.51 a | 43.45 ± 5.11 e | 152.39 ± 12.49 a | 528.67 ± 33.12 a |
| OPO-L | 151.38 ± 7.34 b | 72.82 ± 6.18 d | 137.72 ± 8.73 b | 451.91 ± 25.87 b |
| OPO-H | 122.03 ± 6.70 c | 93.45 ± 4.18 c | 107.81 ± 8.26 c | 348.75 ± 20.89 c |
| Captopril | 96.33 ± 3.89 d | 108.37 ± 5.65 b | 91.32 ± 4.77 d | 287.93 ± 24.62 d |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.-J.; Wang, J.; Cheng, Y.-J.; Tan, X.; Zhai, Y.-L.; Wang, Q.; Gao, F.-J.; Liu, G.-L.; Zhao, X.; Wang, H. Prophylactic Effects of Polymethoxyflavone-Rich Orange Peel Oil on Nω-Nitro-L-Arginine-Induced Hypertensive Rats. Appl. Sci. 2018, 8, 752. https://doi.org/10.3390/app8050752
Li G-J, Wang J, Cheng Y-J, Tan X, Zhai Y-L, Wang Q, Gao F-J, Liu G-L, Zhao X, Wang H. Prophylactic Effects of Polymethoxyflavone-Rich Orange Peel Oil on Nω-Nitro-L-Arginine-Induced Hypertensive Rats. Applied Sciences. 2018; 8(5):752. https://doi.org/10.3390/app8050752
Chicago/Turabian StyleLi, Gui-Jie, Jun Wang, Yu-Jiao Cheng, Xiang Tan, Yu-Lin Zhai, Qiang Wang, Fang-Jin Gao, Guang-Lan Liu, Xin Zhao, and Hua Wang. 2018. "Prophylactic Effects of Polymethoxyflavone-Rich Orange Peel Oil on Nω-Nitro-L-Arginine-Induced Hypertensive Rats" Applied Sciences 8, no. 5: 752. https://doi.org/10.3390/app8050752