Rapid and Quantitative Determination of Soil Water-Soluble Nitrogen Based on Surface-Enhanced Raman Spectroscopy Analysis
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Sample Preparation
2.2. Nano-Sol Substrate Preparation
2.3. Experimental Instrument
2.4. Raman Spectra Acquisition
2.5. Spectral Preprocessing Methods
2.6. Modeling Methods
2.7. Model Evaluation Index
3. Results and Discussion
3.1. The Urea SERS
3.2. The Comparation of Three Nanoenhancers
3.3. 400–1800 cm−1 Soil SERS Analysis
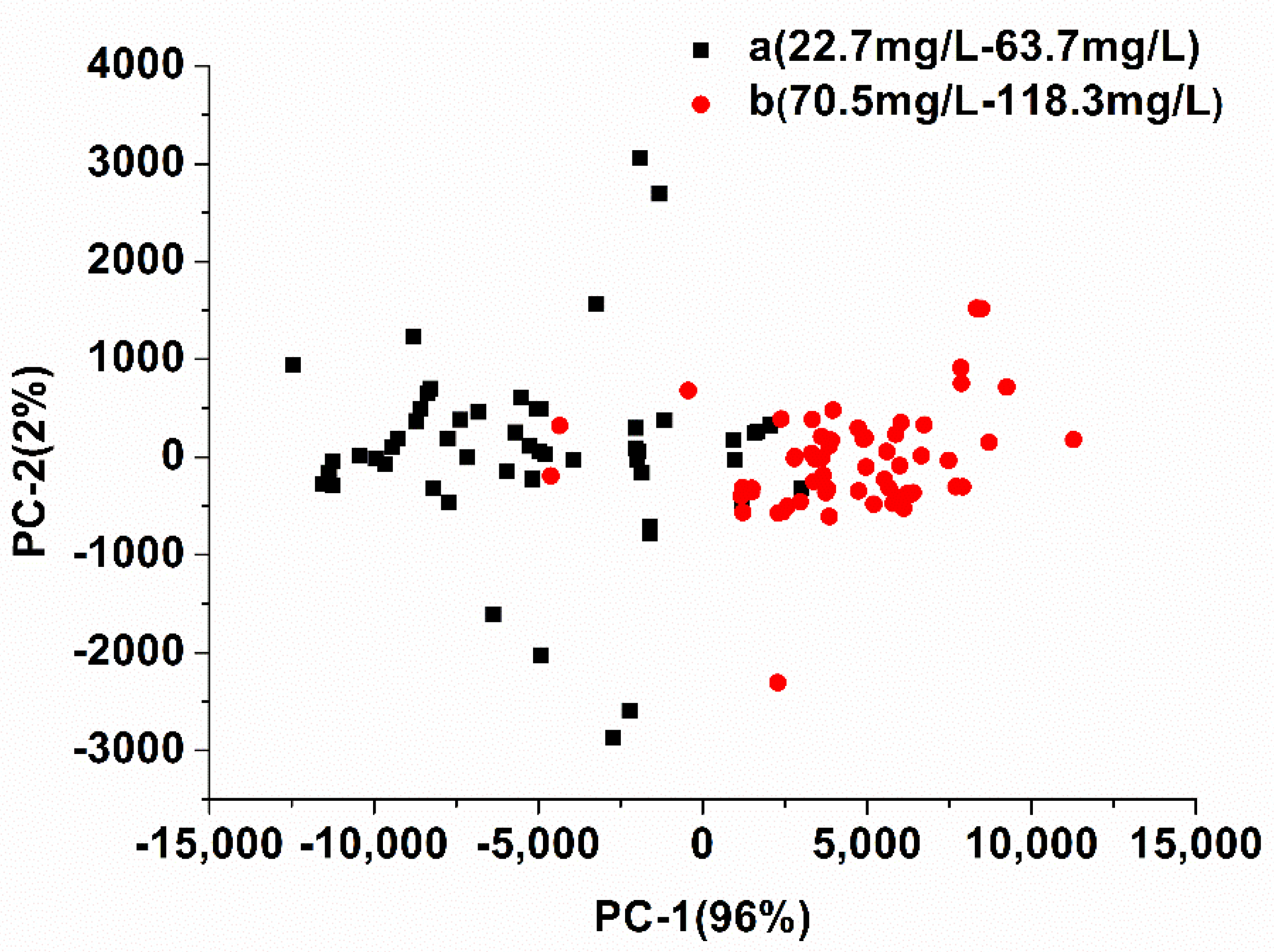
3.4. Model Analysis
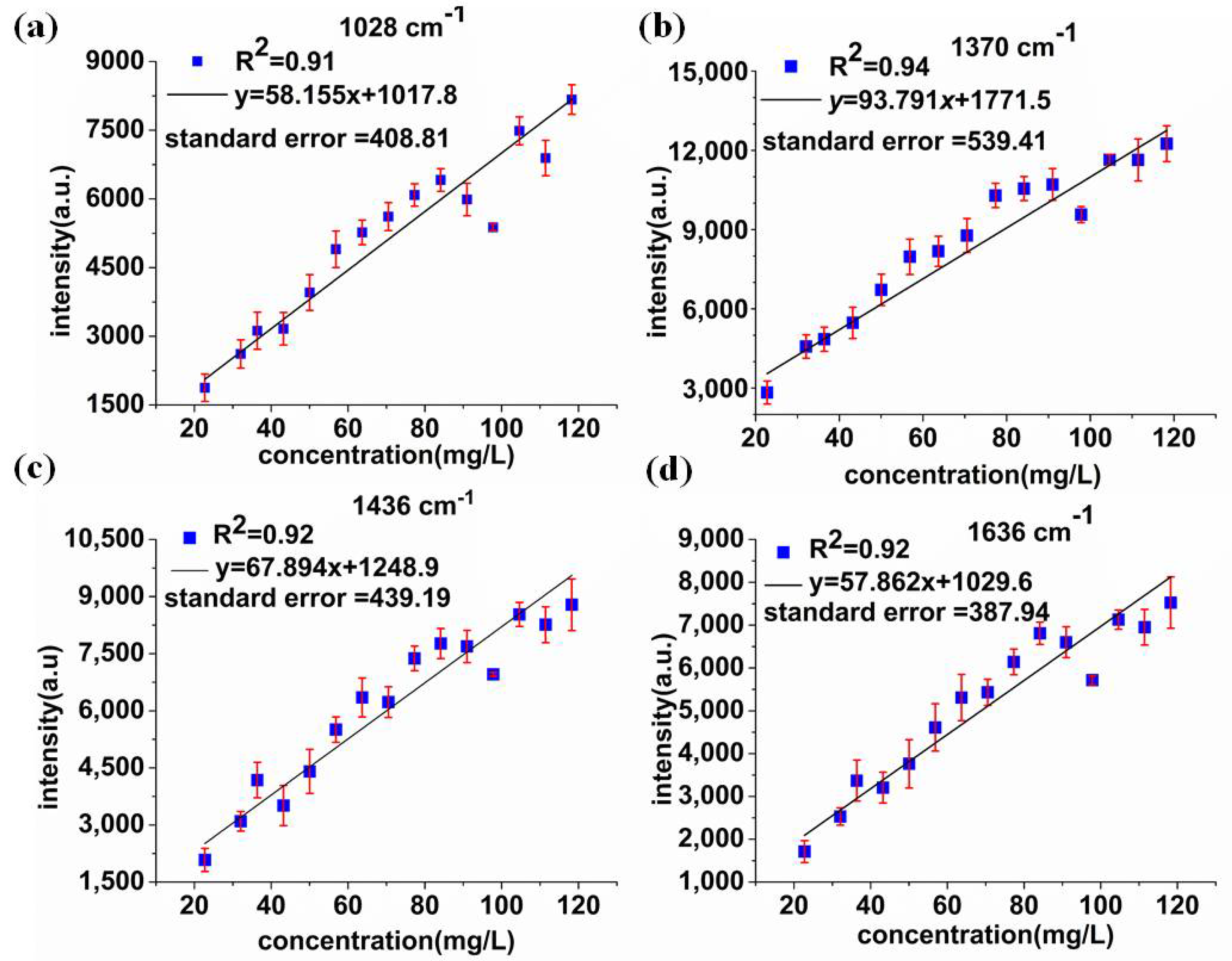
3.5. SERS Characteristic Peaks Model Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Anderson, R.H.; Fuhlendorf, S.D.; Engle, D.M. Soil water-soluble nitrogen Availability in Tallgrass Prairie Under the Fire–Grazing Interaction. Rangel. Ecol. Manag. 2006, 59, 625–631. [Google Scholar] [CrossRef]
- Veuger, B.; Oevelen, D.V.; Middelburg, J.J. Fate of microbial nitrogen, carbon, hydrolysable amino acids, monosaccharides, and fatty acids in sediment. Geochim. Cosmochim. Acta 2012, 83, 217–233. [Google Scholar] [CrossRef]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171. [Google Scholar] [CrossRef]
- Vozniuk, S.T.; Truskavets’Kyi, R.S. Methods of agrochemical analysis of peat soils. Ahrokhimiia Hruntozn 1972, 21, 18–24. [Google Scholar]
- Cherlo, S.K.R.; Devaki, K.; Pushpavanam, S. Phase transfer catalysis of alkaline hydrolysis of n-butyl acetate: Comparison of performance of batch and micro-reactors. Chem. Eng. Proc. 2010, 49, 484–489. [Google Scholar] [CrossRef]
- Önal, A. A review: Current analytical methods for the determination of biogenic amines in foods. Food Chem. 2007, 103, 1475–1486. [Google Scholar] [CrossRef]
- Bevilacqua, M.; Bucci, R.; Materazzi, S.; Marini, F. Application of near infrared (NIR) sensors coupled to chemometrics for dried egg-pasta characterization and egg content quantification. Food Chem. 2013, 140, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Filippi, J.J.; Belhassen, E.; Baldovini, N.; Brevard, H.; Meierhenrich, U.J. Qualitative and quantitative analysis of vetiver essential oils by comprehensive two-dimensional gas chromatography and comprehensive two-dimensional gas chromatography/mass spectrometry. J. Chromatogr. A 2013, 1288, 127–148. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xiao, S.P.; Nie, P.C.; Dong, T.; Qu, F.F.; Lin, L. Research on the optimum water content of detecting soil nitrogen using near infrared sensor. Sensors 2017, 17, 2045. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.C.; Dong, T.; He, Y.; Qu, F.F. Detection of soil nitrogen using near infrared sensors based on soil pretreatment and algorithms. Sensors 2017, 17, 1102. [Google Scholar] [CrossRef] [PubMed]
- Ru, E.C.L.; Etchegoin, P.G.; Meyer, M. Enhancement factor distribution around a single surface-enhanced Raman scattering hot spot and its relation to single molecule detection. J. Chem. Phys. 2006, 125, 1102. [Google Scholar] [CrossRef]
- Doering, W.E.; Nie, S. Single-Molecule and Single-Nanoparticle SERS: Examining the Roles of Surface Active Sites and Chemical Enhancement. J. Phys. Chem. B 2002, 106, 311–317. [Google Scholar] [CrossRef]
- Torres, E.L.; Winefordner, J.D. Trace determination of nitrogen-containing drugs by surface enhanced Raman scattering spectrometry on silver colloids. Anal. Chem. 1987, 59, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Yang, K.; Zhou, G.; Huang, W.E.; Zhu, Y. Surface-enhanced raman spectroscopy combined with stable isotope probing to monitor nitrogen assimilation at both bulk and single-cell level. Anal. Chem. 2017, 89, 5793–5800. [Google Scholar] [CrossRef] [PubMed]
- Vlasov, I.I.; Ralchenko, V.G.; Goovaerts, E.; Saveliev, A.V.; Kanzyuba, M.V. Bulk and surface-enhanced raman spectroscopy of nitrogen-doped ultrananocrystalline diamond films. Phys. Status Solidi 2006, 203, 3028–3035. [Google Scholar] [CrossRef]
- Uehara, J. An Investigation of Adsorption and Orientation of Some Nitrogen Compounds at Iron Surface in an Acid Solution by Surface-Enhanced Raman Scattering Spectroscopy. J. Electrochem. Soc. 1990, 137, 2677–2683. [Google Scholar] [CrossRef]
- Ding, L.; Fang, Y. An investigation of the surface-enhanced raman scattering (sers) effect from laser irradiation of ag nanoparticles prepared by trisodium citrate reduction method. Appl. Surf. Sci. 2007, 253, 4450–4455. [Google Scholar] [CrossRef]
- Su, Q.; Ma, X.; Dong, J.; Jiang, C.; Qian, W. A reproducible sers substrate based on electrostatically assisted aptes-functionalized surface-assembly of gold nanostars. ACS Appl. Mater. Interfaces 2011, 3, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Parvinnia, E.; Sabeti, M.; Jahromi, M.Z.; Boostani, R. Classification of eeg signals using adaptive weighted distance nearest neighbor algorithm. J. King Saud Univ. Comput. Inf. Sci. 2014, 26, 1–6. [Google Scholar] [CrossRef]
- Gorry, P.A. General least-squares smoothing and differentiation by the convolution (Savitzky-Golay) method. Anal. Chem. 1990, 62, 570–573. [Google Scholar] [CrossRef]
- Isaksson, T.; Næs, T. The Effect of Multiplicative Scatter Correction (MSC) and Linearity Improvement in NIR Spectroscopy. Appl. Spectrosc. 1988, 42, 1273–1284. [Google Scholar] [CrossRef]
- Wu, D.; He, Y.; Feng, S.; Sun, D.W. Study on infrared spectroscopy technique for fast measurement of protein content in milk powder based on ls-svm. J. Food Eng. 2008, 84, 124–131. [Google Scholar] [CrossRef]
- Wen, L.; Bai, Z.; Bai, J.; Guo, Z. Decomposition kinetics of hydrogen bonds in coal by a new method of in-situ diffuse reflectance FT-IR. J. Fuel Chem. Technol. 2011, 39, 321–327. [Google Scholar]
- Gong, Q.J.; Qiao, J.L.; Du, L.M.; Dong, C.; Jin, W.J. Recognition and simultaneous determination of ofloxacin enantiomers by synchronization–1st derivative fluorescence spectroscopy. Talanta 2000, 53, 359–365. [Google Scholar] [CrossRef]
- Trygg, J.; Wold, S. PLS regression on wavelet compressed NIR spectra. Chemom. Intell. Lab. Syst. 1998, 42, 209–220. [Google Scholar] [CrossRef]
- He, Y.; Li, X.; Deng, X. Discrimination of varieties of tea using near infrared spectroscopy by principal component analysis and BP model. J. Food Eng. 2007, 79, 1238–1242. [Google Scholar] [CrossRef]
- Wu, D.; Zhou, Z.; Feng, S.; He, Y. Uninformation Variable Elimination and Successive Projections Algorithm in Mid-Infrared Spectral Wavenumber Selection. In Proceedings of the 2009 2nd International Congress on Image and Signal Processing, Tianjin, China, 17–19 October 2009; pp. 1–5. [Google Scholar]
- Nie, P.C.; Dong, T.; He, Y.; Qu, F.F. The Effects of Drying Temperature on Nitrogen Concentration Detection in Calcium Soil Studied by NIR Spectroscopy. Appl. Sci. 2018, 8, 269. [Google Scholar] [CrossRef]
- Rousseau, B.; Van Alsenoy, C.; Keuleers, R.; Desseyn, H.O. Solids modeled by Ab-initio crystal field methods. part 17. study of the structure and vibrational spectrum of urea in the gas phase and in its P421m crystal phase. J. Phys. Chem. A 1998, 102, 6540–6548. [Google Scholar] [CrossRef]
- Wang, Y.F.; Feng, M.; Wang, J.; Yan, C.C.; Guo, J.H.; Fan, X.J.; Lan, G.X. Lattice Vibrational Spectral Study of Urea and Fe-urea Complexes. Chin. J. Light Scatt. 2005, 7, 103–108. [Google Scholar]
- Duncan, J.L. Normal coordinates for the planar vibrations of urea using 15N and 18O frequency shift data. Spectrochim. Acta Part A Mol. Spectrosc. 1971, 27, 1197–1205. [Google Scholar] [CrossRef]
- Xiao, Li.; Jun, Z.; Wei, X.; Hui, J.; Wang, X.; Yang, B.; Bing, Z.; Bo, L.; Yukihiro, O. Mercaptoacetic acid-capped silver nanoparticles colloid: formation, morphology, and sers activity. Langmuir 2003, 19, 4285–4290. [Google Scholar]
- Lindberg, W.; Persson, J.A.; Wold, S. Partial least-squares method for spectrofluorimetric analysis of mixtures of humic acid and lignin sulfonate. Anal. Chem. 1983, 55, 643–648. [Google Scholar] [CrossRef]
- Lin, L.; Wu, R.; Liu, M.; Wang, X.; Yan, L. Surface-enhanced raman spectroscopy analysis of thiabendazole pesticide. Spectrosc. Spectr. Anal. 2015, 35, 404–408. [Google Scholar]
- Yan, X.; Zhang, Y.; Liu, J. Research progress on the evaluation of soil fertility quality. Hunan Agric. Sci. 2008, 5, 82–85. [Google Scholar]
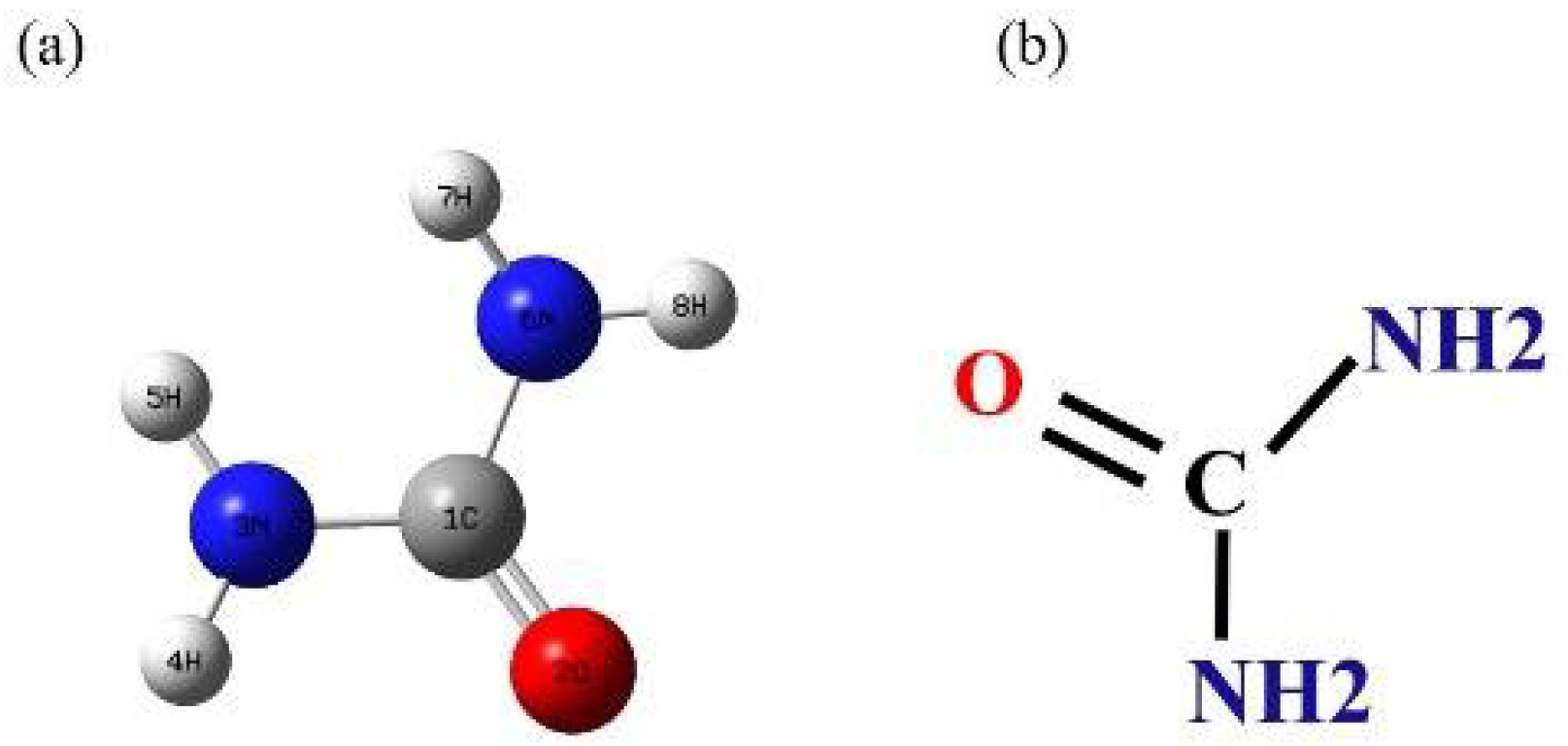
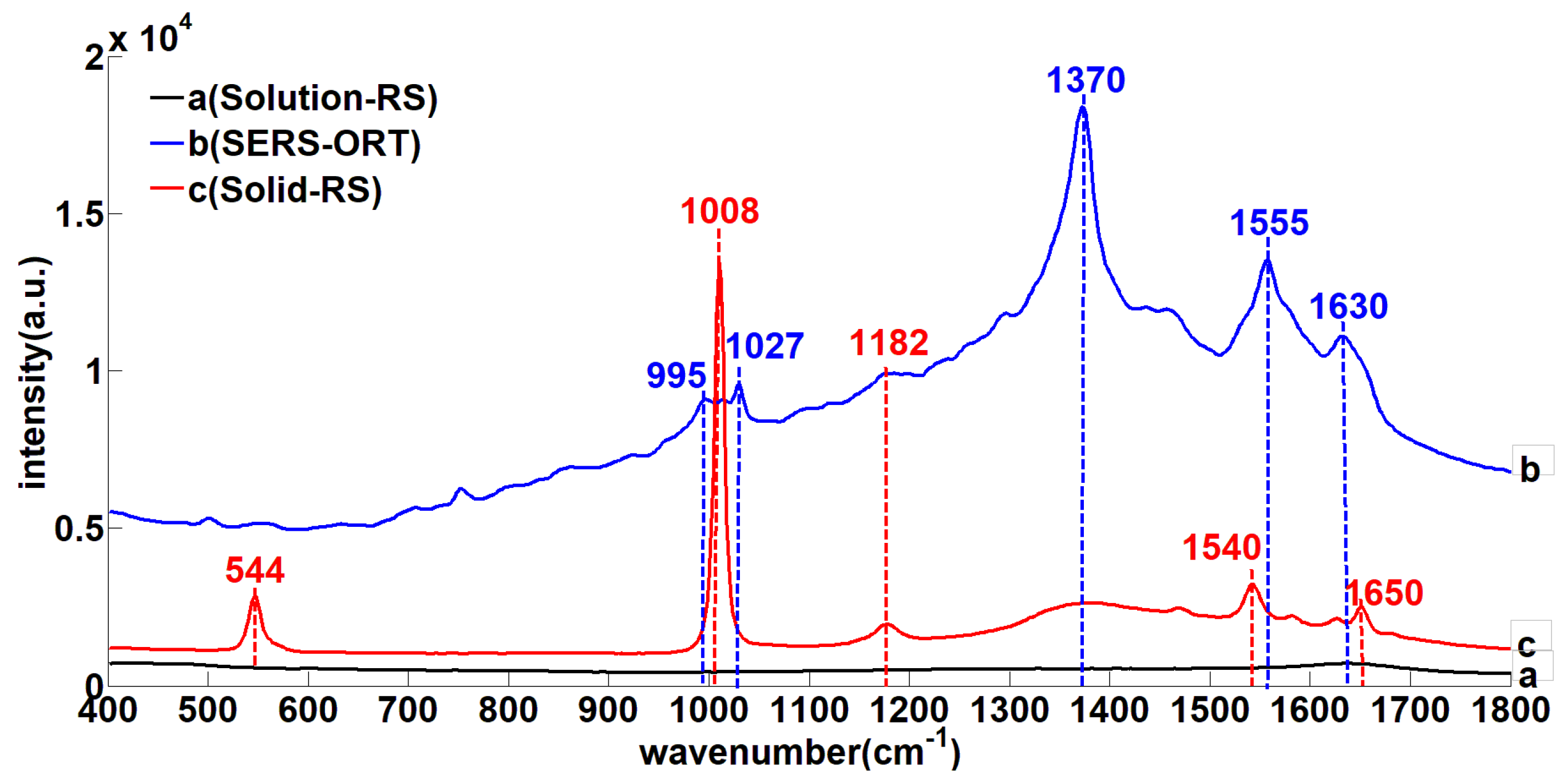
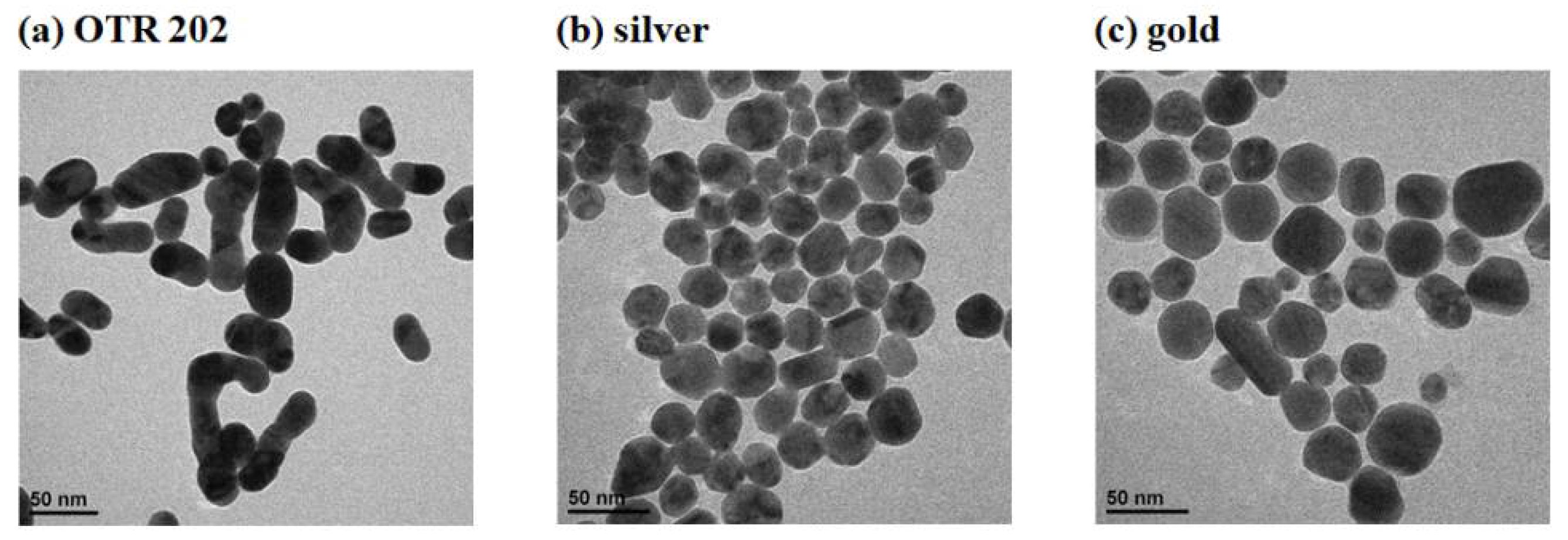
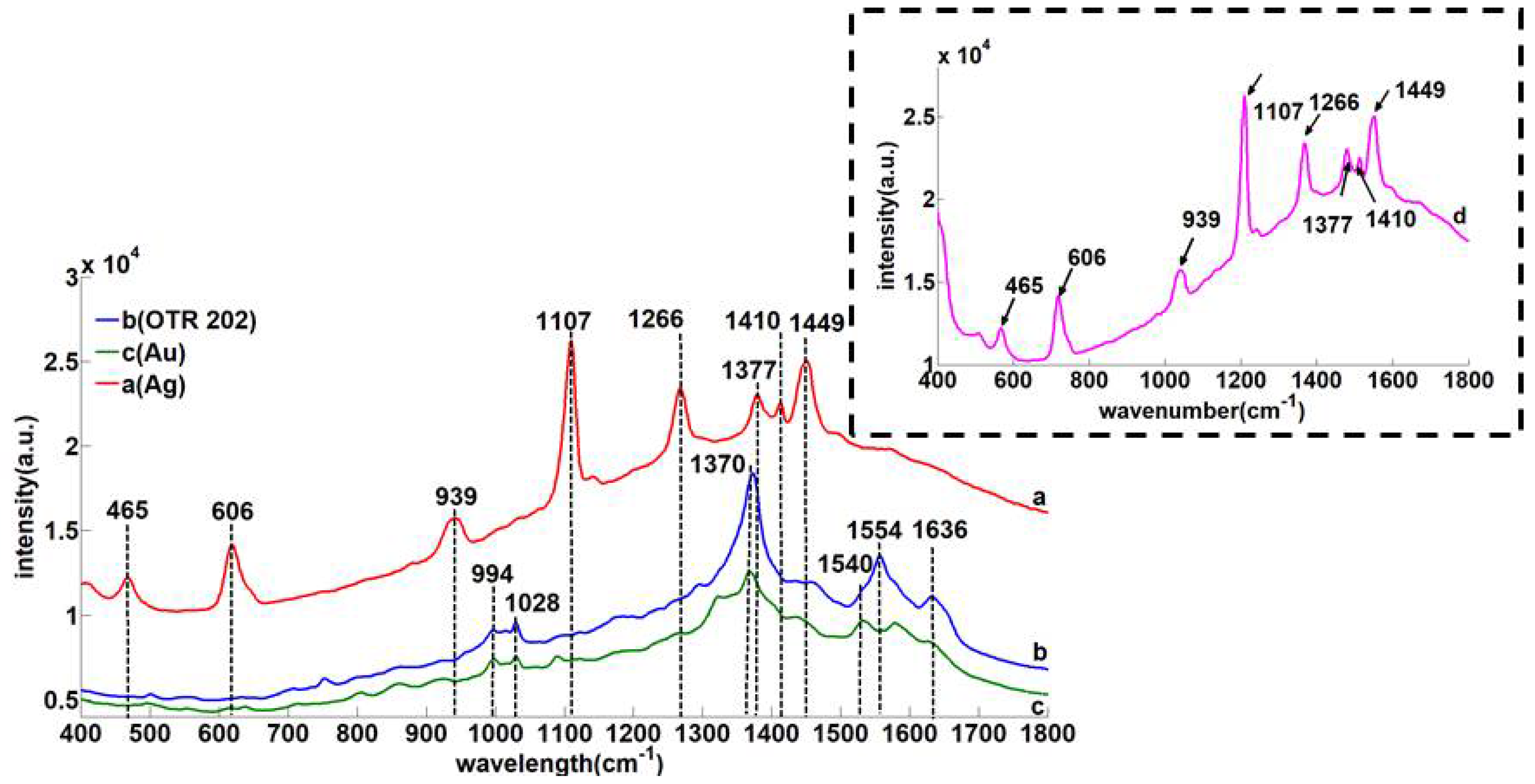
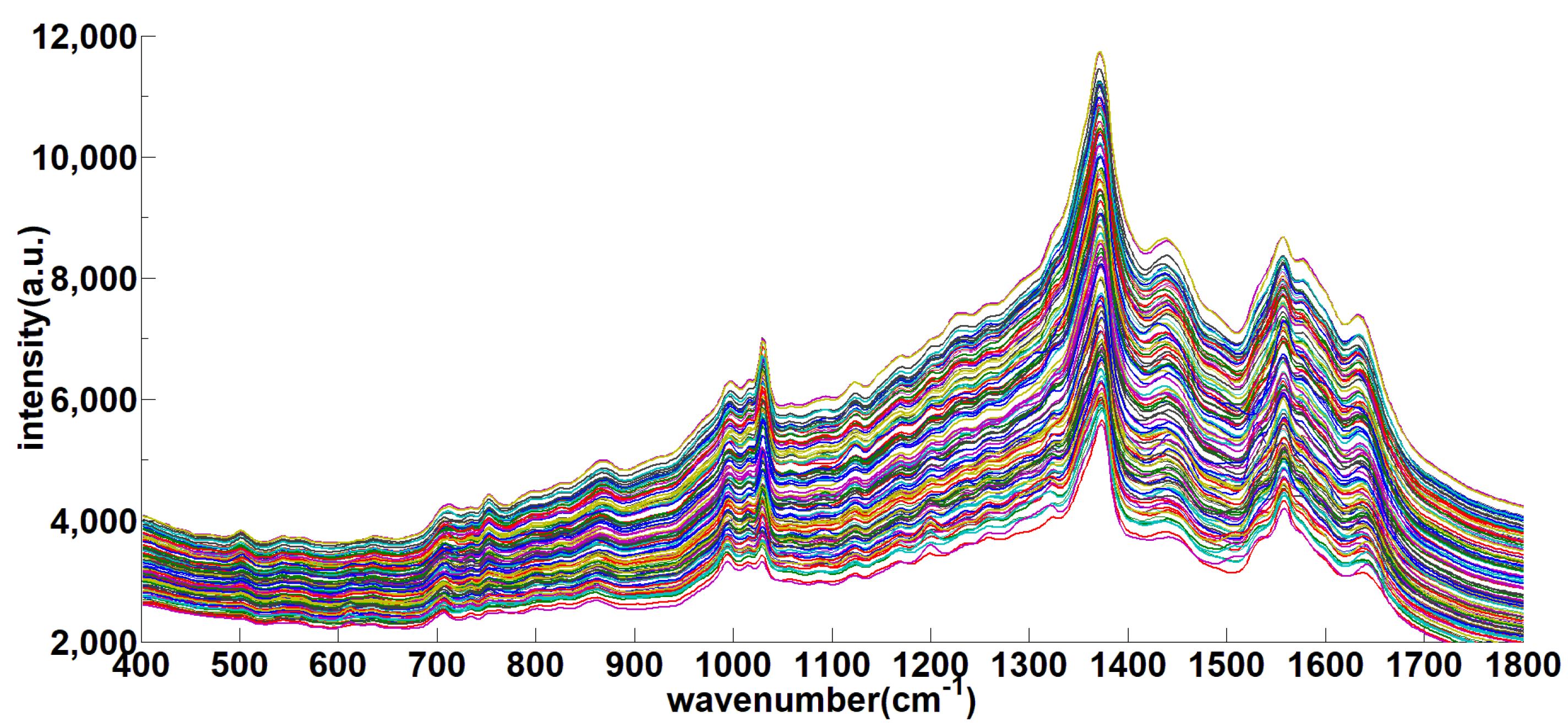
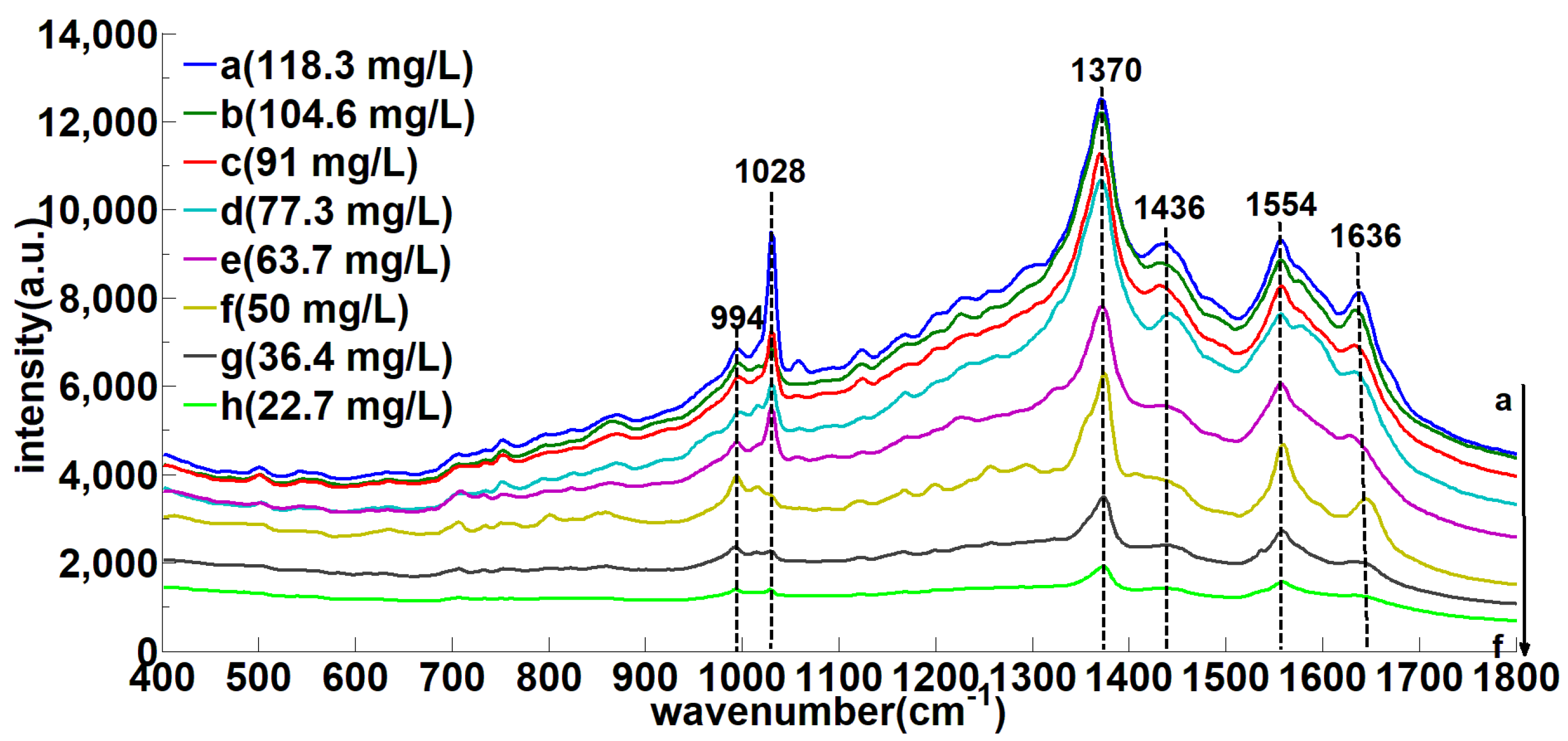
| Solid-RS (cm−1) | SERS (cm−1) | Assignment |
|---|---|---|
| 544 (s) | - | δ(C–N) |
| - | 995 (m) | υ (N–H) |
| 1008 (vs) | 1027(m) | υ(C–N) |
| 1182 (m) | 1182 (m) | ρ (NH2) |
| - | 1370 (vs) | υ (N–H) |
| - | - | δ(N–H) |
| 1540 (m) | 1555 (s) | υ (C=O) |
| - | 1630 (s) | υ (N–H) |
| 1650 (m) | - | δ (NH2) |
| Preprocessing | Principal Components | Calibration Set | Prediction Set | |||
|---|---|---|---|---|---|---|
| RMSEc (mg/L) | RMSEp (mg/L) | RPD | ||||
| RAW | 5 | 0.90 | 9.16 | 0.90 | 9.36 | 3.10 |
| S-G | 5 | 0.91 | 9.17 | 0.90 | 9.17 | 3.00 |
| MSC | 5 | 0.90 | 9.28 | 0.90 | 8.98 | 3.03 |
| SNV | 5 | 0.91 | 9.19 | 0.88 | 9.16 | 3.24 |
| DT | 5 | 0.90 | 9.21 | 0.90 | 9.18 | 3.08 |
| 1st-der | 5 | 0.87 | 9.40 | 0.91 | 8.76 | 3.34 |
| Calibration Set | Prediction Set | Wavenumber (cm−1) | |||
|---|---|---|---|---|---|
| (mg/L) | (mg/L) | RPD | |||
| 0.84 | 12.27 | 0.87 | 11.03 | 2.56 | 994, 1028, 1370, 1436, 1636 |
| 0.78 | 13.57 | 0.77 | 13.07 | 1.73 | 994 |
| 0.81 | 13.42 | 0.81 | 12.61 | 2.16 | 1028 |
| 0.88 | 10.82 | 0.86 | 10.41 | 2.52 | 1370 |
| 0.86 | 12.11 | 0.86 | 11.50 | 2.51 | 1436 |
| 0.87 | 11.21 | 0.85 | 11.54 | 2.44 | 1636 |
| Category | Calibration Set | Prediction Set | ||||
|---|---|---|---|---|---|---|
| Number | Identification Number | Accuracy | Number | Identification Number | Accuracy | |
| all | 75 | 75 | 100% | 45 | 39 | 86.67% |
| low | 35 | 35 | 100% | 21 | 19 | 90.48% |
| high | 40 | 40 | 100% | 24 | 20 | 83.33% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, T.; Xiao, S.; He, Y.; Tang, Y.; Nie, P.; Lin, L.; Qu, F.; Luo, S. Rapid and Quantitative Determination of Soil Water-Soluble Nitrogen Based on Surface-Enhanced Raman Spectroscopy Analysis. Appl. Sci. 2018, 8, 701. https://doi.org/10.3390/app8050701
Dong T, Xiao S, He Y, Tang Y, Nie P, Lin L, Qu F, Luo S. Rapid and Quantitative Determination of Soil Water-Soluble Nitrogen Based on Surface-Enhanced Raman Spectroscopy Analysis. Applied Sciences. 2018; 8(5):701. https://doi.org/10.3390/app8050701
Chicago/Turabian StyleDong, Tao, Shupei Xiao, Yong He, Yu Tang, Pengcheng Nie, Lei Lin, Fangfang Qu, and Shaoming Luo. 2018. "Rapid and Quantitative Determination of Soil Water-Soluble Nitrogen Based on Surface-Enhanced Raman Spectroscopy Analysis" Applied Sciences 8, no. 5: 701. https://doi.org/10.3390/app8050701






