Performance Evaluation of a Chinese Lean Iron Ore as the Oxygen Carrier in Multi and Short-Time Redox Cycles
Abstract
:1. Introduction
2. Experimental
2.1. Experimental Material
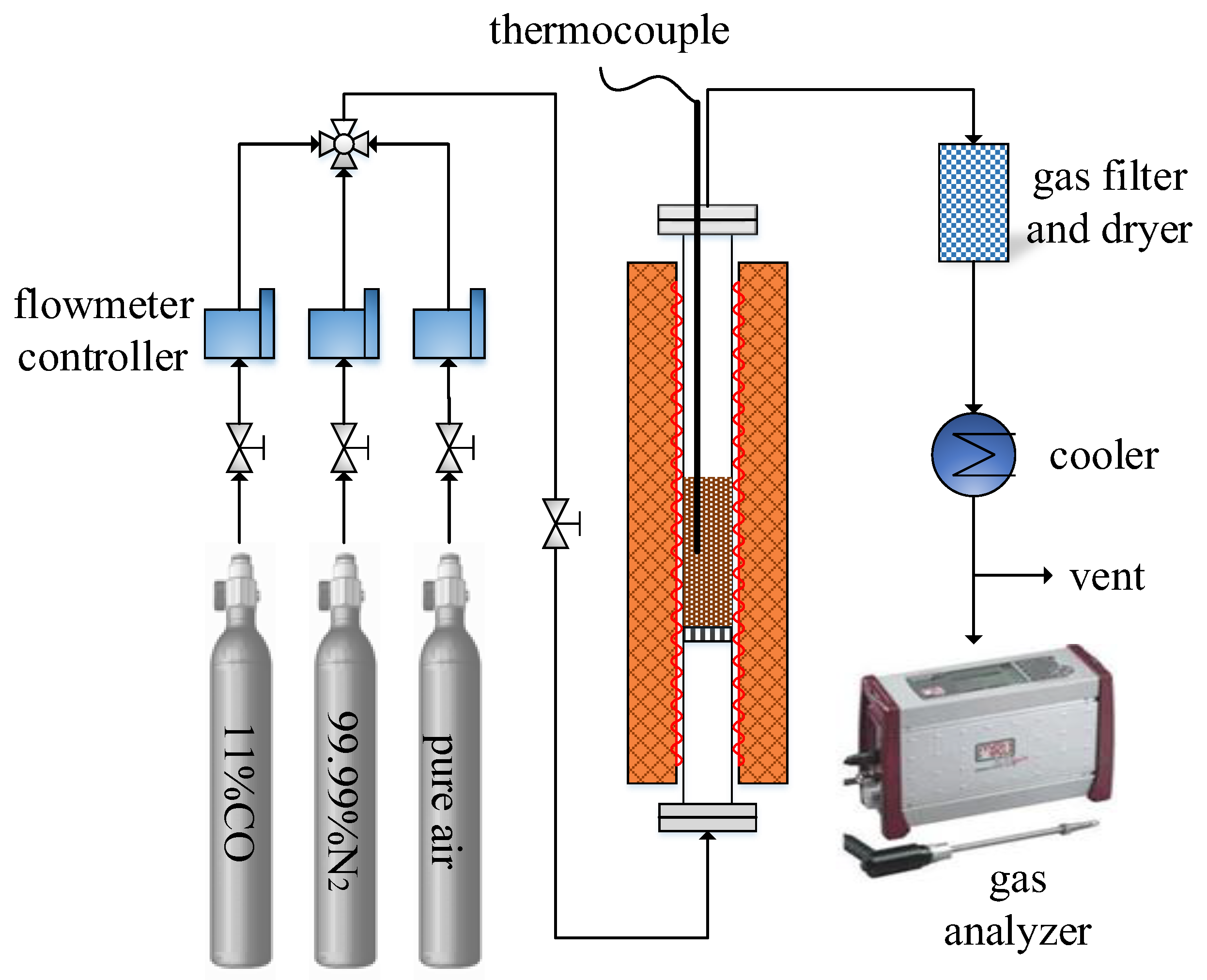
2.2. Experimental Setup and Procedure
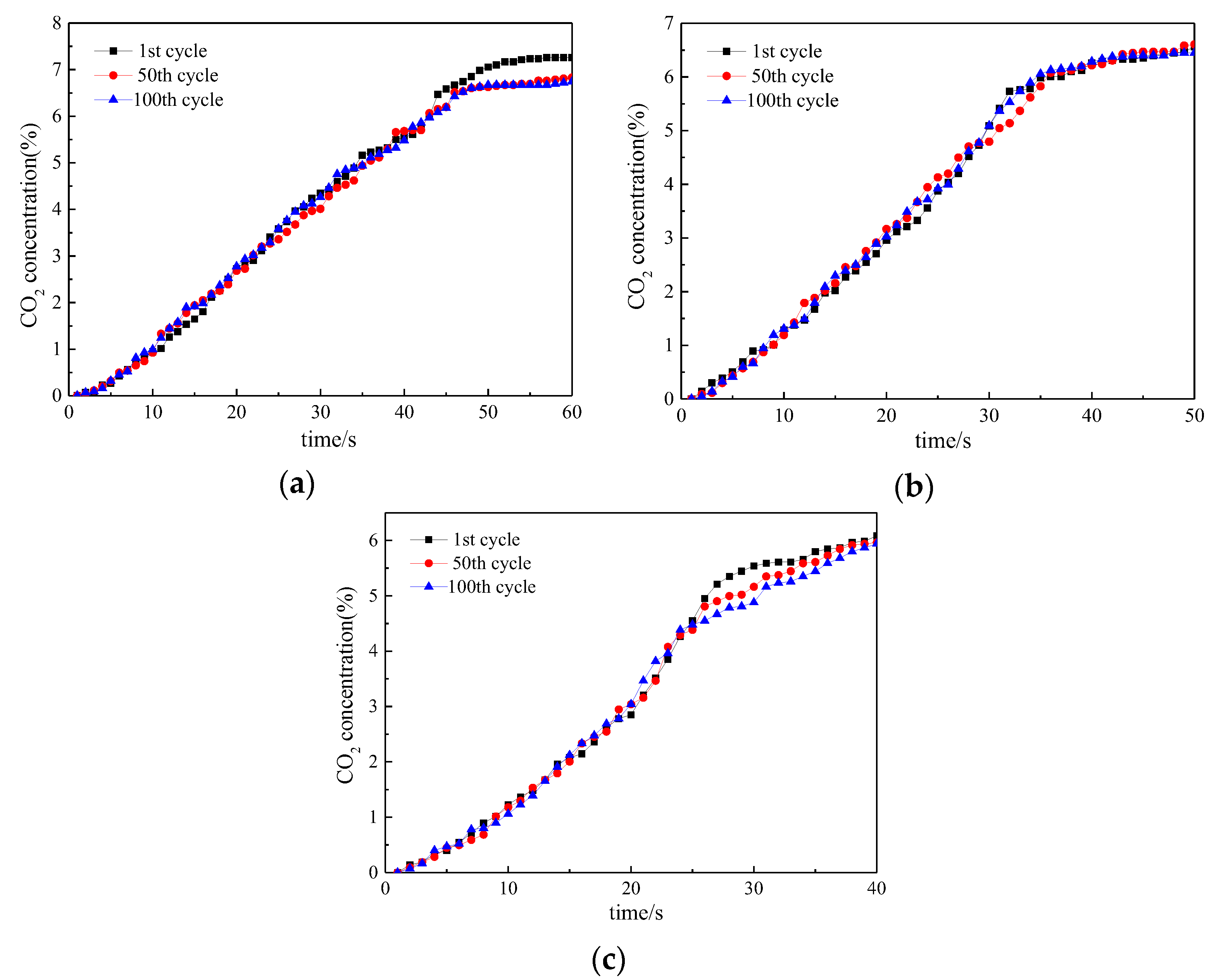
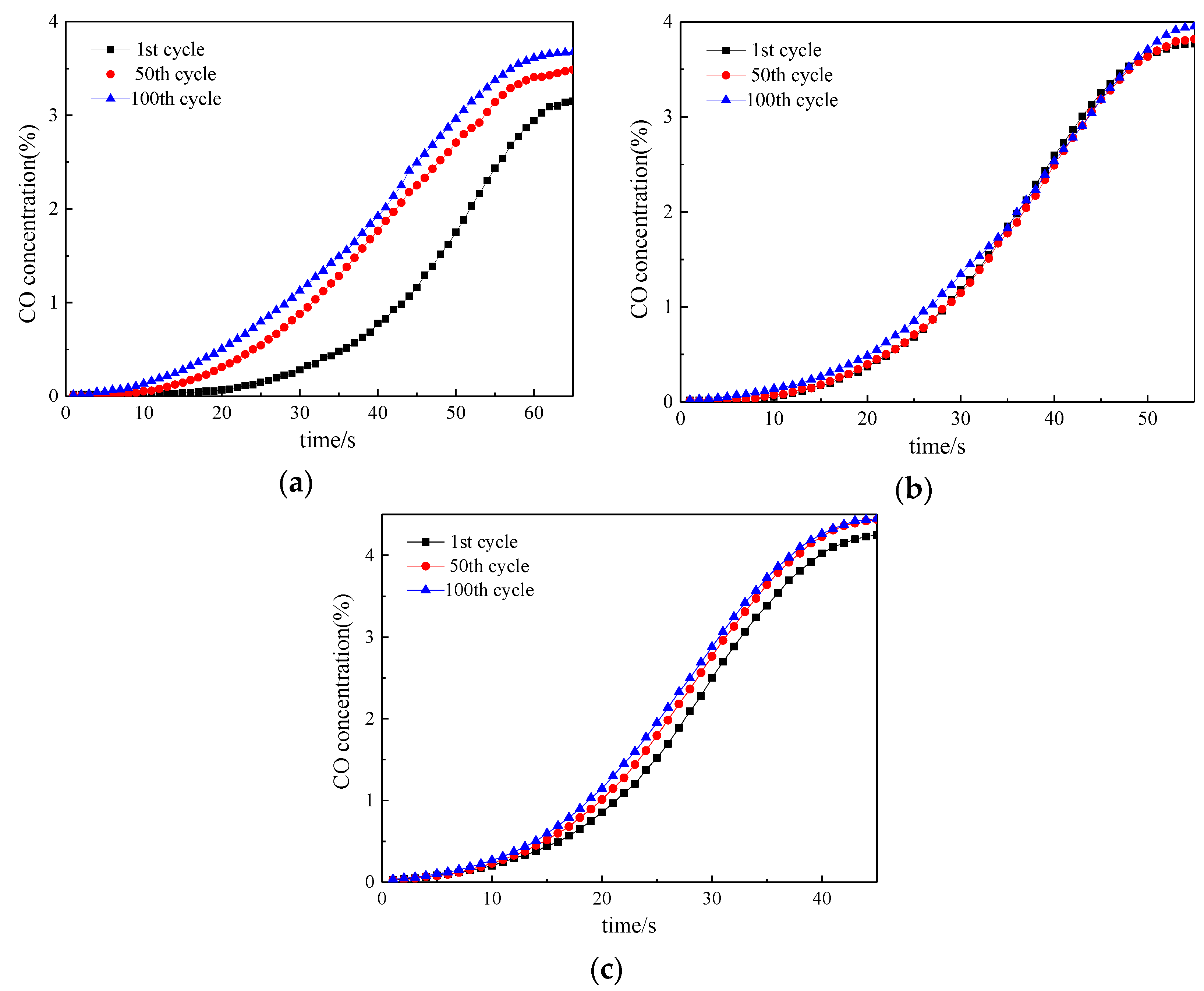
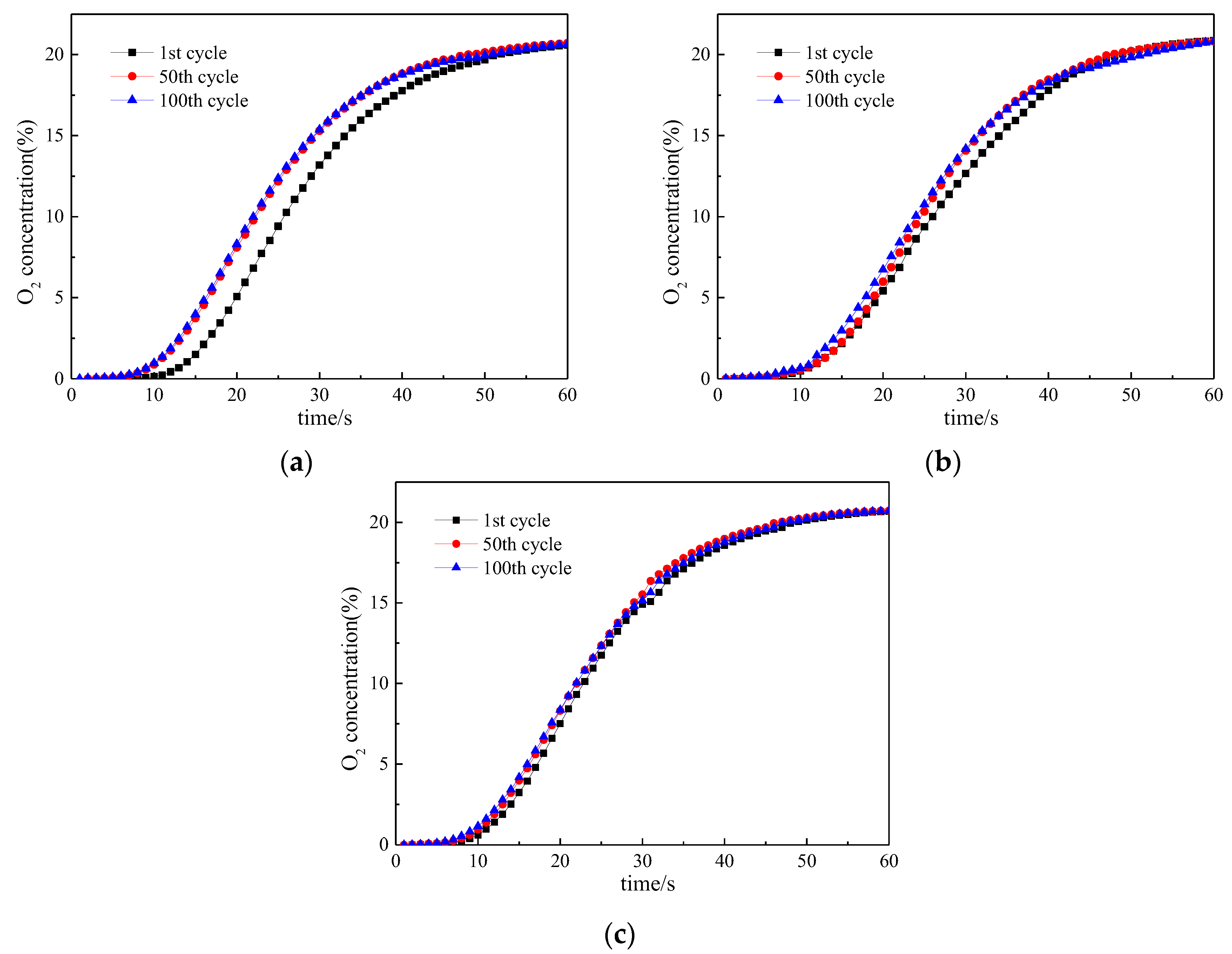
3. Reactivity of Oxygen Carrier with Different Reaction Times
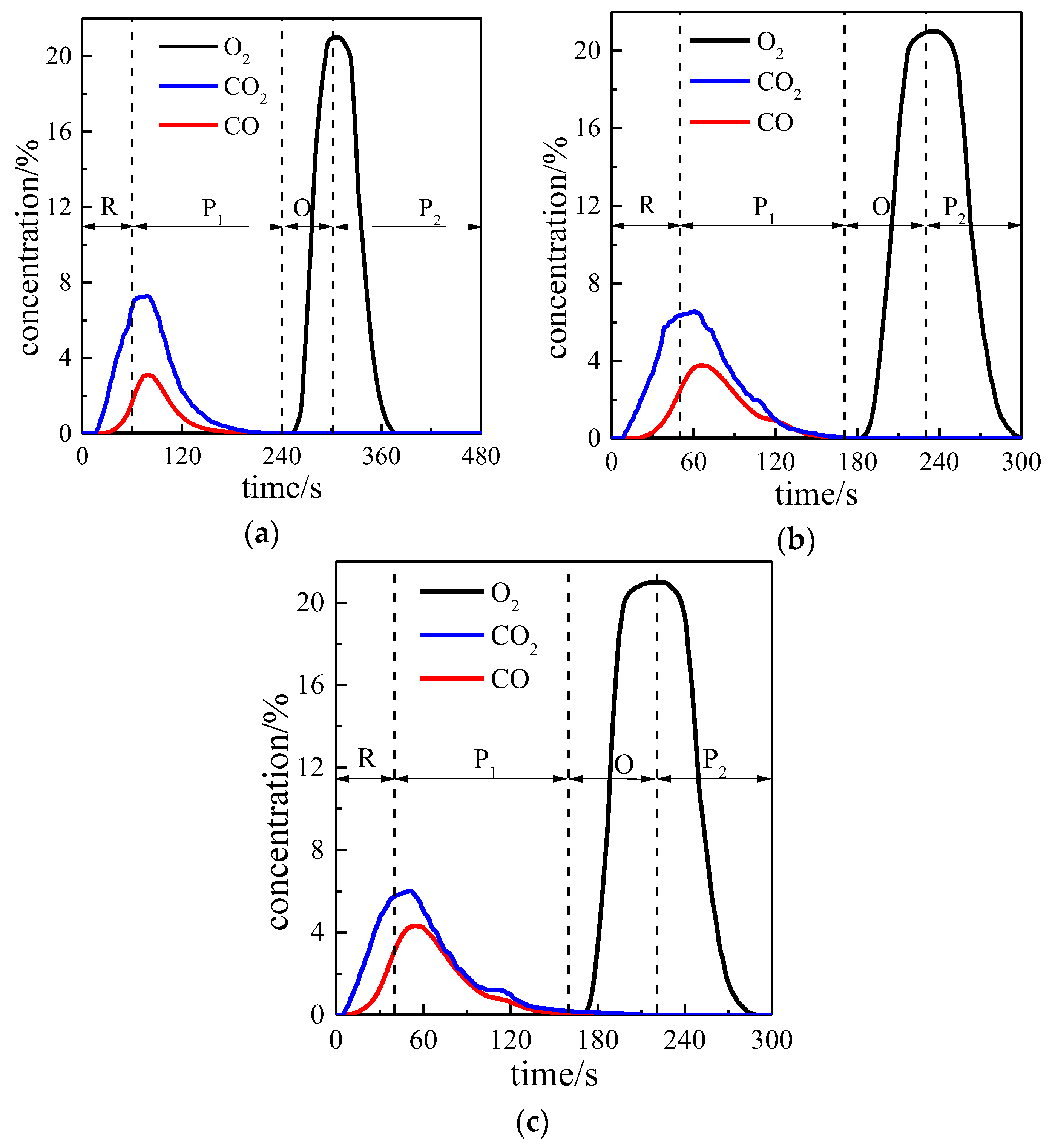
3.1. Reactivity in a Single Cycle
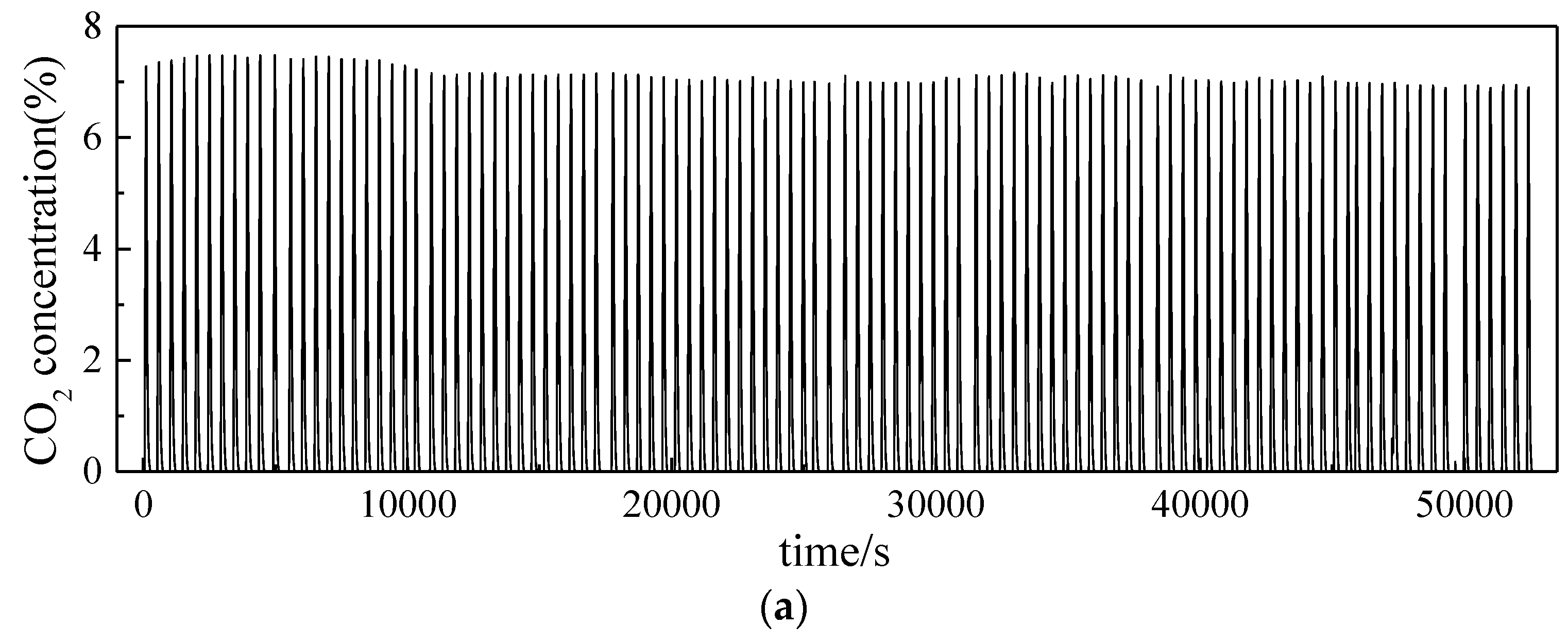
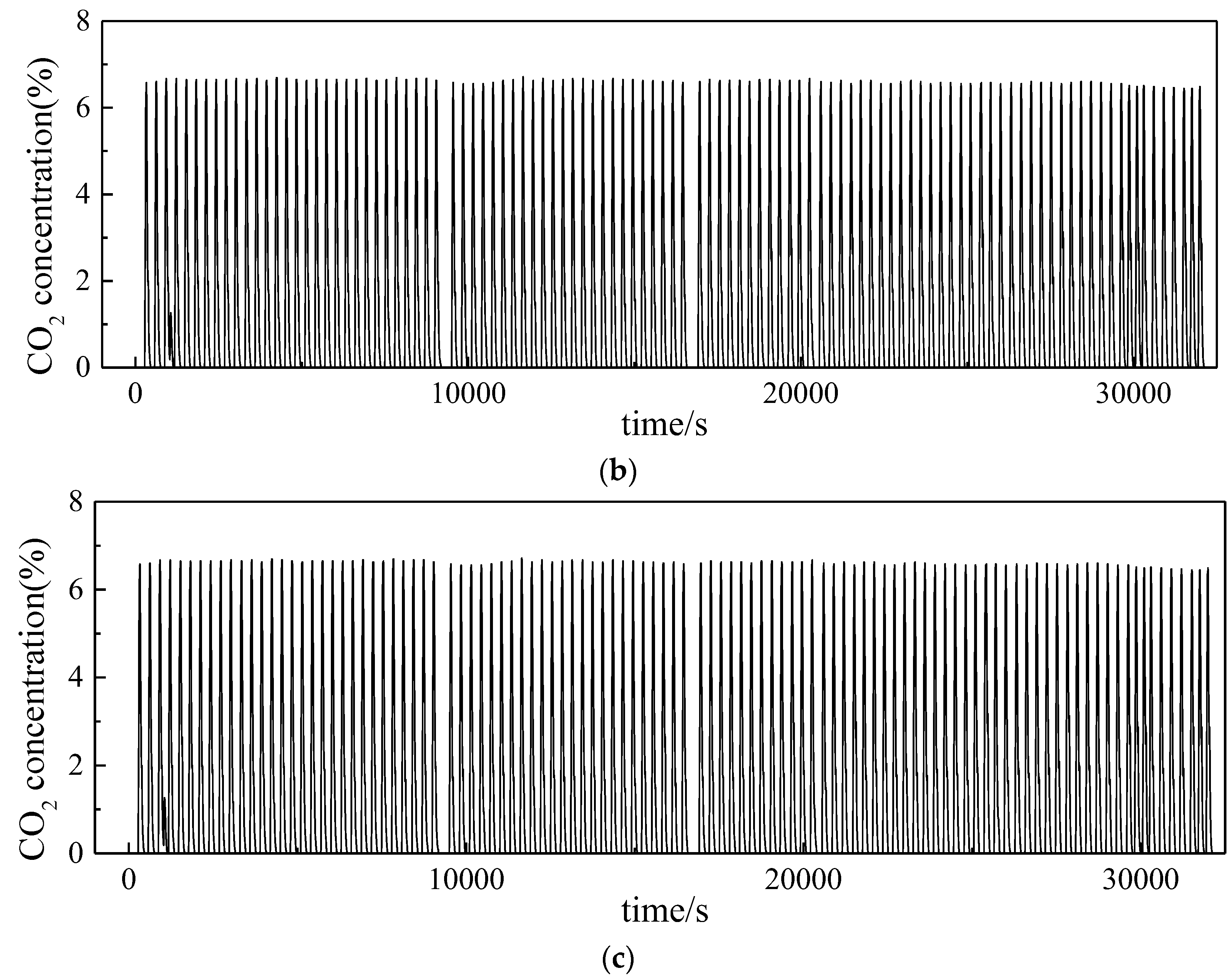
3.2. Investigation on Multi Cycles
3.3. Lifetime Assessment of Oxygen Carrier
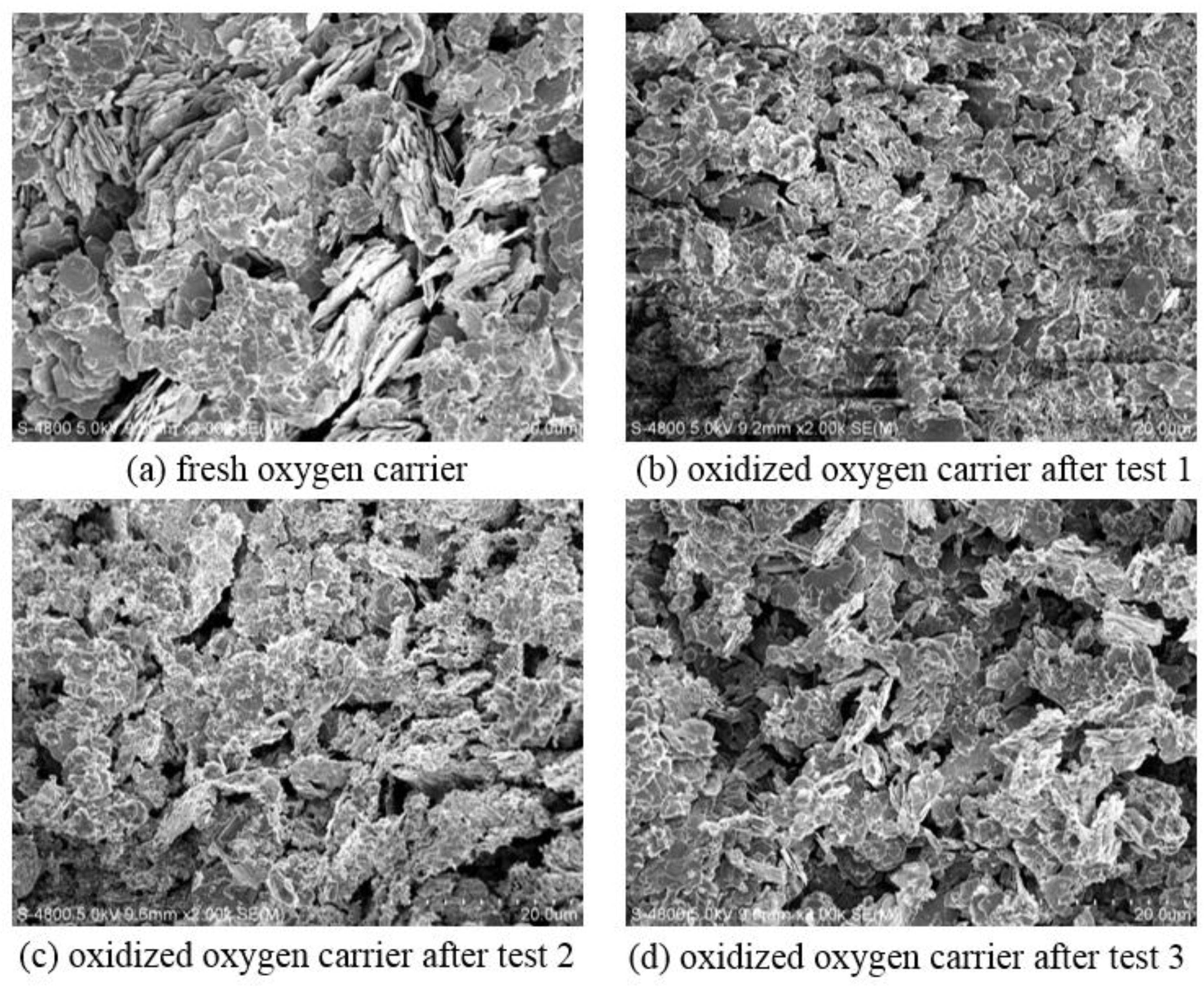
4. Analyses and Discussion
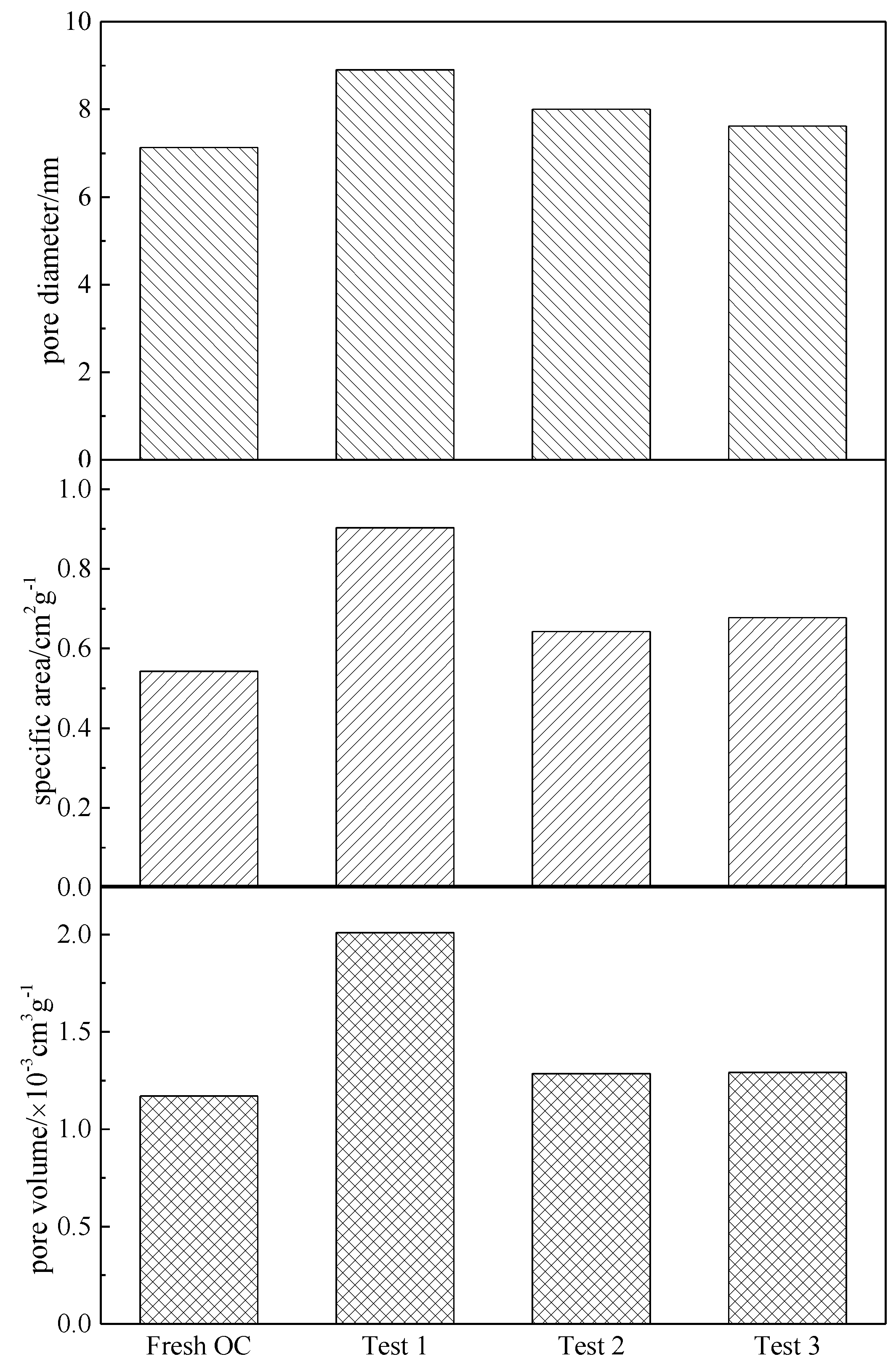
4.1. SEM Analysis
4.2. XRD and BET Analyses
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lewis, W.K.; Gilliland, E.R. Productions of Pure Carbon Dioxide. U.S. Patent No. 2,665,972, 12 January 1954. [Google Scholar]
- Richter, H.J.; Knoche, K.F. Reversibility of Combustion Processes; ACS Publications: Washington, DC, USA, 1983. [Google Scholar]
- Ishida, M.; Zheng, D.; Akehata, T. Evaluation of a chemical-looping-combustion power-generation system by graphic exergy analysis. Energy 1987, 12, 147–154. [Google Scholar] [CrossRef]
- Ishida, M.; Jin, H. A new advanced power-generation system using chemical-looping combustion. Energy 1994, 19, 415–422. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, G.; Deng, Z.; Zhao, K.; He, F.; Chen, D.; Wei, G.; Zhang, A.; Zhao, Z.; Li, H. Investigation on gasification performance of sewage sludge using chemical looping gasification with iron ore oxygen carrier. Int. J. Hydrogen Energy 2017, 42, 25474–25491. [Google Scholar] [CrossRef]
- Riley, J.; Siriwardane, R.; Tian, H.; Benincosa, W.; Poston, J. Kinetic analysis of the interactions between calcium ferrite and coal char for chemical looping gasification applications: Identifying reduction routes and modes of oxygen transfer. Appl. Energy 2017, 201, 94–110. [Google Scholar] [CrossRef]
- Shi, B.; Wu, E.; Wu, W. Novel design of chemical looping air separation process for generating electricity and oxygen. Energy 2017, 134, 449–457. [Google Scholar] [CrossRef]
- Nandy, A.; Loha, C.; Gu, S.; Sarkar, P.; Karmakar, M.K.; Chatterjee, P.K. Present status and overview of Chemical Looping Combustion technology. Renew. Sustain. Energy Rev. 2016, 59, 597–619. [Google Scholar] [CrossRef]
- Wang, K.; Yu, Q.; Qin, Q. The thermodynamic method for selecting oxygen carriers used for chemical looping air separation. J. Therm. Anal. Calorim. 2013, 112, 747–753. [Google Scholar] [CrossRef]
- Gauthier, T.; Yazdanpanah, M.; Forret, A.; Amblard, B.; Lambert, A.; Bertholin, S. CLC, a promising concept with challenging development issues. Powder Technol. 2017, 316, 3–17. [Google Scholar] [CrossRef]
- Kwak, B.S.; Park, N.; Ryu, S.O.; Baek, J.; Ryu, H.; Kang, M. Improved reversible redox cycles on MTiOx (M = Fe, Co, Ni, and Cu) particles afforded by rapid and stable oxygen carrier capacity for use in chemical looping combustion of methane. Chem. Eng. J. 2017, 309, 617–627. [Google Scholar] [CrossRef]
- Kwak, B.S.; Park, N.; Baek, J.; Ryu, H.; Kang, M. Improvement of reduction and oxidation performance of MMgOx (M = Fe, Co, Ni, and Cu) particles for chemical looping combustion. Powder Technol. 2017, 312, 237–247. [Google Scholar] [CrossRef]
- Rydén, M.; Hanning, M.; Corcoran, A.; Lind, F. Oxygen Carrier Aided Combustion (OCAC) of wood chips in a semi-commercial circulating fluidized bed boiler using manganese ore as bed material. Appl. Sci. 2016, 6, 347. [Google Scholar] [CrossRef]
- Frick, V.; Rydén, M.; Leion, H. Investigation of Cu–Fe and Mn–Ni oxides as oxygen carriers for chemical-looping combustion. Fuel Process. Technol. 2016, 150, 30–40. [Google Scholar] [CrossRef]
- Azimi, G.; Leion, H.; Mattisson, T.; Rydén, M.; Snijkers, F.; Lyngfelt, A. Mn–Fe Oxides with Support of MgAl2O4, CeO2, ZrO2 and Y2O3–ZrO2 for Chemical-Looping Combustion and Chemical-Looping with Oxygen Uncoupling. Ind. Eng. Chem. Res. 2014, 53, 10358–10365. [Google Scholar] [CrossRef]
- Rydén, M.; Lyngfelt, A.; Mattisson, T. CaMn0.875Ti0.125O3 as oxygen carrier for chemical-looping combustion with oxygen uncoupling (CLOU)—Experiments in a continuously operating fluidized-bed reactor system. Int. J. Greenh. Gas Control 2011, 5, 56–66. [Google Scholar] [CrossRef]
- Zhang, Y.; Doroodchi, E.; Moghtaderi, B. Comprehensive Study of Fe2O3/Al2O3 Reduction with Ultralow Concentration Methane under Conditions Pertinent to Chemical Looping Combustion. Energy Fuel 2015, 29, 1951–1960. [Google Scholar] [CrossRef]
- Wang, B.; Yan, R.; Lee, D.H.; Zheng, Y.; Zhao, H.; Zheng, C. Characterization and evaluation of Fe2O3/Al2O3 oxygen carrier prepared by sol–gel combustion synthesis. J. Anal. Appl. Pyrol. 2011, 91, 105–113. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, H.; Wei, Y.; Zheng, C. Self-assembly template combustion synthesis of a core–shell CuO@TiO2–Al2O3 hierarchical structure as an oxygen carrier for the chemical-looping processes. Combust. Flame 2015, 162, 3030–3045. [Google Scholar] [CrossRef]
- Zhang, Y.; Doroodchi, E.; Moghtaderi, B.; Han, X.; Liu, Y. Hydrogen Production from Ventilation Air Methane in a Dual-Loop Chemical Looping Process. Energy Fuel 2016, 30, 4372–4380. [Google Scholar] [CrossRef]
- Song, H.; Doroodchi, E.; Moghtaderi, B. Redox Characteristics of Fe–Ni/SiO2 Bimetallic Oxygen Carriers in CO under Conditions Pertinent to Chemical Looping Combustion. Energy Fuel 2012, 26, 75–84. [Google Scholar] [CrossRef]
- Adánez-Rubio, I.; Arjmand, M.; Leion, H.; Gayán, P.; Abad, A.; Mattisson, T.; Lyngfelt, A. Investigation of Combined Supports for Cu-Based Oxygen Carriers for Chemical-Looping with Oxygen Uncoupling (CLOU). Energy Fuel 2013, 27, 3918–3927. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Chen, L.; Qian, D.; Neathery, J.K.; Kozo, S.; Liu, K. Investigation of a Canadian Ilmenite as an Oxygen Carrier for Chemical Looping Combustion. Energy Fuel 2013, 27, 5987–5995. [Google Scholar] [CrossRef]
- Knutsson, P.; Linderholm, C. Characterization of ilmenite used as oxygen carrier in a 100 kW chemical-looping combustor for solid fuels. Appl. Energy 2015, 157, 368–373. [Google Scholar] [CrossRef]
- Demirel, Y.; Matzen, M.; Winters, C.; Gao, X. Capturing and using CO2 as feedstock with chemical looping and hydrothermal technologies. Int. J. Energy Res. 2015, 39, 1011–1047. [Google Scholar] [CrossRef]
- Mendiara, T.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Abad, A.; Adánez, J. On the use of a highly reactive iron ore in Chemical Looping Combustion of different coals. Fuel 2014, 126, 239–249. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Jin, B.; Zhao, J.; Sun, C.; Snape, C.E. Experimental Evaluation of a Chinese Sulfur-Containing Lean Iron Ore as the Oxygen Carrier for Chemical-Looping Combustion. Ind. Eng. Chem. Res. 2016, 55, 428–435. [Google Scholar] [CrossRef]
- Sundqvist, S.; Arjmand, M.; Mattisson, T.; Rydén, M.; ALyngfelt, A. Screening of different manganese ores for chemical-looping combustion (CLC) and chemical-looping with oxygen uncoupling (CLOU). Int. J. Greenh. Gas Control 2015, 43, 179–188. [Google Scholar] [CrossRef]
- Arjmand, M.; Leion, H.; Mattisson, T.; Lyngfelt, A. Investigation of different manganese ores as oxygen carriers in chemical-looping combustion (CLC) for solid fuels. Appl. Energy 2014, 113, 1883–1894. [Google Scholar] [CrossRef]
- Wang, K.; Tian, X.; Zhao, H. Sulfur behavior in chemical-looping combustion using a copper ore oxygen carrier. Appl. Energy 2016, 166, 84–95. [Google Scholar] [CrossRef]
- Matzen, M.; Pinkerton, J.; Wang, X.; Demirel, Y. Use of natural ores as oxygen carriers in chemical looping combustion: A review. Int. J. Greenh. Gas Control 2017, 65, 1–14. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H. Application of CaO-Decorated Iron Ore for Inhibiting Chlorobenzene during in Situ Gasification Chemical Looping Combustion of Plastic Waste. Energy Fuel 2016, 30, 5999–6008. [Google Scholar] [CrossRef]
- Leion, H.; Lyngfelt, A.; Johansson, M.; Jerndal, E.; Mattisson, T. The use of ilmenite as an oxygen carrier in chemical-looping combustion. Chem. Eng. Res. Des. 2008, 86, 1017–1026. [Google Scholar] [CrossRef]
- Leion, H.; Mattisson, T.; Lyngfelt, A. Use of ores and industrial products as oxygen carriers in chemical-looping combustion. Energy Fuel 2009, 23, 2307–2315. [Google Scholar] [CrossRef]
- Chen, L.; Bao, J.; Kong, L.; Combs, M.; Nikolic, H.S.; Fan, Z.; Liu, K. Activation of ilmenite as an oxygen carrier for solid-fueled chemical looping combustion. Appl. Energy 2017, 197, 40–51. [Google Scholar] [CrossRef]
- Tian, H.; Siriwardane, R.; Simonyi, T.; Poston, J. Natural Ores as Oxygen Carriers in Chemical Looping Combustion. Energy Fuel 2013, 27, 4108–4118. [Google Scholar] [CrossRef]
- Schwebel, G.L.; Filippou, D.; Hudon, G.; Tworkowski, M.; Gipperich, A.; Krumm, W. Experimental comparison of two different ilmenites in fluidized bed and fixed bed chemical-looping combustion. Appl. Energy 2014, 113, 1902–1908. [Google Scholar] [CrossRef]
- Jiang, S.; Shen, L.; Niu, X.; Ge, H.; Gu, H. Chemical Looping Co-combustion of Sewage Sludge and Zhundong Coal with Natural Hematite as the Oxygen Carrier. Energy Fuel 2016, 30, 1720–1729. [Google Scholar] [CrossRef]
- Wang, X.; Jin, B.; Zhu, X.; Liu, H. Experimental Evaluation of a Novel 20 kWth in Situ Gasification Chemical Looping Combustion Unit with an Iron Ore as the Oxygen Carrier. Ind. Eng. Chem. Res. 2016, 55, 11775–11784. [Google Scholar] [CrossRef]
- Xiao, R.; Chen, L.; Saha, C.; Zhang, S.; Bhattacharya, S. Pressurized chemical-looping combustion of coal using an iron ore as oxygen carrier in a pilot-scale unit. Int. J. Greenh. Gas Control 2012, 10, 363–373. [Google Scholar] [CrossRef]
- Adanez, J.; Cuadrat, A.; Abad, A.; Gayan, P.; de Diego, L.F.; Garcia-Labiano, F. Ilmenite Activation during Consecutive Redox Cycles in Chemical-Looping Combustion. Energy Fuel 2010, 24, 1402–1413. [Google Scholar] [CrossRef]
- Schwebel, G.L.; Leion, H.; Krumm, W. Comparison of natural ilmenites as oxygen carriers in chemical-looping combustion and influence of water gas shift reaction on gas composition. Chem. Eng. Res. Des. 2012, 90, 1351–1360. [Google Scholar] [CrossRef]
- Gu, H.; Shen, L.; Xiao, J.; Zhang, S.; Song, T. Chemical Looping Combustion of Biomass/Coal with Natural Iron Ore as Oxygen Carrier in a Continuous Reactor. Energy Fuel 2011, 25, 446–455. [Google Scholar] [CrossRef]
- Bao, J.; Li, Z.; Sun, H.; Cai, N. Experiment and rate equation modeling of Fe oxidation kinetics in chemical looping combustion. Combust. Flame 2013, 160, 808–817. [Google Scholar] [CrossRef]
- Mendiara, T.; Abad, A.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Adánez, J. Use of an Fe-Based Residue from Alumina Production as an Oxygen Carrier in Chemical-Looping Combustion. Energy Fuel 2012, 26, 1420–1431. [Google Scholar] [CrossRef]
- Bao, J.; Li, Z.; Cai, N. Interaction between iron-based oxygen carrier and four coal ashes during chemical looping combustion. Appl. Energy 2014, 115, 549–558. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, H.; Zheng, Y.; Liu, Z.; Zheng, C. Chemical Looping Combustion of Petroleum Coke with CuFe2O4 as Oxygen Carrier. Chem. Eng. J. 2013, 36, 1488–1495. [Google Scholar]




| Component | Fe2O3 | SiO2 | Al2O3 | CaO | MgO | SO3 | Others |
|---|---|---|---|---|---|---|---|
| Mass Fraction/% | 44.16 | 18.93 | 2.98 | 17.09 | 7.43 | 2.35 | 7.06 |
| Test Number | Test 1 | Test 2 | Test 3 |
|---|---|---|---|
| Reducing gas | 11% CO | 11% CO | 11% CO |
| Oxidization gas | Pure air | Pure air | Pure air |
| Sweep gas | 99.99% N2 | 99.99% N2 | 99.99% N2 |
| Reduction temperature (K) | 1223 | 1223 | 1223 |
| Oxidization temperature (K) | 1223 | 1223 | 1223 |
| Gas flow (L/min) | 1 | 1 | 1 |
| CO injection time (s) | 60 | 50 | 40 |
| The first purge time (s) | 180 | 120 | 120 |
| Air injection time (s) | 60 | 60 | 60 |
| The second purge time (s) | 180 | 70 | 80 |
| Time of a single cycle (s) | 480 | 300 | 300 |
| Test | /L | /L | Conversion of CO |
|---|---|---|---|
| 1 | 0.021 | 0.089 | 80.9% |
| 2 | 0.025 | 0.067 | 73.9% |
| 3 | 0.028 | 0.045 | 61.6% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, X.; Hu, X.; Shao, Y.; Jin, Z.; Jin, B. Performance Evaluation of a Chinese Lean Iron Ore as the Oxygen Carrier in Multi and Short-Time Redox Cycles. Appl. Sci. 2018, 8, 682. https://doi.org/10.3390/app8050682
Wang X, Wang X, Hu X, Shao Y, Jin Z, Jin B. Performance Evaluation of a Chinese Lean Iron Ore as the Oxygen Carrier in Multi and Short-Time Redox Cycles. Applied Sciences. 2018; 8(5):682. https://doi.org/10.3390/app8050682
Chicago/Turabian StyleWang, Xudong, Xiaojia Wang, Xiaoyu Hu, Yali Shao, Zhaoyang Jin, and Baosheng Jin. 2018. "Performance Evaluation of a Chinese Lean Iron Ore as the Oxygen Carrier in Multi and Short-Time Redox Cycles" Applied Sciences 8, no. 5: 682. https://doi.org/10.3390/app8050682





