Photooxidation of p-Arsanilic Acid in Aqueous Solution by UV/Persulfate Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Photochemical Experiments
2.3. Analytical Methods
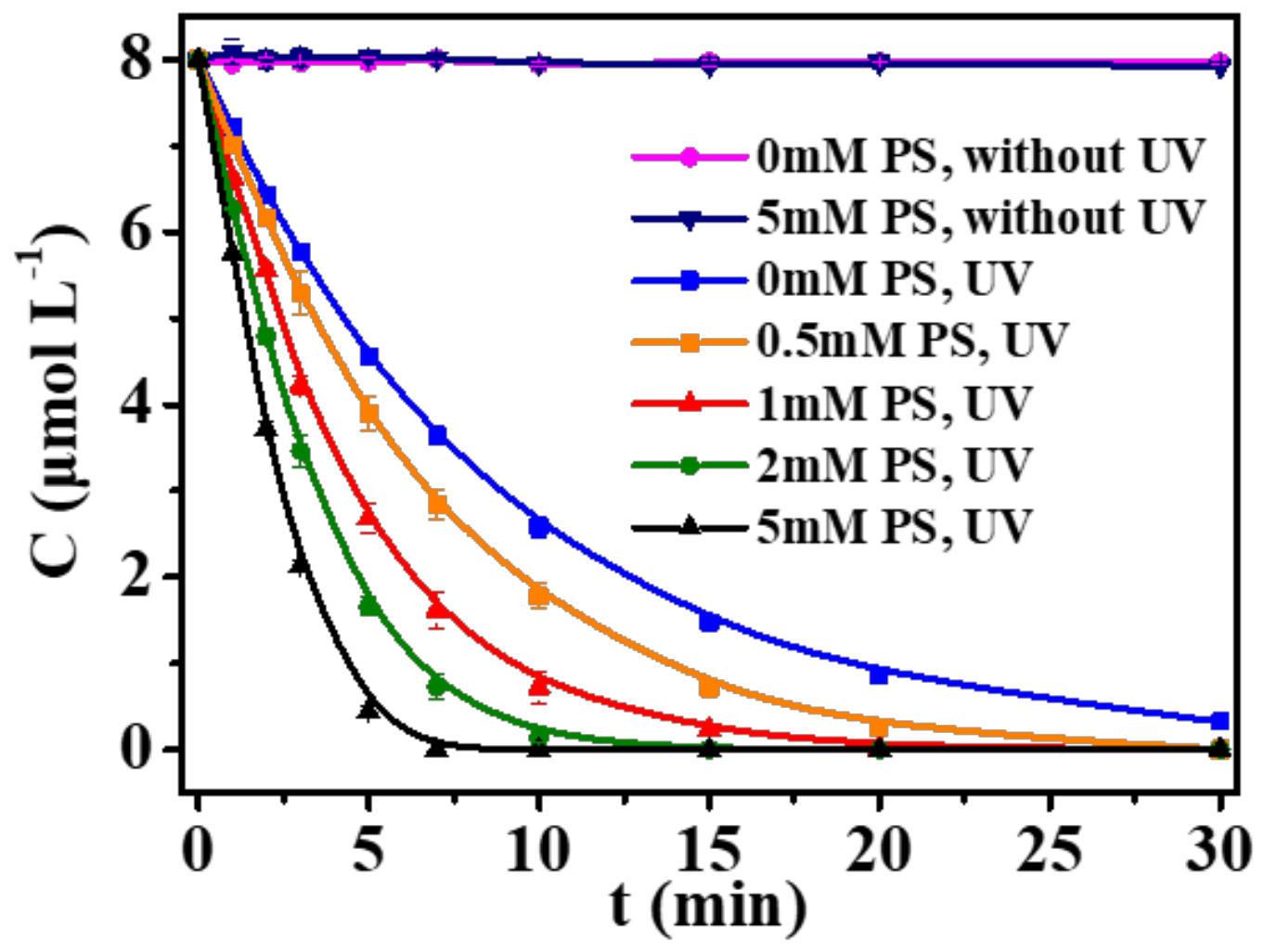
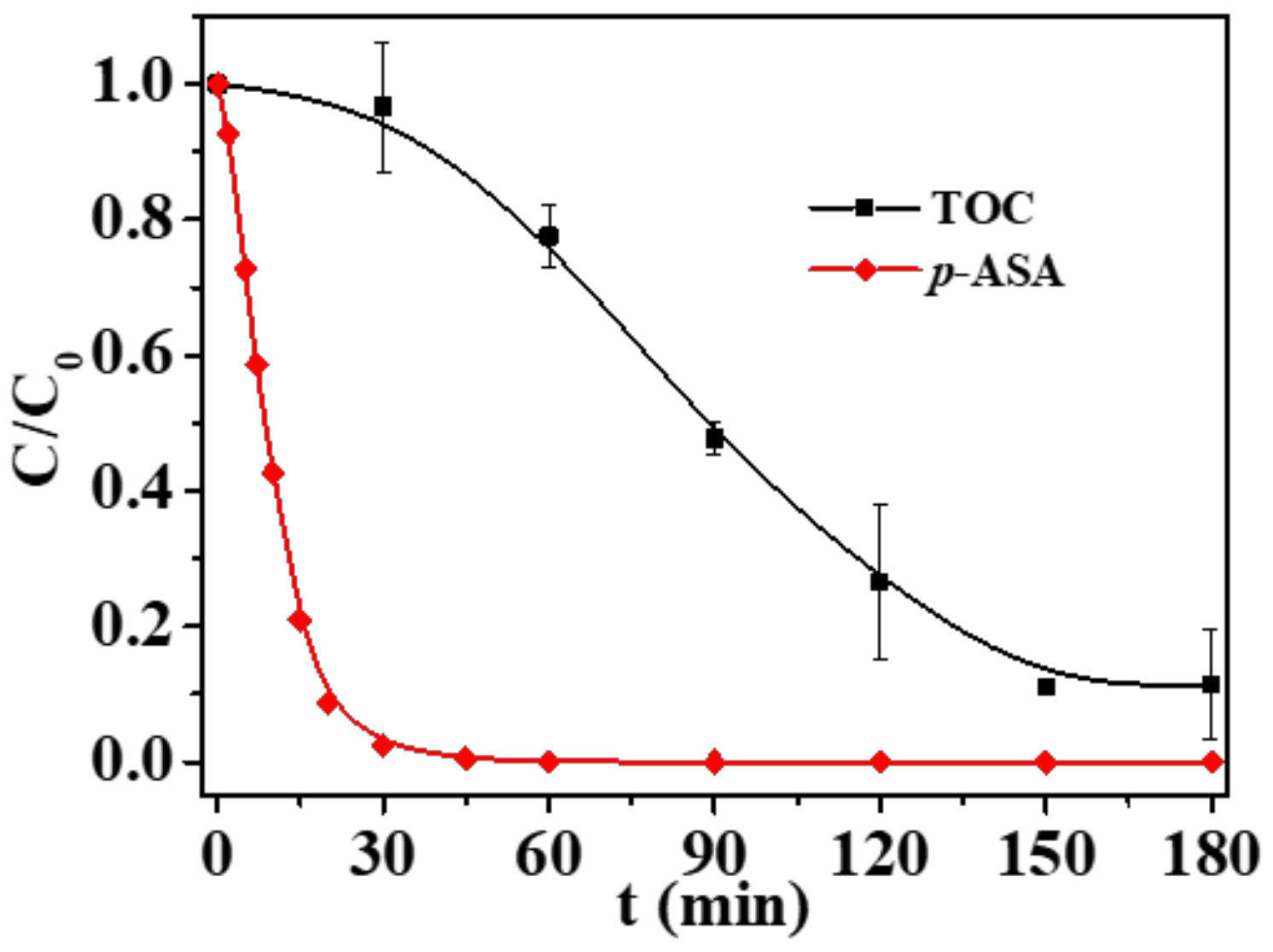
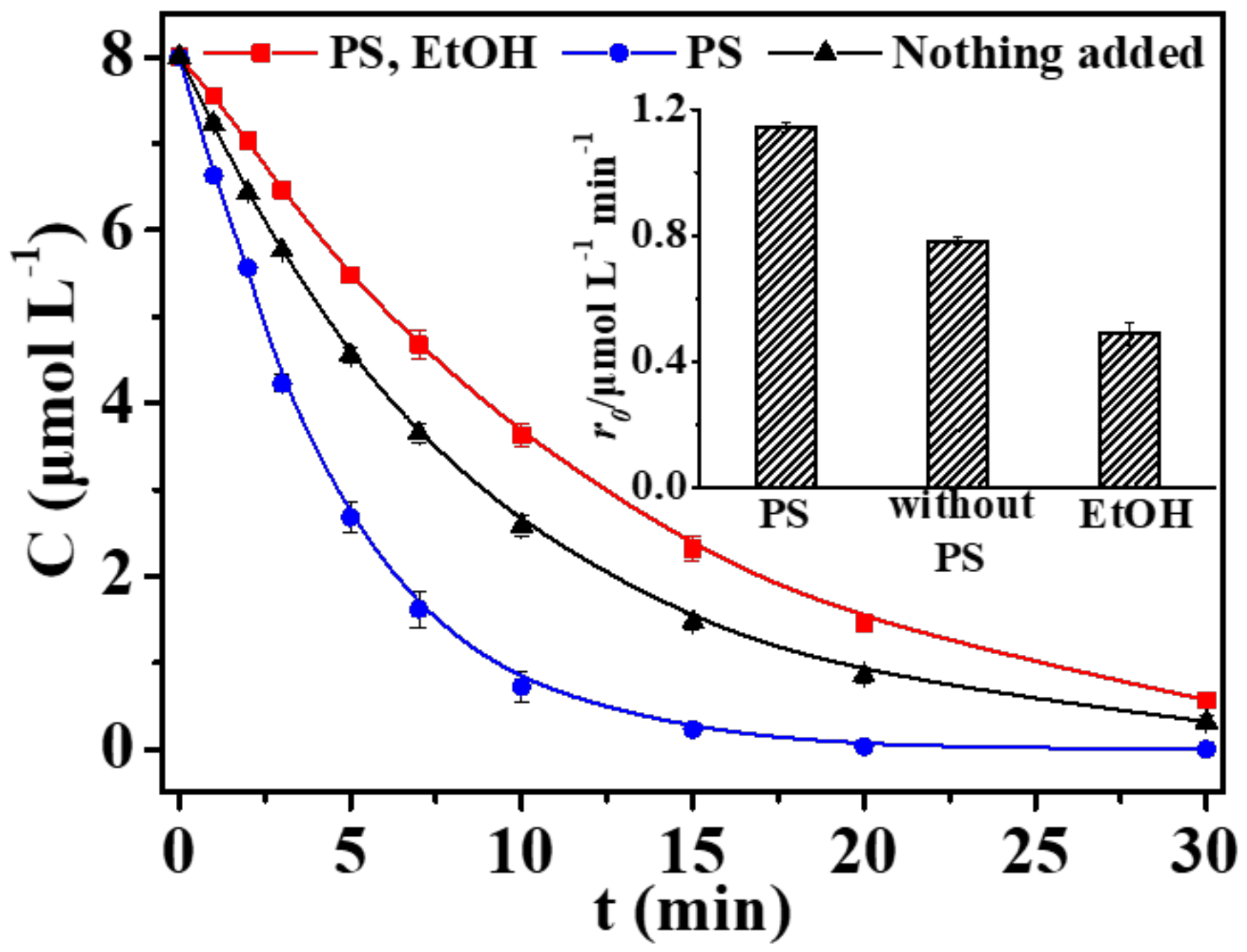
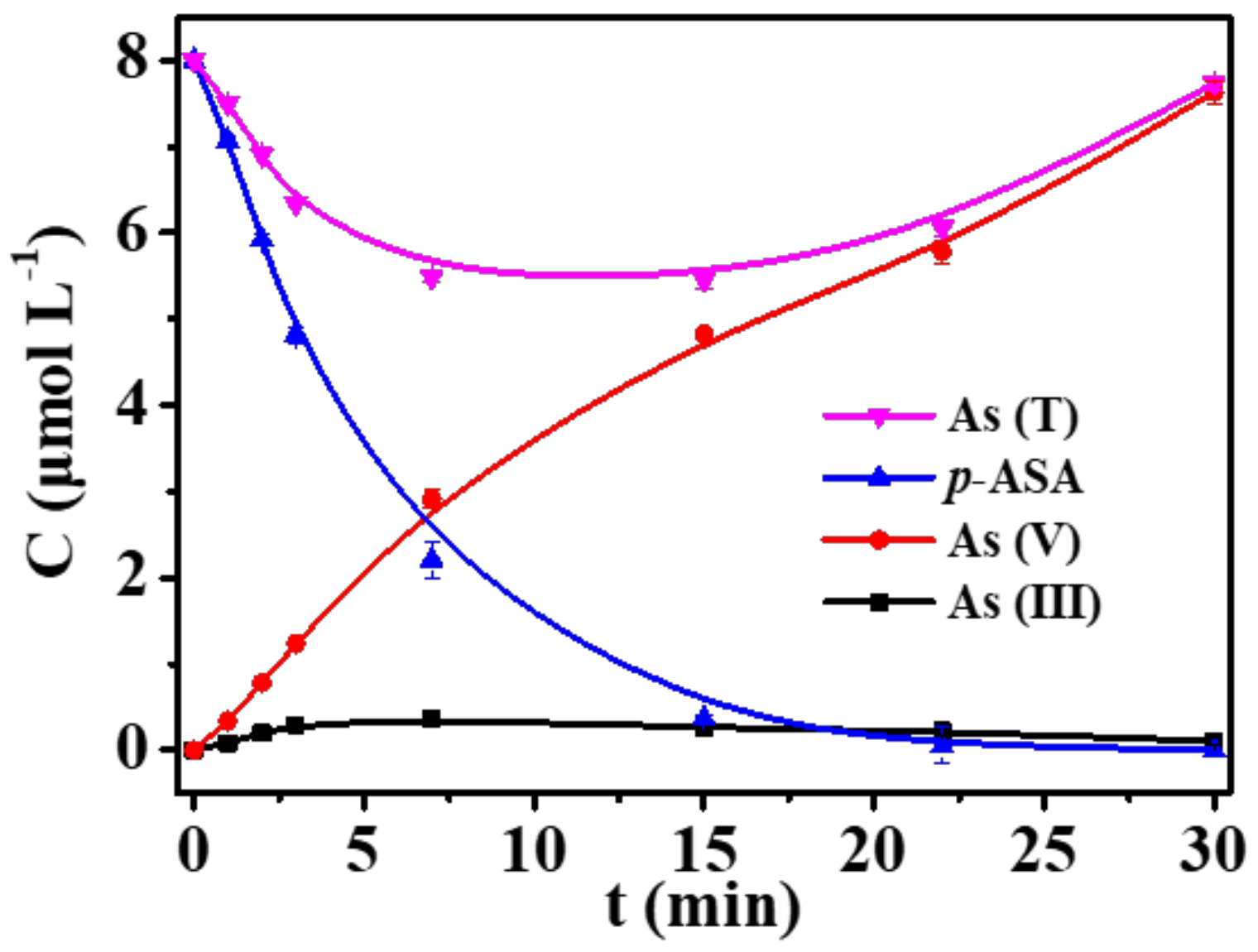
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Christen, K. Chickens, manure, and arsenic. Environ. Sci. Technol. 2001, 35, 184A–185A. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, W.; Hu, Y.; Cheng, H. Extraction and detection of organoarsenic feed additives and common arsenic species in environmental matrices by HPLC-ICP-MS. Microchem. J. 2013, 108, 38–45. [Google Scholar] [CrossRef]
- Mangalgiri, K.P.; Adak, A.; Blaney, L. Organoarsenicals in poultry litter: Detection, fate, and toxicity. Environ. Int. 2015, 75, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Aschbacher, P.W.; Feil, V.J. Fate of [14C]Arsanilic Acid in Pigs and Chickens. J. Agric. Food Chem. 1991, 39, 146–149. [Google Scholar] [CrossRef]
- Liu, Q.; Peng, H.; Lu, X.; Le, X.C. Enzyme-assisted extraction and liquid chromatography mass spectrometry for the determination of arsenic species in chicken meat. Anal. Chim. Acta 2015, 888, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Hu, B.; Liu, Q.; Yang, Z.; Lu, X.; Huang, R.; Li, X.F.; Zuidhof, M.J.; Le, X.C. Liquid chromatography combined with atomic and molecular mass spectrometry for speciation of arsenic in chicken liver. J. Chromatogr. A 2014, 1370, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Stolz, J.F.; Perera, E.; Kilonzo, B.; Kail, B.; Crable, B.; Fisher, E.; Ranganathan, M.; Wormer, L.; Basu, P. Biotransformation of 3-nitro-4-hydroxybenzene arsonic acid (Roxarsone) and release of inorganic arsenic by clostridium species. Environ. Sci. Technol. 2007, 41, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Shi, Y.; Kong, D.; Lu, J. Degradation of roxarsone in a sulfate radical mediated oxidation process and formation of polynitrated by-products. RSC Adv. 2016, 6, 82040–82048. [Google Scholar] [CrossRef]
- Garbarino, J.R.; Bednar, A.J.; Rutherford, D.W.; Beyer, R.S.; Wershaw, R.L. Environmental fate of roxarsone in poultry litter I. Degradation of roxarsone during composting. Environ. Sci. Technol. 2003, 37, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Bednar, A.J.; Garbarino, J.R.; Ferrer, I.; Rutherford, D.W.; Wershaw, R.L.; Ranville, J.F.; Wildeman, T.R. Photodegradation of roxarsone in poultry litter leachates. Sci. Total Environ. 2003, 302, 237–245. [Google Scholar] [CrossRef]
- Jones, F.T. A broad view of arsenic. Poult. Sci. 2007, 86, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Woolson, E.A. Fate of arsenicals in different environmental substrates. Environ. Health Perspect. 1977, 19, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.D.; Wang, Y.J.; Liu, C.; Qin, W.X.; Zhou, D.M. Kinetics, intermediates and acute toxicity of arsanilic acid photolysis. Chemosphere 2014, 107, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Hu, Y.; Cheng, H. Rapid degradation of p-arsanilic acid with simultaneous arsenic removal from aqueous solution using Fenton process. Water Res. 2016, 89, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, J.; Chen, W.; Yu, Y.; Liu, Z.; Li, J.; Wu, F. Multiple transformation pathways of p-arsanilic acid to inorganic arsenic species in water during UV disinfection. J. Environ. Sci. (China) 2016, 47, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Czaplicka, M.; Bratek, Ł.; Jaworek, K.; Bonarski, J.; Pawlak, S. Photo-oxidation of p-arsanilic acid in acidic solutions: Kinetics and the identification of by-products and reaction pathways. Chem. Eng. J. 2014, 243, 364–371. [Google Scholar] [CrossRef]
- Czaplicka, M.; Jaworek, K.; Bąk, M. Study of photodegradation and photooxidation of p-arsanilic acid in water solutions at pH = 7: Kinetics and by-products. Environ. Sci. Pollut. Res. 2015, 22, 16927–16935. [Google Scholar] [CrossRef] [PubMed]
- Koda, E.; Miszkowska, A.; Sieczka, A. Levels of Organic Pollution Indicators in Groundwater at the Old Landfill and Waste Management Site. Appl. Sci. 2017, 7, 638. [Google Scholar] [CrossRef]
- Wang, A.; Teng, Y.; Hu, X.; Wu, L.; Huang, Y.; Luo, Y.; Christie, P. Photodegradation of diphenylarsinic acid by UV-C light: Implication for its remediation. J. Hazard. Mater. 2016, 308, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.S.; Huang, C.P. Mineralization of aniline in aqueous solution by electrochemical activation of persulfate. Chemosphere 2015, 125, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.S.; Jhou, Y.C.; Huang, C.P. Mineralization of dinitrotoluenes in industrial wastewater by electro-activated persulfate oxidation. Chem. Eng. J. 2014, 252, 166–172. [Google Scholar] [CrossRef]
- Wang, C.W.; Liang, C. Oxidative degradation of TMAH solution with UV persulfate activation. Chem. Eng. J. 2014, 254, 472–478. [Google Scholar] [CrossRef]
- Pari, S.; Wang, I.A.; Liu, H.Z.; Wong, B.M. Sulfate radical oxidation of aromatic contaminants: A detailed assessment of density functional theory and high-level quantum chemical methods. Environ. Sci. Process. Impacts 2018, 19, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Kang, J.; Wang, Y.; Indrawirawan, S.; Wang, S. Insights into Heterogeneous Catalysis of Persulfate Activation on Dimensional-Structured Nanocarbons. ACS Catal. 2015, 5, 4629–4636. [Google Scholar] [CrossRef]
- Huang, W.; Bianco, A.; Brigante, M.; Mailhot, G. UVA-UVB activation of hydrogen peroxide and persulfate for advanced oxidation processes: Efficiency, mechanism and effect of various water constituents. J. Hazard. Mater. 2018, 347, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Olmez-Hanci, T.; Arslan-Alaton, I. Comparison of sulfate and hydroxyl radical based advanced oxidation of phenol. Chem. Eng. J. 2013, 224, 10–16. [Google Scholar] [CrossRef]
- Xu, J.; Ding, W.; Wu, F.; Mailhot, G.; Zhou, D.; Hanna, K. Rapid catalytic oxidation of arsenite to arsenate in an iron(III)/sulfite system under visible light. Appl. Catal. B Environ. 2016, 186, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhang, P. Photodegradation of perfluorooctanoic acid in water under irradiation of 254 nm and 185 nm light by use of persulfate. Water Sci. Technol. 2006, 54, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.Q.; Gao, N.Y.; Deng, Y.; Yang, Y.Q.; Ma, Y. Ultraviolet (UV) light-activated persulfate oxidation of sulfamethazine in water. Chem. Eng. J. 2012, 195–196, 248–253. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, Y.; Huang, W.; Huang, S. Degradation kinetics and mechanism of aniline by heat-assisted persulfate oxidation. J. Environ. Sci. 2012, 24, 821–826. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Xie, X.F.; Huang, W.L.; Huang, S. Degradation of aniline by Fe2+-activated persulfate oxidation at ambient temperature. J. Cent. South Univ. 2013, 20, 1010–1014. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiang, Q.; Glatt, H.; Platt, K.L.; Goldstein, B.D.; Witz, G. Studies on pathways of ring opening of benzene in a Fenton system. Free Radic. Biol. Med. 1995, 18, 411–419. [Google Scholar] [CrossRef]
- Olmez-Hanci, T.; Arslan-Alaton, I.; Genc, B. Bisphenol A treatment by the hot persulfate process: Oxidation products and acute toxicity. J. Hazard. Mater. 2013, 263, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.M.; Fernández, G.; Klamerth, N.; Maldonado, M.I.; Álvarez, P.M.; Malato, S. Efficiency of different solar advanced oxidation processes on the oxidation of bisphenol A in water. Appl. Catal. B Environ. 2010, 95, 228–237. [Google Scholar] [CrossRef]
- Liu, H.Z.; Bruton, T.A.; Li, W.; Buren, J.V.; Prasse, C.; Doyle, F.M.; Sedlak, D.L. Oxidation of benzene by persulfate in the presence of Fe(III)- and Mn(IV)-containing oxides: Stoichiometric efficiency and transformation products. Environ. Sci. Technol. 2016, 50, 890–898. [Google Scholar] [CrossRef] [PubMed]
| [M + H]+ | Structure |
|---|---|
| 122.9 |  |
| 123.7 |  |
| 190.7 |  |
| 278.9 |  |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, X.; Xu, J.; Pozdnyakov, I.P.; Liu, Z. Photooxidation of p-Arsanilic Acid in Aqueous Solution by UV/Persulfate Process. Appl. Sci. 2018, 8, 615. https://doi.org/10.3390/app8040615
Shen X, Xu J, Pozdnyakov IP, Liu Z. Photooxidation of p-Arsanilic Acid in Aqueous Solution by UV/Persulfate Process. Applied Sciences. 2018; 8(4):615. https://doi.org/10.3390/app8040615
Chicago/Turabian StyleShen, Xiangyi, Jing Xu, Ivan P. Pozdnyakov, and Zizheng Liu. 2018. "Photooxidation of p-Arsanilic Acid in Aqueous Solution by UV/Persulfate Process" Applied Sciences 8, no. 4: 615. https://doi.org/10.3390/app8040615





