Riding the Plane Wave: Considerations for In Vivo Study Designs Employing High Frame Rate Ultrasound
Abstract
:1. Introduction
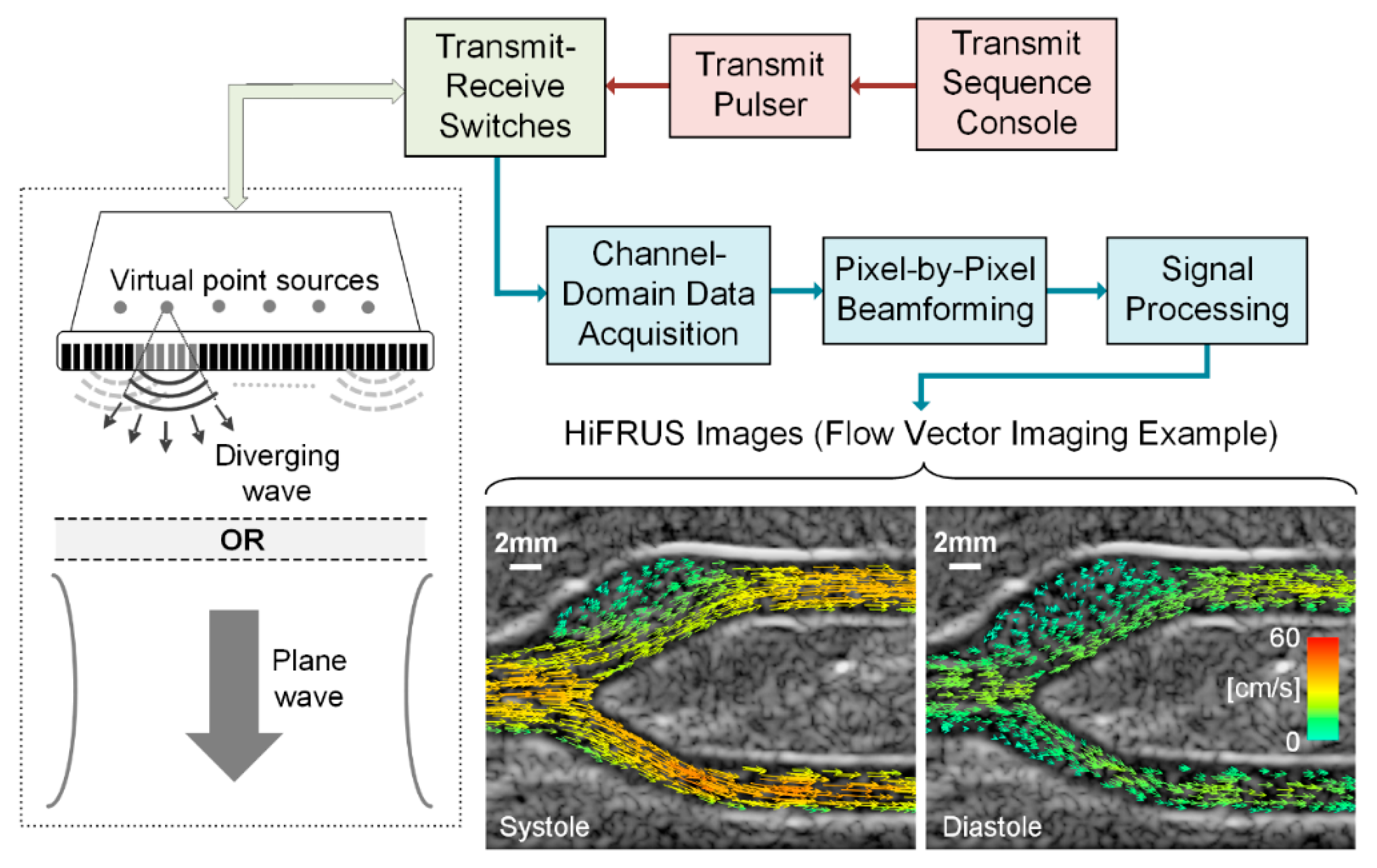
2. A Synopsis of High Frame Rate Ultrasound Technology
3. Framework for In Vivo Cardiovascular Studies
3.1. Validation of Methods Prior to In Vivo Data Collection
3.2. Standards in Technical Reporting
3.3. Human Considerations
4. Research Example: Neurovascular Control and Complex Blood Flow
5. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Quiñones, M.A.; Otto, C.M.; Stoddard, M.; Waggoner, A.; Zoghbi, W.A. Recommendations for quantification of Doppler echocardiography: A report from the Doppler quantification task force of the nomenclature and standards committee of the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2002, 15, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Rooke, T.W.; Hirsch, A.T.; Misra, S.; Sidawy, A.N.; Beckman, J.A.; Findeiss, L.K.; Golzarian, J.; Gornik, H.L.; Halperin, J.L.; Jaff, M.R.; et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (Updating the 2005 guideline). Catheter. Cardiovasc. Interv. 2012, 79, 501–531. [Google Scholar] [CrossRef] [PubMed]
- Steel, R.; Ramnarine, K.V.; Davidson, F.; Fish, P.J.; Hoskins, P.R. Angle-independent estimation of maximum velocity through stenoses using vector Doppler ultrasound. Ultrasound Med. Biol. 2003, 29, 575–584. [Google Scholar] [CrossRef]
- Oates, C.P.; Naylor, A.R.; Hartshorne, T.; Charles, S.M.; Fail, T.; Humphries, K.; Aslam, M.; Khodabakhsh, P. Joint recommendations for reporting carotid ultrasound investigations in the United Kingdom. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Tempel, D.; Van Haperen, R.; Van Der Baan, A.; Grosveld, F.; Daemen, M.J.A.P.; Krams, R.; De Crom, R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 2006, 113, 2744–2753. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.A.; Nikolov, S.; Yu, A.C.H.; Garcia, D. Ultrasound vector flow imaging—Part II: Parallel systems. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 63, 1722–1732. [Google Scholar] [CrossRef] [PubMed]
- Yiu, B.Y.S.; Lai, S.S.M.; Yu, A.C.H. Vector projectile imaging: Time-resolved dynamic visualization of complex flow patterns. Ultrasound Med. Biol. 2014, 40, 2295–2309. [Google Scholar] [CrossRef] [PubMed]
- Yiu, B.Y.S.; Yu, A.C.H. High-frame-rate ultrasound color-encoded speckle imaging of complex flow dynamics. Ultrasound Med. Biol. 2013, 39, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.M.; Pedersen, M.M.; Hansen, K.L.; Nielsen, M.B.; Jensen, J.A. Demonstration of a vector velocity technique. Ultraschall Med. 2011, 32, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.K.; Chee, A.J.Y.; Yiu, B.Y.S.; Tsang, A.C.O.; Chow, K.W.; Yu, A.C.H. Wall-less flow phantoms with tortuous vascular geometries: Design principles and a patient-specific model fabrication example. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; del Alamo, J.C.; Tanne, D.; Yotti, R.; Cortina, C.; Bertrand, E.; Antoranz, J.C.; Perez-David, E.; Rieu, R.; Fernandez-Aviles, F.; et al. Two-dimensional intraventricular flow mapping by digital processing conventional color-doppler echocardiography images. IEEE Trans. Med. Imaging 2010, 29, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Hendabadi, S.; Bermejo, J.; Benito, Y.; Yotti, R.; Fernández-Avilés, F.; Del Álamo, J.C.; Shadden, S.C. Topology of blood transport in the human left ventricle by novel processing of doppler echocardiography. Ann. Biomed. Eng. 2013, 41, 2603–2616. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.A.; Nikolov, S.I.; Yu, A.C.H.; Garcia, D. Ultrasound vector flow imaging—Part I: Sequential systems. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 63, 1704–1721. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.P.; Pedrizzetti, G.; Kilner, P.J.; Kheradvar, A.; Ebbers, T.; Tonti, G.; Fraser, A.G.; Narula, J. Emerging trends in CV flow visualization. JACC Cardiovasc. Imaging 2012, 5, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Goddi, A.; Fanizza, M.; Bortolotto, C.; Raciti, M.V.; Fiorina, I.; He, X.; Du, Y.; Calliada, F. Vector flow imaging techniques: An innovative ultrasonographic technique for the study of blood flow. J. Clin. Ultrasound 2017, 45, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Shattuck, D.P.; Weinshenker, M.D.; Smith, S.W.; von Ramm, O.T. Explososcan: A parallel processing technique for high speed ultrasound imaging with linear phased arrays. J. Acoust. Soc. Am. 1984, 75, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Tanter, M.; Fink, M. Ultrafast imaging in biomedical ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014, 61, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.A.; Holm, O.; Jensen, L.J.; Bendsen, H.; Nikolov, S.I.; Tomov, B.G.; Munk, P.; Hansen, M.; Salomonsen, K.; Hansen, J.; et al. Ultrasound research scanner for real-time synthetic aperture data acquisition. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005, 52, 881–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.Y.; Cheng, J.; Wang, J. High frame rate imaging system for limited diffraction array beam imaging with square-wave aperture weightings. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2006, 53, 1796–1811. [Google Scholar] [CrossRef] [PubMed]
- Tortoli, P.; Bassi, L.; Boni, E.; Dallai, A.; Guidi, F.; Ricci, S. ULA-OP: An advanced open platform for ultrasound research. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 2207–2216. [Google Scholar] [CrossRef] [PubMed]
- Daigle, R.E. Ultrasound Imaging System with Pixel Oriented Processing. U.S. Patent 8,287,456B2, 16 October 2012. [Google Scholar]
- Jensen, J.A.; Holten-Lund, H.; Nilsson, R.T.; Hansen, M.; Larsen, U.D.; Domsten, R.P.; Tomov, B.G.; Stuart, M.B.; Nikolov, S.I.; Pihl, M.J.; et al. SARUS: A synthetic aperture real-time ultrasound system. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 1838–1852. [Google Scholar] [CrossRef] [PubMed]
- Boni, E.; Bassi, L.; Dallai, A.; Guidi, F.; Meacci, V.; Ramalli, A.; Ricci, S.; Tortoli, P. ULA-OP 256: A 256-channel open scanner for development and real-time implementation of new ultrasound methods. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 63, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.; Kremkau, F. Medical ultrasound systems. Interface Focus 2011, 1, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Boni, E.; Bassi, L.; Dallai, A.; Meacci, V.; Ramalli, A.; Scaringella, M.; Guidi, F.; Ricci, S.; Tortoli, P. Architecture of an ultrasound system for continuous real-time high frame rate imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- So, H.; Chen, J.; Yiu, B.; Yu, A. Medical ultrasound imaging: To GPU or not to GPU? IEEE Micro 2011, 31, 54–65. [Google Scholar] [CrossRef] [Green Version]
- Yiu, B.Y.S.; Tsang, I.K.H.; Yu, A.C.H. GPU-based beamformer: Fast realization of plane wave compounding and synthetic aperture imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58, 1698–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Arguedas, C.J.; Romero-Laorden, D.; Martinez-Graullera, O.; Perez-Lopez, M.; Gomez-Ullate, L. An ultrasonic imaging system based on a new SAFT approach and a GPU beamformer. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Yiu, B.Y.S.; Yu, A.C.H. GPU-based minimum variance beamformer for synthetic aperture imaging of the eye. Ultrasound Med. Biol. 2015, 41, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.W.; Hsu, K.H.; Li, P.C. Graphics processing unit-based high-frame-rate color doppler ultrasound processing. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 1856–1860. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, S.; Palmeri, M.; Nightingale, K. GPU-based real-time small displacement estimation with ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Chee, A.J.Y.; Yiu, B.Y.S.; Yu, A.C.H. A GPU-parallelized Eigen-based clutter filter framework for ultrasound color flow imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Broxvall, M.; Emilsson, K.; Thunberg, P. Fast GPU based adaptive filtering of 4D echocardiography. IEEE Trans. Med. Imaging 2012, 31, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Tanter, M.; Bercoff, J.; Sandrin, L.; Fink, M. Ultrafast compound imaging for 2-D motion vector estimation: Application to transient elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2002, 49, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, M.; Walczak, M.; Witek, B.; Kulesza, P.; Sielewicz, K. Modular & scalable ultrasound platform with GPU processing. In Proceedings of the 2012 IEEE International Ultrasonics Symposium (IUS), Dresden, Germany, 7–10 October 2012. [Google Scholar] [CrossRef]
- Cheung, C.; Yu, A.; Salimi, N.; Yiu, B.; Tsang, I.; Kerby, B.; Azar, R.; Dickie, K. Multi-channel pre-beamformed data acquisition system for research on advanced ultrasound imaging methods. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 243–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bercoff, J. Ultrafast ultrasound imaging. In Ultrasound Imaging-Medical Applications; Minin, I.V., Minin, O.V., Eds.; InTech: New York, NY, USA, 2011; pp. 3–24. [Google Scholar]
- Hansen, K.L.; Møller-Sørensen, H.; Pedersen, M.M.; Hansen, P.M.; Kjaergaard, J.; Lund, J.T.; Nilsson, J.C.; Jensen, J.A.; Nielsen, M.B. First report on intraoperative vector flow imaging of the heart among patients with healthy and diseased aortic valves. Ultrasonics 2015, 56, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Goddi, A.; Bortolotto, C.; Fiorina, I.; Raciti, M.V.; Fanizza, M.; Turpini, E.; Boffelli, G.; Calliada, F. High-frame rate vector flow imaging of the carotid bifurcation. Insights Imaging 2017, 8, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.A.; Nikolov, S.I.; Gammelmark, K.L.; Pedersen, M.H. Synthetic aperture ultrasound imaging. Ultrasonics 2006, 44, e5–e15. [Google Scholar] [CrossRef] [PubMed]
- Montaldo, G.; Tanter, M.; Bercoff, J.; Benech, N.; Fink, M. Coherent plane-wave compounding for very high frame rate ultrasonography and transient elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Nikolov, S.I.; Jensen, J.A. In-vivo synthetic aperture flow imaging in medical ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2003, 50, 848–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yiu, B.Y.S.; Yu, A.C.H. Least-squares multi-angle Doppler estimators for plane-wave vector flow imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 63, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.H.G.; Saris, A.E.C.M.; Vaka, N.R.; Nillesen, M.M.; de Korte, C.L. Ultrafast vascular strain compounding using plane wave transmission. J. Biomech. 2014, 47, 815–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruizinga, P.; Mastik, F.; van den Oord, S.C.H.; Schinkel, A.F.L.; Bosch, J.G.; de Jong, N.; van Soest, G.; van der Steen, A.F.W. High-definition imaging of carotid artery wall dynamics. Ultrasound Med. Biol. 2014, 40, 2392–2403. [Google Scholar] [CrossRef] [PubMed]
- Kruizinga, P.; Mastik, F.; Bosch, J.G.; De Jong, N.; Van Der Steen, A.F.W.; Van Soest, G. Measuring submicrometer displacement vectors using high-frame-rate ultrasound imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2015, 62, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; He, Q.; Huang, C.; Liu, K.; Shao, J.; Luo, J. High frame rate and high line density ultrasound imaging for local pulse wave velocity estimation using motion matching: A feasibility study on vessel phantoms. Ultrasonics 2016, 67, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Strachinaru, M.; Bosch, J.G.; van Dalen, B.M.; van Gils, L.; van der Steen, A.F.W.; de Jong, N.; Geleijnse, M.L.; Vos, H.J. Cardiac shear wave elastography using a clinical ultrasound system. Ultrasound Med. Biol. 2017, 43, 1596–1606. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Yiu, B.Y.S.; Yu, A.C.H. Vector flow visualization of urinary flow dynamics in a bladder outlet obstruction model. Ultrasound Med. Biol. 2017, 43, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Cikes, M.; Tong, L.; Sutherland, G.R.; D’hooge, J. Ultrafast cardiac ultrasound imaging: Technical principles, applications, and clinical benefits. JACC Cardiovasc. Imaging 2014, 7, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Ramalli, A.; Tortoli, P.; Fradella, G.; Caciolli, S.; Luo, J.; D’hooge, J. Wide-angle tissue doppler imaging at high frame rate using multi-line transmit beamforming: An experimental validation in vivo. IEEE Trans. Med. Imaging 2016, 35, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Faurie, J.; Baudet, M.; Assi, K.C.; Auger, D.; Gilbert, G.; Tournoux, F.; Garcia, D. Intracardiac vortex dynamics by high-frame-rate Doppler vortography—In vivo comparison with vector flow mapping and 4-D flow MRI. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 64, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberge, J.; Lovstakken, L.; Fadnes, S.; Rodriguez-Morales, A.; Vierendeels, J.; Segers, P.; Swillens, A. Assessing the performance of ultrafast vector flow imaging in the neonatal heart via multiphysics modeling and in vitro experiments. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 63, 1772–1785. [Google Scholar] [CrossRef] [PubMed]
- Tremblay-Darveau, C.; Williams, R.; Milot, L.; Bruce, M.; Burns, P.N. Visualizing the tumor microvasculature with a nonlinear plane-wave Doppler imaging scheme based on amplitude modulation. IEEE Trans. Med. Imaging 2016, 35, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.; Provost, J.; Tanter, M.; Pernot, M. 4D ultrafast ultrasound flow imaging: In vivo quantification of arterial volumetric flow rate in a single heartbeat. Phys. Med. Biol. 2016, 61, L48–L61. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.; Udesen, J.; Gran, F.; Jensen, J.; Bachmann Nielsen, M. In-vivo examples of flow patterns with the fast vector velocity ultrasound method. Ultraschall Med. 2009, 30, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Rossini, L.; Martinez-Legazpi, P.; Vu, V.; Fernández-Friera, L.; Pérez del Villar, C.; Rodríguez-López, S.; Benito, Y.; Borja, M.G.; Pastor-Escuredo, D.; Yotti, R.; et al. A clinical method for mapping and quantifying blood stasis in the left ventricle. J. Biomech. 2016, 49, 2152–2161. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.L.; Møller-Sørensen, H.; Kjaergaard, J.; Jensen, M.B.; Lund, J.T.; Pedersen, M.M.; Lange, T.; Jensen, J.A.; Nielsen, M.B. Analysis of systolic backflow and secondary helical blood flow in the ascending aorta using vector flow imaging. Ultrasound Med. Biol. 2016, 42, 899–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, K.L.; Udesen, J.; Oddershede, N.; Henze, L.; Thomsen, C.; Jensen, J.A.; Nielsen, M.B. In vivo comparison of three ultrasound vector velocity techniques to MR phase contrast angiography. Ultrasonics 2009, 49, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Swillens, A.; Segers, P.; Torp, H.; Løvstakken, L. Two-dimensional blood velocity estimation with ultrasound: Speckle tracking versus crossed-beam vector doppler based on flow simulations in a carotid bifurcation model. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2010, 57, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Chee, A.J.Y.; Ho, C.K.; Yiu, B.Y.S.; Yu, A.C.H. Walled Carotid Bifurcation Phantoms for Imaging Investigations of Vessel Wall Motion and Blood Flow Dynamics. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 63, 1852–1864. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, P.R. Simulation and Validation of Arterial Ultrasound Imaging and Blood Flow. Ultrasound Med. Biol. 2008, 34, 693–717. [Google Scholar] [CrossRef] [PubMed]
- Greyling, A.; van Mil, A.C.C.M.; Zock, P.L.; Green, D.J.; Ghiadoni, L.; Thijssen, D.H. Adherence to guidelines strongly improves reproducibility of brachial artery flow-mediated dilation. Atherosclerosis 2016, 248, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Failla, M.; Grappiolo, A.; Emanuelli, G.; Vitale, G.; Fraschini, N.; Bigoni, M.; Grieco, N.; Denti, M.; Giannattasio, C.; Mancia, G. Sympathetic tone restrains arterial distensibility of healthy and atherosclerotic subjects. J. Hypertens. 1999, 17, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Swierblewska, E.; Hering, D.; Kara, T.; Kunicka, K.; Kruszewski, P.; Bieniaszewski, L.; Boutouyrie, P.; Somers, V.K.; Narkiewicz, K. An independent relationship between muscle sympathetic nerve activity and pulse wave velocity in normal humans. J. Hypertens. 2010, 28, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Van Bortel, L.M.; Laurent, S.; Boutouyrie, P.; Chowienczyk, P.; Cruickshank, J.K.; De Backer, T.; Filipovsky, J.; Huybrechts, S.; Mattace-Raso, F.U.S.; Protogerou, A.D.; et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J. Hypertens. 2012, 30, 445–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thijssen, D.H.J.; Black, M.A.; Pyke, K.E.; Padilla, J.; Atkinson, G.; Harris, R.A.; Parker, B.; Widlansky, M.E.; Tschakovsky, M.E.; Green, D.J. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H2–H12. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, C.M.; Aznaouridis, K.A.; Karatzis, E.N.; Karatzi, K.N.; Stamatelopoulos, K.S.; Vamvakou, G.; Lekakis, J.P.; Mavrikakis, M.E. Effect of coffee on endothelial function in healthy subjects: The role of caffeine. Clin. Sci. 2005, 109, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Hijmering, M.L.; de Lange, D.W.; Lorsheyd, A.; Kraaijenhagen, R.J.; van de Wiel, A. Binge drinking causes endothelial dysfuntion, which is not prevented by wine polyphenols: A small trial in healthy volunteers. Neth. J. Med. 2007, 65, 29–35. [Google Scholar] [PubMed]
- Dawson, E.A.; Whyte, G.P.; Black, M.A.; Jones, H.; Hopkins, N.; Oxborough, D.; Gaze, D.; Shave, R.E.; Wilson, M.; George, K.P.; et al. Changes in vascular and cardiac function after prolonged strenuous exercise in humans. J. Appl. Physiol. 2008, 105, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Tinken, T.M.; Thijssen, D.H.J.; Hopkins, N.; Black, M.A.; Dawson, E.A.; Minson, C.T.; Newcomer, S.C.; Laughlin, M.H.; Cable, N.T.; Green, D.J. Impact of shear rate modulation on vascular function in humans. Hypertension 2009, 54, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Doonan, R.J.; Hausvater, A.; Scallan, C.; Mikhailidis, D.P.; Pilote, L.; Daskalopoulou, S.S. The effect of smoking on arterial stiffness. Hypertens. Res. 2010, 33, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, K.D.; Robertson, I.K.; Ball, M.J. Acute effects of food on postprandial blood pressure and measures of arterial stiffness in healthy humans. Am. J. Clin. Nutr. 2009, 90, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Novak, V.; Novak, P. Influence of respiration on heart rate and blood pressure fluctuations. J. Appl. Physiol. 1993, 74, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Ginghina, C.; Beladan, C.C.; Iancu, M.; Calin, A.; Popescu, B.A. Respiratory maneuvers in echocardiography: A review of clinical applications. Cardiovasc. Ultrasound 2009, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Cinthio, M.; Ahlgren, Å.R.; Bergkvist, J.; Jansson, T.; Persson, H.W.; Lindström, K. Longitudinal movements and resulting shear strain of the arterial wall. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H394–H402. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; Jüni, P.; Altman, D.; Egger, M.; Chan, A.; Altman, D.; Glasziou, P.; Meats, E.; et al. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Hines, E.A.; Brown, G.E. The cold pressor test for measuring the reactibility of the blood pressure: Data concerning 571 normal and hypertensive subjects. Am. Heart J. 1936, 11, 1–9. [Google Scholar] [CrossRef]
- Saab, P.G.; Llabre, M.M.; Hurwitz, B.E.; Schneiderman, N.; Wohlgemuth, W.; Durel, L.A.; Massie, C.; Nagel, J. The cold pressor test: Vascular and myocardial response patterns and their stability. Psychophysiology 1993, 30, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Kline, K.A.; Saab, P.G.; Llabre, M.M.; Spitzer, S.B.; Evans, J.D.; McDonald, P.A.G.; Schneiderman, N. Hemodynamic response patterns: Responder type differences in reactivity and recovery. Psychophysiology 2002, 39, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Flück, D.; Ainslie, P.N.; Bain, A.R.; Wildfong, K.W.; Morris, L.E.; Fisher, J.P. Extra- and intracranial blood flow regulation during the cold pressor test: Influence of age. J. Appl. Physiol. 2017, 123, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Au, J.S.; Bochnak, P.A.; Valentino, S.E.; Cheng, J.L.; Stöhr, E.J. Cardiac and haemodynamic influence on carotid artery longitudinal wall motion. Exp. Physiol. 2018, 103, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Lo, A.; Oehley, M.; Bartlett, A.; Adams, D.; Blyth, P.; Al-Ali, S. Anatomical variations of the common carotid artery bifurcation. AZN J. Surg. 2006, 76, 970–972. [Google Scholar] [CrossRef] [PubMed]
- Schulz, U.G.R.; Rothwell, P.M. Major variation in carotid bifurcation anatomy: A possible risk factor for plaque development? Stroke 2001, 32, 2522–2529. [Google Scholar] [CrossRef] [PubMed]
- Hertzog, M.A. Considerations in determining sample size for pilot studies. Res. Nurs. Health 2008, 31, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Pepe, M.S. Receiver operating characteristic methodology. J. Am. Stat. Assoc. 2000, 95, 308–311. [Google Scholar] [CrossRef]

| HiFRUS Parameter | Value |
|---|---|
| Scanning system | SonixTouch |
| Array pitch | 0.3048 mm |
| Probe frequency | 5 MHz |
| Emission method | Plane wave excitation |
| Transmit pulse duration | 2 cycles |
| Pulse repetition frequency | 10 kHz |
| Steering angles | −10°, 0°, +10° |
| Slow-time window size (or ensemble length) | 128 samples (12.8 ms) |
| Slow-time window step size | 4 samples (0.4 ms) |
| Effective frame rate | 833 fps |
| Scanning location | Left of image aligned 1 cm proximal to the carotid bifurcation |
| Collection duration | 3 s (16 GB on-board memory) |
| Recommendation | Reason |
|---|---|
| Pre-visit instructions | |
| 2 h fasted | Altered sympathetic activation |
| 6 h refrain from caffeine | Altered sympathetic activation |
| 12 h refrain from smoking | Acute effects on vascular structure and function |
| 12 h refrain from moderate-to-vigorous physical activity | Acute effects on vascular structure and function |
| 12 h refrain from alcohol | Acute effects on vascular structure and function |
| Record of current medications | Various acute and chronic effects on the vasculature |
| Participant preparation | |
| Assign unlinked participant ID | Ethical considerations for sensitive health information |
| 10 min rest period | Altered sympathetic activation upon arrival to the lab |
| Resting heart rate recording | Detail of the hemodynamic environment |
| Resting blood pressure recording | Detail of the hemodynamic environment |
| Probe holder placement | Reduction of motion artifacts |
| Breath hold during acquisition | Reduction of motion artifacts |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Au, J.S.; Hughson, R.L.; Yu, A.C.H. Riding the Plane Wave: Considerations for In Vivo Study Designs Employing High Frame Rate Ultrasound. Appl. Sci. 2018, 8, 286. https://doi.org/10.3390/app8020286
Au JS, Hughson RL, Yu ACH. Riding the Plane Wave: Considerations for In Vivo Study Designs Employing High Frame Rate Ultrasound. Applied Sciences. 2018; 8(2):286. https://doi.org/10.3390/app8020286
Chicago/Turabian StyleAu, Jason S., Richard L. Hughson, and Alfred C. H. Yu. 2018. "Riding the Plane Wave: Considerations for In Vivo Study Designs Employing High Frame Rate Ultrasound" Applied Sciences 8, no. 2: 286. https://doi.org/10.3390/app8020286





