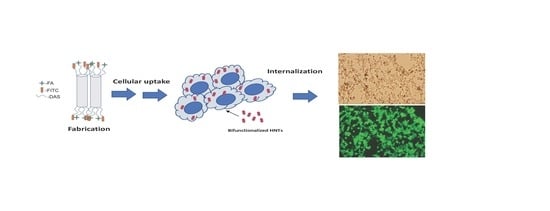
Bi-Functionalized Clay Nanotubes for Anti-Cancer Therapy
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. HNT-DAS-FA/FITC Synthesis
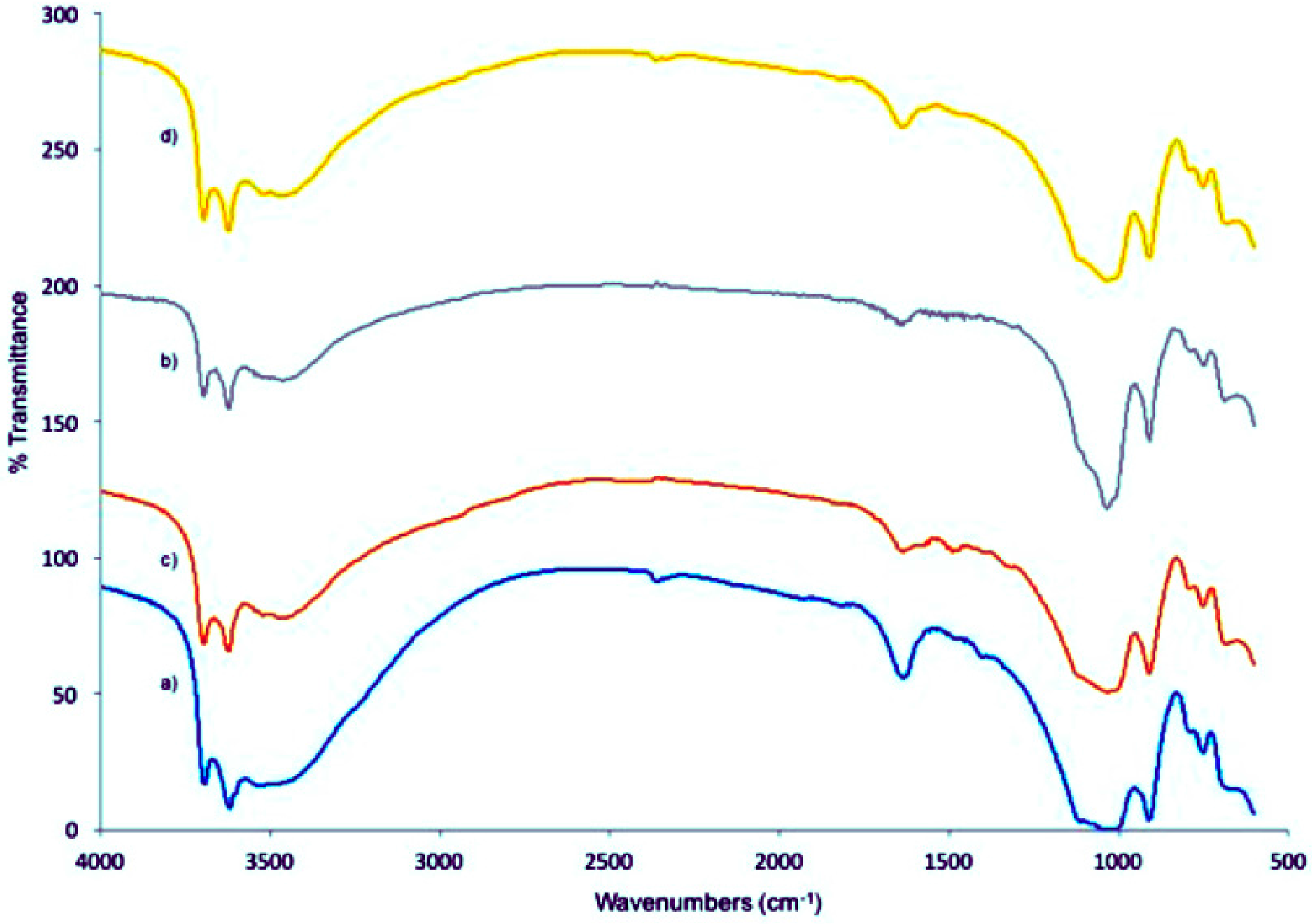
2.2.2. HNT-DAS-FA/FITC Characterization
2.2.3. Cell Culture
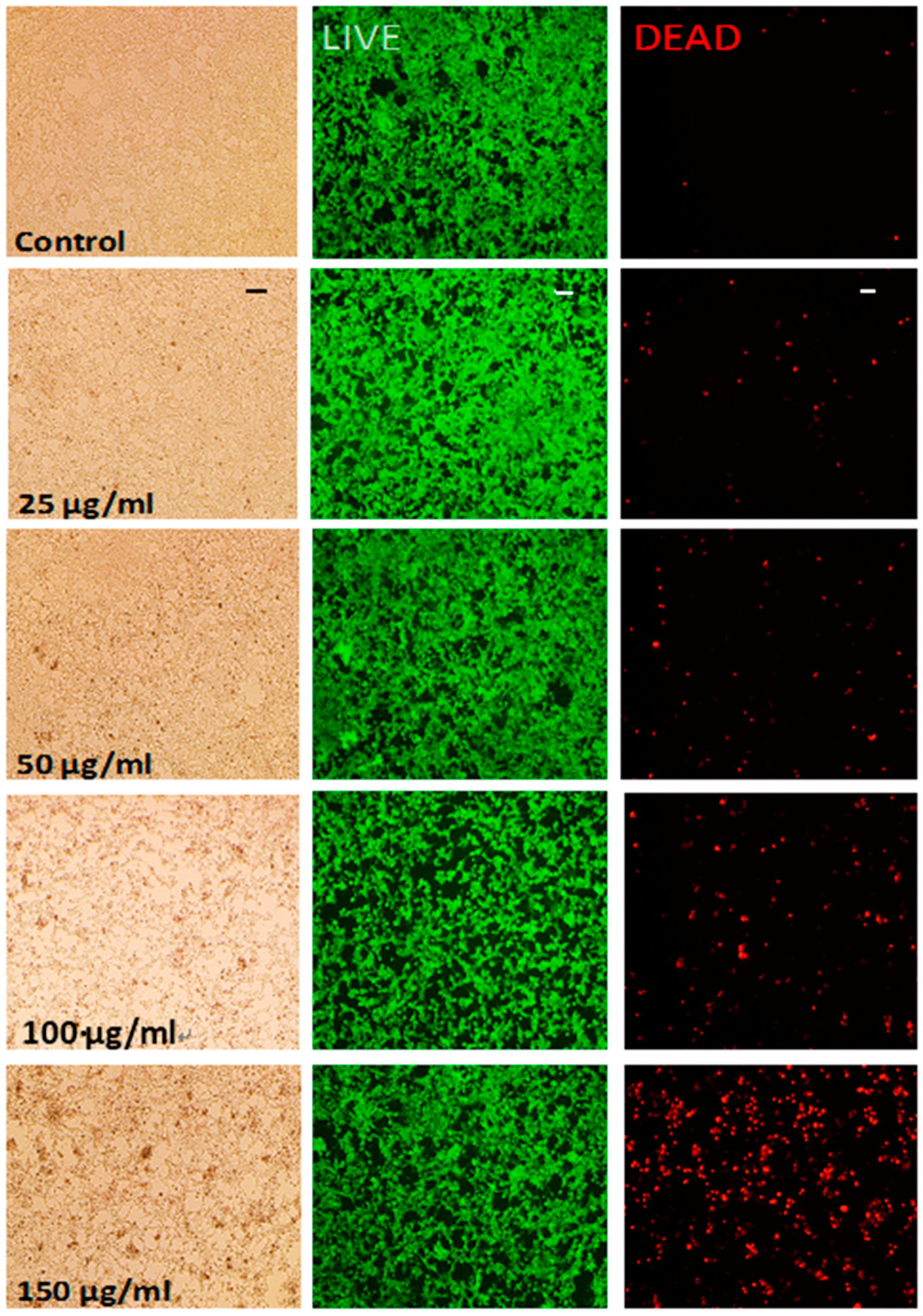
2.2.4. Live/Dead Cytotoxicity Assay
3. Results and Discussion
3.1. Fabrication and Analysis of the HNT-DAS-FA/FITC Complex
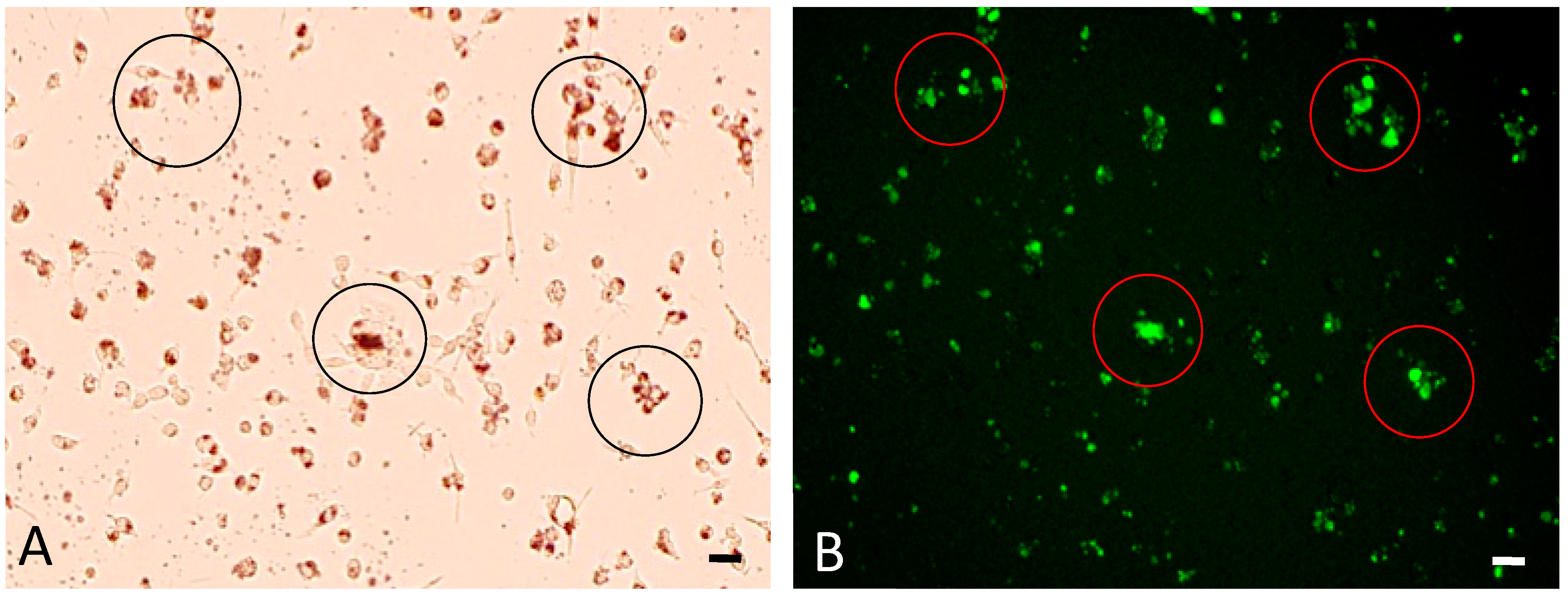
3.2. Cellular Uptake of bHNTs
3.3. Cytotoxicity Testing of bHNTs
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, X.D.; Liu, H.; Zhai, G. Advanced nanocarriers based on heparin and its derivatives for cancer management. Biomacromolecules 2014, 16, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Lee, Y.K.; Hah, H.J.; Ethirajan, M.; Pandey, R.K.; Kopelman, R. Multifunctional biodegradable polyacrylamide nanocarriers for cancer theranostics—A “see and treat” strategy. ACS Nano 2012, 6, 6843–6851. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.J.; Morton, S.W.; Ben-Akiva, E.; Dreaden, E.C.; Shopswitz, K.E.; Hammond, P.T. Layer-by-layer nanoparticles for systemic codelivery of an anticancer drug and siRNA for potential triple-negative breast cancer treatment. ACS Nano 2013, 7, 9571–9584. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Morton, S.W.; Shopsowitz, K.E.; Choi, J.-H.; Deng, Z.J.; Cho, N.-J.; Hammond, P.T. Bimodal tumor-targeting from microenvironment responsive hyaluronan layer-by-layer (LbL) nanoparticles. ACS Nano 2014, 8, 8374–8382. [Google Scholar] [CrossRef] [PubMed]
- Weissig, V.P.; Murdock, N. Nanopharmaceuticals (part I): Products on the market. Int. J. Nanomed. 2014, 9, 4357–4373. [Google Scholar] [CrossRef] [PubMed]
- Green, M.R.; Manikhas, G.M.; Orlov, S.V.; Afanasyev, B.V.; Makhson, A.M.; Bhar, P.; Hawkins, M.J. Abraxane, a novel cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann. Oncol. 2006, 17, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.Z.; Li, Q.; Shen, X.; Mu, C.; Cai, K. Dendrimerlike mesopourous silica nanoparticles as pH-responsive nanocontainers for targeted drug delivery and bioimaging. ACS Appl. Mater. Interfaces 2015, 7, 7357–7372. [Google Scholar] [CrossRef] [PubMed]
- Surapaneni, M.S.D.; Das, S.K.; Das, N.G. Designing paclitaxel drug delivery systems aimed at improved patient outcomes: Current status and challenges. SRN Pharmacol. 2012, 2012, 623139. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zheng, J. Clearance pathways and tumor targeting of imaging nanoparticles. ACS Nano 2015, 9, 6655–6674. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.K.; Park, J.; Jon, S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics 2012, 2, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Luk, B.T.; Zhang, L. Current advances in polymer-based nanotheranostics for cancer treatment and Diagnosis. ACS Appl. Mater. Interfaces 2014, 6, 21859–21873. [Google Scholar] [CrossRef] [PubMed]
- Bharali, D.J.; Yalcin, M.; Darwish, N.H.; Debreli Coskun, M.; Keating, K.A.; Lin, H.Y.; Davis, P.J.; Mousa, S.A. Targeted delivery of paclitaxel and doxorubicin to cancer xenografts via the nanoparticle of nano-diamino-tetrac. Int. J. Nanomed. 2017, 12, 1305–1315. [Google Scholar]
- Shimizu, T.; Tolcher, A.W.; Papadopoulos, K.P.; Beeram, M.; Rasco, D.W.; Smith, L.S.; Gunn, S.; Smetzer, L.; Mays, T.A.; Kaiser, B.; et al. The clinical effect of the dual-targeting strategy involving PI3K/AKT/mTOR and RAS/MEK/ERK pathways in patients with advanced cancer. Clin. Cancer Res. 2012, 18, 2316–2325. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.T.; Terywin, B.G.; Lin, S.Y. Effect of surface functionalization of MCM-41-type mesoporous silica nanoparticles on the endocytosis by human cancer cells. J. Am. Chem. Soc. 2006, 128, 14792–14793. [Google Scholar] [CrossRef] [PubMed]
- Shchukin, D.G.; Mohwald, H. Surface-engineered nanocontainers for entrapment of corrosion inhibitors. Adv. Funct. Mater. 2007, 17, 1451–1458. [Google Scholar] [CrossRef]
- Du, M.; Guo, B.; Liu, M.; Jia, D. Thermal decomposition and oxidation ageing behaviour of polypropylene/halloysite nanotube nanocomposites. Polym. Polym. Compos. 2007, 15, 321–328. [Google Scholar]
- Ravtani, D.; Agrawal, Y.K. Multifarious applications of halloysite nanotubes: A review. Rev. Adv. Mater. Sci. 2012, 30, 282–295. [Google Scholar]
- Gupta, N.; Lin, T.C.; Shapiro, M. Clay-epoxy nanocomposites: Processing and properties. JOM 2007, 59, 61–65. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Leng, F.; Huang, L.; Wang, Z.; Tian, W. Recent advances on surface modification of halloysite nanotubes for multifunctional applications. Appl. Sci. 2017, 7, 1215. [Google Scholar] [CrossRef]
- Sun, L.; Boyer, C.B.; Grimes, R.; Mills, D.K. Drug coated clay nanoparticles for delivery of chemotherapeutics. Curr. Nanosci. 2016, 12, 207–214. [Google Scholar] [CrossRef]
- Lvov, Y.; Wang, W.; Zhang, L.; Fakhrullin, R. Halloysite clay nanotubes for loading and sustained release of functional compounds. Adv. Mater. 2016, 28, 1227–1250. [Google Scholar]
- Patel, S.; Jammaladaka, U.; Sun, L.; Tappa, K.; Mills, D.K. Sustained release of antibacterial agents from doped halloysite nanotubes. Bioengineering 2016, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Abdullayev, E.; Hollister, A.; Mills, D.K.; Lvov, Y.M. Clay nanotube/poly(methyl methacrylate) bone cement composites with sustained antibiotic release. Macromol. Mater. Eng. 2012, 297, 645–653. [Google Scholar] [CrossRef]
- Vergaro, V.; Abdullayev, E.; Lvov, Y.M.; Zeitoun, A.; Cingolani, R.; Rinaldi, R.; Leporatti, S. Cytocompatibility and uptake of halloysite clay nanotubes. Biomacromolecules 2010, 11, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Kryuchkova, M.; Danilushkina, A.; Lvov, Y.; Fakhrullin, R. Evaluation of toxicity of nanoclays and graphene oxide in vivo: A Paramecium caudatum study. Environ. Sci. Nano 2016, 3, 442–452. [Google Scholar] [CrossRef]
- Nicholson, J.C.; Weisman, J.A.; Boyer, C.G.; Wilson, C.J.; Mills, D.K. Dry sintered metal coating of halloysite nanotubes. Appl. Sci. 2012, 6, 265–275. [Google Scholar] [CrossRef]
- Cervini-Silva, J.; Nieto-Camacho, A.; Ramírez-Apan, M.T. The anti-inflammatory properties of different naturally-occurring halloysites. In Natural Mineral Nanotubes: Properties and Application; Pasbakhsh, P., Churchman, C.J., Eds.; Apple Academic Press: Oakville, ON, Canada; Waretown, NJ, USA, 2015; Chapter 24; pp. 449–460. [Google Scholar]
- Wu, W.; Cao, X.; Luo, J.; He, G.; Zhang, Y. Morphology, thermal, and mechanical properties of poly(butylene succinate) reinforced with halloysite nanotube. Polym. Compos. 2014, 35, 847–855. [Google Scholar] [CrossRef]
- Arat, R.; Uyanik, N. Surface modification of nanoclays with co-polymers. Nat. Res. 2017, 8, 159–171. [Google Scholar] [CrossRef]
- Joo, Y.; Jeon, Y.; Lee, S.U.; Sohn, D. Aggregation and stabilization of carboxylic acid functionalized halloysite nanotubes (HNT-COOH). J. Phys. Chem. C 2012, 116, 18230–18235. [Google Scholar] [CrossRef]
- Du, M.; Guo, B.; Jia, D. Thermal stability and flame retardant effects of halloysite nanotubes on poly(propylene). Eur. Polym. J. 2006, 42, 1362–1369. [Google Scholar] [CrossRef]
- Carli, L.N.; Daitx, T.S.; Soares, G.; Crespo, J.S.; Mauler, R.S. PHBV nanocomposites based on organomodified montmorillonite and halloysite: The effect of clay type on themorphology and thermal and mechanical properties. Compos. Part A 2011, 42, 1601–1608. [Google Scholar] [CrossRef]
- Liu, M.; Jia, Z.; Jia, D.; Zhou, C. Recent advance in research on halloysite nanotubes-polymer nanocomposite. Prog. Polym. Sci. 2014, 39, 1498–1525. [Google Scholar] [CrossRef]
- Rooj, S.; Das, A.; Thakur, V.; Mahaling, R.N.; Bhowmick, A.K.; Heinrich, G. Preparation and properties of natural nanocomposites based on natural rubber and naturally occurring halloysite nanotubes. Mater. Des. 2010, 31, 2151–2156. [Google Scholar] [CrossRef]
- Prashantha, K.; Lacrampe, M.F.; Krawczak, P. Processing and characterization of halloysite nanotubes filled polypropylene nanocomposites based on masterbatch route: Effect of halloysite treatment on structural and mechanical properties. Express Polym. Lett. 2011, 5, 295–307. [Google Scholar] [CrossRef]
- Massaro, M.; Lazzara, G.; Milioto, S.; Noto, R.; Riela, S. Covalently modified halloysite clay nanotubes: Synthesis, properties, biological and medical applications. J. Mater. Chem. B 2017, 5, 2867–2882. [Google Scholar] [CrossRef]
- Yah, W.O.; Takahara, A.; Lvov, Y. Selective modification of halloysite lumen with octadecylphosphonic acid: New Iinorganic tubular micelle. J. Am. Chem. Soc. 2012, 134, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Vigneron, N. Human tumor antigens and cancer immunotherapy. BioMed Res. Int. 2015, 2015, 948501. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Low, P.S. Folate-mediated targeting of antineoplastic drugs, imaging agents, and nucleic acids to cancer cells. J. Control. Release 1998, 53, 39–48. [Google Scholar] [CrossRef]
- Sudimack, J.; Lee, R.J. Targeted drug delivery via the folate receptor. Adv. Drug Deliv. Rev. 2000, 41, 147–162. [Google Scholar] [CrossRef]
- Qiao, J.; Mu, X.; Qi, L.; Deng, J.; Mao, L. Folic acid-functionalized fluorescent gold nanoclusters with polymers as linkers for cancer cell imaging. Chem. Commun. 2013, 49, 8030–8032. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Southern, P.D.; Liu, Z.; Green, M.E.R.; Hook, J.M.; Antill, S.J.; Kepert, C.J. Functionalization of halloysite clay nanotubes by grafting with γ-aminopropyltriethoxysilane. J. Phys. Chem. C 2009, 112, 15742–15751. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, T.; Han, L.; Wu, Y.; Song, H.; Bai, L.; Ba, X. Selective modification of halloysite nanotubes with 1-pyrenylboronic acid: A novel fluorescence probe with highly selective and sensitive response to hyperoxide. ACS Appl. Mater. Interfaces 2015, 7, 23805–23811. [Google Scholar] [CrossRef] [PubMed]
- Barrientos-Ramirez, S.; Ramires, G.M.; Ramos-Fernandez, E.V.; Sepulveda-Escribano, A.; Pastor-Blas, M.M.; Gonzalex-Montiel, A. Surface modification of natural halloysite clay nanotubes with aminosilanes. Application as catalyst supports in the atom transfer dadical polymerization of methyl methacrylate. Appl. Catal. A Gen. 2011, 406, 22–33. [Google Scholar] [CrossRef]
- Andreia, F.; Peixoto, A.C.F.; Pereira, C.; Pires, J.; Freire, C. Physiochemical characterization of organosilylated halloysite clay nanotubes. Microporous Mesoporous Mater. 2016, 219, 145–154. [Google Scholar]
- Nalinkanth, G.; Veerabadran, D.M.; Torchilin, V.; Price, R.R.; Lvov, Y. Organized shells on clay nanotubes for controlled release of macromolecules. Macromol. J. 2008, 9, 99–103. [Google Scholar]
- Jin, Y.; Yendluri, R.; Chen, B.; Wang, J.; Lvov, Y. Composite microparticles of halloysite clay nanotubes bound by calcium carbonate. J. Colloid Interface Sci. 2016, 466, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.R.; Shoaib, M.H.; Azhar, M.; Um, S.H.; Yousuf, R.I.; Hashmi, S.; Dar, A. In vitro assessment of cytotoxicity of halloysite nanotubes against HepG2, HCT116 and human peripheral blood lymphocytes. Colloids Surf. B 2015, 135, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, R.; Liu, M.; Yan, C.; Shan, A. Adsorption of modified halloysite nanotubes in vitro and the protective effect in rats exposed to zearalenone. Arch. Anim. Nutr. 2014, 68, 320–335. [Google Scholar] [CrossRef] [PubMed]
- Bellani, L.; Giorgetti, S.; Riela, G.; Lazzara, A.; Scialabba, M.; Massaro, M. Ecotoxicity of halloysite nanotube—Supported palladium nanoparticles in Raphanus sativus L. Environ. Toxicol. Chem. 2016, 35, 2503–2510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fakhrullina, G.I.; Akhatova, F.S.; Lvov, Y.; Fakhrullin, R.F. Toxicity of halloysite clay nanotubes in vivo: A Caenorhabditis elegans study. Environ. Sci. Nano 2015, 2, 54–59. [Google Scholar] [CrossRef]
- Lvov, Y.M.; DeVillers, M.M.; Fakhrullin, R.F. The application of halloysite tubule nanoclay in drug delivery. Expert Opin. Drug Deliv. 2016, 13, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Panariti, A.; Miserocchi, G.; Rivolta, I. The effect of nanoparticle uptake on cellular behavior: Disrupting or enabling functions? Nanotechnol. Sci. Appl. 2012, 5, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Huy, Y.; Chen, J.; Li, X.; Huang, S.; Li, Y.; Xu, J.; Zhong, S. Multifunctional halloysite nanotubes for targeted delivery and controlled release of doxorubicin in vitro and in-vivo studies. Nanotechnology 2017, 28, 375101. [Google Scholar] [CrossRef] [PubMed]
- Leporatti, S. Halloysite clay nanotubes as nano-bazookas for drug delivery. Polym. Int. 2017, 66, 1111–1118. [Google Scholar] [CrossRef]
- Tully, J.; Yendluri, R.; Lvov, Y. Halloysite clay nanotubes for enzyme immobilization. Biomacromolecules 2015, 17, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Keeney, M.; Jiang, X.Y.; Yamane, M.; Lee, M.; Goodman, S.; Yang, F. Nanocoating for biomolecule delivery using layer-by-layer self-assembly. J. Mater. Chem. B 2017, 3, 8757–8770. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Niu, M.; Gong, R.; Shi, D.; Chen, L.; Zhang, L.; Lvov, Y. Electrospun microfiber membranes embedded with drug-loaded clay nanotubes for sustained antimicrobial protection. ACS Nano 2015, 9, 1600–1612. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fernández, A.; Peña-Parás, L.; Vidaltamayo, R.; Cué-Sampedro, R.; Mendoza-Martínez, A.; Zomosa-Signoret, V.C.; Rivas-Estilla, A.M.; Riojas, P. Synthesization, Characterization, and in vitro Evaluation of Cytotoxicity of Biomaterials Based on Halloysite Nanotubes. Materials 2014, 7, 7770–7780. [Google Scholar] [CrossRef] [PubMed]
- Bugatti, V.; Viscusi, G.; Naddeo, C.; Gorrasi, G. Nanocomposites based on PCL and halloysite nanotubes filled with lysozyme: Effect of draw ratio on the physical properties and release analysis. Nanomaterials 2017, 7, 213. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhang, J.; Wang, A. In situ generation of sodium alginate/hydroxyapatite/halloysite nanotubes nanocomposite hydrogel beads as drug-controlled release matrices. J. Mater. Chem. 2013, 1, 6261–6270. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grimes, W.R.; Luo, Y.; McFarland, A.W., Jr.; Mills, D.K. Bi-Functionalized Clay Nanotubes for Anti-Cancer Therapy. Appl. Sci. 2018, 8, 281. https://doi.org/10.3390/app8020281
Grimes WR, Luo Y, McFarland AW Jr., Mills DK. Bi-Functionalized Clay Nanotubes for Anti-Cancer Therapy. Applied Sciences. 2018; 8(2):281. https://doi.org/10.3390/app8020281
Chicago/Turabian StyleGrimes, William R., Yangyang Luo, Antwine W. McFarland, Jr., and David K. Mills. 2018. "Bi-Functionalized Clay Nanotubes for Anti-Cancer Therapy" Applied Sciences 8, no. 2: 281. https://doi.org/10.3390/app8020281