1H-NMR Spectroscopy: A Possible Approach to Advanced Bitumen Characterization for Industrial and Paving Applications
Abstract
:1. Introduction
1.1. Background
1.2. Objectives
2. Materials and Methods
3. Experimental Work
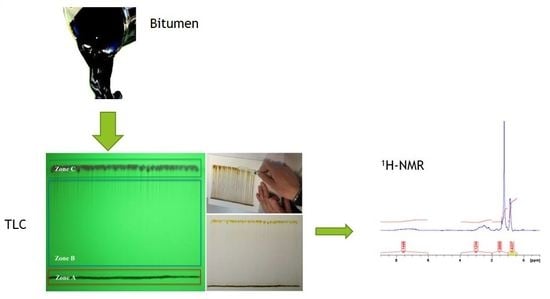
3.1. Bitumen Separation Techniques
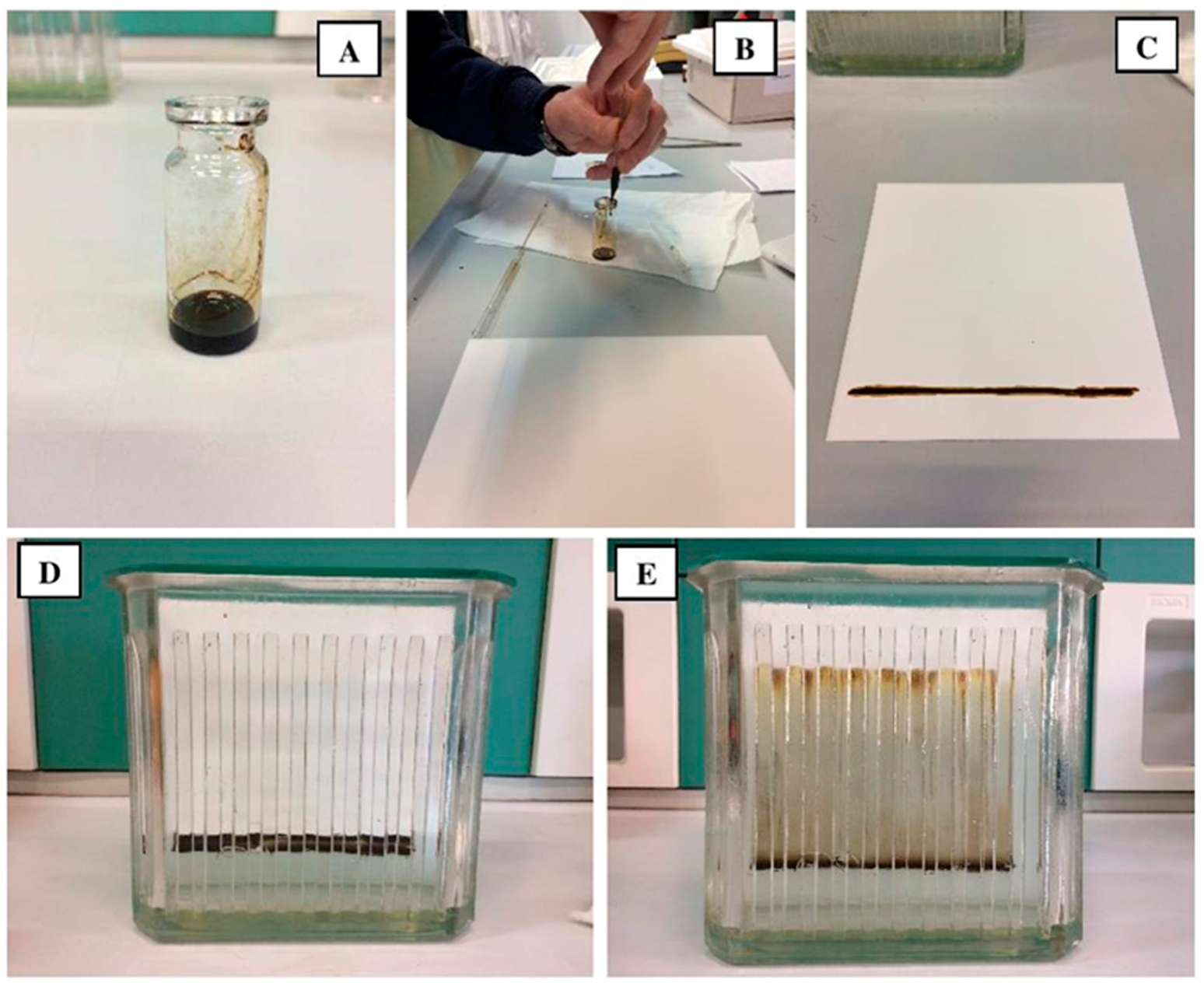
3.1.1. Asphaltenes and Maltenes Separation (Modified Conventional Method)
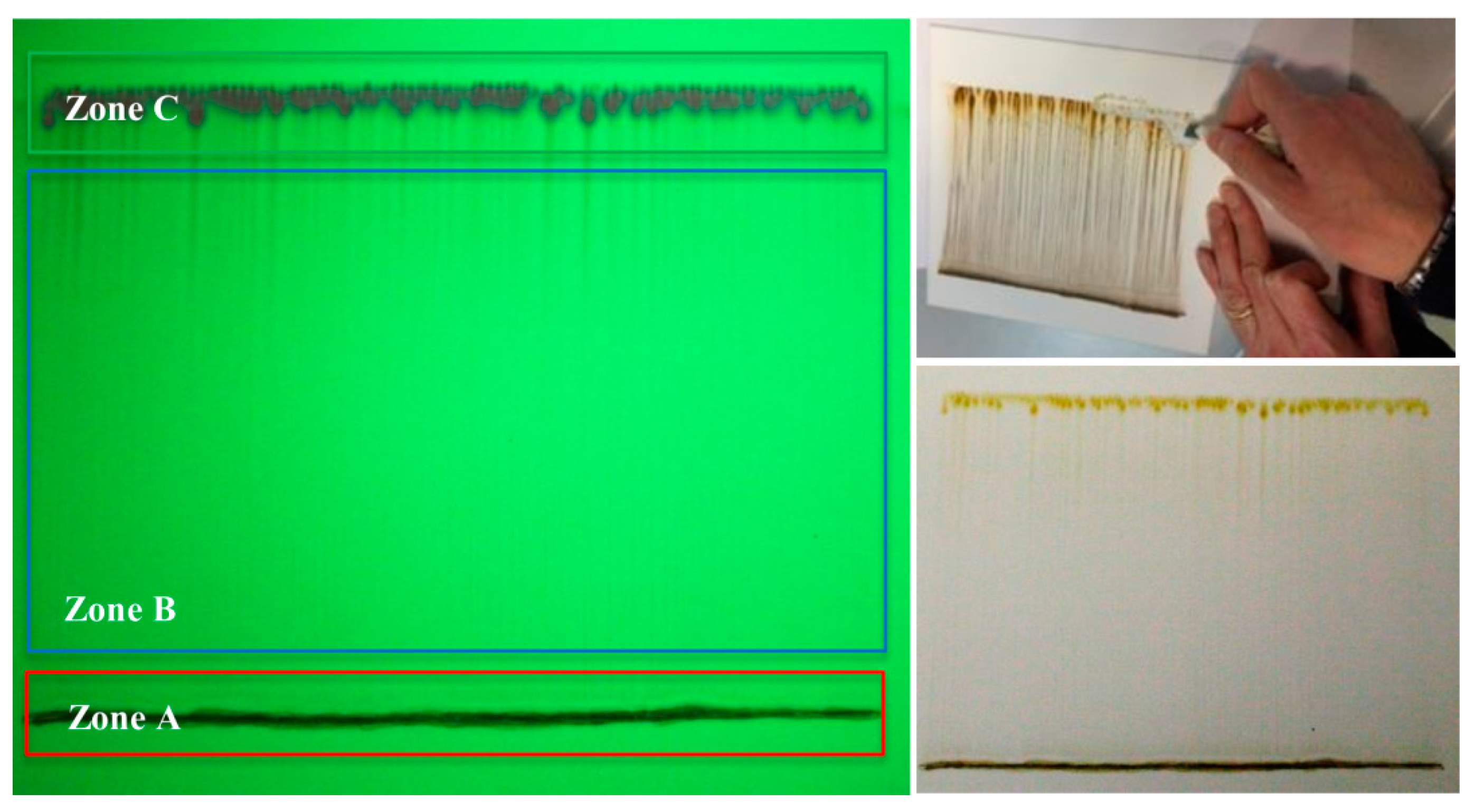
3.1.2. Thin Layer Chromatography (TLC)
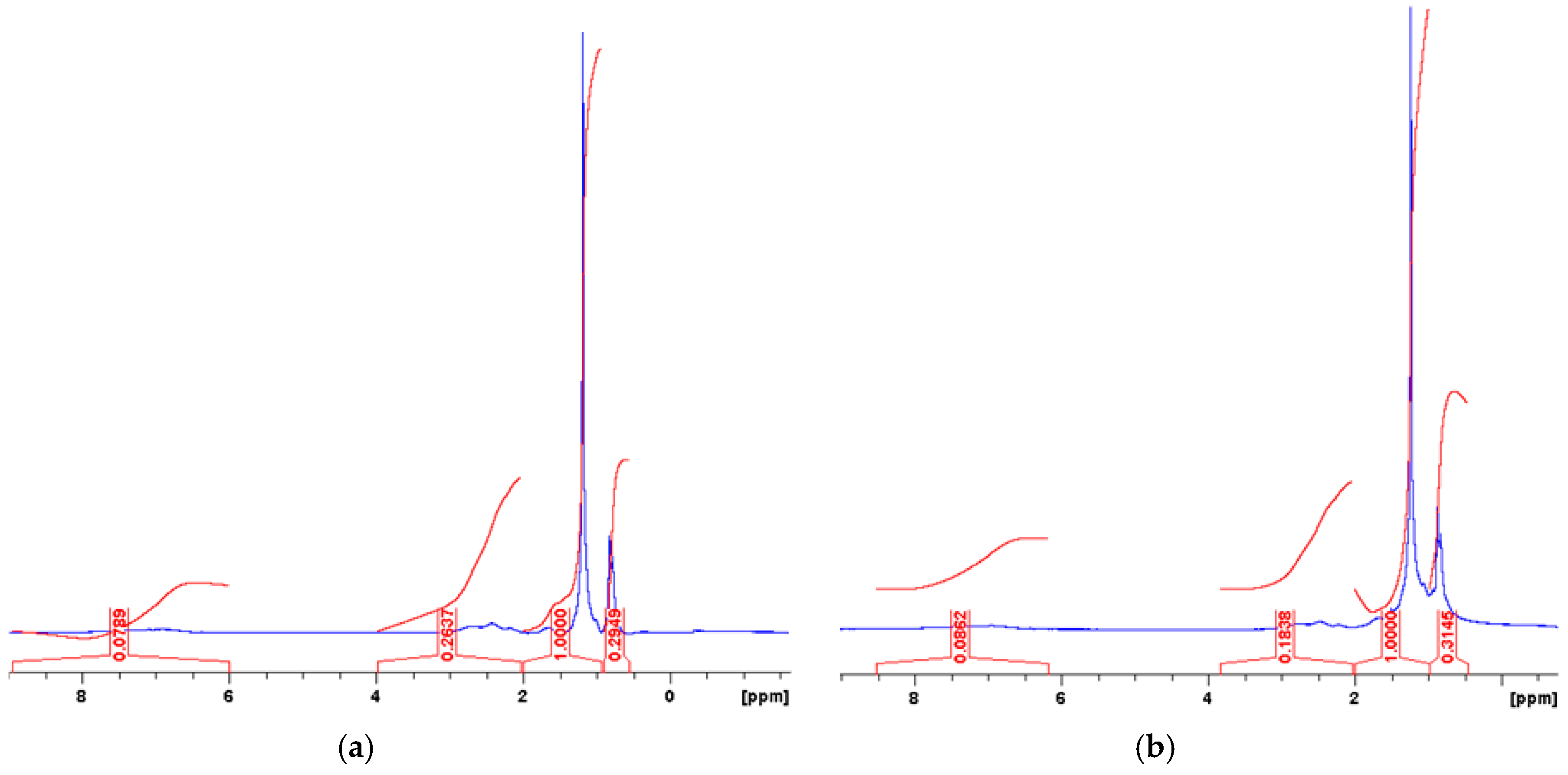
3.2. Nuclear Magnetic Resonance Spectroscopy (NMR)
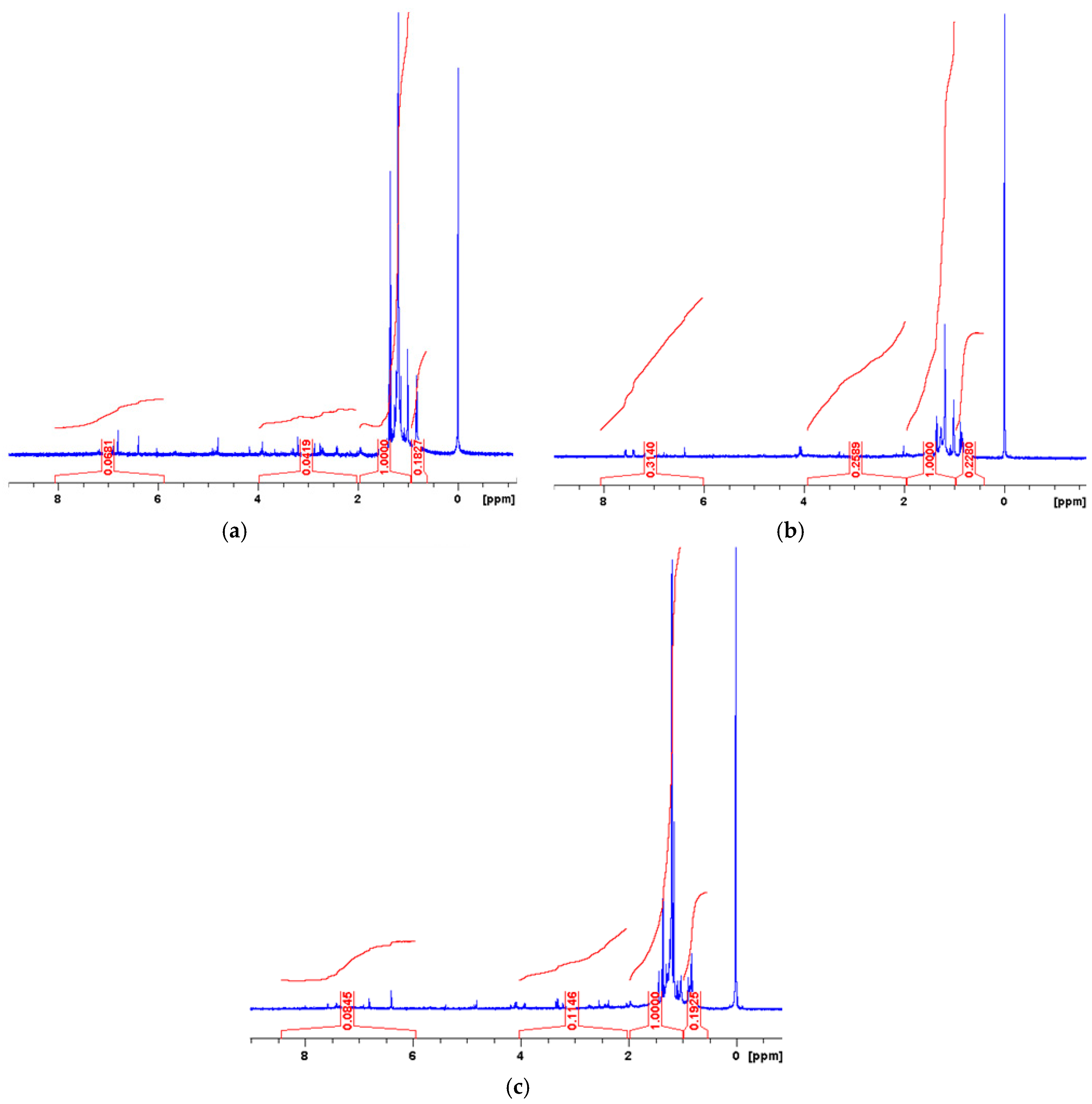
4. Results and Discussion
5. Conclusions and Remarks
- Asphaltene and maltene fractions of bitumens can be successfully separated by means of TLC techniques and be used for further characterization tests in the laboratory.
- The choice of solvents determination, mobile phases and support material are crucial to obtain a consistent separation of the binder components.
- 1H-NMR provides a wider perspective on the chemical characteristics of the various binders than traditional SARA analysis.
- Although within the proposed technique unconventional laboratory work is required, the obtained data provide a comprehensive understanding on the chemo-mechanical nature of the binders.
- The acquired knowledge will be at the basis of any prospective alteration/modification of binders for commercial purposes.
Author Contributions
Conflicts of Interest
References
- Asphalt Institute. Superpave Asphalt Binder Specification (SP-1), 3rd ed.; Asphalt Institute: Lexington, KY, USA, 2003. [Google Scholar]
- Read, J.; Whiteoak, D. The Shell Bitumen Handbook, 5th ed.; Thomas Telford Publishing: London, UK, 2003. [Google Scholar]
- Handle, F.; Harir, M.; Fussl, J.; Koyun, A.N.; Grossegger, D.; Hertkorn, N.; Eberhardsteiner, L.; Hofko, B.; Hospodka, M.; Blab, R.; et al. Tracking Aging of Bitumen and Its Saturate, Aromatic, Resin, and Asphaltene Fractions Using High-Field Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Fuels 2017, 31, 4771–4779. [Google Scholar] [CrossRef]
- Robertson, R.E. Chemical Properties of Asphalts and Their Relationship to Pavement Performance; Strategic Highway Research Program National Research Council: Washington, DC, USA, 1991. [Google Scholar]
- Gentile, L.; Filippelli, L.; Oliviero Rossi, C.; Baldino, N.; Ranieri, G.A. Rheological and H-NMR Spin-Spin relaxation time for the evaluation of the effects of PPA addition on bitumen. Mol. Cryst. Liq. Cryst. 2012, 558, 54–63. [Google Scholar] [CrossRef]
- Baldino, N.; Gabriele, D.; Oliviero Rossi, C.; Seta, L.; Lupi, F.R.; Caputo, P.; Falvo, T. Rheological effects on bitumen of polyphosphoric acid (PPA) addition. Constr. Build. Mater. 2013, 40, 397–404. [Google Scholar] [CrossRef]
- Masson, J.-F.; Leblond, V.; Margeson, J. Bitumen morphologies by phase-detection atomic force microscopy. J. Microsc. 2006, 221, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Nciri, N.; Song, S.; Kim, N.; Cho, N. Chemical Characterization of Gilsonite Bitumen. J. Pet. Environ. Biotechnol. 2014, 5, 1000193. [Google Scholar] [CrossRef]
- Aroulanda, C.; Celebre, G.; De Luca, G.; Longeri, M. Molecular ordering and structure of quasi-spherical solutes by liquid crystal NMR and Monte Carlo simulations: The case of norbornadiene. J. Phys. Chem. B 2006, 110, 10485–10496. [Google Scholar] [CrossRef] [PubMed]
- Celebre, G.; Concistrè, M.; De Luca, G.; Longeri, M.; Pileio, G. Intrinsic information content of NMR dipolar couplings: A conformational investigation of 1,3-butadiene in a nernatic phase. ChemPhysChem 2006, 7, 1930–1943. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Longeri, M.; Pileio, G.; Lantto, P. NMR spectroscopy investigation of the cooperative nature of the internal rotational motions in acetophenone. ChemPhysChem 2005, 6, 2086–2098. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, A.S.; Grishaev, A.; Roche, J.; Zasloff, M.; Bax, A. Improved Cross Validation of a Static Ubiquitin Structure Derived from High Precision Residual Dipolar Couplings Measured in a Drug-Based Liquid Crystalline Phase. J. Am. Chem. Soc. 2014, 136, 3752–3755. [Google Scholar] [CrossRef] [PubMed]
- Majid, A.; Pihillagawa, I. Potential of NMR spectroscopy in the characterization of non-conventional oils. J. Fuels 2014, 390261. [Google Scholar] [CrossRef]
- Nciri, N.; Kim, N.; Cho, N. New insights into the effects of styrene-butadiene-styrene polymer modifier on the structure, properties, and performance of asphalt binder: The case of AP-5 asphalt and solvent deasphalting pitch. Mater. Chem. Phys. 2017, 193, 477–495. [Google Scholar] [CrossRef]
- Poveda, J.; Molina, D.; Pantoja-Agreda, E. 1H- and 13C-NMR structural characterization of Asphaltenes from vacuum residua modified by Thermal cracking. J. Oil Gas Altern. Energy Sources 2014, 5, 49–60. [Google Scholar] [CrossRef]
- Maiuolo, L.; Bortolini, O.; De Nino, A.; Russo, B.; Gavioli, R.; Sforza, F. Modified N,O-Nucleosides: Design, Synthesis, and Anti-tumour Activity. Aust. J. Chem. 2014, 67, 670–674. [Google Scholar] [CrossRef]
- De Nino, A.; Bortolini, O.; Maiuolo, L.; Garofalo, A.; Russo, B.; Sindona, G. A sustainable procedure for highly enantioselective organocatalyzed Diels-Alder cycloadditions in homogeneous ionic liquid/water phase. Tetrahedron Lett. 2011, 52, 1415–1417. [Google Scholar] [CrossRef]
- Lee, H.-J.; Koung, F.-P.; Kwon, K.-R.; Kang, D.-I.; Cohen, L.; Yang, P.-Y.; Yoo, H.-S. Comparative Analysis of the Bufonis Venenum by Using TLC, HPLC, and LC-MS for Different Extraction Methods. J. Pharmacopunct. 2012, 15, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Dolowy, M.; Pyka, A. Chromatographic Methods in the Separation of Long-Chain Mono- and Polyunsaturated Fatty Acids. J. Chem. 2015, 120830. [Google Scholar] [CrossRef]
- Masson, J.-F.; Price, T.; Collins, P. Dynamics of bitumen fractions by Thin-Layer Chromatography/Flame Ionization detection. Energy Fuels 2001, 15, 955–960. [Google Scholar] [CrossRef]
- Dunn, K.; Chilingarian, G.V.; Yen, T.F. Bitumens: Liquid Chromatography; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Christopher, J.; Sarpal, A.S.; Kapur, G.S.; Krishna, A.; Tyagi, B.R.; Jain, M.C.; Jain, S.K.; Bhatnagar, A.K. Chemical structure of bitumen-derived asphaltenes by nuclear magnetic resonance spectroscopy and X-ray diffractometry. Fuel 1996, 75, 999–1008. [Google Scholar] [CrossRef]
- Oyekunle, L.O. Certain Relationships between Chemical Composition and Properties of Petroleum Asphalts from Different Origin. Oil Gas Sci. Technol. Rev. IFP 2006, 61, 433–441. [Google Scholar] [CrossRef]
- Preethi, J.; Harita, B.; Rajesh, T. Review on Thin Layer Chromatography. J. Formul. Sci. Bioavailab. 2017, 1, 1–4. [Google Scholar]
- Zander, M.; Marsh, H.; Rodríguez-Reinoso, F. Chemistry and properties of coal-tar and petroleum pitch. In Sciences of Carbon Materials; Marsh, H., Rodríguez-Reinoso, F., Eds.; Universidad de Alicante, Secretariado de Publicaciones: Alicante, Spain, 2000; pp. 205–257. [Google Scholar]
- Çubuk, M.; Gürü, M.; Kürşat Çubuk, M. Improvement of bitumen performance with epoxy resin. Fuel 2009, 88, 1324–1328. [Google Scholar] [CrossRef]

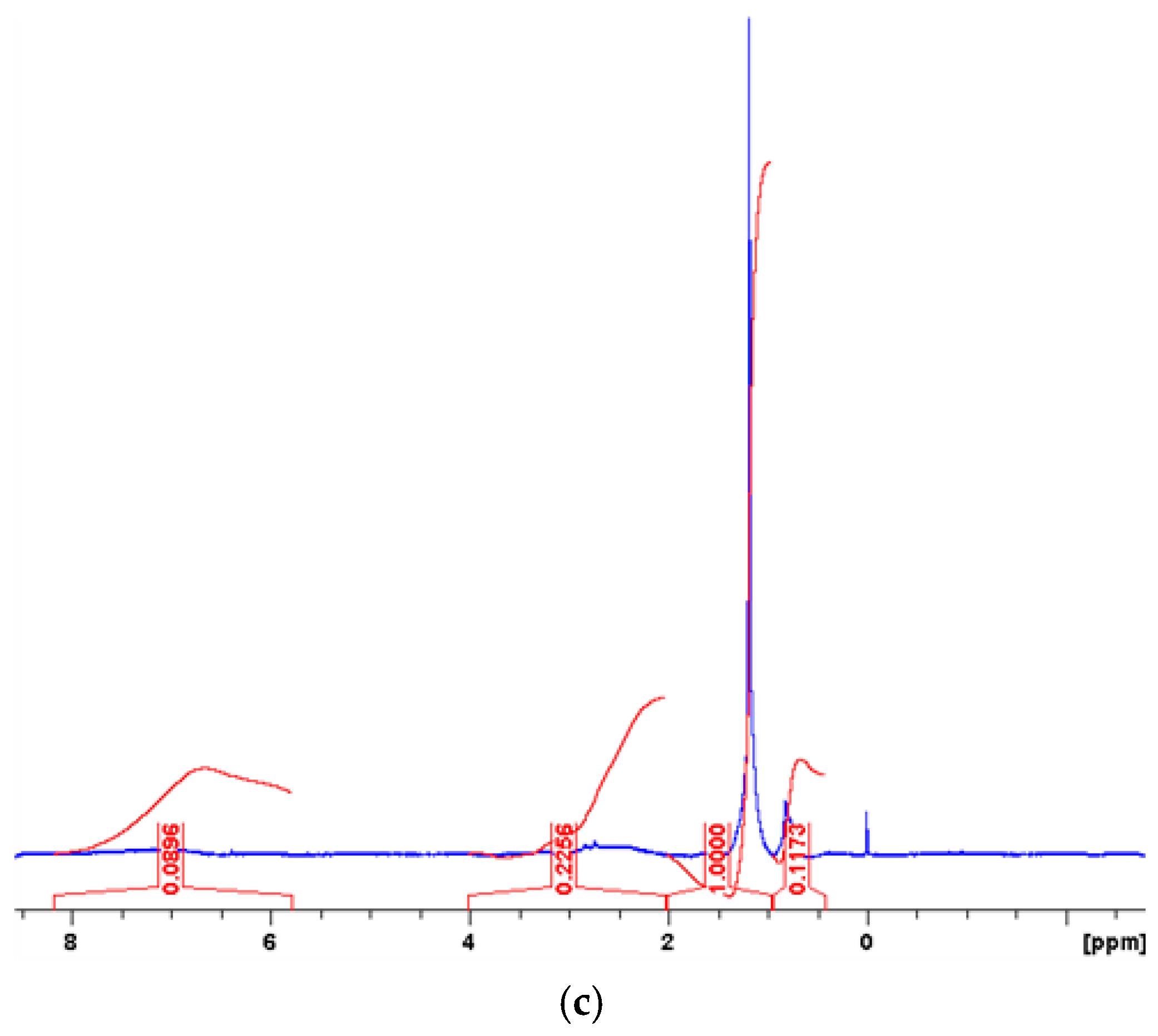

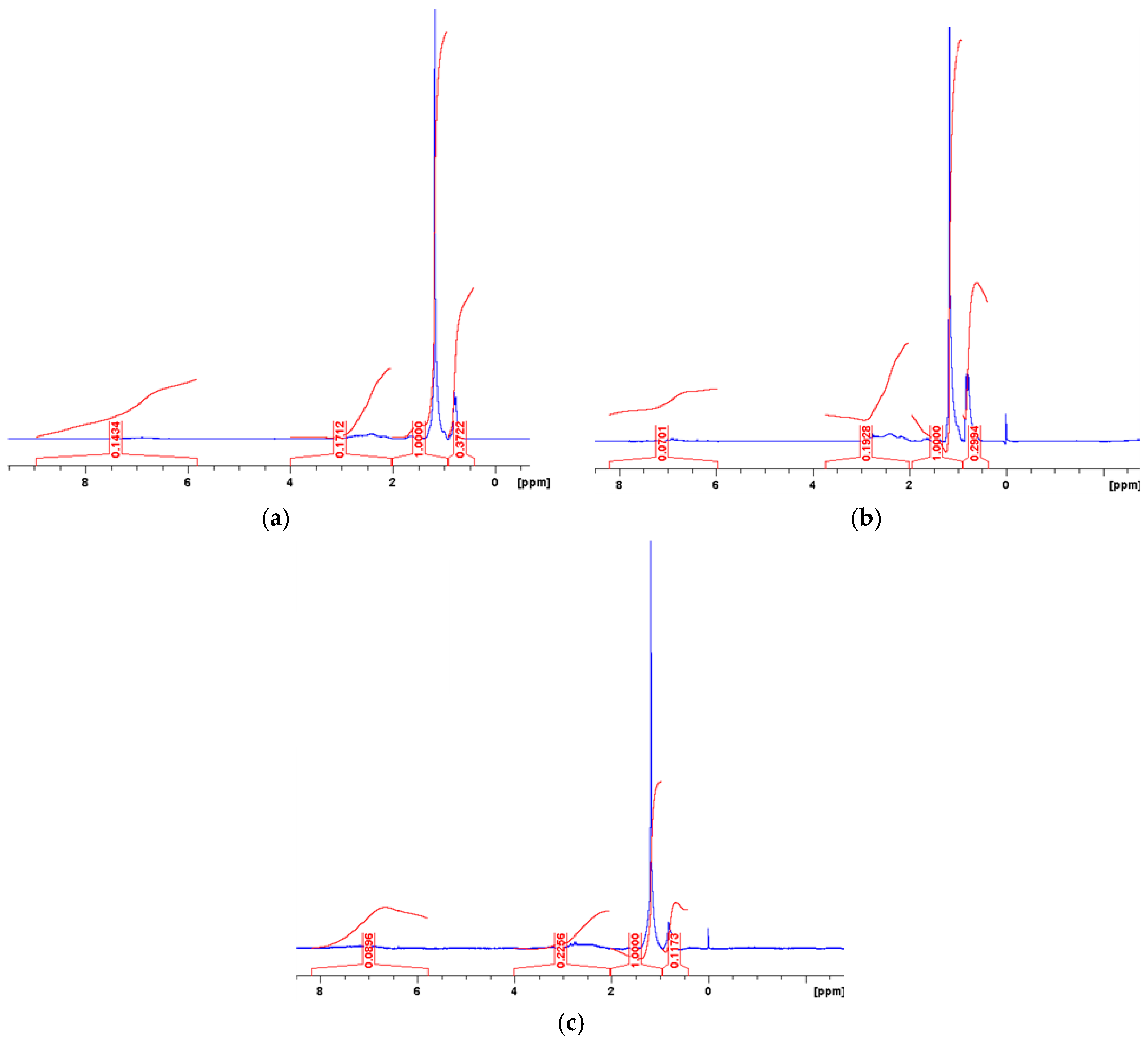

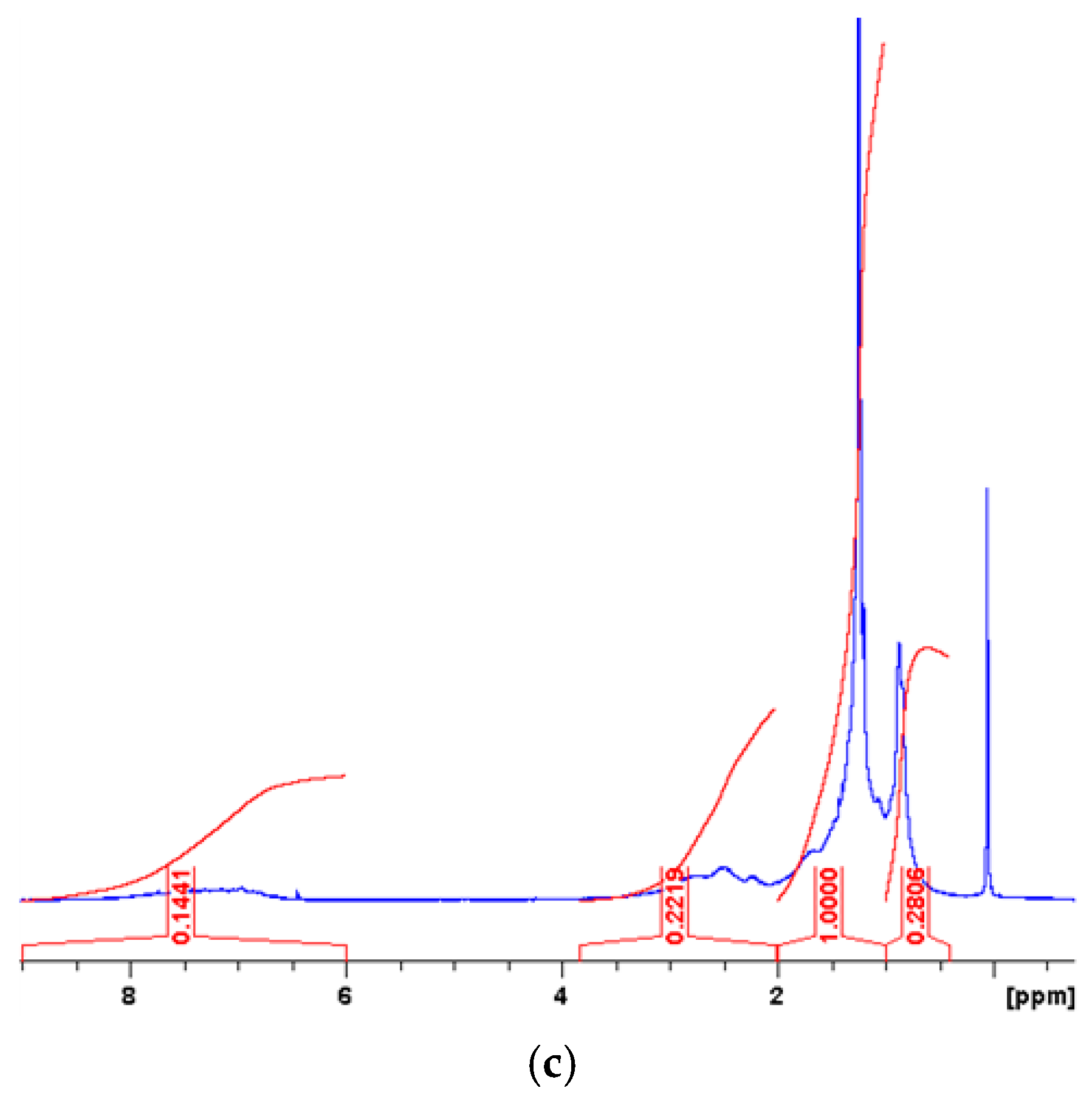
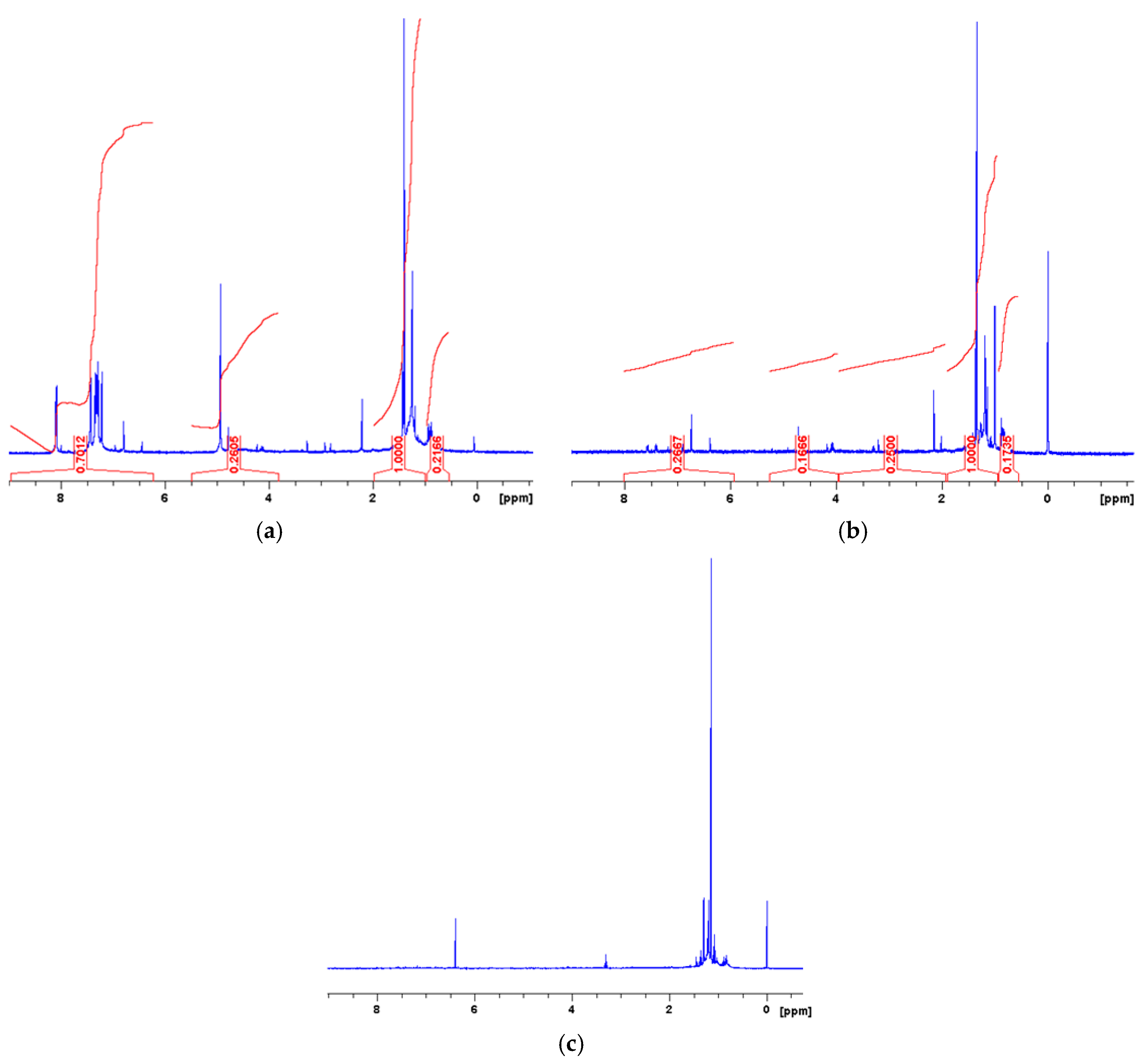
| Measured Properties | Standard | Unit | Paving Grade | Paving Grade | Industrial |
|---|---|---|---|---|---|
| 70/100 | 160/220 | 160/220 | |||
| Penetration at 25 °C | EN 1426 | 0.1 mm | 70–100 | 160–220 | 170 |
| Softening point (R&B) | EN 1427 | °C | 43–51 | 35–43 | 35–43 |
| Flash point | EN 2592 | °C | ≥230 | ≥220 | 271 |
| Solubility | EN 12592 | % (m/m) | ≥99 | ≥99 | ≥99 |
| Mass change at 163 °C | EN 12607-1 | % | ≤0.8 | ≤1 | ≤1 |
| Retained penetration | EN 1426 | % | ≥46 | ≥37 | ≥37 |
| Increase in softening point | EN 1427 | °C | ≤9 | ≤11 | ≤12 |
| Parameter | Chemical Shift | Type of Protons |
|---|---|---|
| Har | 6.0–9.0 | Aromatic hydrogen |
| Hol | 4.0–6.0 | Olefinic hydrogen |
| Hα | 2.0–4.0 | Aliphatic Hydrogen on Cα to aromatic rings |
| Hᵦ | 1.0–2.0 | Aliphatic Hydrogen on Cᵦ and the CH2 beyond the Cᵦ to aromatic rings |
| Hγ | 0.5–1.0 | Aliphatic Hydrogen on Cγ and the CH3 beyond the Cγ to aromatic rings |
| Bitumen | Hydrogen Distribution ±0.05 | |||
|---|---|---|---|---|
| Har | Hα | Hβ | Hγ | |
| Paving 70/100 | 8.00 | 3.86 | 64.38 | 23.73 |
| Paving 160/220 | 8.32 | 3.80 | 64.19 | 23.66 |
| Industrial 160/220 | 4.82 | 16.10 | 61.07 | 18.00 |
| Bitumen | Hydrogen Distribution of Maltenes ±0.05 | Hydrogen Distribution of Asphaltenes ±0.05 | ||||||
|---|---|---|---|---|---|---|---|---|
| Har | Hα | Hβ | Hγ | Har | Hα | Hβ | Hγ | |
| Paving 70/100 | 4.49 | 12.34 | 64.01 | 19.16 | 14.25 | 15.75 | 61.80 | 8.19 |
| Paving 160/220 | 7.43 | 11.77 | 60.55 | 20.24 | 18.30 | 16.77 | 55.47 | 9.31 |
| Industrial 160/220 | 7.54 | 11.61 | 61.00 | 19.86 | 19.58 | 8.19 | 60.79 | 11.44 |
| Bitumen | Hydrogen Distribution of Zone C ±0.05 | Hydrogen Distribution of Zone B ±0.05 | ||||||
|---|---|---|---|---|---|---|---|---|
| Har | Hα | Hβ | Hγ | Har | Hol/Hα | Hβ | Hγ | |
| Paving70/100 | 3.48 | 7.80 | 62.27 | 26.45 | 22.47 | 8.97/13.46 | 53.86 | 9.34 |
| Paving 160/220 | 8.49 | 7.30 | 58.73 | 25.47 | 32.19 | 11.95 Hol | 45.91 | 9.94 |
| Industrial 160/220 | 8.75 | 13.48 | 60.73 | 17.04 | - | - | 92.62 | 7.38 |
| Bitumen | Hydrogen Distribution of Zone A ±0.05 | Hydrogen Distribution of Zones B + A ±0.05 | ||||||
| Har | Hα | Hβ | Hγ | Har | Hol/Hα | Hβ | Hγ | |
| Paving70/100 | 17.43 | 14.38 | 55.53 | 12.66 | 19.17 | 17.69 | 52.27 | 10.57 |
| Paving 160/220 | 5.27 | 3.24 | 77.36 | 14.13 | 18.73 | 7.59 | 61.64 | 12.03 |
| Industrial 160/220 | 6.07 | 8.23 | 71.86 | 13.84 | - | - | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliviero Rossi, C.; Caputo, P.; De Luca, G.; Maiuolo, L.; Eskandarsefat, S.; Sangiorgi, C. 1H-NMR Spectroscopy: A Possible Approach to Advanced Bitumen Characterization for Industrial and Paving Applications. Appl. Sci. 2018, 8, 229. https://doi.org/10.3390/app8020229
Oliviero Rossi C, Caputo P, De Luca G, Maiuolo L, Eskandarsefat S, Sangiorgi C. 1H-NMR Spectroscopy: A Possible Approach to Advanced Bitumen Characterization for Industrial and Paving Applications. Applied Sciences. 2018; 8(2):229. https://doi.org/10.3390/app8020229
Chicago/Turabian StyleOliviero Rossi, Cesare, Paolino Caputo, Giuseppina De Luca, Loredana Maiuolo, Shahin Eskandarsefat, and Cesare Sangiorgi. 2018. "1H-NMR Spectroscopy: A Possible Approach to Advanced Bitumen Characterization for Industrial and Paving Applications" Applied Sciences 8, no. 2: 229. https://doi.org/10.3390/app8020229