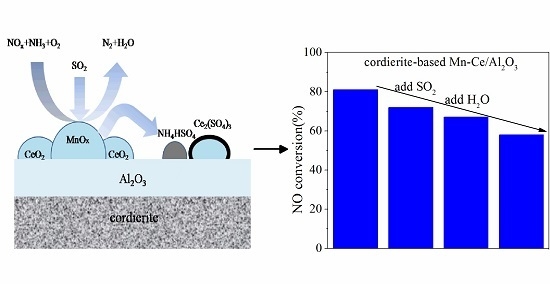
Poisoning Effect of SO2 on Honeycomb Cordierite-Based Mn–Ce/Al2O3Catalysts for NO Reduction with NH3 at Low Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalytic Activity Evaluation
2.3. Catalyst Characterization
3. Results and Discussion
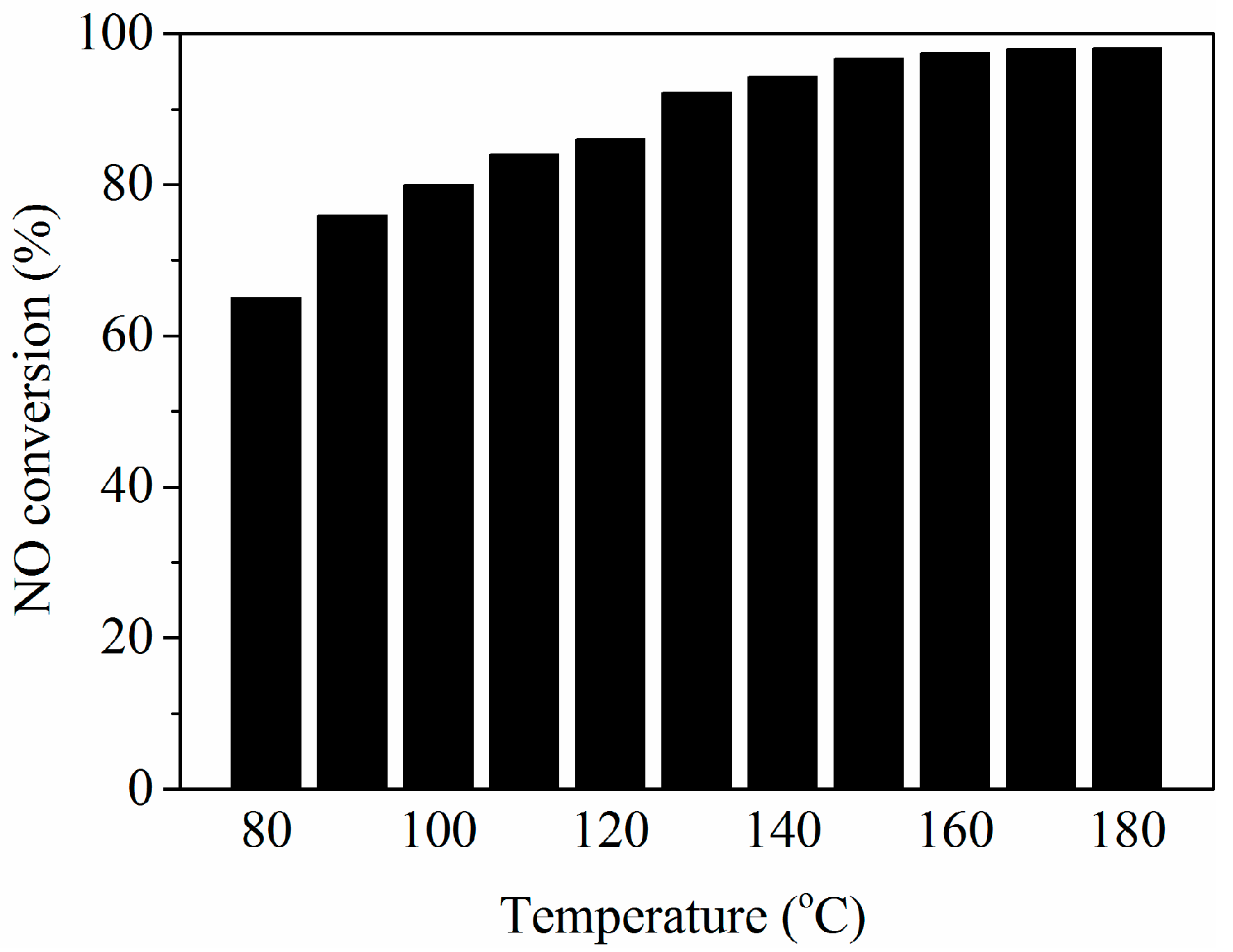
3.1. NH3-SCR Activity Results
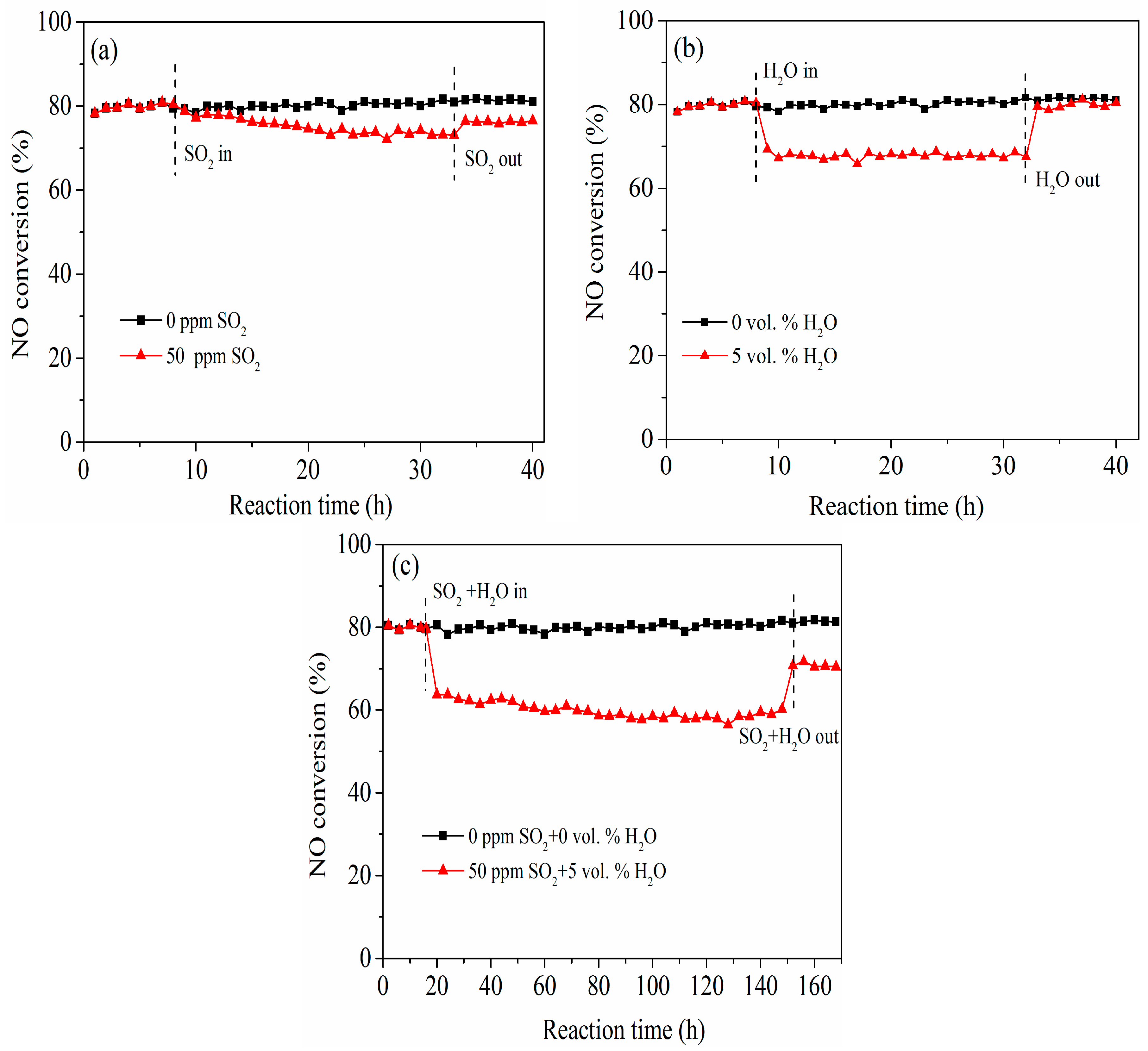
3.2. Effects of SO2 and H2O on NO Removal Activity
3.3. Physico-Chemical Characterization of Honeycomb Cordierite-Based Mn–Ce/Al2O3 Catalysts
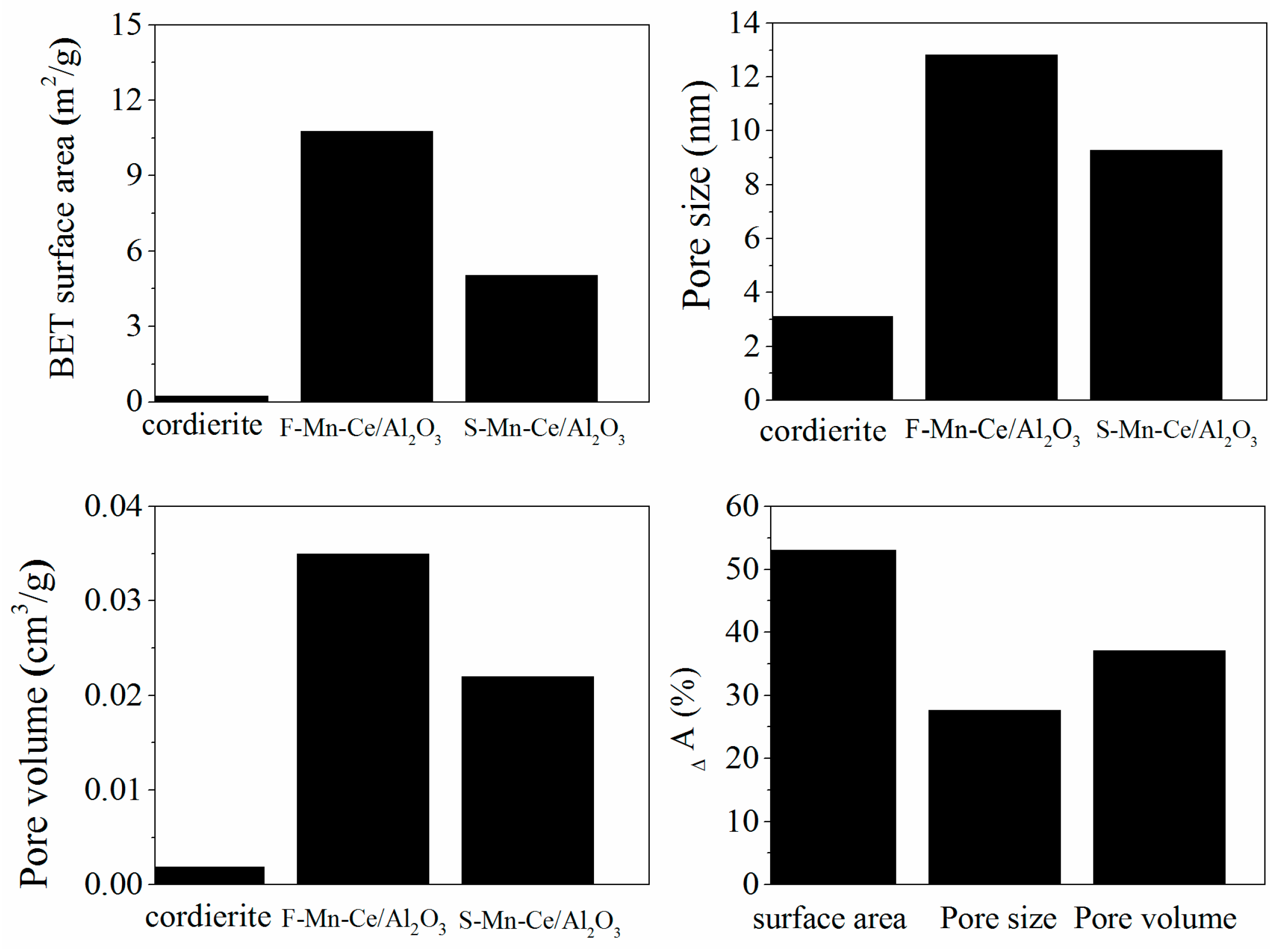
3.3.1. BET, SEM-EDS Mapping
3.3.2. XPS
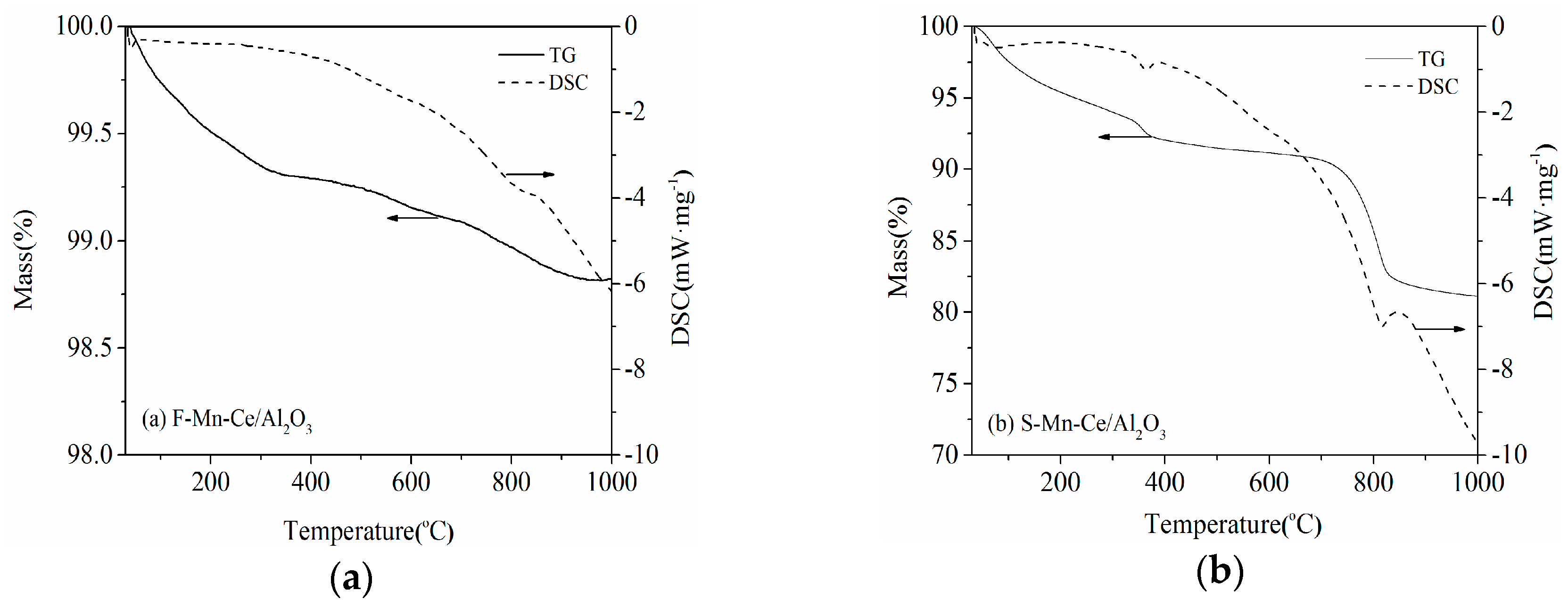
3.3.3. TG-DTA
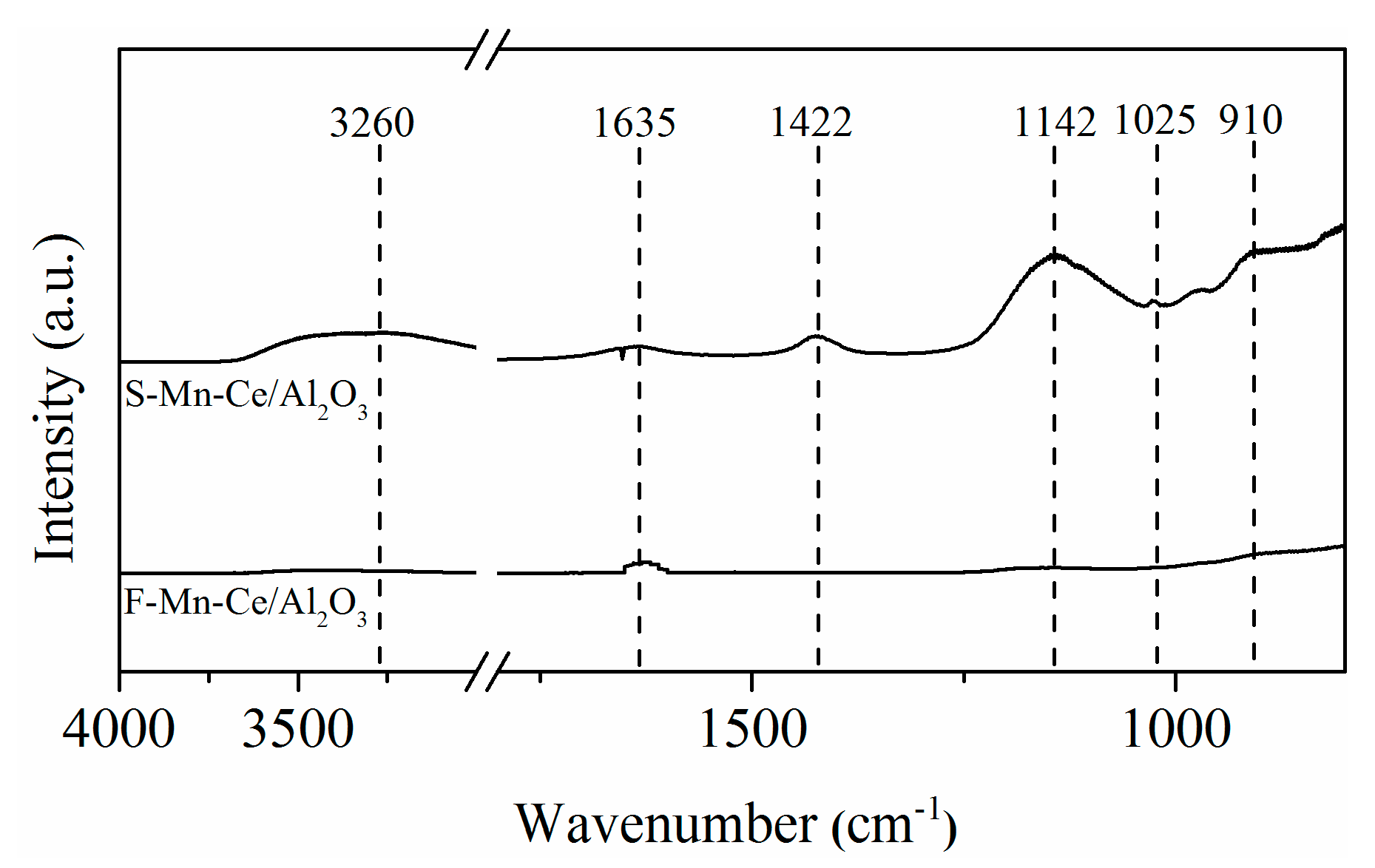
3.3.4. FT-IR Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, Z.; Yang, Y.; Zhang, S.; Zhu, T.; Zhu, J.; Wang, J. Selective catalytic reduction of NOx with NH3 over Mn-Ce mixed oxide catalyst at low temperatures. Catal. Today 2013, 216, 76–81. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, H. Low-temperature selective catalytic reduction of NOx with NH3 over Fe–Cu mixed oxide/ZSM-5 catalysts containing Fe2CuO4 phase. Res. Chem. Intermed. 2015, 41, 4961–4975. [Google Scholar] [CrossRef]
- Boningari, T.; Smirniotis, P.G. Impact of nitrogen oxides on the environment and human health: Mn-based materials for the NOx abatement. Curr. Opin. Chem. Eng. 2016, 13, 133–141. [Google Scholar] [CrossRef]
- Shen, B.; Liu, T.; Zhao, N.; Yang, X.; Deng, L. Iron-doped Mn-Ce/TiO2 catalyst for low temperature selective catalytic reduction of NO with NH3. J. Environ. Sci. 2010, 22, 1447–1454. [Google Scholar] [CrossRef]
- Zhang, Q.; Qiu, C.; Xu, H.; Lin, T.; Lin, Z.; Gong, M.; Chen, Y. Low-temperature selective catalytic reduction of NO with NH3 over monolith catalyst of MnOx/CeO2–ZrO2–Al2O3. Catal. Today 2011, 175, 171–176. [Google Scholar] [CrossRef]
- Liang, Q.; Li, J.; He, H.; Liang, W.; Zhang, T.; Fan, X. Effects of SO2 on the low temperature selective catalytic reduction of NO by NH3 over CeO2-V2O5-Wo3/TiO2 catalysts. Front. Environ. Sci. Eng. 2017, 11, 4. [Google Scholar] [CrossRef]
- Li, J.; Chang, H.; Ma, L.; Hao, J.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Sheng, Z.; Hu, Y.; Xue, J.; Wang, X.; Liao, W. SO2 poisoning and regeneration of Mn-Ce/TiO2 catalyst for low temperature NOx reduction with NH3. J. Rare Earths 2012, 30, 676–682. [Google Scholar] [CrossRef]
- Huang, J.; Tong, Z.; Huang, Y.; Zhang, J. Selective catalytic reduction of NO with NH3 at low temperatures over iron and manganese oxides supported on mesoporous silica. Appl. Catal. B Environ. 2008, 78, 309–314. [Google Scholar] [CrossRef]
- Liu, C.; Shi, J.W.; Gao, C.; Niu, C. Manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3: A review. Appl. Catal. A Gen. 2016, 522, 54–69. [Google Scholar] [CrossRef]
- Wu, Z.; Jin, R.; Liu, Y.; Wang, H. Ceria modified MnOx/TiO2 as a superior catalyst for NO reduction with NH3 at low-temperature. Catal. Commun. 2008, 9, 2217–2220. [Google Scholar] [CrossRef]
- Zhang, L.; Cui, S.; Guo, H.; Ma, X.; Luo, X. The influence of K+ cation on the MnOx-CeO2/TiO2 catalysts for selective catalytic reduction of NOx with NH3 at low temperature. J. Mol. Catal. A Chem. 2014, 390, 14–21. [Google Scholar] [CrossRef]
- Jin, Q.; Shen, Y.; Zhu, S. Effect of fluorine additive on CeO2ZrO2)/TiO2 for selective catalytic reduction of NO by NH3. J. Colloid Interface Sci. 2016, 487, 401. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Su, S.; Xiang, J.; Wang, P.Y.; Hu, S.; Sun, L.S.; Zhang, A.C. The activity and mechanism study of Fe-Mn-Ce/gamma-Al2O3 catalyst for low temperature selective catalytic reduction of NO with NH3. Fuel 2015, 139, 232–239. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, H.; Dong, L. Ceria-based catalysts for low-temperature selective catalytic reduction of NO with NH3. Catal. Sci. Technol. 2016, 6, 1248–1264. [Google Scholar] [CrossRef]
- Smirniotis, P.G.; Peña, D.A.; Uphade, B.S. Low-Temperature Selective Catalytic Reduction (SCR) of NO with NH3 by Using Mn, Cr, and Cu Oxides Supported on Hombikat TiO2. Angew. Chem. Int. Ed. 2010, 40, 2479–2482. [Google Scholar] [CrossRef]
- Thirupathi, B.; Smirniotis, P.G. Nickel-doped Mn/TiO2 as an efficient catalyst for the low-temperature SCR of NO with NH3: Catalytic evaluation and characterizations. J. Catal. 2012, 288, 74–83. [Google Scholar] [CrossRef]
- Xiong, Y.; Tang, C.; Yao, X.; Zhang, L.; Li, L.; Wang, X.; Deng, Y.; Gao, F.; Lin, D. Effect of metal ions doping (M = Ti4+, Sn4+) on the catalytic performance of MnOx/CeO2 catalyst for low temperature selective catalytic reduction of NO with NH3. Appl. Catal. A Gen. 2015, 495, 206–216. [Google Scholar] [CrossRef]
- Kijlstra, W.S.; Brands, D.S.; Poels, E.K.; Bliek, A. Kinetics of the selective catalytic reduction of NO with NH3 over MnOx/Al2O3 catalysts at low temperature. Catal. Today 1999, 50, 133–140. [Google Scholar] [CrossRef]
- Wu, Z.; Jin, R.; Wang, H.; Liu, Y. Effect of ceria doping on SO2 resistance of Mn/TiO2 for selective catalytic reduction of NO with NH3 at low temperature. Catal. Commun. 2009, 10, 935–939. [Google Scholar] [CrossRef]
- Jin, R.; Liu, Y.; Wu, Z.; Wang, H.; Gu, T. Relationship between SO2 poisoning effects and reaction temperature for selective catalytic reduction of NO over Mn–Ce/TiO2 catalyst. Catal. Today 2010, 153, 84–89. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.; Ma, K.; Zou, W.; Cao, Y.; Xiong, Y.; Tang, C.; Lin, D. Ultra-low loading of copper modified TiO2/CeO2 catalysts for low-temperature selective catalytic reduction of NO by NH3. Appl. Catal. B Environ. 2017, 207, 366–375. [Google Scholar] [CrossRef]
- Mach, P.; Kocian, M. Combination of Experimental and Theoretical Investigations of MnOx/Ce0.9Zr0.1O2 Nanorods for Selective Catalytic Reduction of NO with Ammonia. J. Phys. Chem. C 2013, 117, 9999–10006. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Wang, Y.; Ge, C.Z.; Zhan, L.; Li, C.; Qiao, W.; Ling, L. MnOx–CeO2/activated carbon honeycomb catalyst for selective catalytic reduction of NO with NH3 at low temperatures. Ind. Eng. Chem. Res. 2012, 51, 11667–11673. [Google Scholar] [CrossRef]
- Machida, M.; Daisuke Kurogi, A.; Kijima, T. MnOx−CeO2 binary oxides for catalytic NOx-sorption at low temperatures. Selective reduction of sorbed NOx. Chem. Mater. 2000, 12, 3158–3164. [Google Scholar] [CrossRef]
- Zhou, A.; Yu, D.; Yang, L.; Sheng, Z. Combined effects Na and SO2 in flue gas on Mn-Ce/TiO2 catalyst for low temperature selective catalytic reduction of NO by NH3 simulated by Na2SO4 doping. Appl. Surf. Sci. 2016, 378, 167–173. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, L.; Zhang, S.; Yang, W. Synthesis of a novel polyaniline-intercalated layered manganese oxide nanocomposite as electrode material for electrochemical capacitor. J. Power Sources 2007, 173, 1017–1023. [Google Scholar] [CrossRef]
- Reddy, A.S.; Gopinath, C.S.; Chilukuri, S. Selective ortho -methylation of phenol with methanol over copper manganese mixed-oxide spinel catalysts. J. Catal. 2006, 243, 278–291. [Google Scholar] [CrossRef]
- Venezia, A.M.; Carlo, G.D.; Pantaleo, G.; Liotta, L.F.; Melaet, G.; Kruse, N. Oxidation of CH4 over Pd supported on TiO2-doped SiO2: Effect of Ti(iv) loading and influence of SO2. Appl. Catal. B Environ. 2009, 88, 430–437. [Google Scholar] [CrossRef]
- Kapteijn, F.; Singoredjo, L.; Andreini, A.; Moulijn, J.A. Cheminform abstract: Activity and selectivity of pure manganese oxides in the selective catalytic reduction of nitric oxide with ammonia. Cheminform 1994, 3, 173–189. [Google Scholar] [CrossRef]
- Li, P.; Xin, Y.; Li, Q.; Wang, Z.; Zhang, Z.; Zheng, L. Ce-Ti amorphous oxides for selective catalytic reduction of NO with NH3: Confirmation of Ce-O-Ti active sites. Environ. Sci. Technol. 2012, 46, 9600–9605. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, L.; Tabakova, T.; Pantaleo, G.; Ivanov, I.; Zanella, R.; Paneva, D.; Velinov, N.; Sobczak, J.W.; Lisowski, W.; Avdeev, G. Nano-gold catalysts on Fe-modified ceria for pure hydrogen production via wgs and prox: Effect of preparation method and Fe-doping on the structural and catalytic properties. Appl. Catal. A Gen. 2013, 467, 76–90. [Google Scholar] [CrossRef]
- Xu, W.; He, H.; Yu, Y. Deactivation of a Ce/TiO2 catalyst by SO2 in the selective catalytic reduction of NO by NH3. J. Phys. Chem. C 2009, 113, 4426–4432. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, J.; Zhang, S.; Ma, L.; Woo, S.I. Selective catalytic reduction of NOx by NH3 over MoO3-promoted CeO2/TiO2 catalyst. Catal. Commun. 2014, 46, 90–93. [Google Scholar] [CrossRef]
- Chang, H.; Chen, X.; Li, J.; Ma, L.; Wang, C.; Liu, C.; Schwank, J.W.; Hao, J. Improvement of activity and SO2 tolerance of Sn-modified MnOx–CeO2 catalysts for NH3-SCR at low temperatures. Environ. Sci. Technol. 2013, 47, 5294–5301. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhang, L.; Li, L.; Liu, L.; Cao, Y.; Dong, X.; Gao, F.; Yu, D.; Tang, C.; Chen, Z. Investigation of the structure, acidity, and catalytic performance of CuO/Ti0.95Ce0.05O2 catalyst for the selective catalytic reduction of NO by NH3 at low temperature. Appl. Catal. B Environ. 2014, 150–151, 315–329. [Google Scholar] [CrossRef]
- Chen, B.; Yunsheng, M.A.; Ding, L.; Lingshun, X.U.; Zongfang, W.U.; Yuan, Q.; Huang, W. XPS and TPD study of NO interaction with Cu(111): Role of different oxygen species. Chin. J. Catal. 2013, 34, 964–972. [Google Scholar] [CrossRef]
- Pan, S.; Luo, H.; Li, L.; Wei, Z.; Huang, B. H2O and SO2 deactivation mechanism of MnOx/MWCNTs for low-temperature SCR of NOx with NH3. J. Mol. Catal. A Chem. 2013, 377, 154–161. [Google Scholar] [CrossRef]
- Hino, M.; Kurashige, M.; Matsuhashi, H.; Arata, K. The surface structure of sulfated zirconia: Studies of XPS and thermal analysis. Thermochim. Acta 2006, 441, 35–41. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Jia, Y.; Liu, X.; Zhong, Q. Selective catalytic oxidation of NO with over Ce-doped MnO/TiO catalysts. J. Nat. Gas Chem. 2012, 21, 17–24. [Google Scholar] [CrossRef]
- Alemany, L.J.; Berti, F.; Busca, G.; Ramis, G.; Robba, D.; Toledo, G.P.; Trombetta, M. Characterization and composition of commercial V2O5&z.Sbnd;Wo3&z.Sbnd;TiO2SCR catalysts. Appl. Catal. B Environ. 1996, 10, 299–311. [Google Scholar] [CrossRef]
- Bai, S.; Zhao, J.; Wang, L.; Zhu, Z. SO2-promoted reduction of NO with NH3 over vanadium molecularly anchored on the surface of carbon nanotubes. Catal. Today 2010, 158, 393–400. [Google Scholar] [CrossRef]
- Pietrogiacomi, D.; Magliano, A.; Ciambelli, P.; Sannino, D.; Campa, M.C.; Indovina, V. The effect of sulphation on the catalytic activity of CoOx/ZrO2 for NO reduction with NH3 in the presence of O2. Appl. Catal. B Environ. 2009, 89, 33–40. [Google Scholar] [CrossRef]
- Seo, P.W.; Park, K.H.; Hong, S.C. SO2 durability enhancement of ball milled V/TiO2 catalyst. J. Ind. Eng. Chem. 2010, 16, 283–287. [Google Scholar] [CrossRef]
- Yu, J.; Guo, F.; Wang, Y.; Zhu, J.; Liu, Y.; Su, F.; Gao, S.; Xu, G. Sulfur poisoning resistant mesoporous Mn-base catalyst for low-temperature SCR of NO with NH3. Appl. Catal. B Environ. 2010, 95, 160–168. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; Wiley: Hoboken, NJ, USA, 1986; p. 1122. [Google Scholar] [CrossRef]
- Topsøe, N.Y. Mechanism of the selective catalytic reduction of nitric oxide by ammonia elucidated by in situ online fourier transform infrared spectroscopy. Science 1994, 265, 1217. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Liu, Z.; Zhu, Z.; Liu, Q.; Ge, J.; Huang, Z. Simultaneous removal of SO2 and NOx from flue gas using a CuO/Al2O3 catalyst sorbent: I.Deactivation of SCR activity by SO2 at low temperatures. J. Catal. 2004, 224, 36–41. [Google Scholar] [CrossRef]
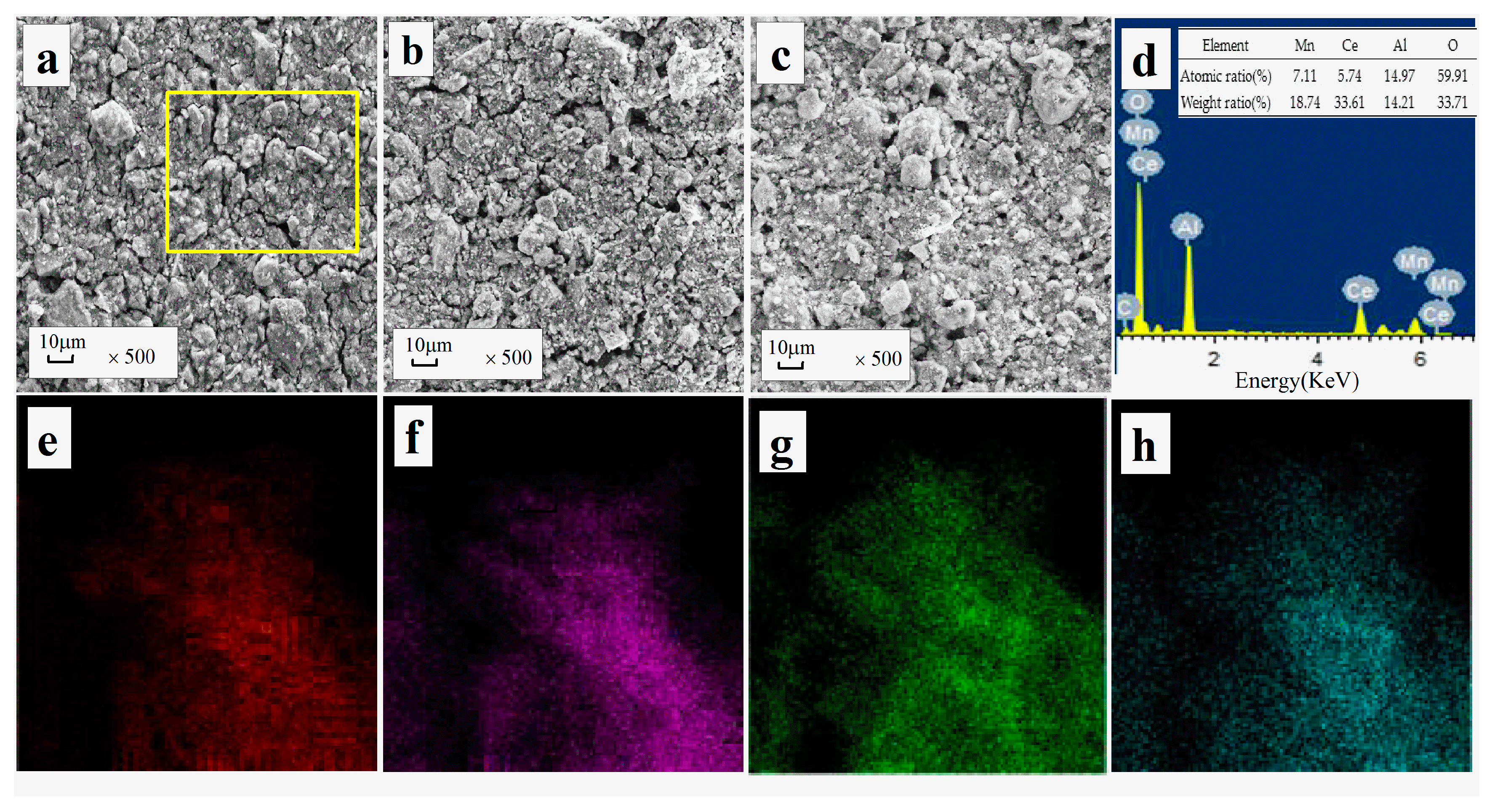
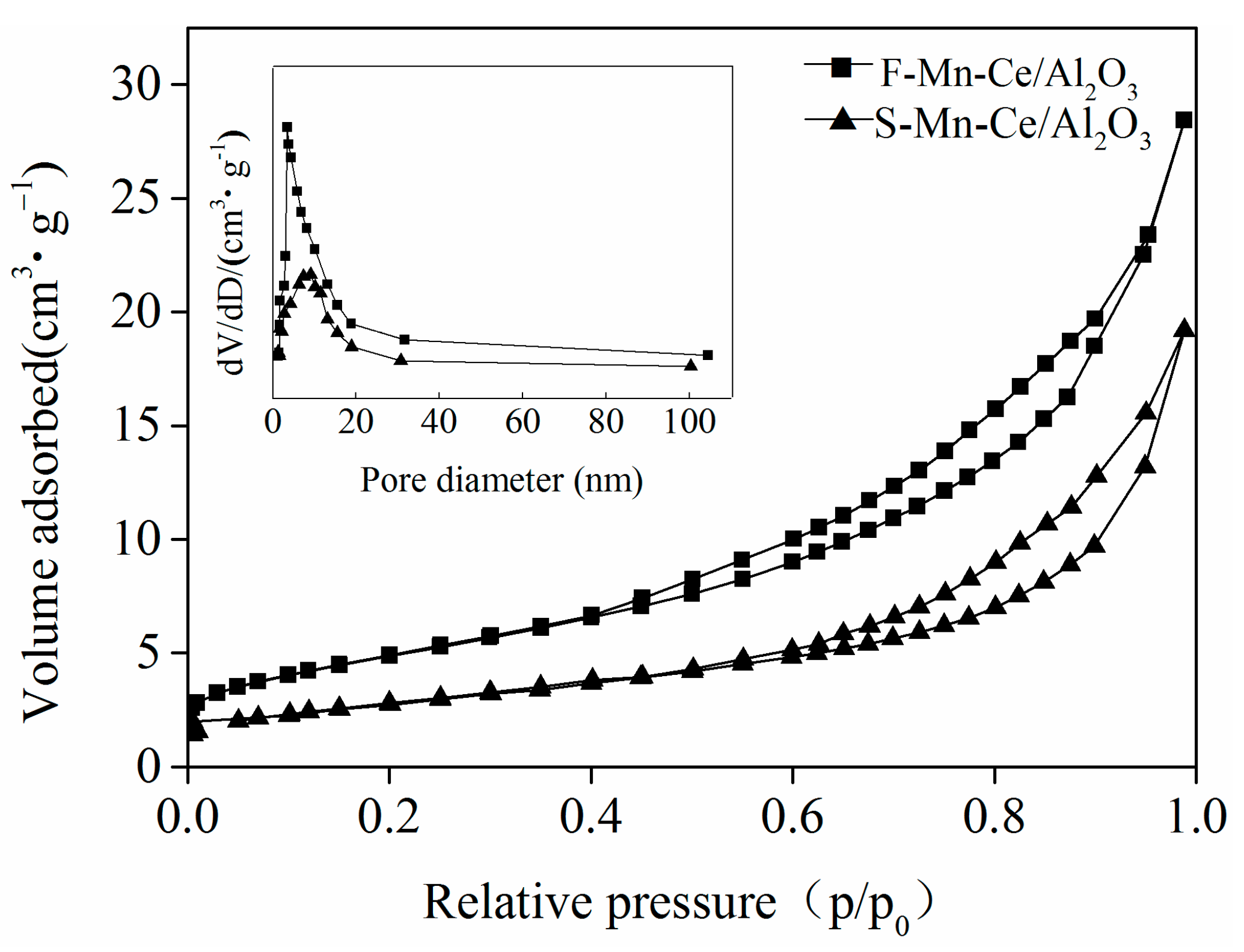
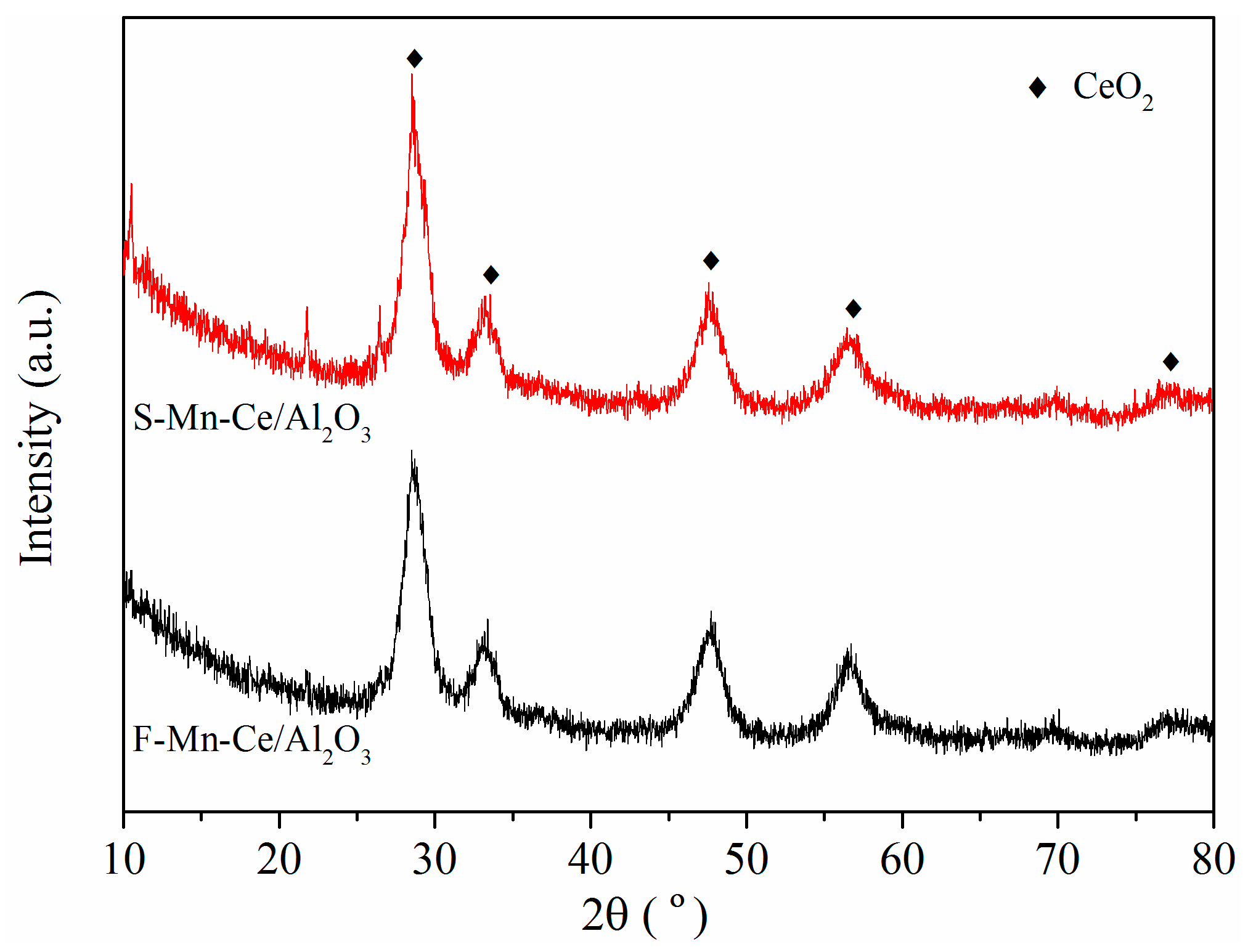
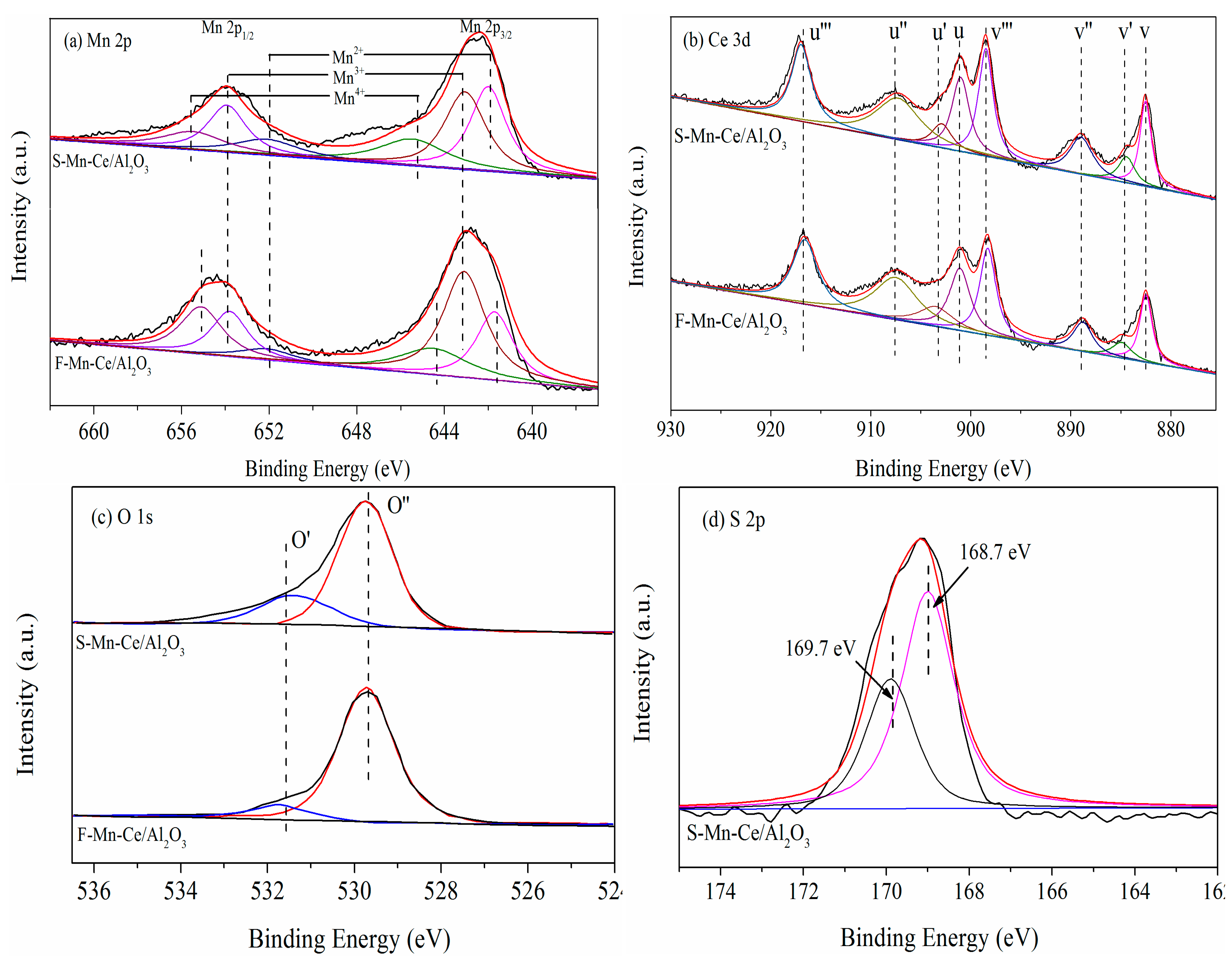
| Samples | Atomic Concentration (at %) | Surface Atomic Ratio (%) | |||||
|---|---|---|---|---|---|---|---|
| Mn | Ce | O | S | Ce3+/(Ce3+ + Ce4+) | Mn4+/(Mn2+ + Mn3+ + Mn4+) | O′/O′ + O′′ | |
| F-Mn–Ce/Al2O3 | 7.31 | 2.49 | 55.21 | 0 | 11.6 | 35.6 | 15.8 |
| S-Mn–Ce/Al2O3 | 4.05 | 1.99 | 66.75 | 3.01 | 12.7 | 26.74 | 30.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Zhang, C.; Zhao, Y.; Yan, X.; Cao, P. Poisoning Effect of SO2 on Honeycomb Cordierite-Based Mn–Ce/Al2O3Catalysts for NO Reduction with NH3 at Low Temperature. Appl. Sci. 2018, 8, 95. https://doi.org/10.3390/app8010095
Wang C, Zhang C, Zhao Y, Yan X, Cao P. Poisoning Effect of SO2 on Honeycomb Cordierite-Based Mn–Ce/Al2O3Catalysts for NO Reduction with NH3 at Low Temperature. Applied Sciences. 2018; 8(1):95. https://doi.org/10.3390/app8010095
Chicago/Turabian StyleWang, Chengzhi, Cheng Zhang, Yonggang Zhao, Xin Yan, and Peng Cao. 2018. "Poisoning Effect of SO2 on Honeycomb Cordierite-Based Mn–Ce/Al2O3Catalysts for NO Reduction with NH3 at Low Temperature" Applied Sciences 8, no. 1: 95. https://doi.org/10.3390/app8010095