The Effects of Remineralization via Fluoride Versus Low-Level Laser IR810 and Fluoride Agents on the Mineralization and Microhardness of Bovine Dental Enamel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Specimens Preparation
2.3. Demineralization
2.4. Remineralization
- Group A: Control. No treatment was given to the enamel (but they underwent the cyclic pH as all the groups).
- Group B: Fluoride (Duraphat varnish). The specimens were cleaned and dried with cotton; material was then applied on the surface and left for 1 min followed by storage in the remineralization solution.
- Group C: Tricalcium phosphate (Clinpro White varnish). The sample was rinsed and dried, and the material was applied to the surface and left for 1 min and stored in a remineralization solution.
- Group D: LLL (IR810 (Quantum)). The surface of the specimens were rinsed and dried, and it was exposed to infrared LLLs for 1 min at 810 nm and 200 mW in continuous wave mode. The window treatment received 6 J of energy.
- Group E: Fluoride + LLL. The sample was rinsed and dried followed by fluoride for 1 min. This was then irradiated on the fluoride for another minute with the laser as mentioned earlier.
- Group F: Tricalcium phosphate + LLL. The window treatment was rinsed, dried, mineralized for 1 min. It was then exposed to another minute of laser-like Group D with the remineralizing agent.
2.5. UV-Vis Spectroscopy for Phosphorus Determination
2.6. Atomic Absorption Spectroscopy for Calcium Determination
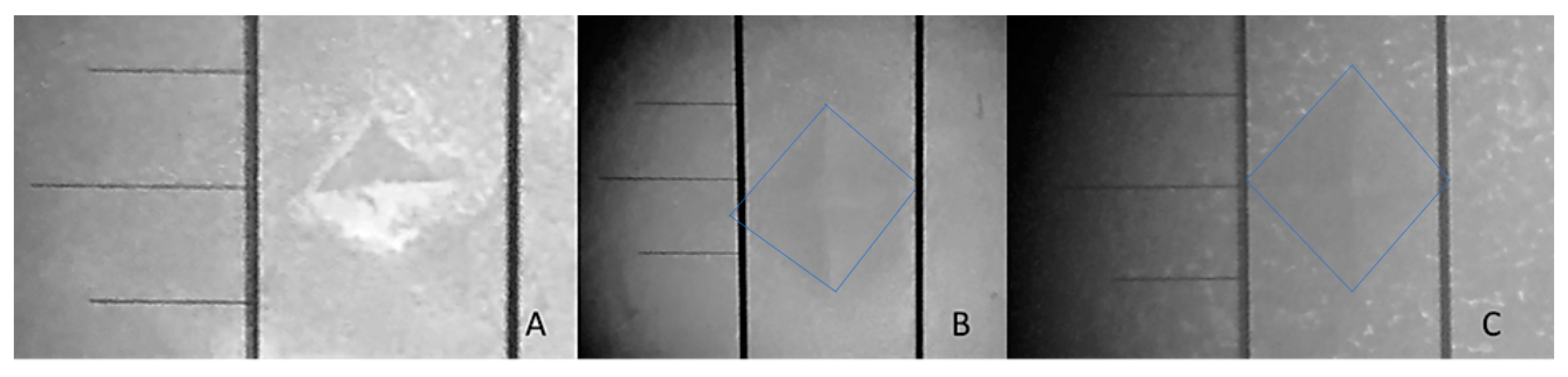
2.7. Surface Micro-Hardness (SMH)
2.8. Statistical Analyses
2.9. Ethical Considerations
3. Results
3.1. Phosphorus and Calcium Determination
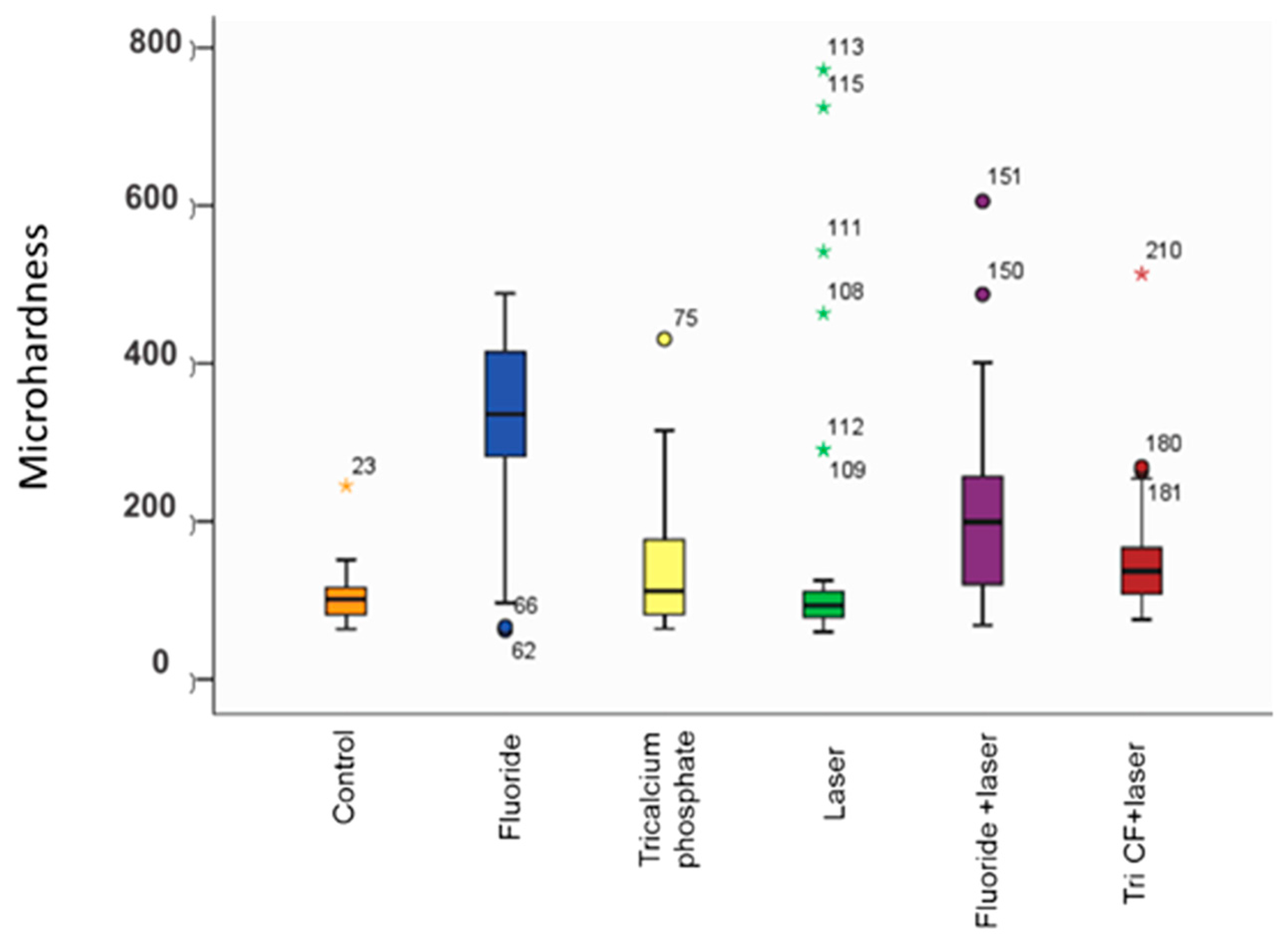
3.2. Enamel Surface Micro-Hardness
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Peters, M. Strategies for noninvasive demineralized tissue repair. Dent. Clin. N. Am. 2010, 54, 507–525. [Google Scholar] [CrossRef] [PubMed]
- Murdoch-Kinch, C.A.; McLean, M.E. Minimally invasive dentistry. J. Am. Dent. Assoc. 2003, 134, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, N.; Cai, F.; Huq, N.; Burrow, M.; Reynolds, E. New Approaches to enhanced remineralization of tooth enamel. J. Dent. Res. 2010, 89, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhou, J.; Watanabe, S.; Watanabe, K. In vitro study of the effects of fluoride-releasing dental materials on remineralization in an enamel erosion model. J. Dent. 2012, 40, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Caruso, S.; Bernardi, S.; Pasini, M.; Giuca, M.R.; Docimo, R.; Continenza, M.A.; Gatto, R. The process of mineralisation in the development of human tooth. Eur. J. Paediatr. Dent. 2016, 17, 322–326. [Google Scholar] [PubMed]
- Lins, R.D.; Dantas, E.M.; Lucena, K.C.; Catão, M.H.; Granville-Garcia, A.F.; Carvalho Neto, L.G. Biostimulation effects of low-power laser in the repair process. An. Bras. Dermatol. 2010, 85, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Matson, J.R.; Matson, E.; Navarro, R.S.; Bocangel, J.S.; Jaeger, R.G.; Eduardo, C.P. Er: YAG laser effects on enamel occlusal fissures: An in vitro study. J. Clin. Laser Med. Surg. 2002, 20, 27–35. [Google Scholar] [CrossRef] [PubMed]
- 3M ESPE. Solutions. Products 3M. Available online: http://solutions.3m.com.mx/wps/portal/3M/es_MX/3MESPE_LA/dental-professionals/ (accessed on 25 November 2014).
- Elkassas, D.; Arafa, A. Remineralizing efficacy of different calcium-phosphate and fluoride based delivery vehicles on artificial caries like enamel lesions. J. Dent. 2014, 42, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Karlinsey, R.; Mackey, A.; Walker, E.; Frederick, K. Surfactant-modified beta-TCP: Structure, properties, and in vitro remineralization of subsurface enamel lesions. J. Mater. Sci. Mater. Med. 2010, 21, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Vlacic, J.; Meyers, I.; Kim, J.; Walsh, L. Laser-activated fluoride treatment of enamel against and artificial caries challenge: Comparison of five wavelengths. Aust. Dent. J. 2007, 52, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Muller, K.; Rodrigues, C.; Nunez, S.; Rocha, R.; Ribeiro, M. Effects of low-power red laser on induced-dental caries in rats. Arch. Oral Biol. 2007, 52, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Laser Systems. Laser Dental Quantum® IR810. Available online: http://www.lasersystems.com.mx/dental.php (accessed on 20 October 2014).
- Heravi, F.; Farzaneh, A.; Mahdieh, M.; Sorouh, B. Comparative evaluation of the effect of Er: YAG laser and low level laser irradiation combined with CCP-ACPF cream on treatment of enamel caries. J. Clin. Exp. Dent. 2014, 6, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Lippert, F.; Lynch, R. Comparison of Knoop and Vickers surface microhardness and transverse microradiography for the study of early caries lesion formation in human and bovine enamel. J. Arch. Oral Biol. 2014, 59, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Monroy, J.M.; Contreras-Bulnes, R.; Olea-Mejía, O.F.; García-Fabila, M.M.; Rodríguez-Vilchis, L.E.; Sánchez-Flores, I.; Centeno-Pedraza, C. Chemical changes associated with increased acid resistance of Er: YAG laser irradiated enamel. Sci. World J. 2014, 1, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lippert, F.; Hara, A. Fluoride dose-response of human and bovine enamel caries lesions under remineralizing conditions. Am. J. Dent. 2012, 25, 205–209. [Google Scholar] [PubMed]
- López, G.; De la Casa, M.; Sáez, M.; López, M. Decalcification of root dentin: Comparison of two methods for identification. Sci. Oral J. 2013, 10, 15–23. [Google Scholar]
- Hjortsjö, C.; Jonski, G.; Young, A.; Saxegaard, E. Etching effect of fluorides on human tooth enamel in vitro. Arch. Oral Biol. 2014, 59, 1328–1333. [Google Scholar] [CrossRef] [PubMed]
- Low, I.; Alhuthali, A. In-situ monitoring of dental erosion in tooth enamel when exposed to soft drinks. Mater. Sci. Eng. 2008, 28, 1322–1325. [Google Scholar] [CrossRef]
| Calcium Content mg/L | Phosphorus Content mg/L | ||||
|---|---|---|---|---|---|
| Groups | Demineral | Remineral | Groups | Demineral | Remineral |
| Before Treatment | After Treatment | Before Treatment | After Treatment | ||
| A (Control) | 3.80 ± 0.67 | 1.00 ± 0.20 | A (Control) | 2.17 ± 0.22 | 0.52 ± 0.05 |
| B (Fluoride) | 3.00 ± 0.63 | 0.88 ± 0.11 * | B (Fluoride) | 1.45 ± 0.24 | 0.40 ± 0.05 * |
| C (TriFC) | 3.75 ± 0.75 | 1.34 ± 0.17 | C (TriFC) | 1.58 ± 0.29 | 0.41 ± 0.07 |
| D (LLL) | 3.02 ± 0.58 | 0.99 ± 0.15 | D (LLL) | 1.94 ± 0.32 | 0.53 ± 0.08 |
| E (Fluoride + LLL) | 2.52 ± 0.90 | 0.96 ± 0.16 | E (Fluoride + LLL) | 1.98 ± 0.19 | 0.42 ± 0.05 |
| F (TriFC + LLL) | 2.70 ± 0.54 | 1.14 ± 0.19 | F (TriFC + LLL) | 1.98 ± 0.25 | 0.43 ± 0.07 |
| Calcium | A | B | C | D | E | F |
| A | - | |||||
| B | 0.018271 | - | ||||
| C | 0.000000 * | 0.000000 * | - | |||
| D | 0.399090 | 0.009474 | 0.000002 * | - | ||
| E | 0.000000 * | 0.000035 * | 0.000000 * | 0.000000 * | - | |
| F | 0.003380 | 0.000001 * | 0.013938 | 0.007088 | 0.000000 | - |
| Phosphorus | A | B | C | D | E | F |
| A | - | |||||
| B | 0.000000 * | - | ||||
| C | 0.000000 * | 0.199111 | - | |||
| D | 0.444079 | 0.000000 * | 0.000000 * | - | ||
| E | 0.000000 * | 0.076646 | 0.279880 | 0.000000 * | - | |
| F | 0.000001 * | 0.019918 | 0.113015 | 0.000002 * | 0.265181 | - |
| Variable | Variable | R | p | |
|---|---|---|---|---|
| Remineralization solution | Calcium ions | Microhardness | −0.268 | 0.0008 |
| Phosphorus ions | Microhardness | −0.208 | <0.002 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara-Carrillo, E.; Lovera-Rojas, N.; Morales-Luckie, R.A.; Robles-Bermeo, N.L.; García-Fabila, M.M.; De la Rosa-Santillana, R.; Medina-Solís, C.E. The Effects of Remineralization via Fluoride Versus Low-Level Laser IR810 and Fluoride Agents on the Mineralization and Microhardness of Bovine Dental Enamel. Appl. Sci. 2018, 8, 78. https://doi.org/10.3390/app8010078
Lara-Carrillo E, Lovera-Rojas N, Morales-Luckie RA, Robles-Bermeo NL, García-Fabila MM, De la Rosa-Santillana R, Medina-Solís CE. The Effects of Remineralization via Fluoride Versus Low-Level Laser IR810 and Fluoride Agents on the Mineralization and Microhardness of Bovine Dental Enamel. Applied Sciences. 2018; 8(1):78. https://doi.org/10.3390/app8010078
Chicago/Turabian StyleLara-Carrillo, Edith, Nayeli Lovera-Rojas, Raúl Alberto Morales-Luckie, Norma Leticia Robles-Bermeo, María Magdalena García-Fabila, Rubén De la Rosa-Santillana, and Carlo Eduardo Medina-Solís. 2018. "The Effects of Remineralization via Fluoride Versus Low-Level Laser IR810 and Fluoride Agents on the Mineralization and Microhardness of Bovine Dental Enamel" Applied Sciences 8, no. 1: 78. https://doi.org/10.3390/app8010078








