Effect of Ultrafast Imaging on Shear Wave Visualization and Characterization: An Experimental and Computational Study in a Pediatric Ventricular Model
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
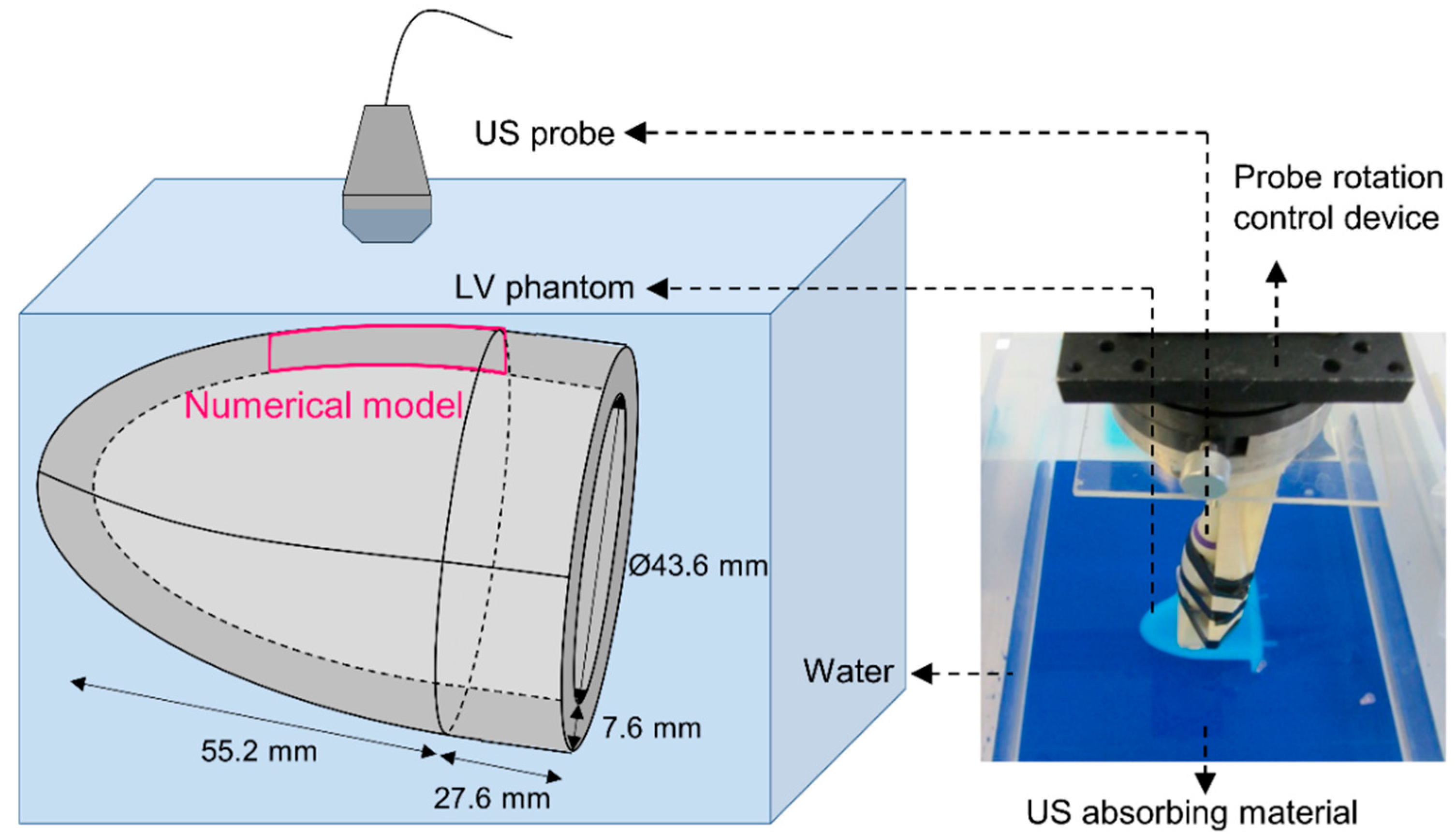
2.1. SWE Experiments
2.2. SWE Multiphysics Model
2.2.1. Pushing Sequence
2.2.2. Imaging Sequence
2.3. Post-Processing
2.3.1. Axial Velocity Estimation
2.3.2. Shear Modulus Estimation
3. Results
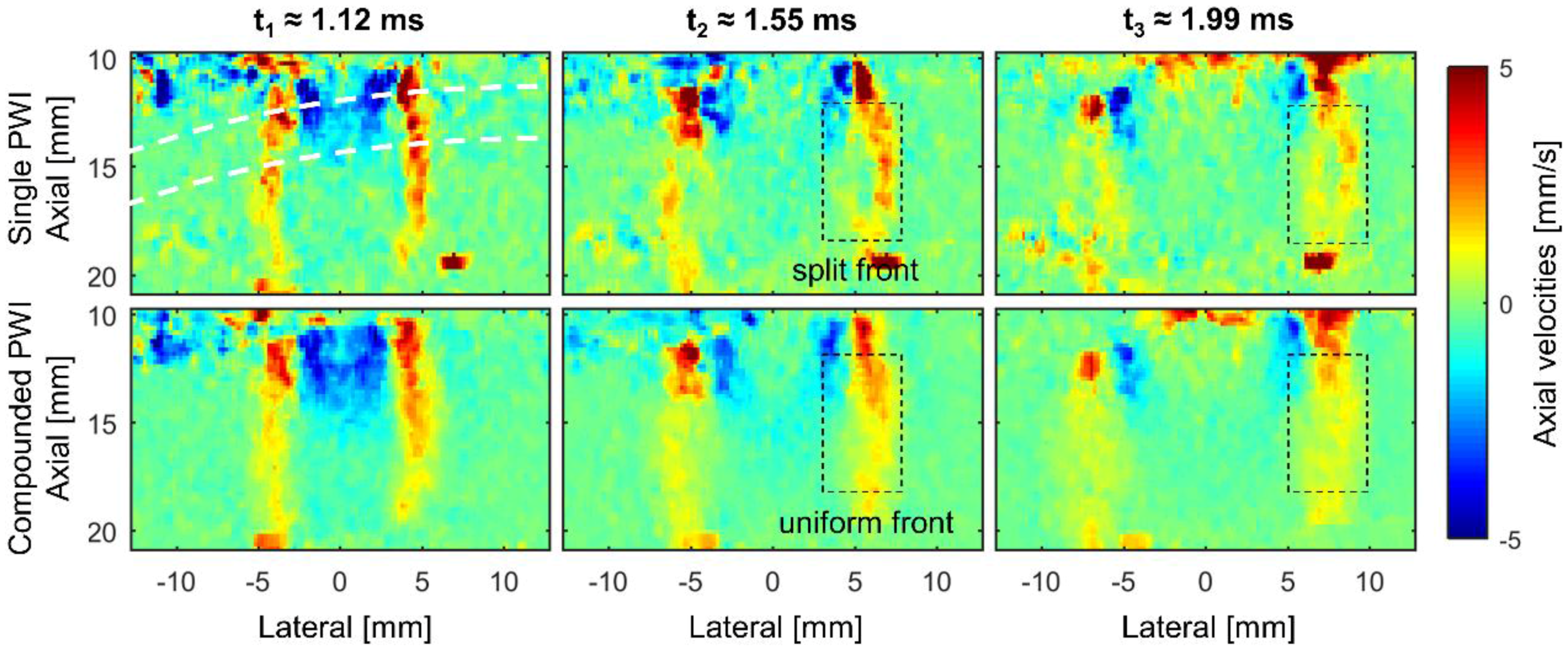
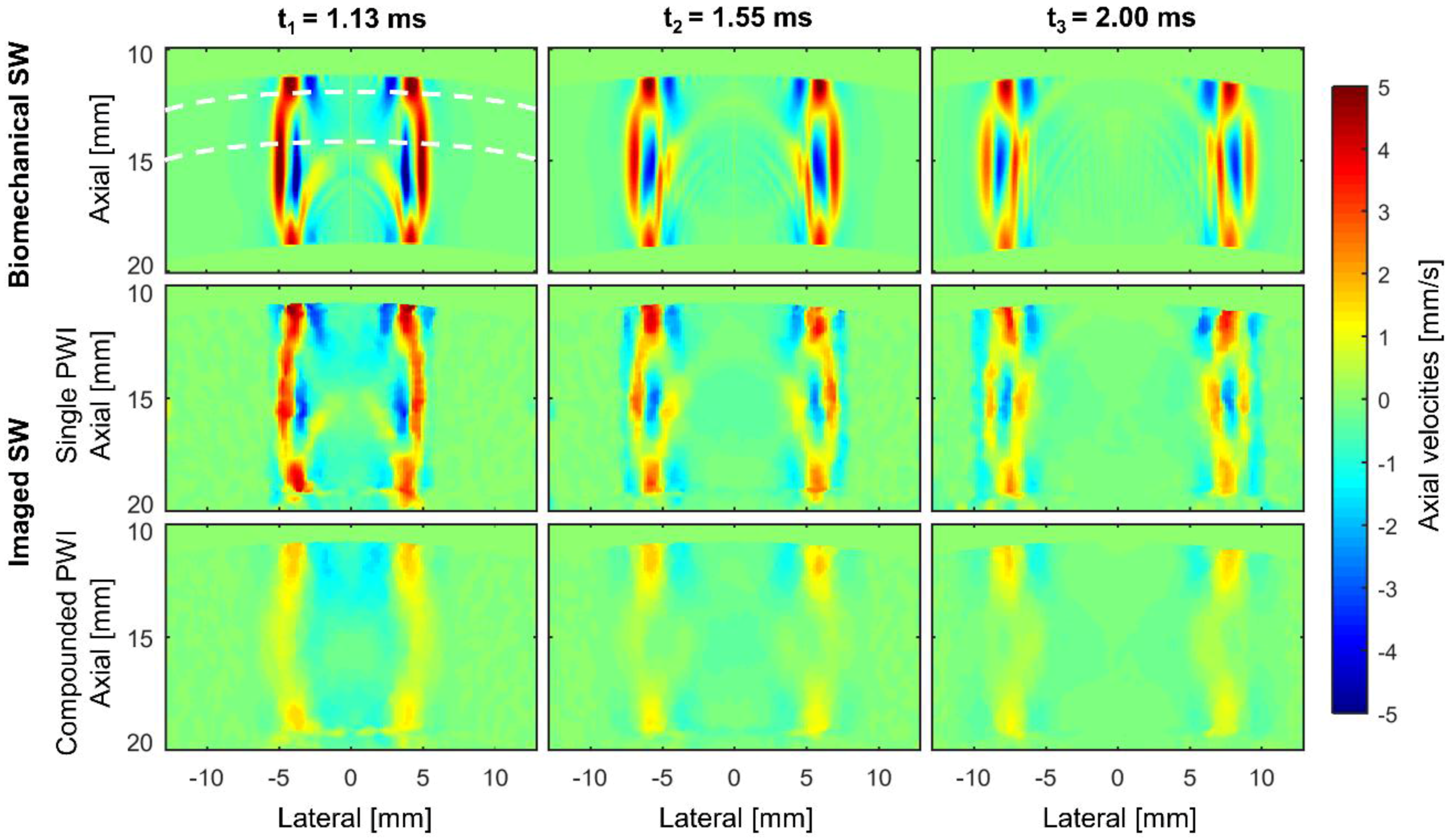
3.1. Analyzing the Shear Wave’s Characteristics in the Time Domain
3.2. Analyzing the Shear Wave’s Characteristics in the Frequency Domain
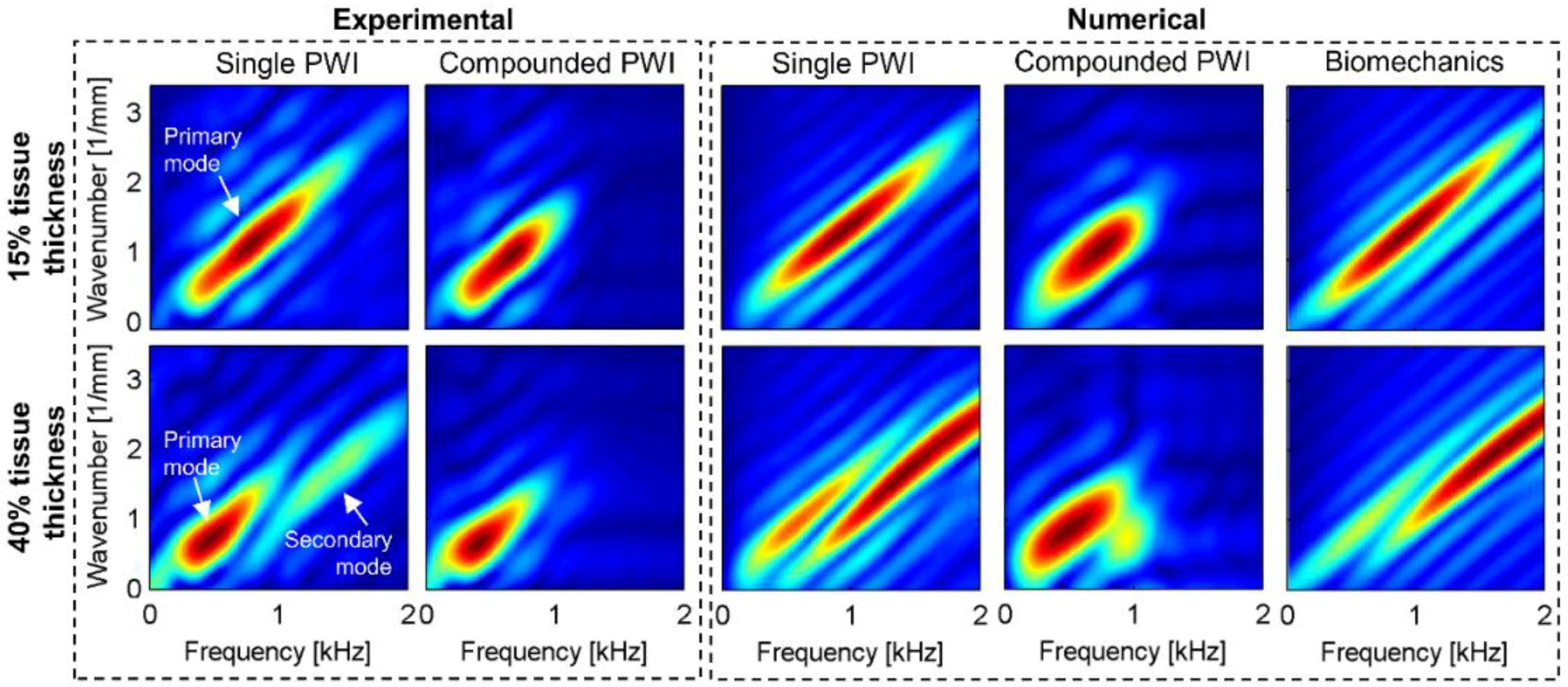
3.2.1. Mode(s) Excitation
3.2.2. Fourier Energy Magnitude
3.2.3. Frequency Content
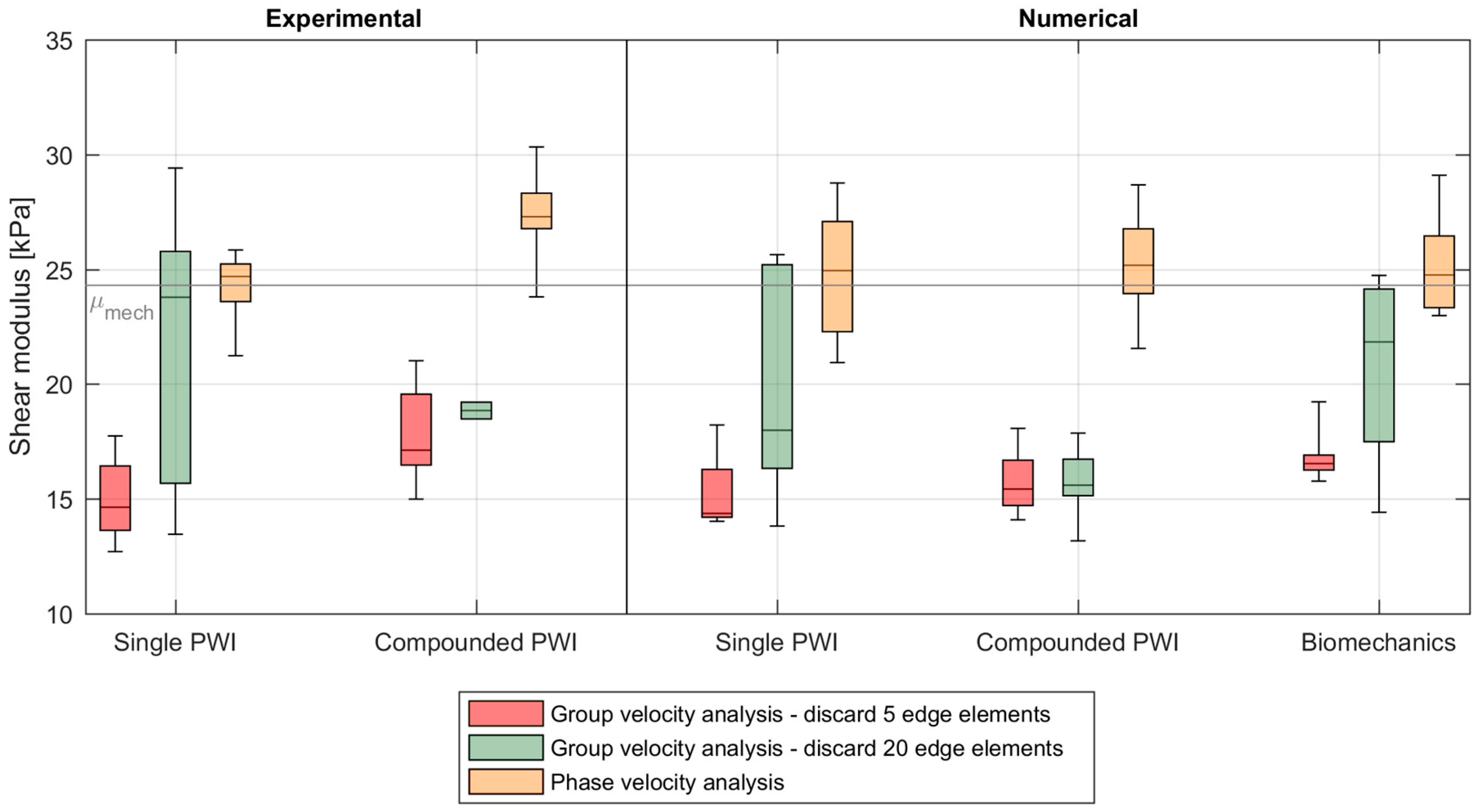
3.3. Shear Wave Speed Analysis
4. Discussion
4.1. Multiphysics Modeling
4.2. Effect of Ultrafast Imaging on SWE in the Studied Left Ventricular Model
4.3. Recommendations and Impact for Other Applications
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bercoff, J. Ultrafast Ultrasound Imaging. In Ultrasound Imaging—Medical Applications; Minin, P.O., Ed.; Intech: Rijeka, Croatia, 2011. [Google Scholar]
- Tanter, M.; Fink, M. Ultrafast imaging in biomedical ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014, 61, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Tanter, M.; Bercoff, J.; Sandrin, L.; Fink, M. Ultrafast Compound Imaging for 2-D Motion Vector Estimation: Application to Transient Elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2002, 49, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Sandrin, L.; Tanter, M.; Catheline, S.; Fink, M. Shear Modulus Imaging with 2-D Transient Elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2002, 49, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Bercoff, J.; Tanter, M.; Fink, M. Supersonic Shear Imaging: A New Technique for Soft Tissue Elasticity Mapping. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Gennisson, J.L.; Deffieux, T.; Fink, M.; Tanter, M. Ultrasound elastography: Principles and techniques. Diagn. Interv. Imaging 2013, 94, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Sarvazyan, A.P.; Rudenko, O.V.; Swanson, S.D.; Fowlkes, J.B.; Emelianov, S.Y. Shear Wave Elasticity Imaging: A New Ultrasonics Technology of Medical Diagnostics. Ultrasound Med. Biol. 1998, 24, 1419–1435. [Google Scholar] [CrossRef]
- Nightingale, K.; Soo, M.S.; Nightingale, R.; Trahey, G. Acoustic Radiation Force Impulse Imaging: In Vivo Demonstration of Clinical Feasibility. Ultrasound Med. Biol. 2002, 28, 227–235. [Google Scholar] [CrossRef]
- Doherty, J.R.; Trahey, G.E.; Nightingale, K.R.; Palmeri, M.L. Acoustic radiation force elasticity imaging in diagnostic ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Montaldo, G.; Tanter, M.; Bercoff, J.; Benech, N.; Fink, M. Coherent Plane-Wave Compounding for Very High Frame Rate Ultrasonography and Transient Elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Bercoff, J.; Montaldo, G.; Loupas, T.; Savery, D.; Mézière, F.; Fink, M.; Tanter, M. Ultrafast Compound Doppler Imaging: Providing Full Blood Flow Characterization. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Papadacci, C.; Pernot, M.; Couade, M.; Fink, M.; Tanter, M. High Contrast Ultrafast Imaging of the Human Heart. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014, 61, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Couture, O.; Bannouf, S.; Montaldo, G.; Aubry, J.F.; Fink, M.; Tanter, M. Ultrafast imaging of ultrasound contrast agents. Ultrasound Med. Biol. 2009, 35, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- Mace, E.; Montaldo, G.; Osmanski, B.F.; Cohen, I.; Fink, M.; Tanter, M. Functional ultrasound imaging of the brain: Theory and basic principles. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Tanter, M.; Bercoff, J.; Athanasiou, A.; Deffieux, T.; Gennisson, J.L.; Montaldo, G.; Muller, M.; Tardivon, A.; Fink, M. Quantitative assessment of breast lesion viscoelasticity: Initial clinical results using supersonic shear imaging. Ultrasound Med. Biol. 2008, 34, 1373–1386. [Google Scholar] [CrossRef] [PubMed]
- Ferraioli, G.; Parekh, P.; Levitov, A.B.; Filice, C. Shear wave elastography for evaluation of liver fibrosis. J. Ultrasound Med. 2014, 33, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Couade, M.; Pernot, M.; Prada, C.; Messas, E.; Emmerich, J.; Bruneval, P.; Criton, A.; Fink, M.; Tanter, M. Quantitative assessment of arterial wall biomechanical properties using shear wave imaging. Ultrasound Med. Biol. 2010, 36, 1662–1676. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.; Couade, M.; Bercoff, J.; Tanter, M. Assessment of Viscous and Elastic Properties of Sub-Wavelength Layered Soft Tissues Using Shear Wave Spectroscopy: Theoretical Framework and In Vitro Experimental Validation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58, 2305–2315. [Google Scholar] [CrossRef] [PubMed]
- Widman, E.; Maksuti, E.; Amador, C.; Urban, M.W.; Caidahl, K.; Larsson, M. Shear Wave Elastography Quantifies Stiffness in Ex Vivo Porcine Artery with Stiffened Arterial Region. Ultrasound Med. Biol. 2016, 42, 2423–2435. [Google Scholar] [CrossRef] [PubMed]
- Caenen, A.; Shcherbakova, D.; Verhegghe, B.; Papadacci, C.; Pernot, M.; Segers, P.; Swillens, A. A Versatile and Experimentally Validated Finite Element Model to Assess the Accuracy of Shear Wave Elastography in a Bounded Viscoelastic Medium. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2015, 62, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, M.L.; Sharma, A.C.; Bouchard, R.R.; Nightingale, R.W.; Nightingale, K.R. A Finite-Element Method Model of Soft Tissue Response to Impulsive Acoustic Radiation Force. IEEE Trans. Ultrason Ferroelectr. Freq. Control 2005, 52, 1699–1712. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Szajewski, B.A.; Hah, Z.; Parker, K.J.; Maniatty, A.M. Modeling shear waves through a viscoelastic medium induced by acoustic radiation force. Int. J. Numer. Methods Biomed. Eng. 2012, 28, 678–696. [Google Scholar] [CrossRef] [PubMed]
- Caenen, A.; Pernot, M.; Shcherbakova, D.A.; Mertens, L.; Kersemans, M.; Segers, P.; Swillens, A. Investigating Shear Wave Physics in a Generic Pediatric Left Ventricular Model via In Vitro experiments and Finite Element Simulations. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.A. Field: A Program for Simulating Ultrasound Systems. In Proceedings of the 10th Nordic-Baltic Conference on Biomedical Imaging, Tampere, Finland, 9–13 June 1996; pp. 351–353. [Google Scholar]
- Jensen, J.A.; Svensen, N.B. Calculation of Pressure Fields from Arbitrarily Shaped, Apodized, and Excited Ultrasound Transducers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1992, 39, 262–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nightingale, K. Acoustic Radiation Force Impulse (ARFI) Imaging: A Review. Curr. Med. Imaging Rev. 2011, 7, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Shutilov, V. Fundamental Physics of Ultrasound; CRC Press: Boca Raton, FL, USA, 1988. [Google Scholar]
- Standard Test Methods for Density and Specific Gravity (Relative Density) of Plastics by Displacement; ASTM Standard D792-08; ASTM International: West Conshohocken, PA, USA, 2008.
- Kamopp, D.C.; Margolis, D.L.; Rosenberg, R.C. Appendix: Typical material property values useful in modeling mechanical, acoustic and hydraulic elements. In System Dynamics: Modeling, Simulation and Control of Mechatronic Systems; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Dendy, P.; Heaton, B. Physics for Diagnostic Radiology; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Schroeder, W.; Martin, K.; Lorensen, B. The Visualization Toolkit, 4th ed.; Kitware Inc.: Clifton Park, NY, USA, 2006. [Google Scholar]
- Shcherbakova, D. A multiphysics model of the mouse aorta for the mice optimization of high-frequency ultrasonic imaging in mice. In Faculty of Engineering and Architecture; Ghent University: Ghent, Belgium, 2012. [Google Scholar]
- Ekroll, I.K.; Swillens, A.; Segers, P.; Dahl, T.; Torp, H.; Lovstakken, L. Simultaneous quantification of flow and tissue velocities based on multi-angle plane wave imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 727–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasai, C.; Namekawa, K.; Koyano, A.; Omoto, R. Real-Time Two-Dimensional Blood Flow Imaging Using an Autocorrelation Technique. IEEE Trans. Sonics Ultrason. 1985, 32, 458–464. [Google Scholar] [CrossRef]
- Lovstakken, L. Signal Processing in Diagnostic Ultrasound: Algorithms for Real-Time Estimation and Visualization of Blood Flow Velocity; Norwegian University of Science and Technology: Trondheim, Norway, 2007. [Google Scholar]
- Palmeri, M.L.; Wang, M.H.; Dahl, J.J.; Frinkley, K.D.; Nightingale, K.R. Quantifying hepatic shear modulus in vivo using acoustic radiation force. Ultrasound Med. Biol. 2008, 34, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.; Nenadic, I.; Urban, M.W.; Greenleaf, J.F. Material property estimation for tubes and arteries using ultrasound radiation force and analysis of propagating modes. J. Acoust. Soc. Am. 2011, 129, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Nenadic, I.Z.; Urban, M.W.; Mitchell, S.A.; Greenleaf, J.F. Lamb wave dispersion ultrasound vibrometry (LDUV) method for quantifying mechanical properties of viscoelastic solids. Phys. Med. Biol. 2011, 56, 2245–2264. [Google Scholar] [CrossRef] [PubMed]
- Kanai, H. Propagation of spontaneously actuated pulsive vibration in human heart wall and in vivo viscoelasticity estimation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005, 52, 1931–1942. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, M.L.; McAleavey, S.A.; Trahey, G.E.; Nightingale, K.R. Ultrasonic Tracking of Acoustic Radiation Force-Induced Displacements in Homogeneous Media. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2006, 53, 1300–1313. [Google Scholar] [CrossRef] [PubMed]
- Rouze, N.C.; Wang, M.H.; Palmeri, M.L.; Nightingale, K.R. Parameters affecting the resolution and accuracy of 2-D quantitative shear wave images. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- Ekroll, I.K.; Voormolen, M.M.; Standal, O.K.V.; Rau, J.M.; Lovstakken, L. Coherent compounding in doppler imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2015, 62, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, M.L.; Deng, Y.; Rouze, N.C.; Nightingale, K.R. Dependence of shear wave spectral content on acoustic radiation force excitation duration and spatial beamwidth. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014. [Google Scholar] [CrossRef]
- Mercado, K.P.; Langdon, J.; Helguera, M.; McAleavey, S.A.; Hocking, D.C.; Dalecki, D. Scholte wave generation during single tracking location shear wave elasticity imaging of engineered tissues. J. Acoust. Soc. Am. 2015, 138, EL138–EL144. [Google Scholar] [CrossRef] [PubMed]
- Couade, M.; Pernot, M.; Messas, E.; Bel, A.; Ba, M.; Hagege, A.; Fink, M.; Tanter, M. In vivo quantitative mapping of myocardial stiffening and transmural anisotropy during the cardiac cycle. IEEE Trans. Med. Imaging 2011, 30, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.N.; Pernot, M.; Couade, M.; Messas, E.; Bruneval, P.; Bel, A.; Hagege, A.; Fink, M.; Tanter, M. Mapping myocardial fiber orientation using echocardiography-based shear wave imaging. IEEE Trans. Med. Imaging 2012, 31, 554–562. [Google Scholar] [PubMed]

| Parameters | Values | |
|---|---|---|
| Pushing sequence | Push frequency f0 | 8 MHz |
| F-number | 2.5 | |
| Apodization | - | |
| Push duration | 250 µs | |
| Imaging sequence | Number of cycles | 2 |
| Emission frequency | 8 MHz | |
| Pulse repetition frequency (PRF) | 6.9 kHz | |
| Imaging depth | 40 mm | |
| F-number on transmit | - | |
| Transmit apodization | - | |
| F-number on receive | 1.2 | |
| Receive apodization | Hanning | |
| Receive bandwidth | 60% |
| Characteristics | Value | |
|---|---|---|
| Water | Density | 1000 kg/m³ |
| Speed of sound [29] | 1480 m/s | |
| Bulk modulus [29] | 2200 MPa | |
| PVA | Density | 1045.5 kg/m³ |
| Speed of sound | 1568 m/s | |
| Young’s Modulus | 73.0 kPa | |
| Attenuation coefficient [30] | 0.4 dB/cm/MHz | |
| Coefficient of Poisson [20] | 0.49999 | |
| Normalized shear modulus | 4.04 × 10−3 | |
| Relaxation time | 99.8 × 10−6 s | |
| Normalized shear modulus | 7.04 × 10−2 | |
| Relaxation time | 77.9 s |
| Acquisition | 15% Tissue Thickness | 40% Tissue Thickness | |
|---|---|---|---|
| Experimental | |||
| Single PWI | 6.52 | 3.42 | |
| Compounded PWI | 1.68 | 1.26 | |
| Numerical | |||
| Single PWI | 5.13 | 3.45 | |
| Compounded PWI | 0.64 | 0.33 | |
| Biomechanics | 33.37 | 31.91 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caenen, A.; Pernot, M.; Kinn Ekroll, I.; Shcherbakova, D.; Mertens, L.; Swillens, A.; Segers, P. Effect of Ultrafast Imaging on Shear Wave Visualization and Characterization: An Experimental and Computational Study in a Pediatric Ventricular Model. Appl. Sci. 2017, 7, 840. https://doi.org/10.3390/app7080840
Caenen A, Pernot M, Kinn Ekroll I, Shcherbakova D, Mertens L, Swillens A, Segers P. Effect of Ultrafast Imaging on Shear Wave Visualization and Characterization: An Experimental and Computational Study in a Pediatric Ventricular Model. Applied Sciences. 2017; 7(8):840. https://doi.org/10.3390/app7080840
Chicago/Turabian StyleCaenen, Annette, Mathieu Pernot, Ingvild Kinn Ekroll, Darya Shcherbakova, Luc Mertens, Abigail Swillens, and Patrick Segers. 2017. "Effect of Ultrafast Imaging on Shear Wave Visualization and Characterization: An Experimental and Computational Study in a Pediatric Ventricular Model" Applied Sciences 7, no. 8: 840. https://doi.org/10.3390/app7080840





