A Review on Nanocomposite Materials for Rechargeable Li-ion Batteries
Abstract
:1. Introduction
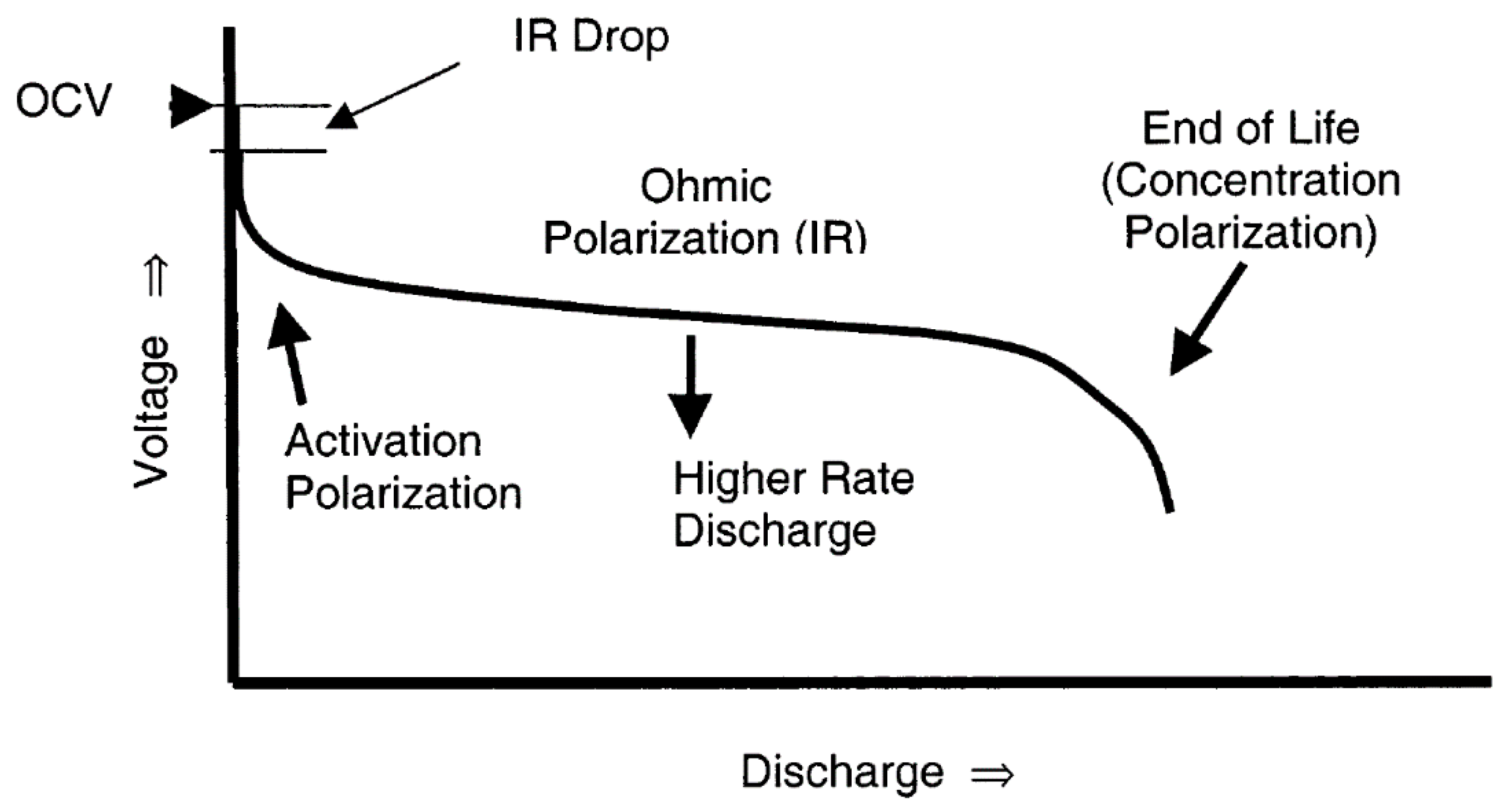
2. Basics of Rechargeable Batteries
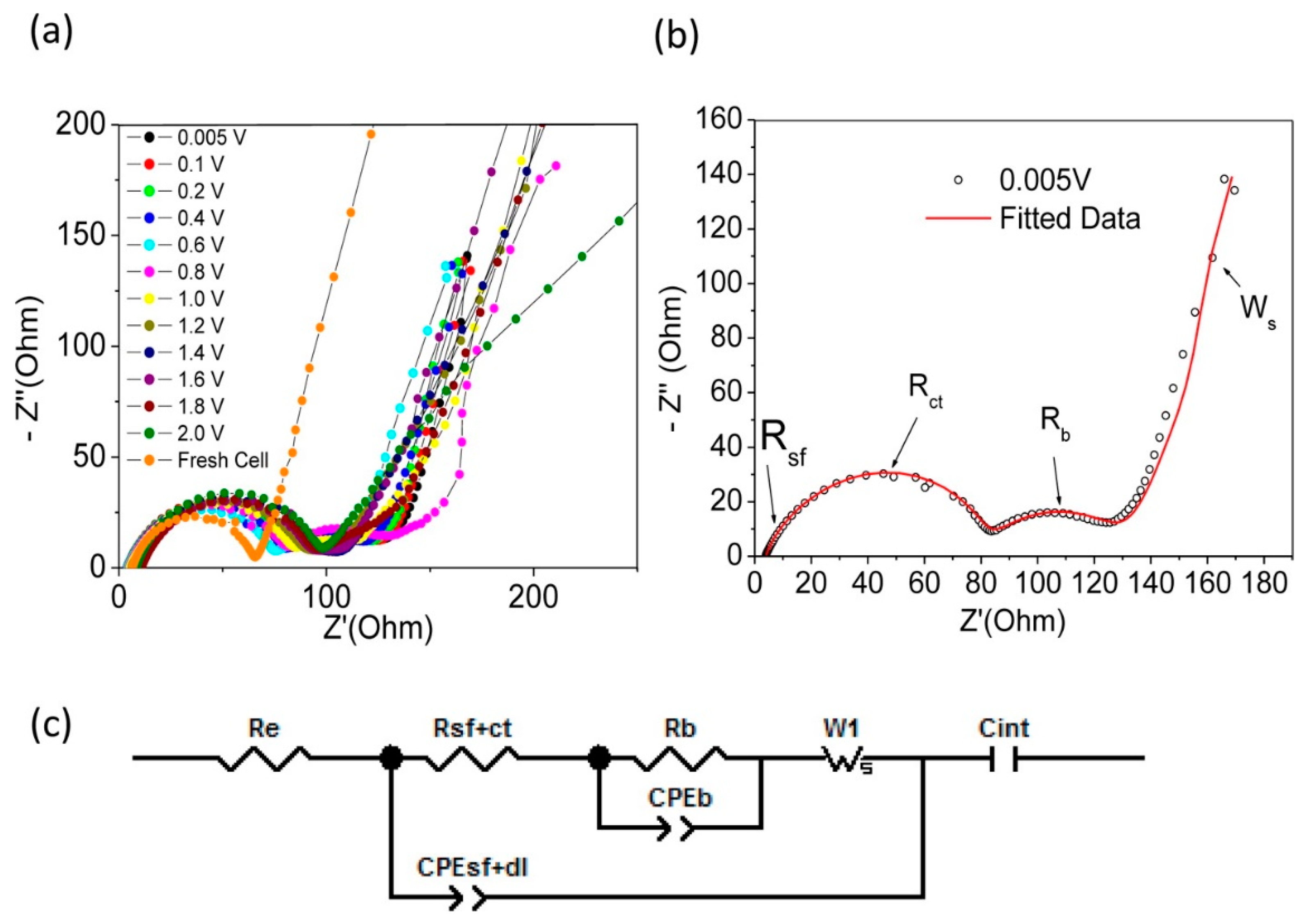
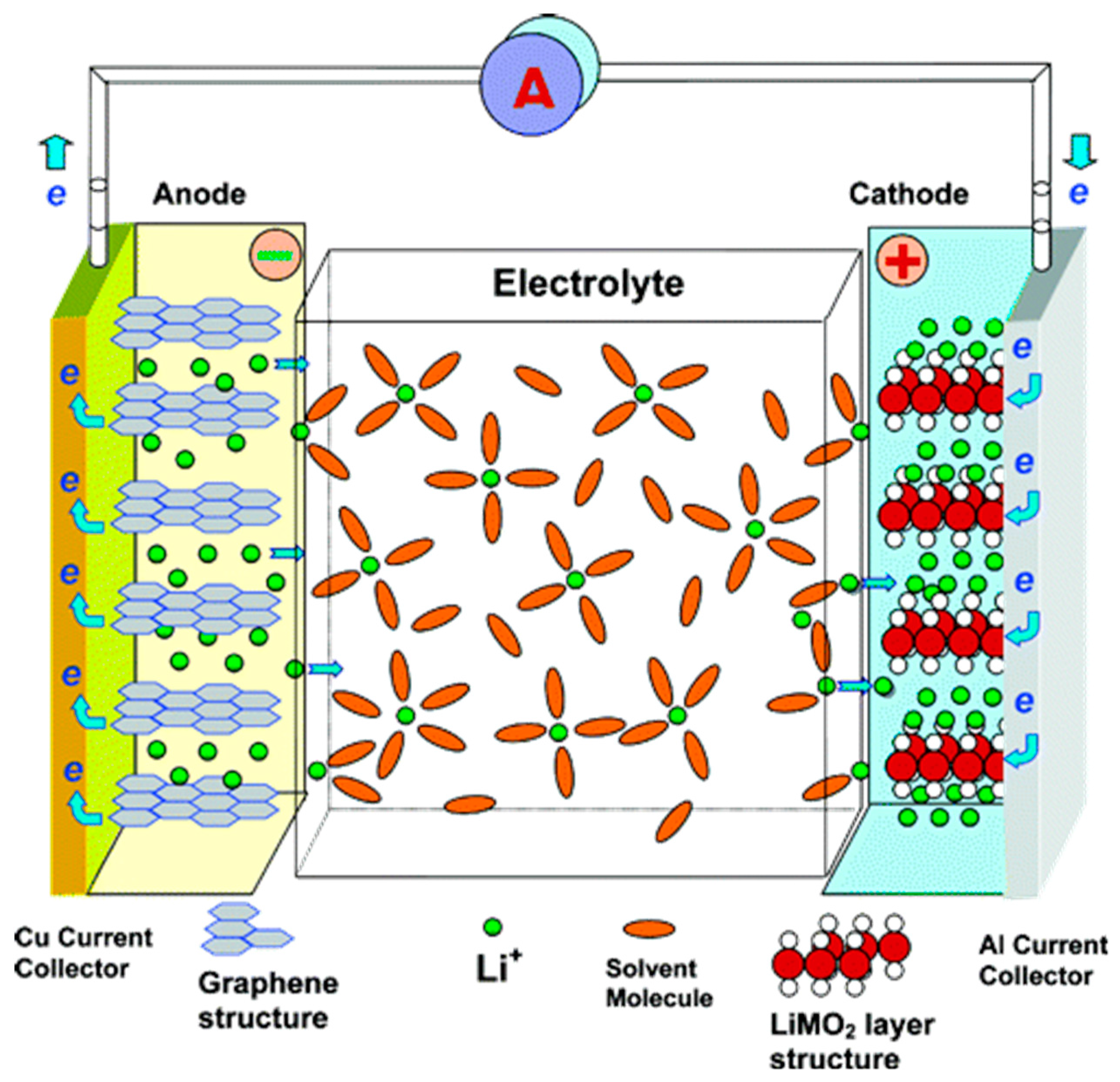
3. Li-ion Batteries
4. Cathode
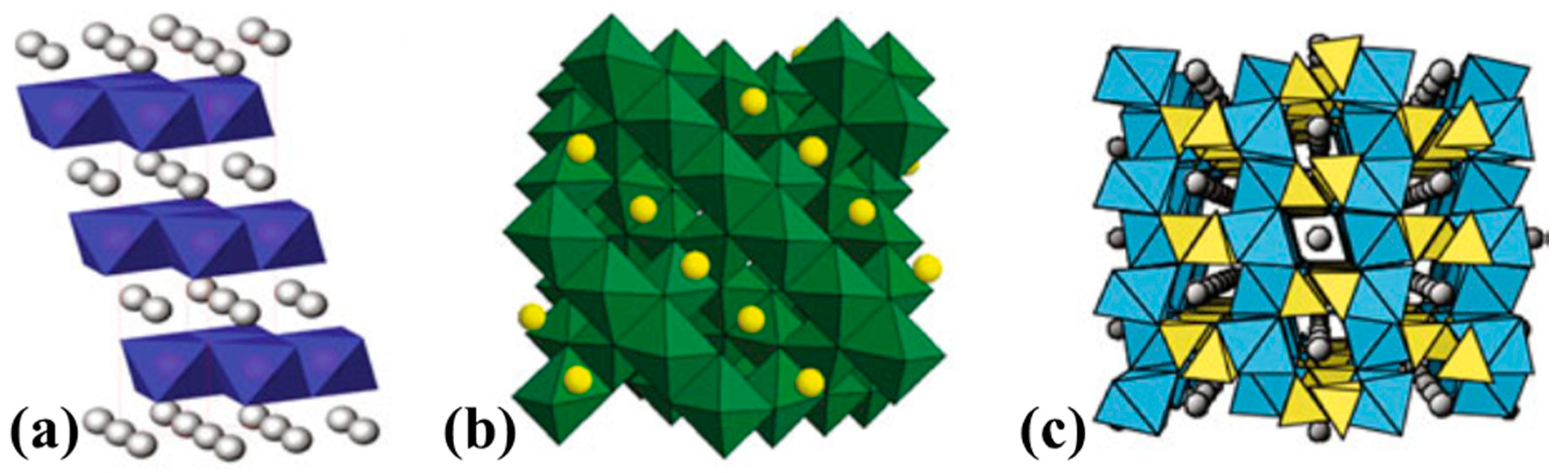
4.1. Layered Oxides, LiMO2 (M = Co, Mn, Ni and Their Mixtures)
4.2. Spinel, LiM2O4 (M = Mn, and Mixtures of Co and Ni)
4.3. Olivine, LiMPO4 (M = Fe, Co, Mn and Their Mixtures)
5. Anode Materials
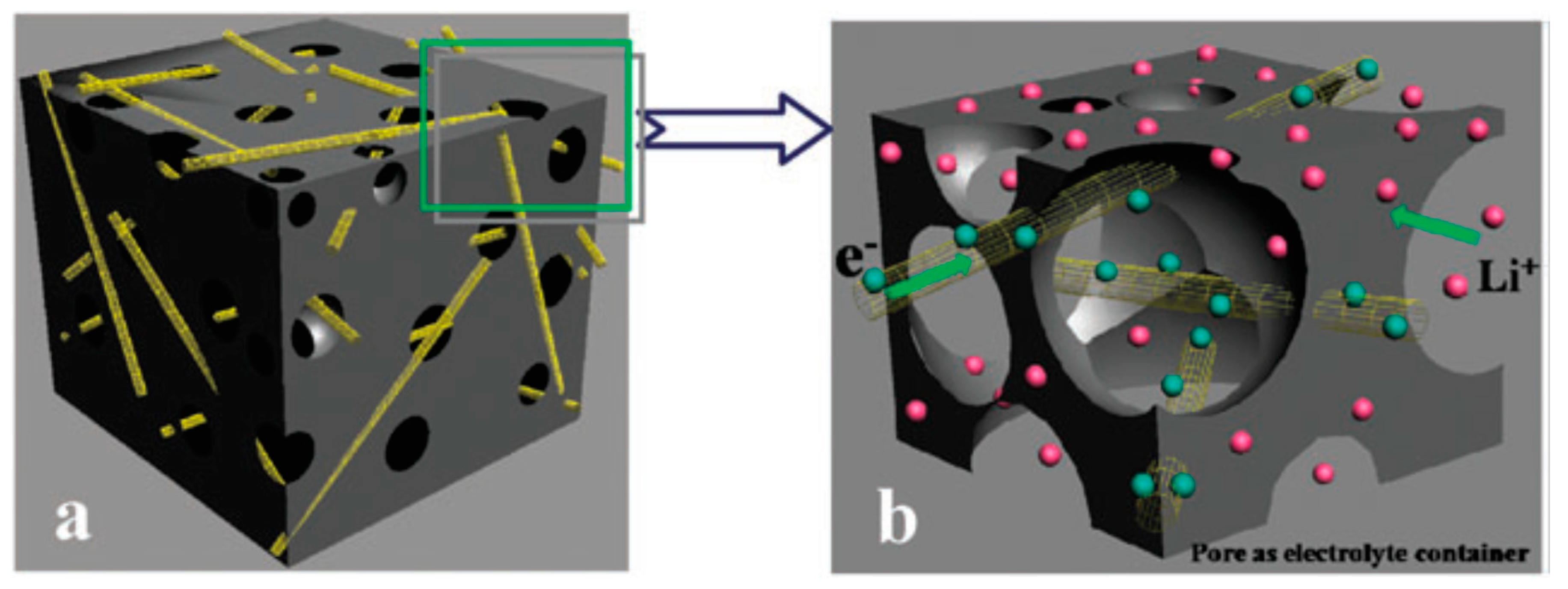
5.1. Carbon Based Anodes
5.2. Alloy Anodes
6. Binder
Separator
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, H.; Cong, T.N.; Yang, W.; Tan, C.; Li, Y.; Ding, Y. Progress in electrical energy storage system: A critical review. Prog. Nat. Sci. 2009, 19, 291–312. [Google Scholar] [CrossRef]
- Weinert, J.X.; Burke, A.F.; Wei, X. Lead-acid and lithium-ion batteries for the Chinese electric bike market and implications on future technology advancement. J. Power Sources 2007, 172, 938–945. [Google Scholar] [CrossRef]
- Frost & Sullivan. Frost & Sullivan Research Service—World Lithium-ion Battery Market. 2009. Available online: http://www.frost.com/prod/servlet/report-brochure.pag?id=N76F-27-00-00-00#Further (accessed on 10 May 2017).
- Ceraolo, M.; Huria, T.; Pede, G.; Vellucci, F. Lithium-ion starting-lighting-ignition batteries: Examining the feasibility. In Proceedings of the 2011 IEEE Vehicle Power and Propulsion Conference (VPPC), Chicago, IL, USA, 6–9 September 2011. [Google Scholar]
- Doeff, M.M. Battery cathodes. In Batteries for Sustainability; Springer: New York, NY, USA, 2013; pp. 5–49. [Google Scholar]
- Battery University. What’s the Best Battery? Available online: http://batteryuniversity.com/learn/archive/whats_the_best_battery (accessed on 9 May 2017).
- Zu, C.X.; Li, H. Thermodynamic analysis on energy densities of batteries. Energy Environ. Sci. 2011, 4, 2614–2624. [Google Scholar] [CrossRef]
- U.S. Department of Energy. The EV Everywhere Grand Challenge: Road to Success; U.S. Department of Energy: Washington, DC, USA, 2014. Available online: http://energy.gov/sites/prod/files/2016/05/f31/eveverywhere_road_to_success.pdf (accessed on 11 May 2017).
- Voelcker, J. Electric-car battery costs: Tesla $190 per kwh for pack, GM $145 for cells. Green Car Reports. 28 April 2016. Available online: http://www.greencarreports.com/news/1103667_electric-car-battery-costs-tesla-190-per-kwh-for-pack-gm-145-for-cells (accessed on 16 June 2017).
- Nykvist, B.; Nilsson, M. Rapidly falling costs of battery packs for electric vehicles. Nat. Clim. Chang. 2015, 5, 329–332. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Fergus, J.W. Recent developments in cathode materials for lithium ion batteries. J. Power Sources 2010, 195, 939–954. [Google Scholar] [CrossRef]
- Tarascon, J.; Guyomard, D. The Li1+xMn2O4/C rocking-chair system: A review. Electrochim. Acta 1993, 38, 1221–1231. [Google Scholar] [CrossRef]
- Reddy, M.; Subba Rao, G.; Chowdari, B. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef] [PubMed]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Zaccaria, R.P.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef]
- Lestriez, B. Functions of polymers in composite electrodes of lithium ion batteries. C. R. Chim. 2010, 13, 1341–1350. [Google Scholar] [CrossRef]
- Chou, S.L.; Pan, Y.; Wang, J.Z.; Liu, H.K.; Dou, S.X. Small things make a big difference: Binder effects on the performance of Li and Na batteries. Phys. Chem. Chem. Phys. 2014, 16, 20347–20359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, M.Y.; Lin, C.E.; Zhu, B.K. Progress in polymeric separators for lithium ion batteries. RSC Adv. 2015, 5, 89848–89860. [Google Scholar] [CrossRef]
- Wakihara, M. Recent developments in lithium ion batteries. Mater. Sci. Eng. R Rep. 2001, 33, 109–134. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Pang, X.; Zhou, Z. Recent progress in high-voltage lithium ion batteries. J. Power Sources 2013, 237, 229–242. [Google Scholar] [CrossRef]
- MIT Electric Vehicle Team. A Guide to Understanding Battery Specifications. Available online: http://web.mit.edu/evt/summary_battery_specifications.pdf (accessed on 7 May 2017).
- Reddy, M.V.; Wei Wen, B.L.; Loh, K.P.; Chowdari, B.V.R. Energy storage studies on InVO4 as high performance anode material for Li-ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 7777–7785. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.; Subba Rao, G.; Chowdari, B. Preparation and characterization of LiNi0.5Co0.5O2 and LiNi0.5Co0.4Al0.1O2 by molten salt synthesis for Li ion batteries. J. Phys. Chem. C 2007, 111, 11712–11720. [Google Scholar]
- Levi, M.; Aurbach, D. Impedance of a single intercalation particle and of non-homogeneous, multilayered porous composite electrodes for Li-ion batteries. J. Phys. Chem. B 2004, 108, 11693–11703. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef] [PubMed]
- Nishi, Y. Lithium ion secondary batteries; past 10 years and the future. J. Power Sources 2001, 100, 101–106. [Google Scholar] [CrossRef]
- Mizushima, K.; Jones, P.; Wiseman, P.; Goodenough, J. LixCoO2 (0 < x < 1): A new cathode material for batteries of high energy density. Solid State Ion. 1981, 3, 171–174. [Google Scholar] [CrossRef]
- Mizushima, K.; Jones, P.; Wiseman, P.; Goodenough, J. LixCoO2 (0< x < −1): A new cathode material for batteries of high energy density. Mater. Res. Bull. 1980, 15, 783–789. [Google Scholar] [CrossRef]
- Zou, H.; Gratz, E.; Apelian, D.; Wang, Y. A novel method to recycle mixed cathode materials for lithium ion batteries. Green Chem. 2013, 15, 1183–1191. [Google Scholar] [CrossRef]
- Thackeray, M.M.; David, W.I.F.; Bruce, P.G.; Goodenough, J.B. Lithium insertion into manganese spinels. Mater. Res. Bull. 1983, 18, 461–472. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.; Goodenough, J. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Brodd, R.J. Batteries for Sustainability: Selected Entries from the Encyclopedia of Sustainability Science and Technology; Springer: New York, NY, USA, 2013. [Google Scholar]
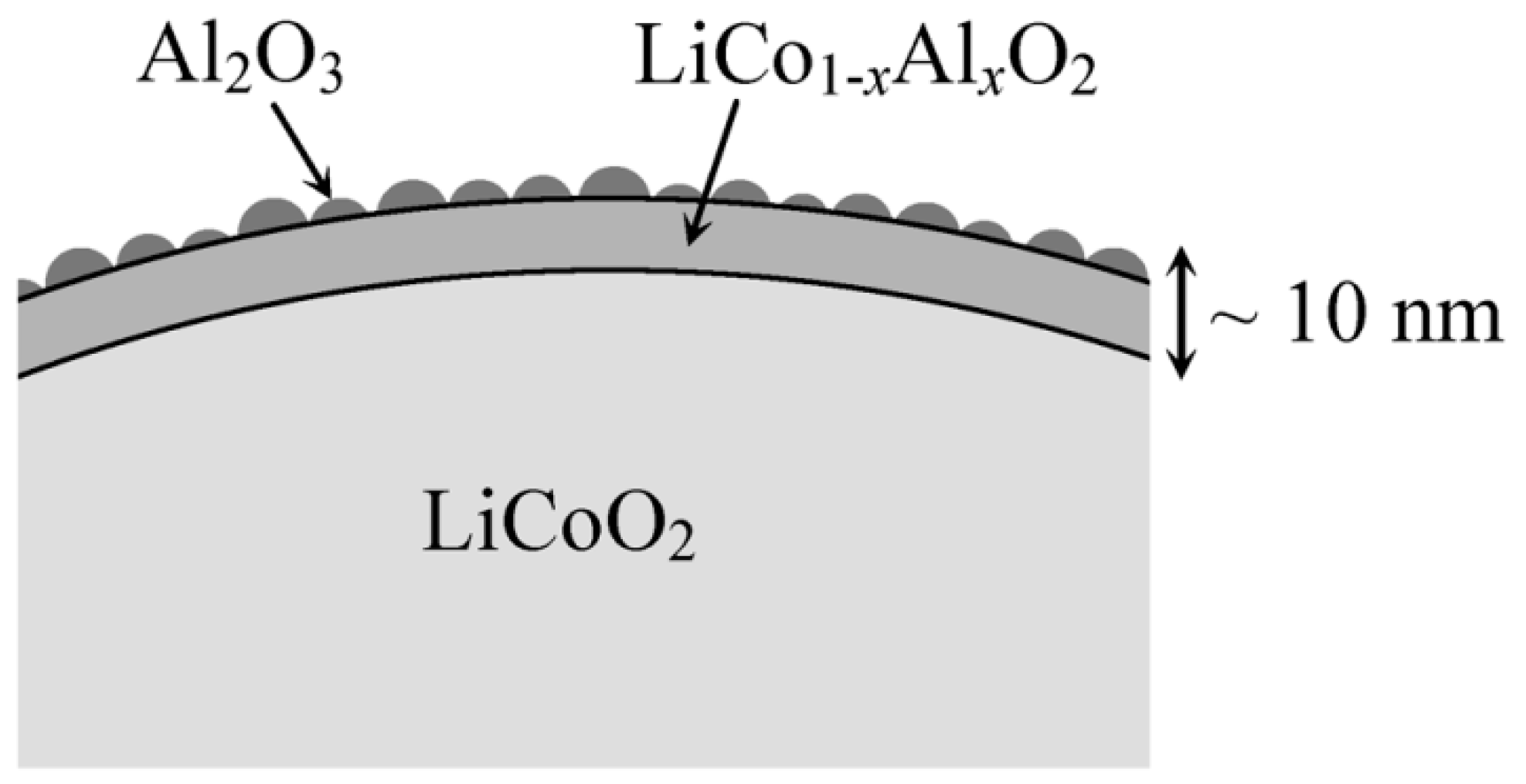
- Cho, J.; Kim, Y.J.; Park, B. Novel LiCoO2 cathode material with Al2O3 coating for a Li ion cell. Chem. Mater. 2000, 12, 3788–3791. [Google Scholar] [CrossRef]
- Chen, Z.; Dahn, J. Methods to obtain excellent capacity retention in LiCoO2 cycled to 4.5 V. Electrochim. Acta 2004, 49, 1079–1090. [Google Scholar] [CrossRef]
- Chebiam, R.; Kannan, A.; Prado, F.; Manthiram, A. Comparison of the chemical stability of the high energy density cathodes of lithium-ion batteries. Electrochem. Commun. 2001, 3, 624–627. [Google Scholar] [CrossRef]
- Cho, J.; Kim, G. Enhancement of thermal stability of LiCoO2 by LiMn2O4 coating. Electrochem. Solid State Lett. 1999, 2, 253–255. [Google Scholar] [CrossRef]
- Cho, J.; Kim, C.S.; Yoo, S.I. Improvement of structural stability of LiCoO2 cathode during electrochemical cycling by sol–gel coating of SnO2. Electrochem. Solid State Lett. 2000, 3, 362–365. [Google Scholar] [CrossRef]
- Chen, Z.; Dahn, J. Effect of a ZrO2 coating on the structure and electrochemistry of LixCoO2 when cycled to 4.5 V. Electrochem. Solid State Lett. 2002, 5, A213–A216. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, C.; Liu, L.; Wu, F.; Chen, L.; Huang, X. Electrochemical evaluation and structural characterization of commercial LiCoO2 surfaces modified with MgO for lithium-ion batteries. J. Electrochem. Soc. 2002, 149, A466–A471. [Google Scholar] [CrossRef]
- Hong, W.; Ming-Cai, C. Modification of LiCoO2 by surface coating with MgO/TiO2/SiO2 for high-performance lithium-ion battery. Electrochem. Solid-State Lett. 2006, 9, A82–A85. [Google Scholar] [CrossRef]
- Chen, Z.; Qin, Y.; Amine, K.; Sun, Y.K. Role of surface coating on cathode materials for lithium-ion batteries. J. Mater. Chem. 2010, 20, 7606–7612. [Google Scholar] [CrossRef]
- Cho, J.; Lee, J.G.; Kim, B.; Park, B. Effect of P2O5 and AlPO4 coating on LiCoO2 cathode material. Chem. Mater. 2003, 15, 3190–3193. [Google Scholar] [CrossRef]
- Lee, J.G.; Kim, T.G.; Park, B. Metal-phosphate coating on LiCoO2 cathodes with high cutoff voltages. Mater. Res. Bull. 2007, 42, 1201–1211. [Google Scholar] [CrossRef]
- Kim, J.; Noh, M.; Cho, J.; Kim, H.; Kim, K.B. Controlled nanoparticle metal phosphates (Metal = Al, Fe, Ce, and Sr) coatings on LiCoO2 cathode materials. J. Electrochem. Soc. 2005, 152, A1142–A1148. [Google Scholar] [CrossRef]
- Tan, K.; Reddy, M.; Rao, G.S.; Chowdari, B. Effect of AlPO4-coating on cathodic behaviour of Li(Ni0.8Co0.2)O2. J. Power Sources 2005, 141, 129–142. [Google Scholar] [CrossRef]
- Sun, Y.K.; Han, J.M.; Myung, S.T.; Lee, S.W.; Amine, K. Significant improvement of high voltage cycling behavior AlF3-coated LiCoO2 cathode. Electrochem. Commun. 2006, 8, 821–826. [Google Scholar] [CrossRef]
- Yang, Z.; Qiao, Q.; Yang, W. Improvement of structural and electrochemical properties of commercial LiCoO2 by coating with LaF3. Electrochim. Acta 2011, 56, 4791–4796. [Google Scholar] [CrossRef]
- Park, J.S.; Mane, A.U.; Elam, J.W.; Croy, J.R. Amorphous metal fluoride passivation coatings prepared by atomic layer deposition on LiCoO2 for Li-ion batteries. Chem. Mater. 2015, 27, 1917–1920. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, N.; Liu, J.; Wang, Z.; Chen, L. Coating material-induced acidic electrolyte improves LiCoO2 performances. Electrochem. Solid State Lett. 2006, 9, A552–A556. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H.; Fu, L.; Liu, H.; Wu, Y.; Rahm, E.; Holze, R.; Wu, H. Cathode materials modified by surface coating for lithium ion batteries. Electrochim. Acta 2006, 51, 3872–3883. [Google Scholar] [CrossRef]
- Cho, J.; Kim, Y.J.; Park, B. LiCoO2 cathode material that does not show a phase transition from hexagonal to monoclinic phase. J. Electrochem. Soc. 2001, 148, A1110–A1115. [Google Scholar] [CrossRef]
- Kim, J.S.; Johnson, C.; Vaughey, J.; Hackney, S.; Walz, K.; Zeltner, W.; Anderson, M.; Thackeray, M. The electrochemical sability of spinel electrodes coated with ZrO2, Al2O3, and SiO2 from colloidal suspensions. J. Electrochem. Soc. 2004, 151, A1755–A1761. [Google Scholar] [CrossRef]
- Dahéron, L.; Dedryvere, R.; Martinez, H.; Flahaut, D.; Ménétrier, M.; Delmas, C.; Gonbeau, D. Possible explanation for the efficiency of Al-based coatings on LiCoO2: Surface properties of LiCo1−xAlxO2 solid solution. Chem. Mater. 2009, 21, 5607–5616. [Google Scholar] [CrossRef]
- Kweon, H.J.; Park, J.; Seo, J.; Kim, G.; Jung, B.; Lim, H.S. Effects of metal oxide coatings on the thermal stability and electrical performance of LiCoO2 in a Li-ion cell. J. Power Sources 2004, 126, 156–162. [Google Scholar] [CrossRef]
- Tan, K.; Reddy, M.; Rao, G.S.; Chowdari, B. High-performance LiCoO2 by molten salt (LiNO3:LiCl) synthesis for Li-ion batteries. J. Power Sources 2005, 147, 241–248. [Google Scholar] [CrossRef]
- Xu, J.; Thomas, H.; Francis, R.W.; Lum, K.R.; Wang, J.; Liang, B. A review of processes and technologies for the recycling of lithium-ion secondary batteries. J. Power Sources 2008, 177, 512–527. [Google Scholar] [CrossRef]
- Gaines, L. The future of automotive lithium-ion battery recycling: Charting a sustainable course. Sustain. Mater. Technol. 2014, 1, 2–7. [Google Scholar] [CrossRef]
- Hirano, A.; Kanno, R.; Kawamoto, Y.; Takeda, Y.; Yamaura, K.; Takano, M.; Ohyama, K.; Ohashi, M.; Yamaguchi, Y. Relationship between non-stoichiometry and physical properties in LiNiO2. Solid State Ion. 1995, 78, 123–131. [Google Scholar] [CrossRef]
- Cho, J.; Kim, T.-J.; Kim, Y.J.; Park, B. High-performance ZrO2-coated LiNiO2 cathode material. Electrochem. Solid State Lett. 2001, 4, A159–A161. [Google Scholar] [CrossRef]
- Dahn, J.; Fuller, E.; Obrovac, M.; Von Sacken, U. Thermal stability of LixCoO2, LixNiO2 and λ-MnO2 and consequences for the safety of Li-ion cells. Solid State Ion. 1994, 69, 265–270. [Google Scholar] [CrossRef]
- Tey, S.L.; Reddy, M.; Subba Rao, G.; Chowdari, B.; Yi, J.; Ding, J.; Vittal, J.J. Synthesis, Structure, and Magnetic Properties of [Li(H2O)M(N2H3CO2)3]·0.5H2O (M = Co, Ni) as Single Precursors to LiMO2 Battery Materials. Chem. Mater. 2006, 18, 1587–1594. [Google Scholar]
- InvestmentMine. Commodity and Metal Prices. Available online: http://www.infomine.com/investment/metal-prices/ (accessed on 10 May 2017).
- Armstrong, A.R.; Bruce, P.G. Synthesis of layered LiMnO2 as an electrode for rechargeable lithium batteries. Nature 1996, 381, 499–500. [Google Scholar] [CrossRef]
- Vitins, G.; West, K. Lithium intercalation into layered LiMnO2. J. Electrochem. Soc. 1997, 144, 2587–2592. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.; Kim, T.J.; Park, B. The effect of a metal-oxide coating on the cycling behavior at 55 °C in orthorhombic LiMnO2 cathode materials. J. Electrochem. Soc. 2002, 149, A288–A292. [Google Scholar] [CrossRef]
- Cho, J.; Kim, Y.J.; Kim, T.J.; Park, B. Enhanced structural stability of ο-LiMnO2 by sol–gel coating of Al2O3. Chem. Mater. 2001, 13, 18–20. [Google Scholar] [CrossRef]
- Chikkannanavar, S.B.; Bernardi, D.M.; Liu, L. A review of blended cathode materials for use in Li-ion batteries. J. Power Sources 2014, 248, 91–100. [Google Scholar] [CrossRef]
- Reddy, M.; Tung, B.D.; Yang, L.; Minh, N.D.Q.; Loh, K.; Chowdari, B. Molten salt method of preparation and cathodic studies on layered-cathode materials Li(Co0.7Ni0.3)O2 and Li(Ni0.7Co0.3)O2 for Li-ion batteries. J. Power Sources 2013, 225, 374–381. [Google Scholar]
- Reddy, M.; Rao, G.S.; Chowdari, B. Synthesis by molten salt and cathodic properties of Li(Ni1/3Co1/3Mn1/3)O2. J. Power Sources 2006, 159, 263–267. [Google Scholar] [CrossRef]
- Reddy, M.; Rao, G.S.; Chowdari, B. Synthesis and electrochemical studies of the 4 V cathode, Li(Ni2/3Mn1/3)O2. J. Power Sources 2006, 160, 1369–1374. [Google Scholar] [CrossRef]
- Zhao, X.; Reddy, M.; Liu, H.; Rao, G.S.; Chowdari, B. Layered Li(Ni0.2Mn0.2Co0.6)O2 synthesized by a molten salt method for lithium-ion batteries. RSC Adv. 2014, 4, 24538–24543. [Google Scholar]
- Tan, T.Q.; Idris, M.S.; Osman, R.A.M.; Reddy, M.; Chowdari, B. Structure and electrochemical behaviour of LiNi0.4Mn0.4Co0.2O2 as cathode material for lithium ion batteries. Solid State Ion. 2015, 278, 43–48. [Google Scholar]
- Doughty, D.; Roth, E.P. A general discussion of Li ion battery safety. Electrochem. Soc. Interface 2012, 21, 37–44. [Google Scholar] [CrossRef]
- Jang, D.H.; Shin, Y.J.; Oh, S.M. Dissolution of spinel oxides and capacity losses in 4 V Li/LixMn2O4 cells. J. Electrochem. Soc. 1996, 143, 2204–2211. [Google Scholar] [CrossRef]
- Amatucci, G.; Blyr, A.; Sigala, C.; Alfonse, P.; Tarascon, J. Surface treatments of Li1+xMn2−xO4 spinels for improved elevated temperature performance. Solid State Ion. 1997, 104, 13–25. [Google Scholar] [CrossRef]
- Tsunekawa, H.; Tanimoto, S.; Marubayashi, R.; Fujita, M.; Kifune, K.; Sano, M. Capacity fading of graphite electrodes due to the deposition of manganese ions on them in Li-ion batteries. J. Electrochem. Soc. 2002, 149, A1326–A1331. [Google Scholar] [CrossRef]
- Arumugam, D.; Kalaignan, G.P. Synthesis and electrochemical characterizations of Nano-SiO2-coated LiMn2O4 cathode materials for rechargeable lithium batteries. J. Electroanal. Chem. 2008, 624, 197–204. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, J.; Guo, S.; He, X.; Jiang, C.; Wan, C. Investigation of SnO2-modified LiMn2O4 composite as cathode material for lithium-ion batteries. Int. J. Electrochem. Sci. 2010, 5, 1113–1126. [Google Scholar]
- Gnanaraj, J.; Pol, V.; Gedanken, A.; Aurbach, D. Improving the high-temperature performance of LiMn2O4 spinel electrodes by coating the active mass with MgO via a sonochemical method. Electrochem. Commun. 2003, 5, 940–945. [Google Scholar] [CrossRef]
- Zhou, W.J.; He, B.L.; Li, H.L. Synthesis, structure and electrochemistry of Ag-modified LiMn2O4 cathode materials for lithium-ion batteries. Mater. Res. Bull. 2008, 43, 2285–2294. [Google Scholar] [CrossRef]
- Huang, S.; Wen, Z.; Yang, X.; Zhu, X.; Lin, B. Synthesis and the Improved High-Rate Performance of LiMn2O4/Ag Composite Cathode for Lithium-Ion Batteries. Electrochem. Solid State Lett. 2006, 9, A443–A447. [Google Scholar] [CrossRef]
- Ding, Y.; Xie, J.; Cao, G.; Zhu, T.; Yu, H.; Zhao, X. Enhanced elevated-temperature performance of Al-doped single-crystalline LiMn2O4 nanotubes as cathodes for lithium ion batteries. J. Phys. Chem. C 2011, 115, 9821–9825. [Google Scholar] [CrossRef]
- Jia, X.; Yan, C.; Chen, Z.; Wang, R.; Zhang, Q.; Guo, L.; Wei, F.; Lu, Y. Direct growth of flexible LiMn2O4/CNT lithium-ion cathodes. Chem. Commun. 2011, 47, 9669–9671. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Kim, G.B.; Lim, H.S.; Kim, C.S.; Yoo, S.I. Improvement of structural stability of LiMn2O4 cathode material on 55 °C cycling by sol–gel coating of LiCoO2. Electrochem. Solid State Lett. 1999, 2, 607–609. [Google Scholar] [CrossRef]
- Kitao, H.; Fujihara, T.; Takeda, K.; Nakanishi, N.; Nohma, T. High-temperature storage performance of Li-ion batteries using a mixture of Li-Mn spinel and Li-Ni-Co-Mn oxide as a positive electrode material. Electrochem. Solid State Lett. 2005, 8, A87–A90. [Google Scholar] [CrossRef]
- Yi, T.F.; Zhu, Y.R.; Zhu, X.D.; Shu, J.; Yue, C.B.; Zhou, A.N. A review of recent developments in the surface modification of LiMn2O4 as cathode material of power lithium-ion battery. Ionics 2009, 15, 779–784. [Google Scholar] [CrossRef]
- Prabu, M.; Reddy, M.; Selvasekarapandian, S.; Rao, G.S.; Chowdari, B. (Li, Al)-co-doped spinel, Li(Li0.1Al0.1Mn1.8)O4 as high performance cathode for lithium ion batteries. Electrochim. Acta 2013, 88, 745–755. [Google Scholar]
- Zhao, X.; Reddy, M.; Liu, H.; Ramakrishna, S.; Rao, G.S.; Chowdari, B.V. Nano LiMn2O4 with spherical morphology synthesized by a molten salt method as cathodes for lithium ion batteries. RSC Adv. 2012, 2, 7462–7469. [Google Scholar] [CrossRef]
- Sakunthala, A.; Reddy, M.; Selvasekarapandian, S.; Chowdari, B.; Selvin, P.C. Synthesis of compounds, Li(MMn11/6)O4 (M = Mn1/6, Co1/6,(Co1/12Cr1/12),(Co1/12Al1/12),(Cr1/12Al1/12)) by polymer precursor method and its electrochemical performance for lithium-ion batteries. Electrochim. Acta 2010, 55, 4441–4450. [Google Scholar] [CrossRef]
- Reddy, M.; Sakunthala, A.; SelvashekaraPandian, S.; Chowdari, B. Preparation, Comparative Energy Storage Properties, and Impedance Spectroscopy Studies of Environmentally Friendly Cathode, Li(MMn11/6) O4 (M = Mn1/6,Co1/6, (Co1/12Cr1/12)). J. Phys. Chem. C 2013, 117, 9056–9064. [Google Scholar] [CrossRef]
- Reddy, M.; Manoharan, S.S.; John, J.; Singh, B.; Rao, G.S.; Chowdari, B. Synthesis, Characterization, and Electrochemical Cycling Behavior of the Ru-Doped Spinel, Li [Mn2−xRux]O4 (x = 0, 0.1, and 0.25). J. Electrochem. Soc. 2009, 156, A652–A660. [Google Scholar] [CrossRef]
- Reddy, M.; Cheng, H.; Tham, J.; Yuan, C.; Goh, H.; Chowdari, B. Preparation of Li(Ni0.5Mn1.5)O4 by polymer precursor method and its electrochemical properties. Electrochim. Acta 2012, 62, 269–275. [Google Scholar]
- Reddy, M.; Raju, M.S.; Sharma, N.; Quan, P.; Nowshad, S.H.; Emmanuel, H.C.; Peterson, V.; Chowdari, B. Preparation of Li1.03Mn1.97O4 and Li1.06Mn1.94O4 by the polymer precursor method and X-ray, neutron diffraction and electrochemical studies. J. Electrochem. Soc. 2011, 158, A1231–A1236. [Google Scholar]
- Wei, Y.; Yan, L.; Wang, C.; Xu, X.; Wu, F.; Chen, G. Effects of Ni doping on [MnO6] octahedron in LiMn2O4. J. Phys. Chem. B 2004, 108, 18547–18551. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Tan, H.; Yang, Z.; Zeng, J. Preparation and doping mode of doped LiMn2O4 for Li-ion batteries. Energies 2013, 6, 1718–1730. [Google Scholar] [CrossRef]
- Masquelier, C.; Croguennec, L. Polyanionic (phosphates, silicates, sulfates) frameworks as electrode materials for rechargeable Li (or Na) batteries. Chem. Rev. 2013, 113, 6552–6591. [Google Scholar] [CrossRef] [PubMed]
- Ravet, N.; Goodenough, J.; Besner, S.; Simoneau, M.; Hovington, P.; Armand, M. Improved iron based cathode material. In Proceedings of the 196th ECS meeting, Honolulu, HI, USA, 17–22 October 1999. [Google Scholar]
- Yamada, A.; Chung, S.C.; Hinokuma, K. Optimized LiFePO4 for lithium battery cathodes. J. Electrochem. Soc. 2001, 148, A224–A229. [Google Scholar] [CrossRef]
- Saravanan, K.; Reddy, M.; Balaya, P.; Gong, H.; Chowdari, B.; Vittal, J.J. Storage performance of LiFePO4 nanoplates. J. Mater. Chem. 2009, 19, 605–610. [Google Scholar] [CrossRef]
- Doeff, M.M.; Hu, Y.; McLarnon, F.; Kostecki, R. Effect of surface carbon structure on the electrochemical performance of LiFePO4. Electrochem. Solid State Lett. 2003, 6, A207–A209. [Google Scholar] [CrossRef]
- Wilcox, J.D.; Doeff, M.M.; Marcinek, M.; Kostecki, R. Factors influencing the quality of carbon coatings on LiFePO4. J. Electrochem. Soc. 2007, 154, A389–A395. [Google Scholar] [CrossRef]
- Zaghib, K.; Shim, J.; Guerfi, A.; Charest, P.; Striebel, K. Effect of carbon source as additives in LiFePO4 as positive electrode for lithium-ion batteries. Electrochem. Solid State Lett. 2005, 8, A207–A210. [Google Scholar] [CrossRef]
- Dominko, R.; Bele, M.; Gaberscek, M.; Remskar, M.; Hanzel, D.; Pejovnik, S.; Jamnik, J. Impact of the carbon coating thickness on the electrochemical performance of LiFePO4/C composites. J. Electrochem. Soc. 2005, 152, A607–A610. [Google Scholar] [CrossRef]
- Hu, Y.; Doeff, M.M.; Kostecki, R.; Finones, R. Electrochemical performance of sol–gel synthesized LiFePO4 in lithium batteries. J. Electrochem. Soc. 2004, 151, A1279–A1285. [Google Scholar] [CrossRef]
- Jinli, Z.; Jiao, W.; Yuanyuan, L.; Ning, N.; Junjie, G.; Feng, Y.; Wei, L. High-performance lithium iron phosphate with phosphorus-doped carbon layers for lithium ion batteries. J. Mater. Chem. A 2015, 3, 2043–2049. [Google Scholar] [CrossRef]
- Zhang, J.; Nie, N.; Liu, Y.; Wang, J.; Yu, F.; Gu, J.; Li, W. Boron and nitrogen codoped carbon layers of LiFePO4 improve the high-rate electrochemical performance for lithium ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 20134–20143. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.P.; Reddy, M.; Adams, S.; Chowdari, B. Preparation, temperature dependent structural, molecular dynamics simulations studies and electrochemical properties of LiFePO4. Mater. Res. Bull. 2015, 66, 71–75. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Hu, Y.; O’Hayre, R.; Shao, Z. A porous LiFePO4 and carbon nanotube composite. Chem. Commun. 2010, 46, 7151–7153. [Google Scholar] [CrossRef] [PubMed]
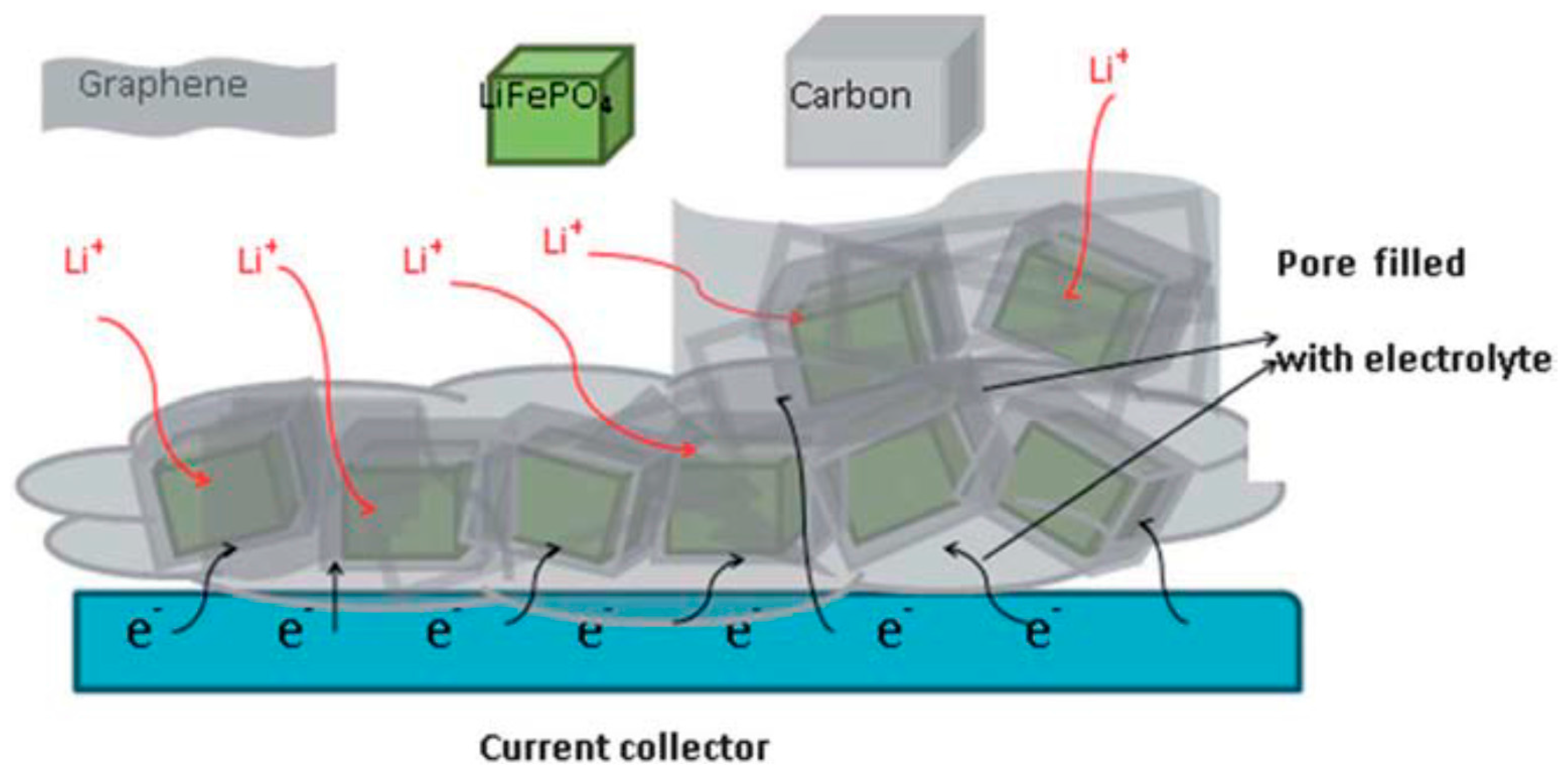
- Yang, J.; Wang, J.; Wang, D.; Li, X.; Geng, D.; Liang, G.; Gauthier, M.; Li, R.; Sun, X. 3D porous LiFePO4/graphene hybrid cathodes with enhanced performance for Li-ion batteries. J. Power Sources 2012, 208, 340–344. [Google Scholar] [CrossRef]
- Shi, Y.; Chou, S.L.; Wang, J.Z.; Wexler, D.; Li, H.J.; Liu, H.K.; Wu, Y. Graphene wrapped LiFePO4/C composites as cathode materials for Li-ion batteries with enhanced rate capability. J. Mater. Chem. 2012, 22, 16465–16470. [Google Scholar] [CrossRef]
- Hu, L.H.; Wu, F.Y.; Lin, C.T.; Khlobystov, A.N.; Li, L.J. Graphene-modified LiFePO4 cathode for lithium ion battery beyond theoretical capacity. Nat. Commun. 2013, 4, 1687. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, J.; Tang, Y.; Wang, D.; Xiao, B.; Li, X.; Li, R.; Liang, G.; Sham, T.K.; Sun, X. In situ self-catalyzed formation of core–shell LiFePO4@CNT nanowires for high rate performance lithium-ion batteries. J. Mater. Chem. A 2013, 1, 7306–7311. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Li, X.; Wang, D.; Liu, J.; Liang, G.; Gauthier, M.; Li, Y.; Geng, D.; Li, R. Hierarchically porous LiFePO4/nitrogen-doped carbon nanotubes composite as a cathode for lithium ion batteries. J. Mater. Chem. 2012, 22, 7537–7543. [Google Scholar] [CrossRef]
- Reddy, M.; Rao, G.S.; Chowdari, B. Long-term cycling studies on 4 V-cathode, lithium vanadium fluorophosphate. J. Power Sources 2010, 195, 5768–5774. [Google Scholar] [CrossRef]
- Hameed, A.S.; Nagarathinam, M.; Reddy, M.; Chowdari, B.; Vittal, J.J. Synthesis and electrochemical studies of layer-structured metastable α i-LiVOPO4. J. Mater. Chem. 2012, 22, 7206–7213. [Google Scholar] [CrossRef]
- Hameed, A.S.; Reddy, M.; Chowdari, B.; Vittal, J.J. Carbon coated Li3V2(PO4)3 from the single-source precursor, Li2(VO)2(HPO4)2(C2O4)·6H2O as cathode and anode materials for Lithium ion batteries. Electrochim. Acta 2014, 128, 184–191. [Google Scholar] [CrossRef]
- Nagarathinam, M.; Saravanan, K.; Phua, E.J.H.; Reddy, M.; Chowdari, B.; Vittal, J.J. Redox-Active Metal-Centered Oxalato Phosphate Open Framework Cathode Materials for Lithium Ion Batteries. Angew. Chem. Int. Ed. 2012, 51, 5866–5870. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.S.; Reddy, M.; Nagarathinam, M.; Runčevski, T.; Dinnebier, R.E.; Adams, S.; Chowdari, B.; Vittal, J.J. Room temperature large-scale synthesis of layered frameworks as low-cost 4 V cathode materials for lithium ion batteries. Sci. Rep. 2015, 5, 16270. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.S.; Reddy, M.; Sarkar, N.; Chowdari, B.; Vittal, J.J. Synthesis and electrochemical investigation of novel phosphite based layered cathodes for Li-ion batteries. RSC Adv. 2015, 5, 60630–60637. [Google Scholar] [CrossRef]
- Hameed, A.S.; Nagarathinam, M.; Schreyer, M.; Reddy, M.; Chowdari, B.; Vittal, J.J. A layered oxalatophosphate framework as a cathode material for Li-ion batteries. J. Mater. Chem. A 2013, 1, 5721–5726. [Google Scholar] [CrossRef]
- Ke, F.S.; Wu, Y.S.; Deng, H. Metal-organic frameworks for lithium ion batteries and supercapacitors. J. Solid State Chem. 2015, 223, 109–121. [Google Scholar] [CrossRef]
- Bai, L.; Tu, B.; Qi, Y.; Gao, Q.; Liu, D.; Liu, Z.; Zhao, L.; Li, Q.; Zhao, Y. Enhanced performance in gas adsorption and Li ion batteries by docking Li+ in a crown ether-based metal-organic framework. Chem. Commun. 2016, 52, 3003–3006. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Singh, U.; Aravindan, V.; Srinivasan, M.; Ogale, S. Synthesis of CuO nanostructures from Cu-based metal organic framework (MOF-199) for application as anode for Li-ion batteries. Nano Energy 2013, 2, 1158–1163. [Google Scholar] [CrossRef]
- Wu, Y.P.; Rahm, E.; Holze, R. Carbon anode materials for lithium ion batteries. J. Power Sources 2003, 114, 228–236. [Google Scholar] [CrossRef]
- Roberts, A.D.; Li, X.; Zhang, H. Porous carbon spheres and monoliths: Morphology control, pore size tuning and their applications as Li-ion battery anode materials. Chem. Soc. Rev. 2014, 43, 4341–4356. [Google Scholar] [CrossRef] [PubMed]
- Buiel, E.; Dahn, J. Li-insertion in hard carbon anode materials for Li-ion batteries. Electrochim. Acta 1999, 45, 121–130. [Google Scholar] [CrossRef]
- Nishimura, K.; Honbo, H.; Takeuchi, S.; Horiba, T.; Oda, M.; Koseki, M.; Muranaka, Y.; Kozono, Y.; Miyadera, H. Design and performance of 10 Wh rechargeable lithium batteries. J. Power Sources 1997, 68, 436–439. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, C.; Wan, C.; Holze, R. Composite materials of silver and natural graphite as anode with low sensibility to humidity. J. Power Sources 2002, 112, 255–260. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, C.; Wan, C.; Tsuchida, E. Composite anode material for lithium ion battery with low sensitivity to water. Electrochem. Commun. 2000, 2, 626–629. [Google Scholar] [CrossRef]
- Yu, P.; Ritter, J.A.; White, R.E.; Popov, B.N. Ni-composite microencapsulated graphite as the negative electrode in lithium-ion batteries I. Initial irreversible capacity study. J. Electrochem. Soc. 2000, 147, 1280–1285. [Google Scholar] [CrossRef]
- Yu, P.; Ritter, J.A.; White, R.E.; Popov, B.N. Ni-composite microencapsulated graphite as the negative electrode in lithium-ion batteries II: Electrochemical impedance and self-discharge studies. J. Electrochem. Soc. 2000, 147, 2081–2085. [Google Scholar] [CrossRef]
- Guo, K.; Pan, Q.; Wang, L.; Fang, S. Nano-scale copper-coated graphite as anode material for lithium-ion batteries. J. Appl. Electrochem. 2002, 32, 679–685. [Google Scholar] [CrossRef]
- Kim, S.-S.; Kadoma, Y.; Ikuta, H.; Uchimoto, Y.; Wakihara, M. Electrochemical performance of natural graphite by surface modification using aluminum. Electrochem. Solid State Lett. 2001, 4, A109–A112. [Google Scholar] [CrossRef]
- Takamura, T.; Sumiya, K.; Suzuki, J.; Yamada, C.; Sekine, K. Enhancement of Li doping/undoping reaction rate of carbonaceous materials by coating with an evaporated metal film. J. Power Sources 1999, 81, 368–372. [Google Scholar] [CrossRef]
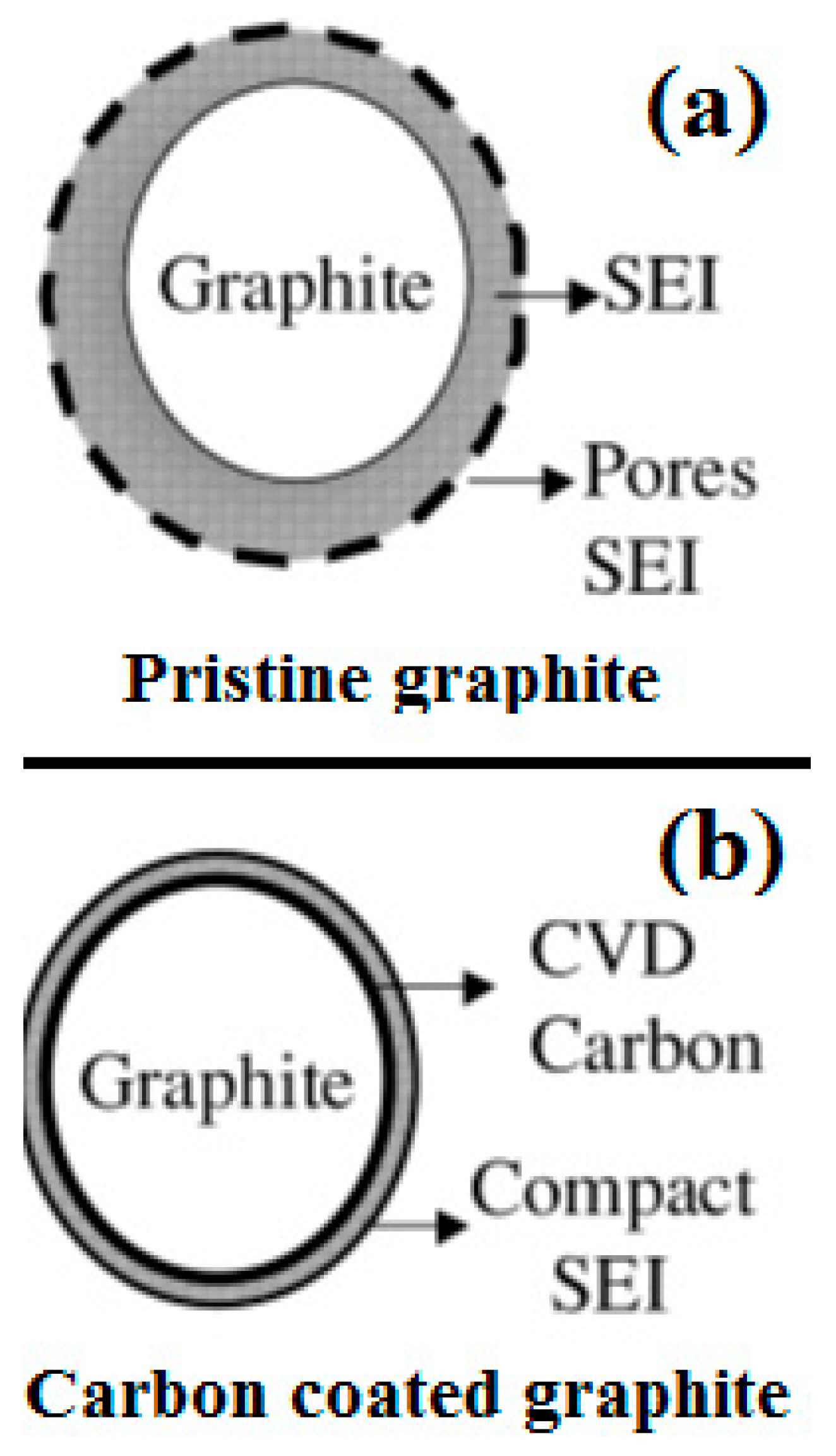
- Yoshio, M.; Wang, H.; Fukuda, K.; Umeno, T.; Abe, T.; Ogumi, Z. Improvement of natural graphite as a lithium-ion battery anode material, from raw flake to carbon-coated sphere. J. Mater. Chem. 2004, 14, 1754–1758. [Google Scholar] [CrossRef]
- Wang, H.; Yoshio, M. Carbon-coated natural graphite prepared by thermal vapor decomposition process, a candidate anode material for lithium-ion battery. J. Power Sources 2001, 93, 123–129. [Google Scholar] [CrossRef]
- Natarajan, C.; Fujimoto, H.; Tokumitsu, K.; Mabuchi, A.; Kasuh, T. Reduction of the irreversible capacity of a graphite anode by the CVD process. Carbon 2001, 39, 1409–1413. [Google Scholar] [CrossRef]
- Yoshio, M.; Wang, H.; Fukuda, K.; Hara, Y.; Adachi, Y. Effect of carbon coating on electrochemical performance of treated natural graphite as lithium-ion battery anode material. J. Electrochem. Soc. 2000, 147, 1245–1250. [Google Scholar] [CrossRef]
- Dey, A.; Sullivan, B. The electrochemical decomposition of propylene carbonate on graphite. J. Electrochem. Soc. 1970, 117, 222–224. [Google Scholar] [CrossRef]
- Jeong, G.; Kim, Y.U.; Kim, H.; Kim, Y.J.; Sohn, H.J. Prospective materials and applications for Li secondary batteries. Energy Environ. Sci. 2011, 4, 1986–2002. [Google Scholar] [CrossRef]
- Aurbach, D.; Zinigrad, E.; Cohen, Y.; Teller, H. A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid State Ion. 2002, 148, 405–416. [Google Scholar] [CrossRef]
- Beattie, S.D.; Larcher, D.; Morcrette, M.; Simon, B.; Tarascon, J.M. Si electrodes for Li-ion batteries-a new way to look at an old problem. J. Electrochem. Soc. 2008, 155, A158–A163. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lee, S.M. Carbon-coated nano-Si dispersed oxides/graphite composites as anode material for lithium ion batteries. Electrochem. Commun. 2004, 6, 465–469. [Google Scholar] [CrossRef]
- Wen, Z.; Yang, J.; Wang, B.; Wang, K.; Liu, Y. High capacity silicon/carbon composite anode materials for lithium ion batteries. Electrochem. Commun. 2003, 5, 165–168. [Google Scholar] [CrossRef]
- Yoshio, M.; Wang, H.; Fukuda, K.; Umeno, T.; Dimov, N.; Ogumi, Z. Carbon-coated Si as a lithium-ion battery anode material. J. Electrochem. Soc. 2002, 149, A1598–A1603. [Google Scholar] [CrossRef]
- Wang, G.; Ahn, J.; Yao, J.; Bewlay, S.; Liu, H. Nanostructured Si–C composite anodes for lithium-ion batteries. Electrochem. Commun. 2004, 6, 689–692. [Google Scholar] [CrossRef]
- Liu, N.; Wu, H.; McDowell, M.T.; Yao, Y.; Wang, C.; Cui, Y. A yolk-shell design for stabilized and scalable Li-ion battery alloy anodes. Nano Lett. 2012, 12, 3315–3321. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Lu, Z.; Zhao, J.; McDowell, M.T.; Lee, H.W.; Zhao, W.; Cui, Y. A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nat. Nanotechnol. 2014, 9, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Mazouzi, D.; Karkar, Z.; Hernandez, C.R.; Manero, P.J.; Guyomard, D.; Roué, L.; Lestriez, B. Critical roles of binders and formulation at multiscales of silicon-based composite electrodes. J. Power Sources 2015, 280, 533–549. [Google Scholar] [CrossRef]
- Huang, Y.H.; Goodenough, J.B. High-rate LiFePO4 lithium rechargeable battery promoted by electrochemically active polymers. Chem. Mater. 2008, 20, 7237–7241. [Google Scholar] [CrossRef]
- Chen, W.M.; Qie, L.; Yuan, L.X.; Xia, S.A.; Hu, X.L.; Zhang, W.X.; Huang, Y.H. Insight into the improvement of rate capability and cyclability in LiFePO4/polyaniline composite cathode. Electrochim. Acta 2011, 56, 2689–2695. [Google Scholar] [CrossRef]
- Tamura, T.; Aoki, Y.; Ohsawa, T.; Dokko, K. Polyaniline as a functional binder for LiFePO4 cathodes in lithium batteries. Chem. Lett. 2011, 40, 828–830. [Google Scholar] [CrossRef]
- Huang, Y.H.; Park, K.S.; Goodenough, J.B. Improving lithium batteries by tethering carbon-coated LiFePO4 to polypyrrole. J. Electrochem. Soc. 2006, 153, A2282–A2286. [Google Scholar] [CrossRef]
- Dominko, R.; Gaberšček, M.; Drofenik, J.; Bele, M.; Pejovnik, S. A novel coating technology for preparation of cathodes in Li-ion batteries. Electrochem. Solid State Lett. 2001, 4, A187–A190. [Google Scholar] [CrossRef]
- Chen, Z.; Christensen, L.; Dahn, J. Comparison of PVDF and PVDF-TFE-P as binders for electrode materials showing large volume changes in lithium-ion batteries. J. Electrochem. Soc. 2003, 150, A1073–A1078. [Google Scholar] [CrossRef]
- Chen, Z.; Christensen, L.; Dahn, J. Large-volume-change electrodes for Li-ion batteries of amorphous alloy particles held by elastomeric tethers. Electrochem. Commun. 2003, 5, 919–923. [Google Scholar] [CrossRef]
- Liu, W.R.; Yang, M.H.; Wu, H.C.; Chiao, S.; Wu, N.L. Enhanced cycle life of Si anode for Li-ion batteries by using modified elastomeric binder. Electrochem. Solid State Lett. 2005, 8, A100–A103. [Google Scholar] [CrossRef]
- Koo, B.; Kim, H.; Cho, Y.; Lee, K.T.; Choi, N.S.; Cho, J. A Highly cross-linked polymeric binder for high-performance silicon negative electrodes in lithium ion batteries. Angew. Chem. Int. Ed. 2012, 51, 8762–8767. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.W.; Jeong, Y.K.; Deniz, E.; AlQaradawi, S.Y.; Choi, J.W.; Coskun, A. Dynamic cross-linking of polymeric binders based on host–guest interactions for silicon anodes in lithium ion batteries. ACS Nano 2015, 9, 11317–11324. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Zhang, Z. Battery separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Ryou, M.H.; Son, B.; Lee, J.N.; Lee, D.J.; Lee, Y.M.; Choi, J.W.; Park, J.K. Co-polyimide-coated polyethylene separators for enhanced thermal stability of lithium ion batteries. Electrochim. Acta 2012, 85, 524–530. [Google Scholar] [CrossRef]
- Liu, H.; Xu, J.; Guo, B.; He, X. Effect of Al2O3/SiO2 composite ceramic layers on performance of polypropylene separator for lithium-ion batteries. Ceram. Int. 2014, 40, 14105–14110. [Google Scholar] [CrossRef]
- Choi, J.A.; Kim, S.H.; Kim, D.W. Enhancement of thermal stability and cycling performance in lithium-ion cells through the use of ceramic-coated separators. J. Power Sources 2010, 195, 6192–6196. [Google Scholar] [CrossRef]
- Ryou, M.H.; Lee, Y.M.; Park, J.K.; Choi, J.W. Mussel-inspired polydopamine-treated polyethylene separators for high-power Li-ion batteries. Adv. Mater. 2011, 23, 3066–3070. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, Y.M.; Bhattacharya, B.; Nho, Y.C.; Park, J.K. Separator grafted with siloxane by electron beam irradiation for lithium secondary batteries. Electrochim. Acta 2009, 54, 4312–4315. [Google Scholar] [CrossRef]
- Ko, J.; Min, B.; Kim, D.W.; Ryu, K.; Kim, K.; Lee, Y.; Chang, S. Thin-film type Li-ion battery, using a polyethylene separator grafted with glycidyl methacrylate. Electrochim. Acta 2004, 50, 367–370. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
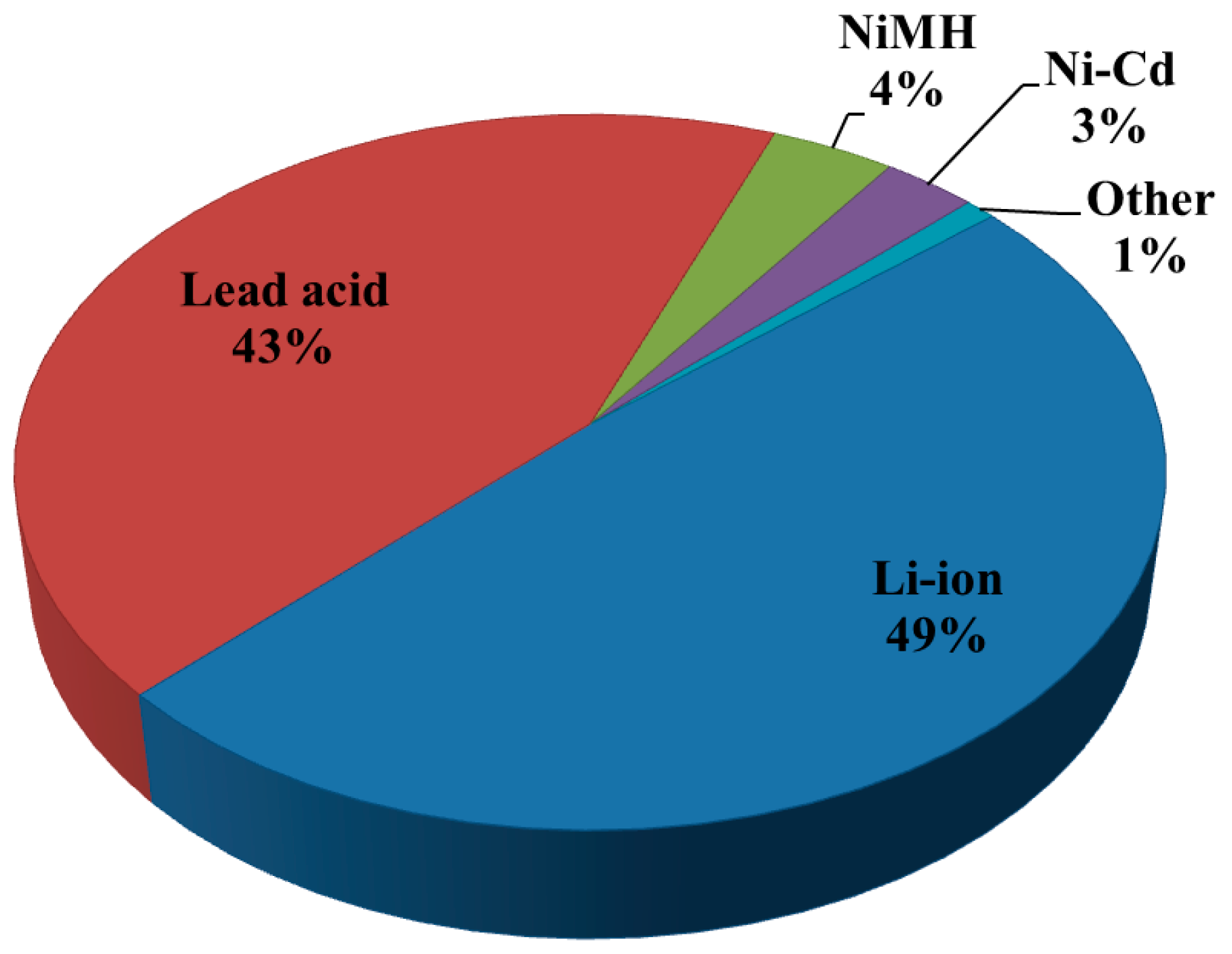
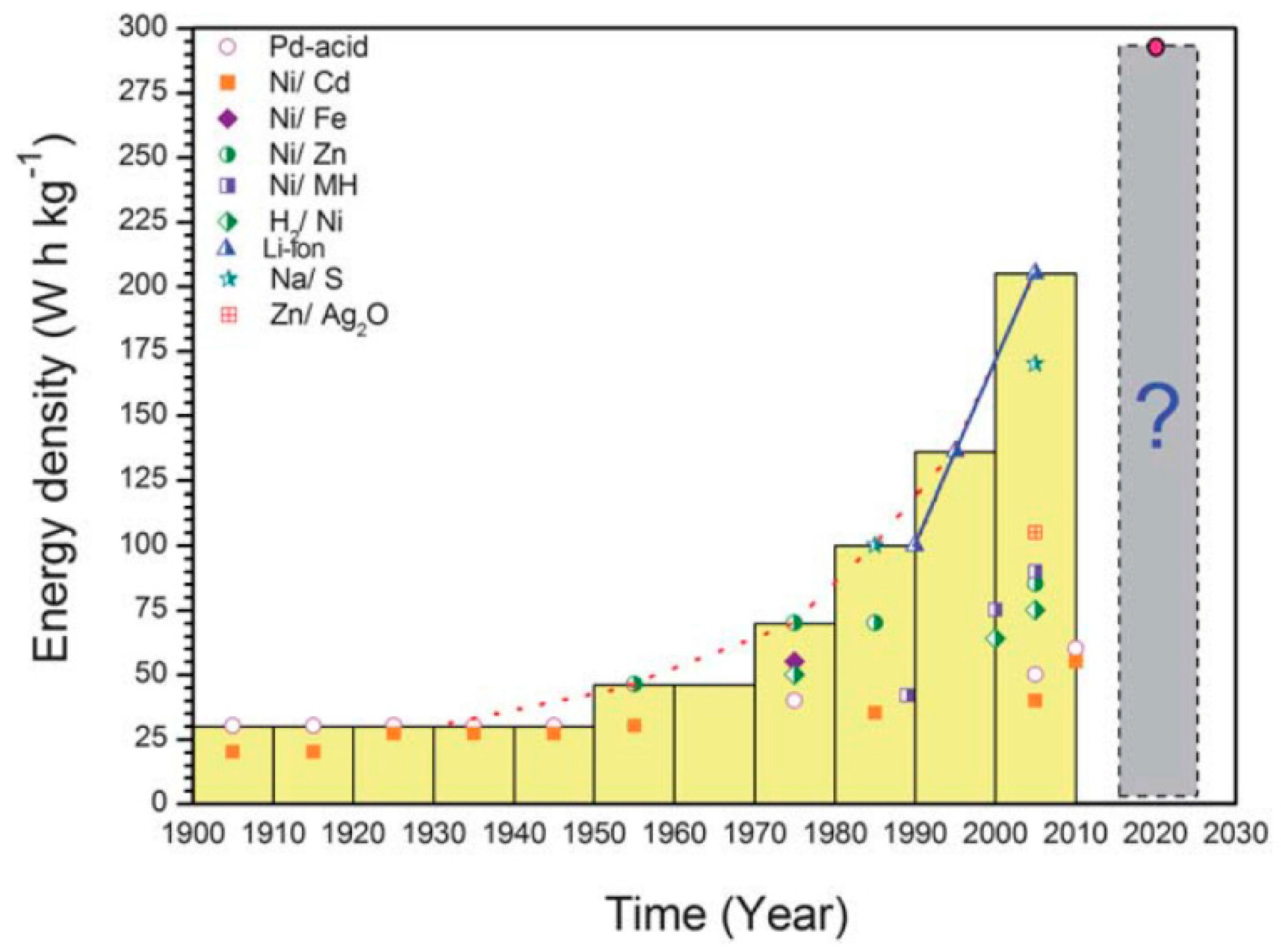
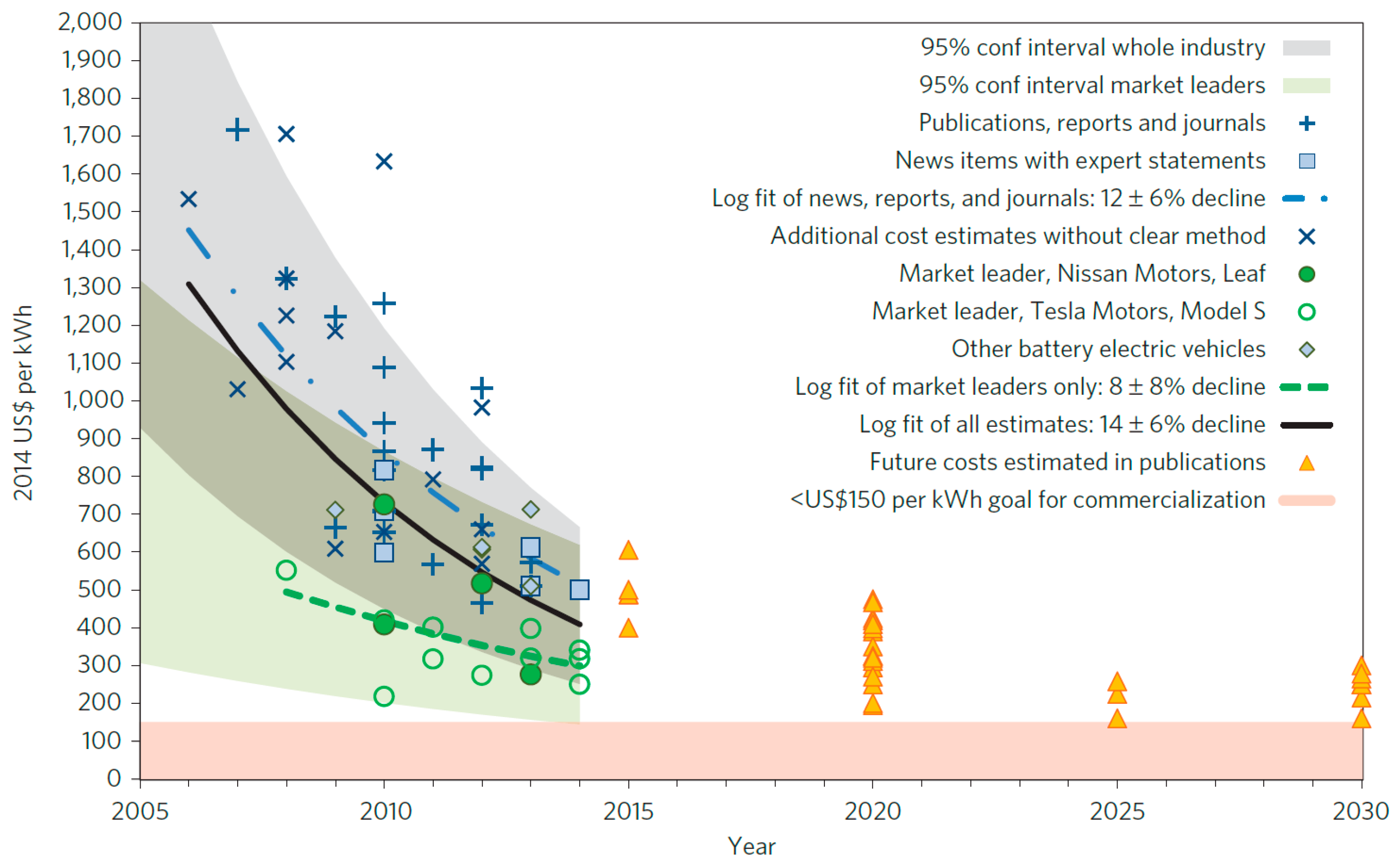
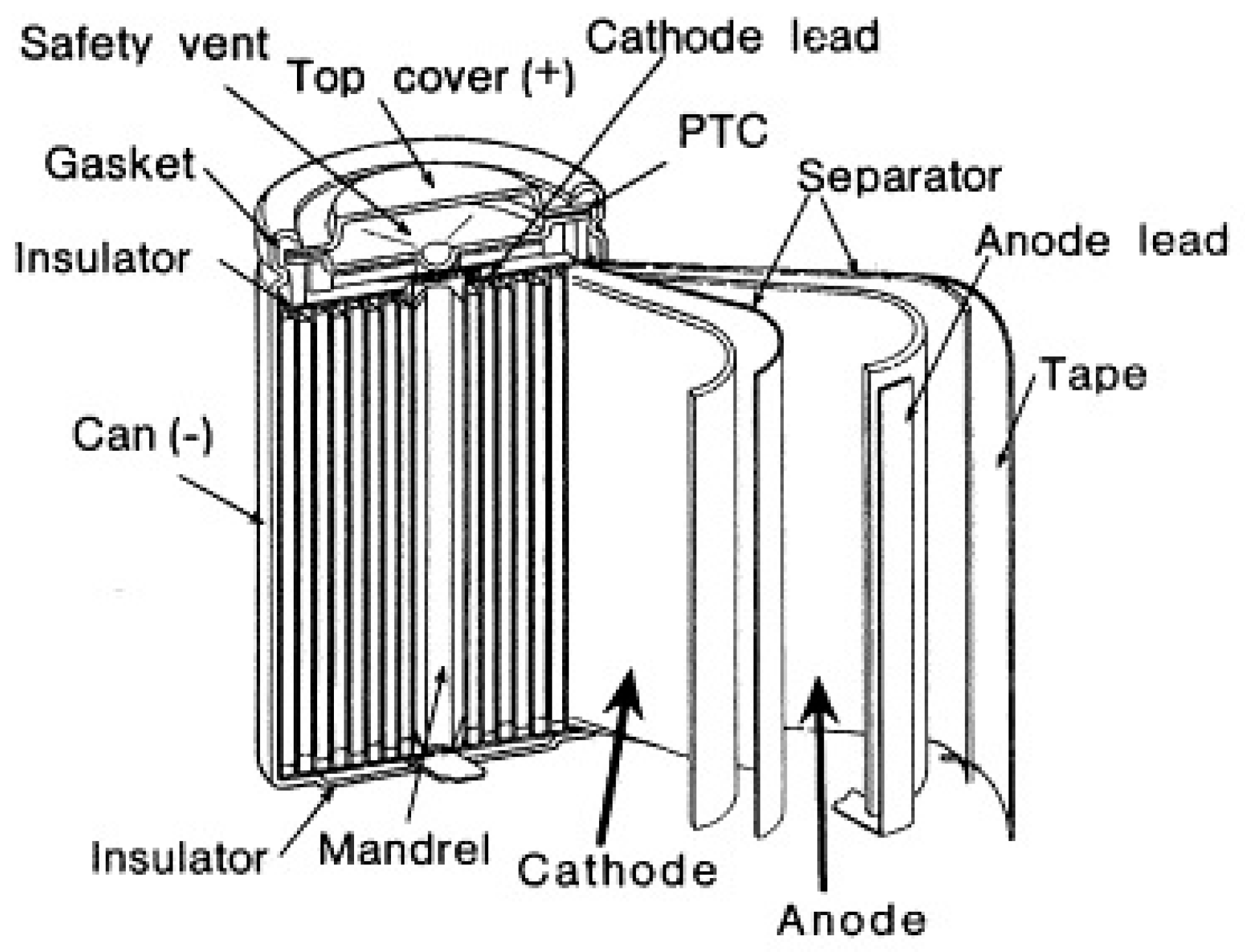
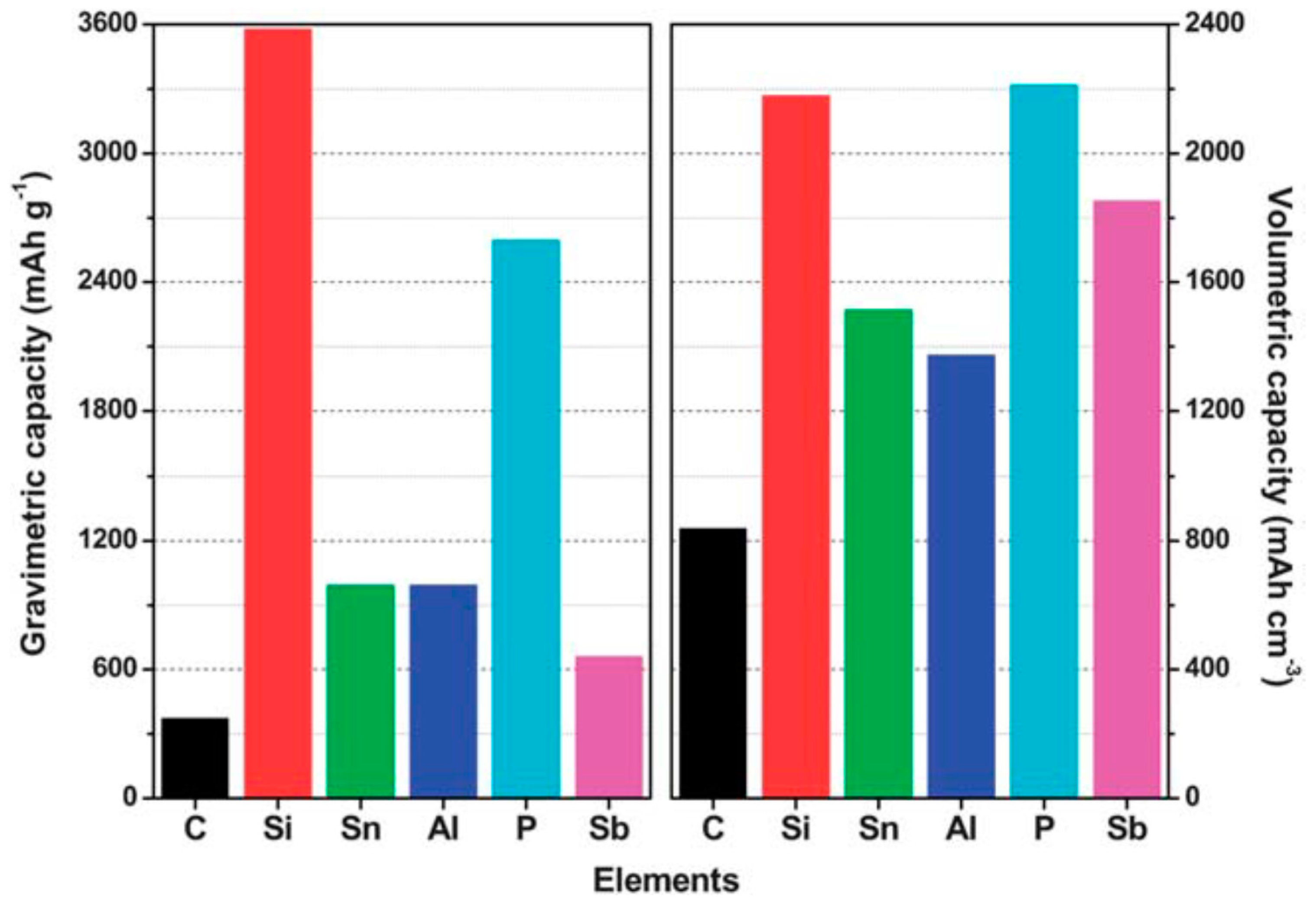
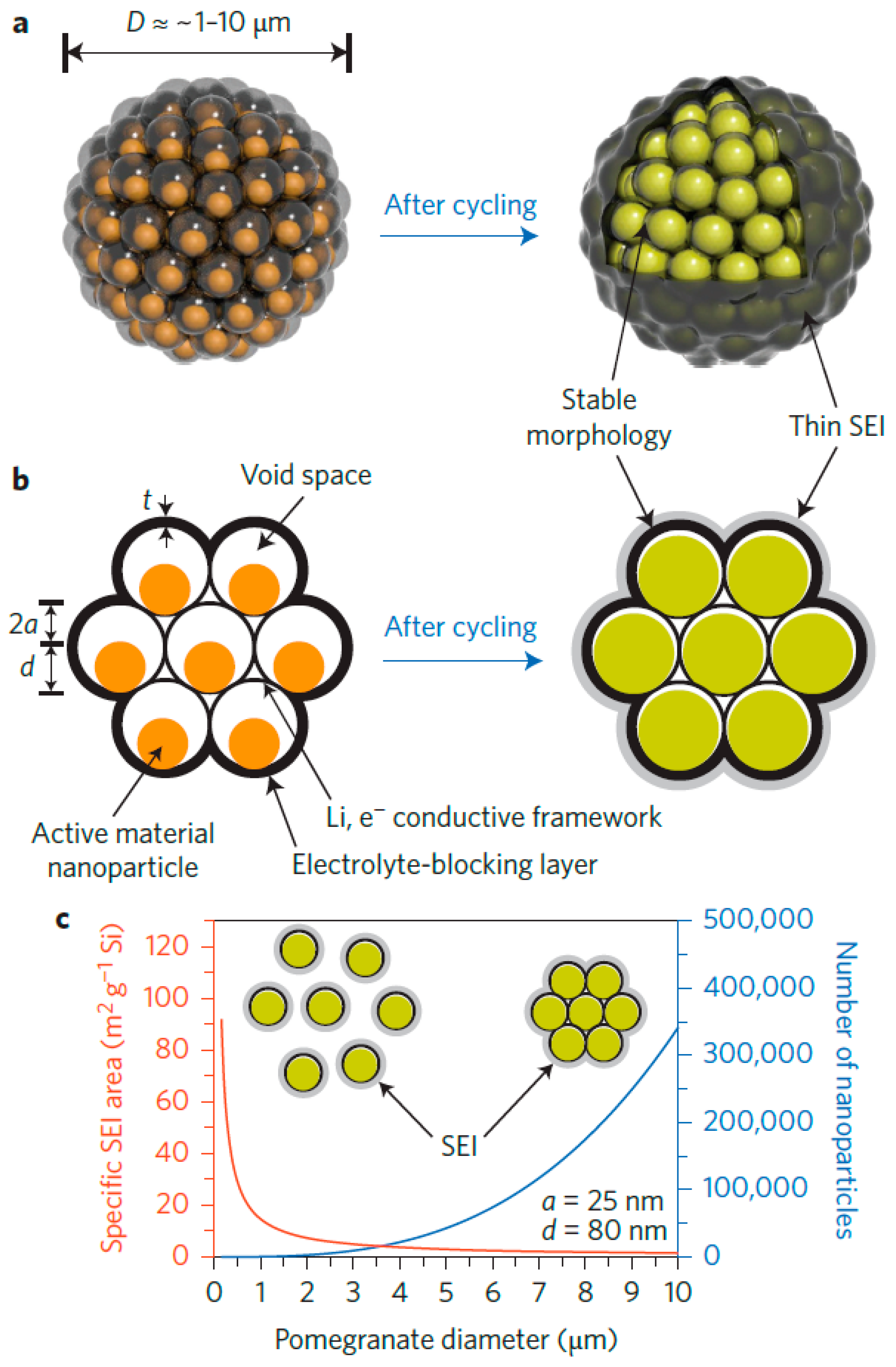
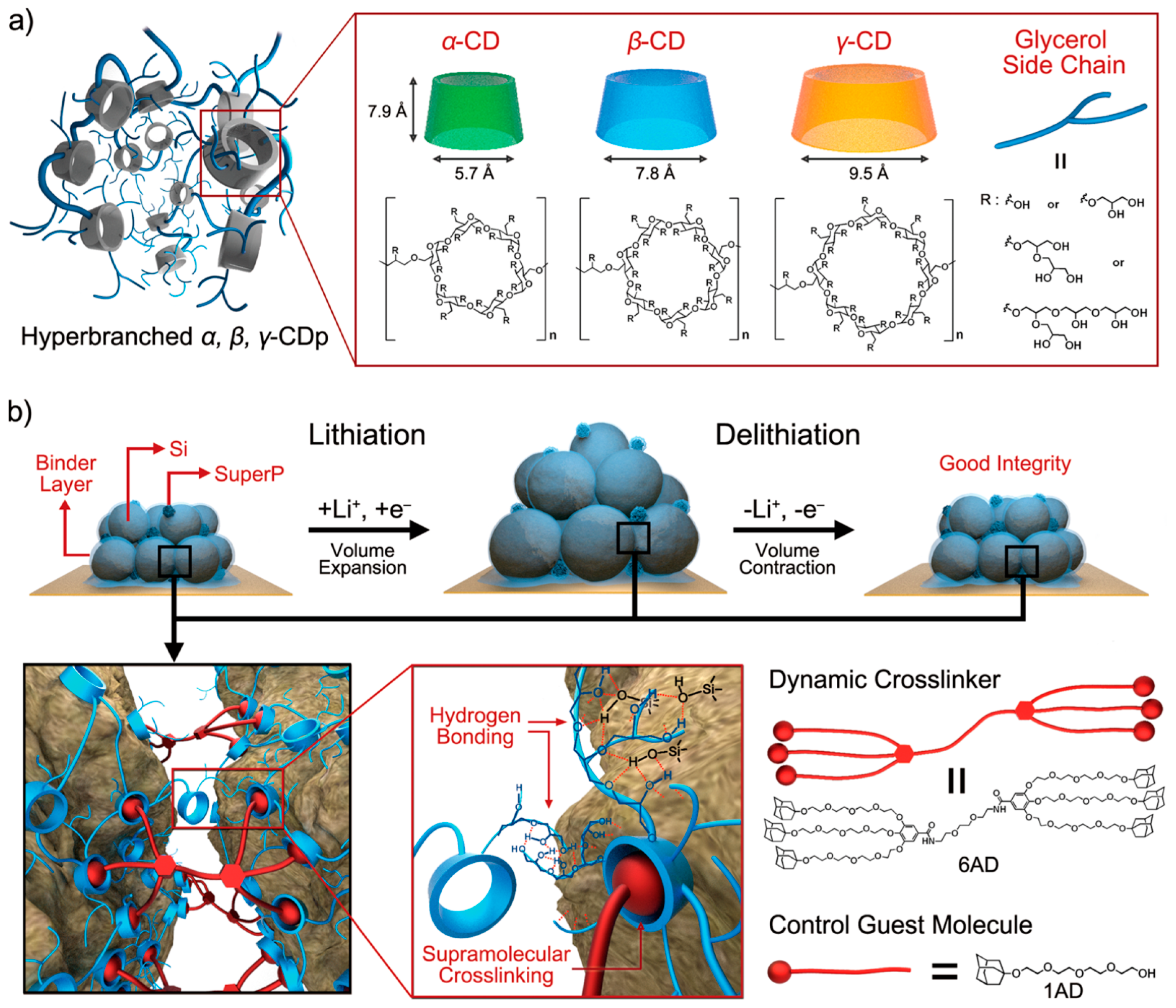
| Properties | Rechargeable Battery Chemistry | |||
|---|---|---|---|---|
| Li-ion | Lead Acid | NiMH | Ni–Cd | |
| Specific energy density (Wh/kg) | 90–190 | 30–55 | 60–120 | 45–80 |
| Cycle life (80% of initial capacity) | 500–2000 | 200–300 | 300–500 | 1000 |
| Cell voltage (V) | 3.3–3.8 | 2.0 | 1.2 | 1.2 |
| Self-discharge/month at 25 °C | <5% | 5–15% | 30% | 20% |
| Safety | Protection circuit is mandatory | Thermally stable | ||
| Commercialized in | 1991 | 1881 | 1990 | 1950 |
| Toxicity | Low | High | Low | High |
| Metrics | Status in 2012 | Target for 2022 |
|---|---|---|
| Battery cost ($/kWh) | 500 | 125 |
| Pack specific energy (Wh/kg) | 80–100 | 250 |
| Pack energy density (Wh/L) | 200 | 400 |
| Pack specific power (W/kg) | 500 | 2000 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirocak, D.E.; Srinivasan, S.S.; Stefanakos, E.K. A Review on Nanocomposite Materials for Rechargeable Li-ion Batteries. Appl. Sci. 2017, 7, 731. https://doi.org/10.3390/app7070731
Demirocak DE, Srinivasan SS, Stefanakos EK. A Review on Nanocomposite Materials for Rechargeable Li-ion Batteries. Applied Sciences. 2017; 7(7):731. https://doi.org/10.3390/app7070731
Chicago/Turabian StyleDemirocak, Dervis Emre, Sesha S. Srinivasan, and Elias K. Stefanakos. 2017. "A Review on Nanocomposite Materials for Rechargeable Li-ion Batteries" Applied Sciences 7, no. 7: 731. https://doi.org/10.3390/app7070731






