Investigation of Catalytic Effects and Compositional Variations in Desorption Characteristics of LiNH2-nanoMgH2
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
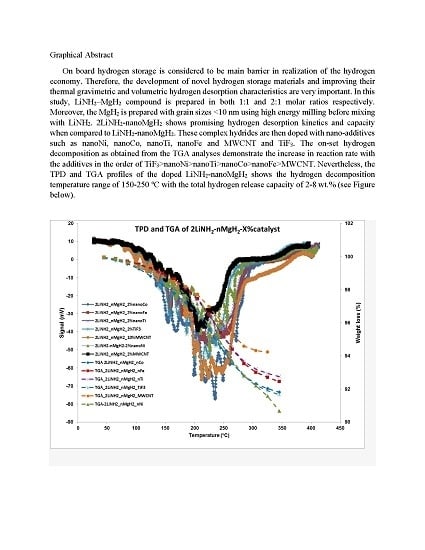
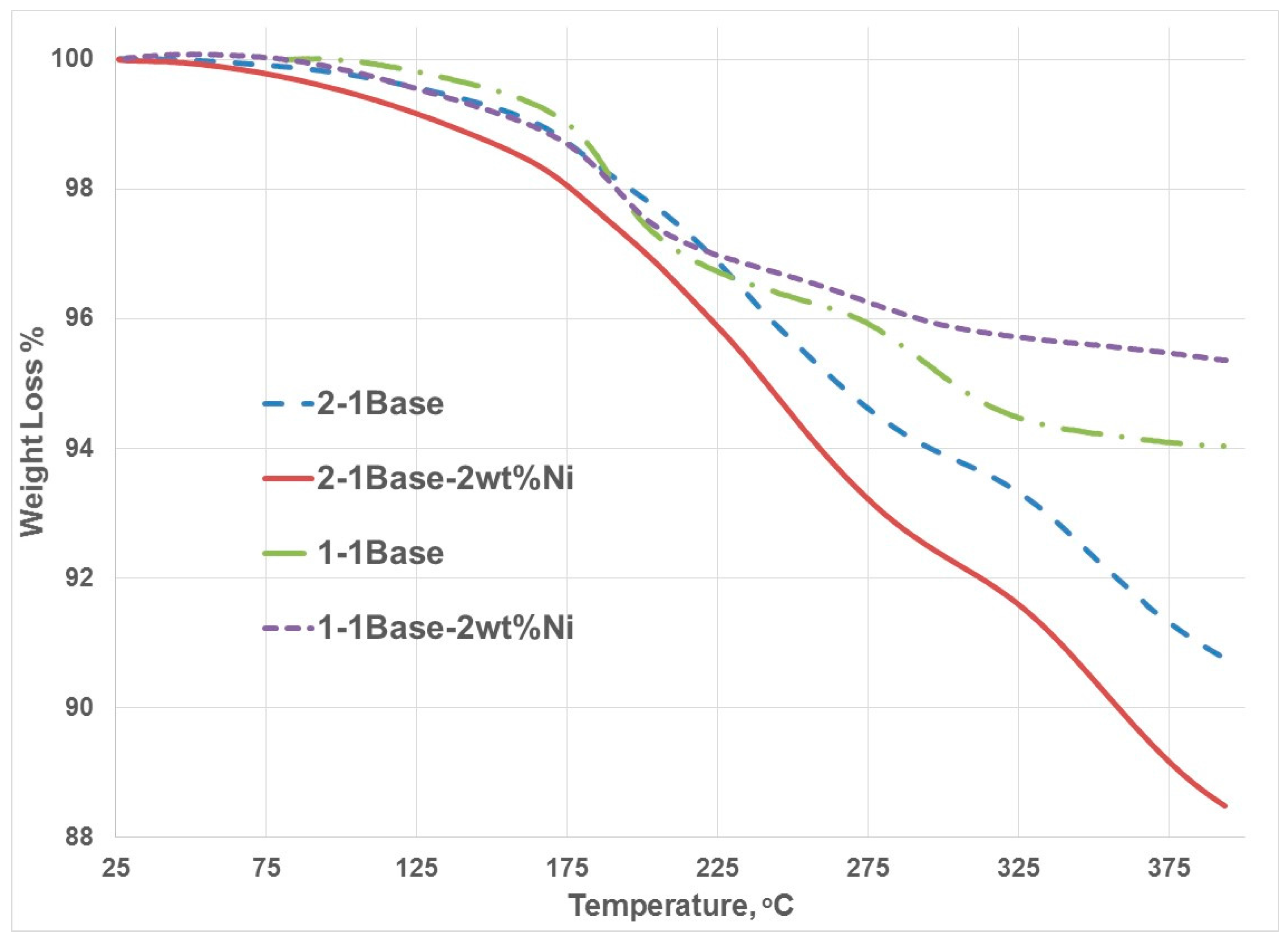
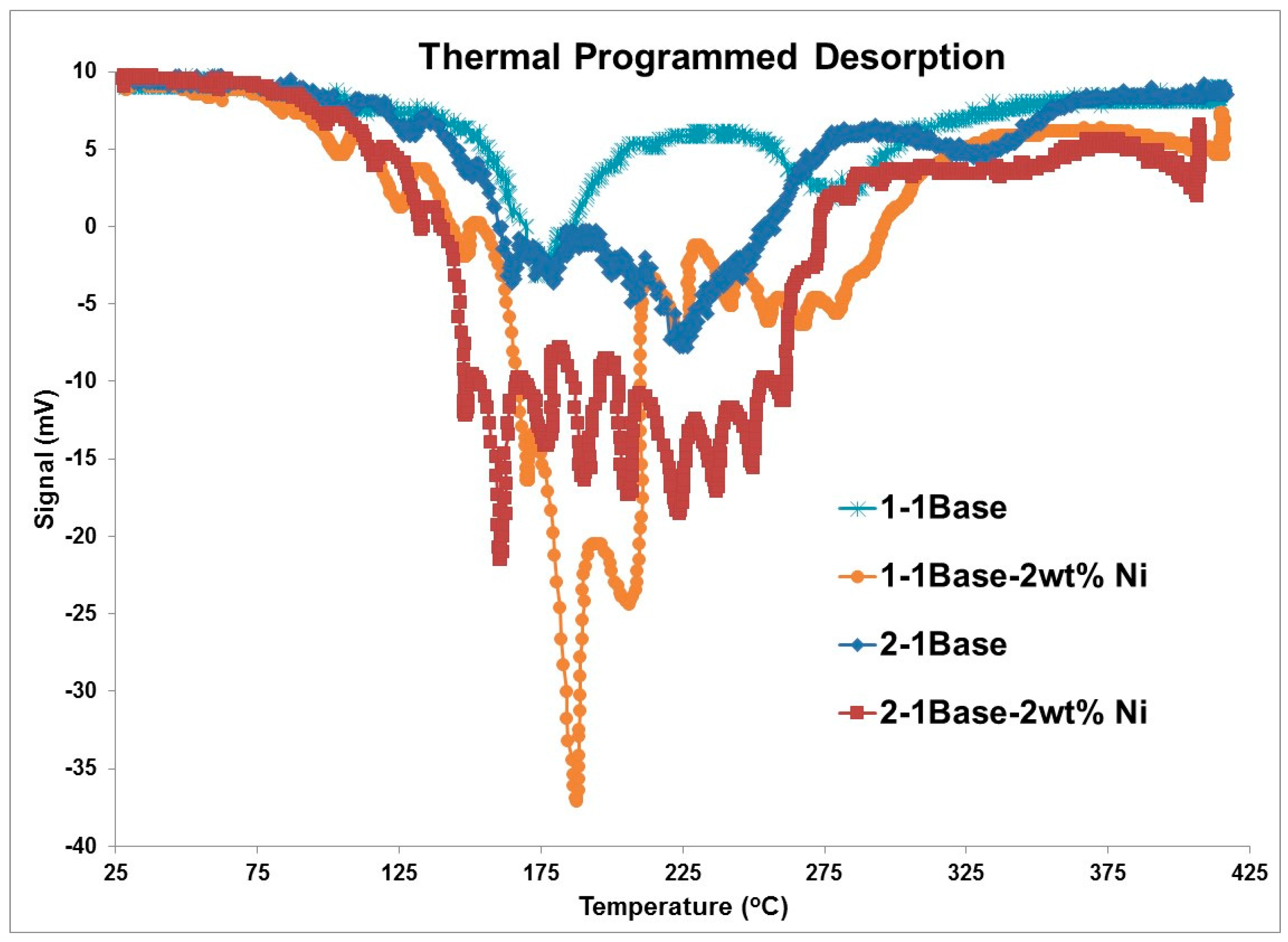
3.1. Thermogravmetric Analysis (TGA) and Thermal Programmed Desorption (TPD)
3.2. Desorption Kinetics Using Sievert’s Type Measurements
3.3. X-ray Diffraction (XRD), Fourier Transform Infrared Spectroscopy (FTIR) Scanning Electron Microscopy (SEM), and Energy Dispersive X-rays (EDX)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BM | Ball Milling |
| CNT | Carbon Nanotube |
| DSC | Differential Scanning Calorimetry |
| DOE | Department of Energy |
| EDX | Energy Dispersive X-rays |
| FTIR | Fourier Transform Infrared Spectroscopy |
| MA | Mechanical Activation |
| MOF | Metal Organic Frameworks |
| MWCNT | Multiwall Carbon Nanotube |
| PCT | Pressure Composition Temperature |
| RPM | revolutions per minute |
| SDT | Simultaneous TGA and DSC |
| SEM | Scanning Electron Microscopy |
| SWCNT | Single Wall Carbon Nanotube |
| TCD | Thermal Conductivity Detector |
| TEM | Transmission Electron Microscopy |
| TGA | Thermogravimetric Analysis |
| TPD | Thermal Program Desorption |
| wt. % | weight percent |
| XRD | X-Ray Diffraction |
References
- Orimo, S.; Nakamori, Y.; Eliseo, J.R.; Zuettel, A.; Jensen, C.M. Complex hydrides for hydrogen storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef] [PubMed]
- Dafert, F.; Miklauz, R. New compounds of nitrogen and hydrogen with lithium. Monatsh. Chem. 1910, 31, 981. [Google Scholar] [CrossRef]
- Ruff, O.; Goeres, H. Li imide and some compounds of N, H and Li. Ber. Dtsch. Chem. Ges. 1910, 44, 502–506. [Google Scholar] [CrossRef]
- Chen, P.; Xiong, Z.; Luo, J.; Lin, J.; Tan, K.L. Interaction of hydrogen with metal nitrides and imides. Nature 2002, 420, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.H.; Ruckenstein, E. Ultrafast reaction between LiH and NH3 during H2 storage in Li3N. J. Phys. Chem. A 2003, 107, 9737–9739. [Google Scholar] [CrossRef]
- Nakamori, Y.; Orimo, S. Destabilization of Li-based complex hydrides. J. Alloys Compd. 2004, 370, 271–275. [Google Scholar] [CrossRef]
- Luo, W. (LiNH2-MgH2): A viable hydrogen storage system. J. Alloys Compd. 2004, 381, 284–287. [Google Scholar] [CrossRef]
- Luo, W.; Rönnebro, E. Towards a viable hydrogen storage system for transportation application. J. Alloys Compd. 2005, 404, 392–395. [Google Scholar] [CrossRef]
- Xiong, Z.; Wu, G.; Hu, J.; Chen, P. Ternary imides for hydrogen storage. Adv. Mater. 2004, 16, 1522–1525. [Google Scholar] [CrossRef]
- Luo, W.; Sickafoose, S. Thermodynamic and structural characterization of the Mg-Li-NH hydrogen storage system. J. Alloys Compd. 2006, 407, 274–281. [Google Scholar] [CrossRef]
- Lohstroh, W.; Fichtner, M. Reaction steps in the Li-Mg-NH hydrogen storage system. J. Alloys Compd. 2007, 446, 332–335. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, C.Z.; Wang, P.; Cheng, H.M. Structure and hydrogen storage property of ball-milled LiNH2/MgH2 mixture. Int. J. Hydrog. Energy 2006, 31, 1236–1240. [Google Scholar] [CrossRef]
- Pottmaier, D.; Dolci, F.; Orlova, M.; Vaughan, G.; Fichtner, M.; Lohstroh, W.; Baricco, M. Hydrogen release and structural transformations in LiNH2-MgH2 systems. J. Alloys Compd. 2010, 509, 719–723. [Google Scholar] [CrossRef]
- Chen, P.; Xiong, Z.; Yang, L.; Wu, G.; Luo, W. Mechanistic investigations on the heterogeneous solid-state reaction of magnesium amides and lithium hydrides. J. Phys. Chem. B 2006, 110, 14221–14225. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, H.; Wang, S.; Liu, X.; Li, Y.; Jiang, L. The desorption kinetics of the Mg(NH2)2 + LiH mixture. Int. J. Hydrog. Energy 2009, 34, 1411–1416. [Google Scholar] [CrossRef]
- Yang, J.; Sudik, A.; Wolverton, C. Activation of hydrogen storage materials in the Li-Mg-NH system: Effect on storage properties. J. Alloys Compd. 2007, 430, 334–338. [Google Scholar] [CrossRef]
- Xiong, Z.; Hu, J.; Wu, G.; Chen, P.; Luo, W.; Gross, K.; Wang, J. Thermodynamic and kinetic investigations of the hydrogen storage in the Li-Mg-NH system. J. Alloys Compd. 2005, 398, 235–239. [Google Scholar] [CrossRef]
- Leng, H.; Ichikawa, T.; Hino, S.; Hanada, N.; Isobe, S.; Fujii, H. New metal-NH system composed of Mg(NH2)2 and LiH for hydrogen storage. J. Phys. Chem. B 2004, 108, 8763–8765. [Google Scholar] [CrossRef]
- Nakamori, Y.; Kitahara, G.; Miwa, K.; Towata, S.; Orimo, S. Reversible hydrogen-storage functions for mixtures of Li3N and Mg3N2. Appl. Phys. A Mater. Sci. Process. 2005, 80, 1–3. [Google Scholar] [CrossRef]
- Leng, H.; Ichikawa, T.; Fujii, H. Hydrogen Storage Properties of Li-Mg-N-H Systems with Different Ratios of LiH/Mg(NH2)2. J. Phys. Chem. B 2006, 110, 12964–12968. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Wu, G.; Hu, J.; Chen, P.; Luo, W.; Wang, J. Investigations on hydrogen storage over Li-Mg-NH complex-the effect of compositional changes. J. Alloys Compd. 2006, 417, 190–194. [Google Scholar] [CrossRef]
- Osborn, W.; Markmaitree, T.; Shaw, L.L. Evaluation of the hydrogen storage behavior of a LiNH2 + MgH2 system with 1:1 ratio. J. Power Sources 2007, 172, 376–378. [Google Scholar] [CrossRef]
- Leng, H.; Ichikawa, T.; Hino, S.; Hanada, N.; Isobe, S.; Fujii, H. Synthesis and decomposition reactions of metal amides in metal-NH hydrogen storage system. J. Power Sources 2006, 156, 166–170. [Google Scholar] [CrossRef]
- Lu, J.; Choi, Y.J.; Fang, Z.Z.; Sohn, H.Y. Effect of milling intensity on the formation of LiMgN from the dehydrogenation of LiNH2-MgH2 (1:1) mixture. J. Power Sources 2010, 195, 1992–1997. [Google Scholar] [CrossRef]
- Leng, H.; Ichikawa, T.; Isobe, S.; Hino, S.; Hanada, N.; Fujii, H. Desorption behaviours from metal-NH systems synthesized by ball milling. J. Alloys Compd. 2005, 404, 443–447. [Google Scholar] [CrossRef]
- Pinkerton, F. Decomposition kinetics of lithium amide for hydrogen storage materials. J. Alloys Compd. 2005, 400, 76–82. [Google Scholar] [CrossRef]
- Tokoyoda, K.; Hino, S.; Ichikawa, T.; Okamoto, K.; Fujii, H. Hydrogen desorption/absorption properties of Li-Ca-NH system. J. Alloys Compd. 2007, 439, 337–341. [Google Scholar] [CrossRef]
- Chu, H.; Xiong, Z.; Wu, G.; He, T.; Wu, C.; Chen, P. Hydrogen storage properties of Li-Ca-NH system with different molar ratios of LiNH2/CaH2. Int. J. Hydrog. Energy 2010, 35, 8317–8321. [Google Scholar] [CrossRef]
- Zaluska, A.; Zaluski, L.; Ström-Olsen, J. Structure, catalysis and atomic reactions on the nano-scale: A systematic approach to metal hydrides for hydrogen storage. Appl. Phys. A Mater. Sci. Process. 2001, 72, 157–165. [Google Scholar] [CrossRef]
- Markmaitree, T.; Ren, R.; Shaw, L.L. Enhancement of lithium amide to lithium imide transition via mechanical activation. J. Phys. Chem. B 2006, 110, 20710–20718. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhong, K.; Luo, K.; Gao, M.; Pan, H.; Wang, Q. Size-Dependent Kinetic Enhancement in Hydrogen Absorption and Desorption of the Li-Mg-N-H System. J. Am. Chem. Soc. 2009, 131, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Varin, R.; Jang, M.; Polanski, M. The effects of ball milling and molar ratio of LiH on the hydrogen storage properties of nanocrystalline lithium amide and lithium hydride (LiNH2 + LiH) system. J. Alloys Compd. 2010, 491, 658–667. [Google Scholar] [CrossRef]
- Shaw, L.L.; Ren, R.; Markmaitree, T.; Osborn, W. Effects of mechanical activation on dehydrogenation of the lithium amide and lithium hydride system. J. Alloys Compd. 2008, 448, 263–271. [Google Scholar] [CrossRef]
- Shahi, R.R.; Yadav, T.; Shaz, M.; Srivastava, O. Effects of mechanical milling on desorption kinetics and phase transformation of LiNH2/MgH2 mixture. Int. J. Hydrog. Energy 2008, 33, 6188–6194. [Google Scholar] [CrossRef]
- Xie, L.; Liu, Y.; Li, G.; Li, X. Improving Hydrogen Sorption Kinetics of the Mg(NH2)2-LiH System by the Tuning Particle Size of the Amide. J. Phys. Chem. C 2009, 113, 14523–14527. [Google Scholar] [CrossRef]
- Osborn, W.; Markmaitree, T.; Shaw, L.L.; Hu, J.Z.; Kwak, J.H.; Yang, Z. Low temperature milling of the LiNH2 + LiH hydrogen storage system. Int. J. Hydrog. Energy 2009, 34, 4331–4339. [Google Scholar] [CrossRef]
- Ikeda, S.; Kuriyama, N.; Kiyobayashi, T. Simultaneous determination of ammonia emission and hydrogen capacity variation during the cyclic testing for LiNH2-LiH hydrogen storage system. Int. J. Hydrog. Energy 2008, 33, 6201–6204. [Google Scholar] [CrossRef]
- Markmaitree, T.; Osborn, W.; Shaw, L.L. Comparisons between MgH2- and LiH-containing systems for hydrogen storage applications. Int. J. Hydrog. Energy 2008, 33, 3915–3924. [Google Scholar] [CrossRef]
- Markmaitree, T.; Osborn, W.; Shaw, L.L. Comparative studies of reaction rates of NH3 with MgH2 and LiH. J. Power Sources 2008, 180, 535–538. [Google Scholar] [CrossRef]
- Luo, W.; Stewart, K. Characterization of NH3 formation in desorption of Li-Mg-NH storage system. J. Alloys Compd. 2007, 440, 357–361. [Google Scholar] [CrossRef]
- Luo, W.; Wang, J.; Stewart, K.; Clift, M.; Gross, K. Li-Mg-NH: Recent investigations and development. J. Alloys Compd. 2007, 446, 336–341. [Google Scholar] [CrossRef]
- Bogdanovic, B.; Schwickardi, M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials. J. Alloys Compd. 1997, 253, 1–9. [Google Scholar] [CrossRef]
- Ichikawa, T.; Isobe, S.; Hanada, N.; Fujii, H. Lithium nitride for reversible hydrogen storage. J. Alloys Compd. 2004, 365, 271–276. [Google Scholar] [CrossRef]
- Isobe, S.; Ichikawa, T.; Hanada, N.; Leng, H.; Fichtner, M.; Fuhr, O.; Fujii, H. Effect of Ti catalyst with different chemical form on Li-NH hydrogen storage properties. J. Alloys Compd. 2005, 404, 439–442. [Google Scholar] [CrossRef]
- Yao, J.; Shang, C.; Aguey-Zinsou, K.; Guo, Z. Desorption characteristics of mechanically and chemically modified LiNH2 and (LiNH2 + LiH). J. Alloys Compd. 2007, 432, 277–282. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, P.; Liu, C.; Cheng, H.M. Improved hydrogen storage performance of Li-Mg-NH materials by optimizing composition and adding single-walled carbon nanotubes. Int. J. Hydrog. Energy 2007, 32, 1262–1268. [Google Scholar] [CrossRef]
- Janot, R.; Eymery, J.B.; Tarascon, J.M. Investigation of the processes for reversible hydrogen storage in the Li-Mg-NH system. J. Power Sources 2007, 164, 496–502. [Google Scholar] [CrossRef]
- Barison, S.; Agresti, F.; Lo Russo, S.; Maddalena, A.; Palade, P.; Principi, G.; Torzo, G. A study of the LiNH2-MgH2 system for solid state hydrogen storage. J. Alloys Compd. 2008, 459, 343–347. [Google Scholar] [CrossRef]
- Wang, J.; Liu, T.; Wu, G.; Li, W.; Liu, Y.; Araújo, C.M.; Scheicher, R.H.; Blomqvist, A.; Ahuja, R.; Xiong, Z. Potassium Modified Mg(NH2)2/2LiH System for Hydrogen Storage. Angew. Chem. Int. Ed. 2009, 48, 5828–5832. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.P.; Wang, P.; Dai, H.B.; Cheng, H.M. Catalytically enhanced dehydrogenation of Li-Mg-NH hydrogen storage material by transition metal nitrides. J. Alloys Compd. 2009, 468, L21–L24. [Google Scholar] [CrossRef]
- Srinivasan, S.S.; Niemann, M.U.; Hattrick-Simpers, J.R.; McGrath, K.; Sharma, P.C.; Goswami, D.Y.; Stefanakos, E.K. Effects of nano additives on hydrogen storage behavior of the complex hydride LiBH4/LiNH2/MgH2. Int. J. Hydrog. Energy 2010, 35, 9646–9652. [Google Scholar] [CrossRef]
- Vittetoe, A.W.; Niemann, M.U.; Srinivasan, S.; McGrath, K.; Kumar, A.; Goswami, D.Y.; Stefanakos, E.K.; Thomas, S. Destabilization of LiAlH4 by nanocrystalline MgH2. Int. J. Hydrog. Energy 2009, 4, 2333–2339. [Google Scholar] [CrossRef]
- Niemann, M.U.; Srinivasan, S.S.; Kumar, A.; Stefanakos, E.K.; Goswami, D.Y.; McGrath, K. Processing analysis of the ternary LiNH2-MgH2-LiBH4 system for hydrogen storage. Int. J. Hydrog. Energy 2009, 34, 8086–8093. [Google Scholar] [CrossRef]
- Markmaitree, T.; Shaw, L.L. Synthesis and hydriding properties of Li2Mg(NH)2. J. Power Sources 2010, 195, 1984–1991. [Google Scholar] [CrossRef]
- Varin, R.A.; Zbroniec, L.; Polanski, M.; Bystrzycki, J. A review of recent advances on the effects of microstructural refinement and nano-catalytic additives on the hydrogen storage properties of metal and complex hydrides. Energies 2010, 4, 1–25. [Google Scholar] [CrossRef]
- Bazzanella, N.; Checchetto, R.; Miotello, A. Atoms and nanoparticles of transition metals as catalysts for hydrogen desorption from magnesium hydride. J. Nanomater. 2011. [Google Scholar] [CrossRef]
- Kojima, Y.; Kawai, Y.; Ohba, N. Hydrogen storage of metal nitrides by a mechanochemical reaction. J. Power Sources 2006, 159, 81–87. [Google Scholar] [CrossRef]
- Maloney, K.; Dopp, R. Advanced Materials for Clean Energy Applications; Online Webinar Event, 26 June 2007; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
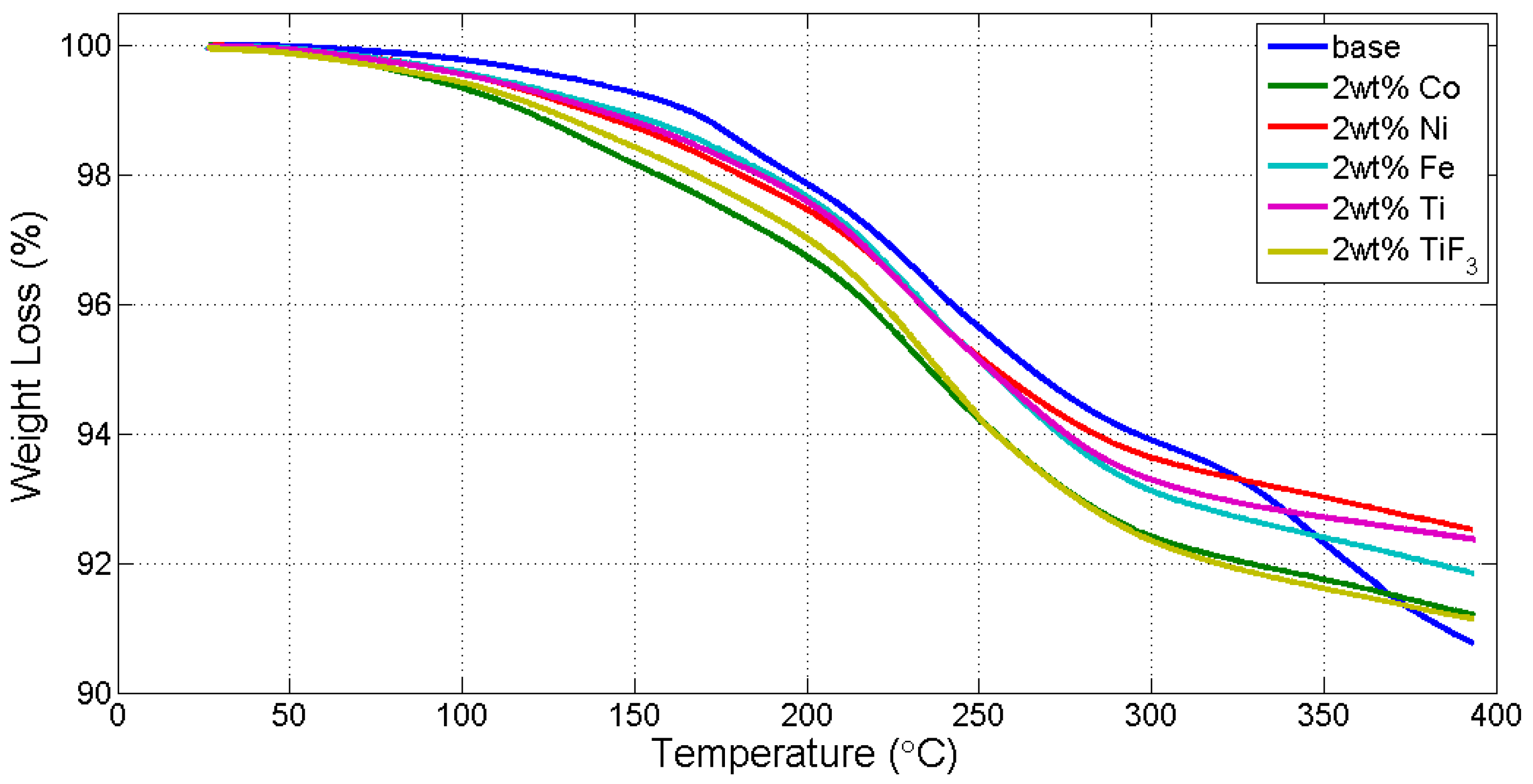
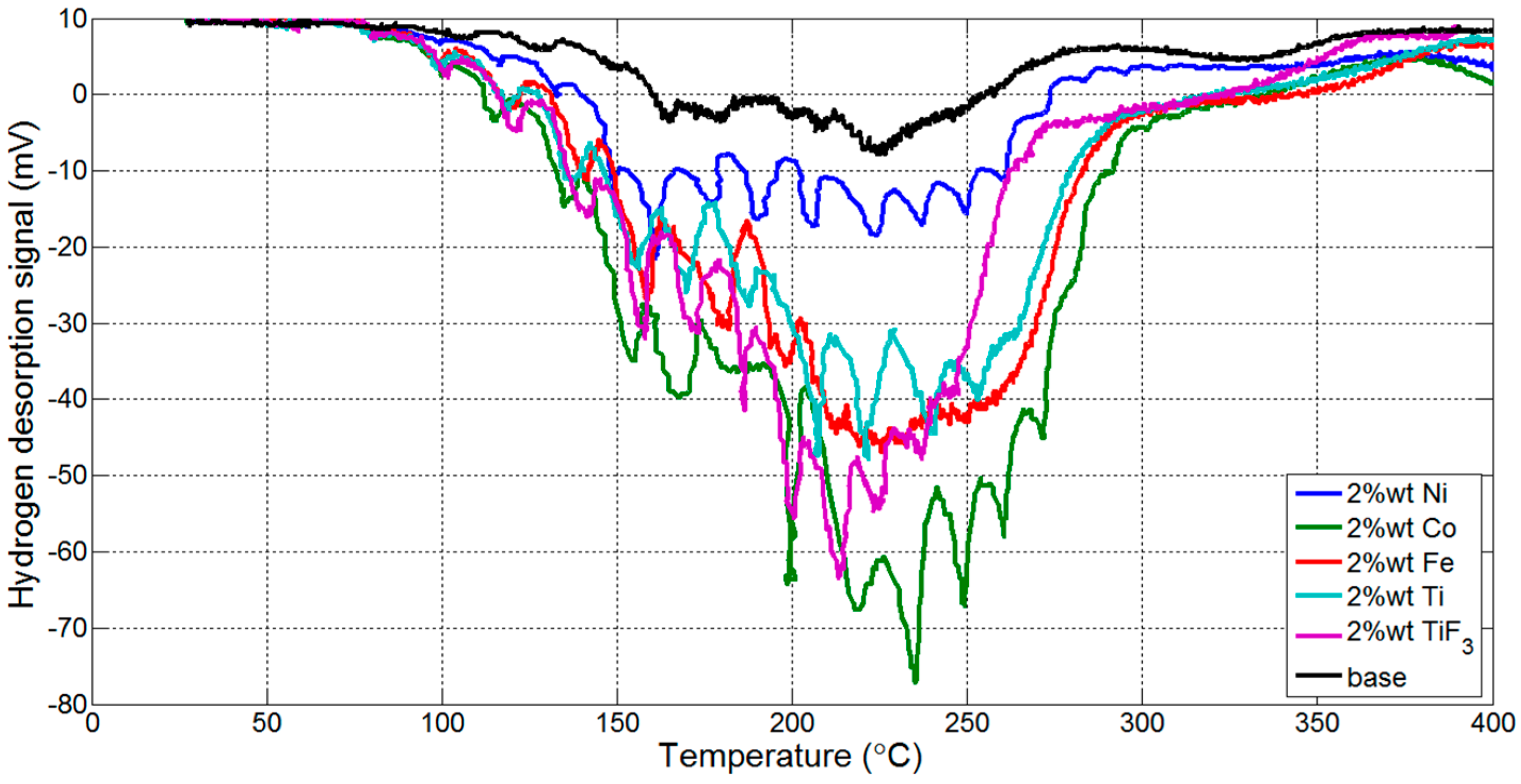


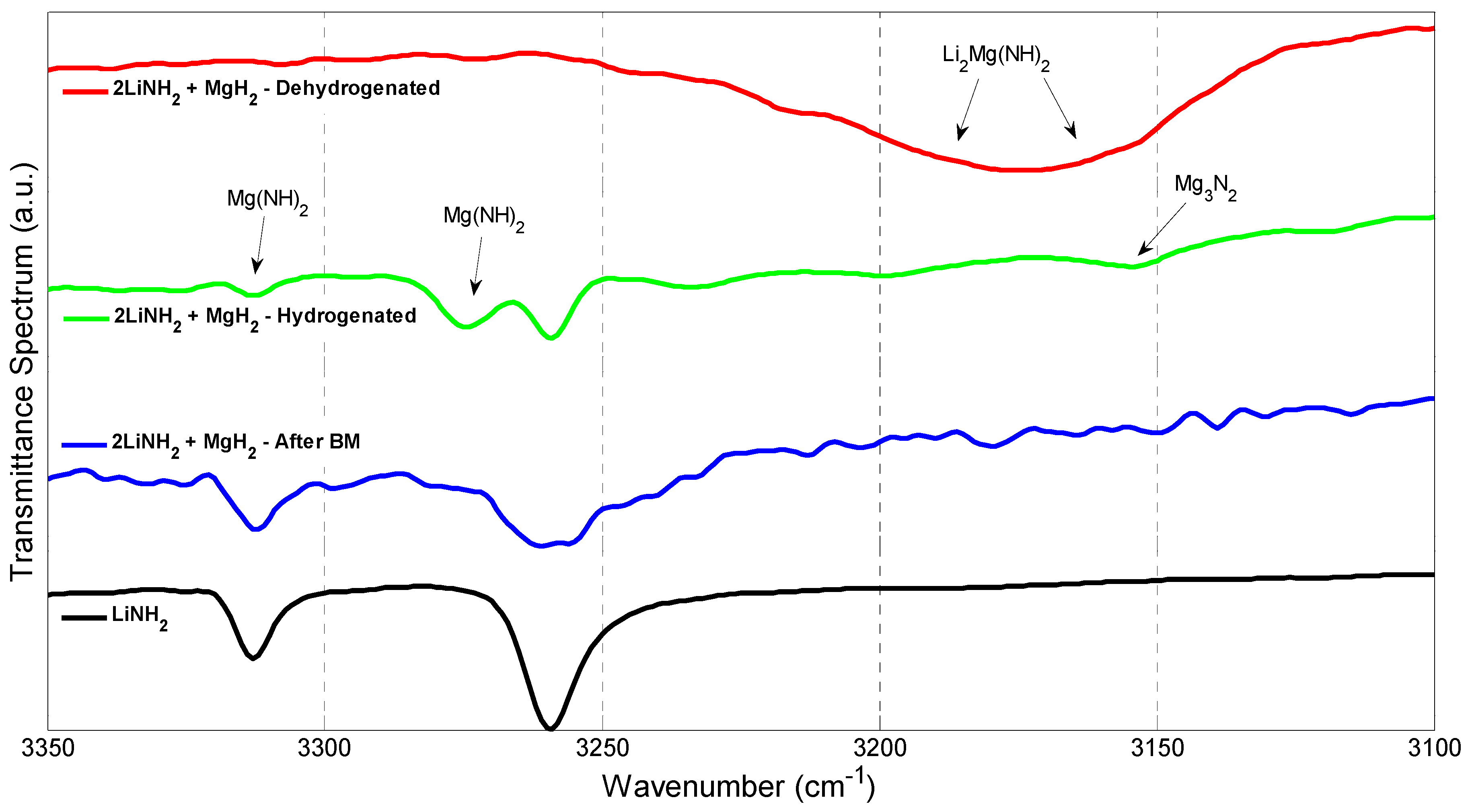

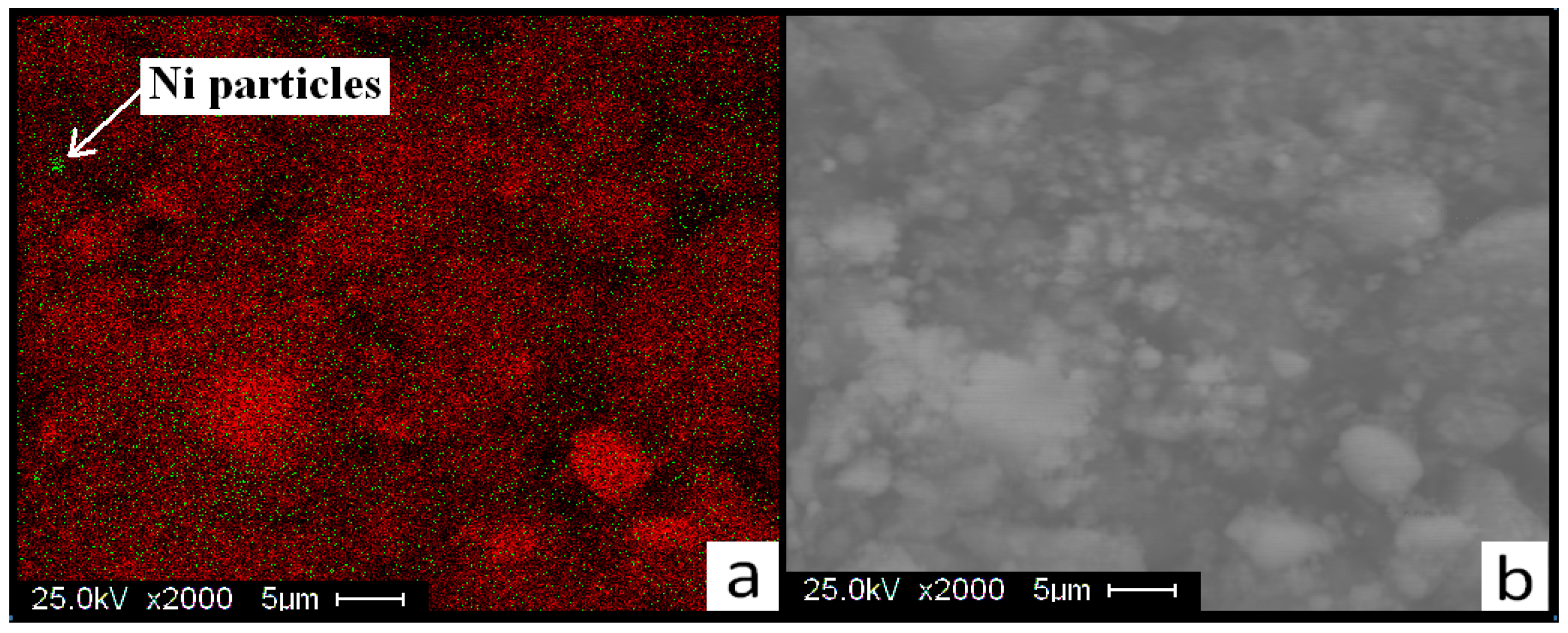
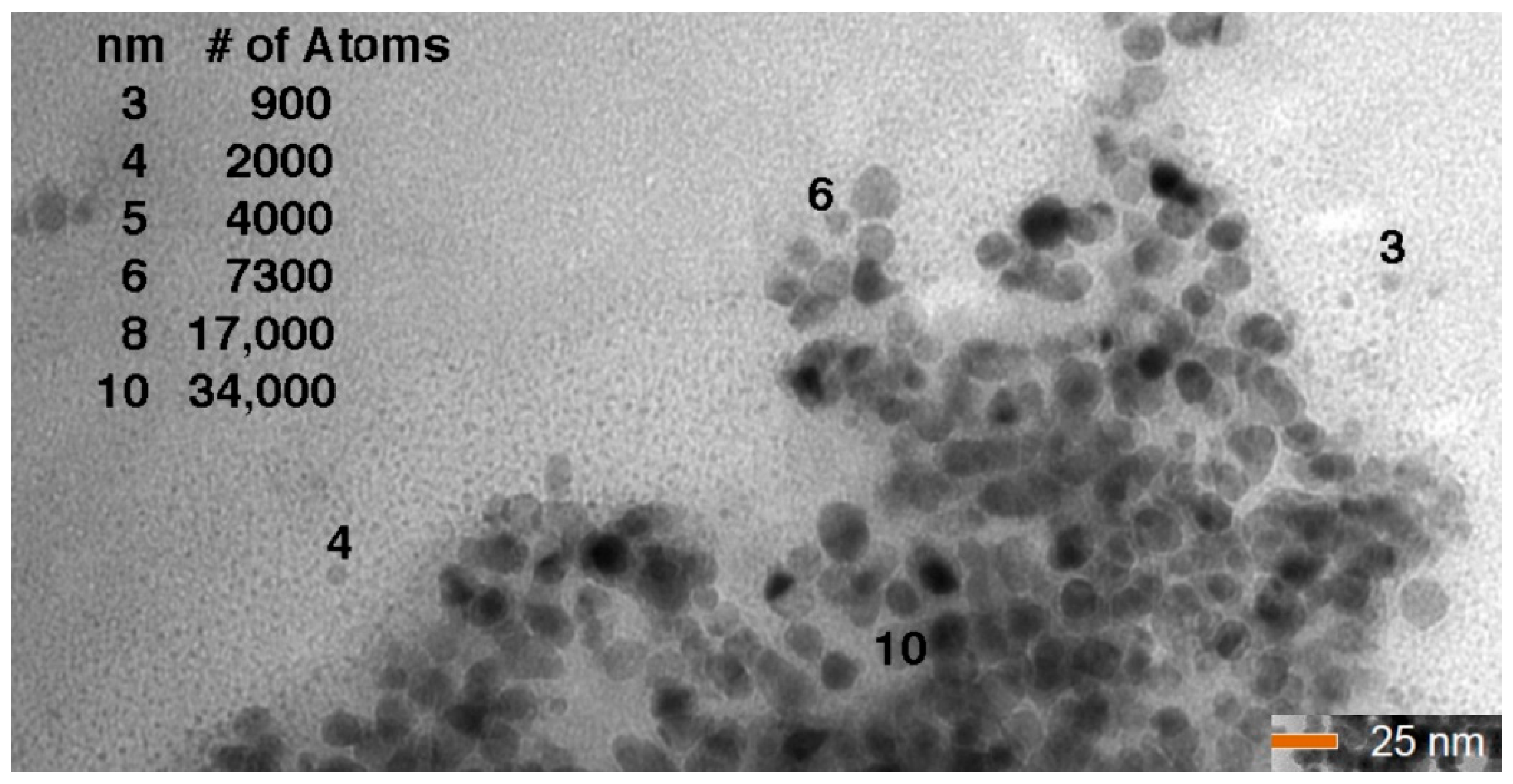
| Reaction Kinetics (wt. %/min) | On-Set Temperature (°C) | ||
|---|---|---|---|
| TiF3 | 0.5816 | nanoCo | 121.96 |
| nanoNi | 0.5330 | TiF3 | 125.99 |
| nanoTi | 0.5312 | nanoTi | 136.65 |
| nanoCo | 0.5255 | nanoFe | 142.86 |
| nanoFe | 0.5113 | nanoNi | 149.30 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srinivasan, S.S.; Demirocak, D.E.; Goswami, Y.; Stefanakos, E. Investigation of Catalytic Effects and Compositional Variations in Desorption Characteristics of LiNH2-nanoMgH2. Appl. Sci. 2017, 7, 701. https://doi.org/10.3390/app7070701
Srinivasan SS, Demirocak DE, Goswami Y, Stefanakos E. Investigation of Catalytic Effects and Compositional Variations in Desorption Characteristics of LiNH2-nanoMgH2. Applied Sciences. 2017; 7(7):701. https://doi.org/10.3390/app7070701
Chicago/Turabian StyleSrinivasan, Sesha S., Dervis Emre Demirocak, Yogi Goswami, and Elias Stefanakos. 2017. "Investigation of Catalytic Effects and Compositional Variations in Desorption Characteristics of LiNH2-nanoMgH2" Applied Sciences 7, no. 7: 701. https://doi.org/10.3390/app7070701