Mechanically Strong CaSiO3 Scaffolds Incorporating B2O3-ZnO Liquid Phase
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials and Processing
2.2. Physical and Chemical Properties Characterization
2.3. Mechanical Properties Assessment
2.4. Bioactivity and Biodegradability Assessment
2.5. Cytocompatibility and Cell Viability Assessment
3. Results and Discussion
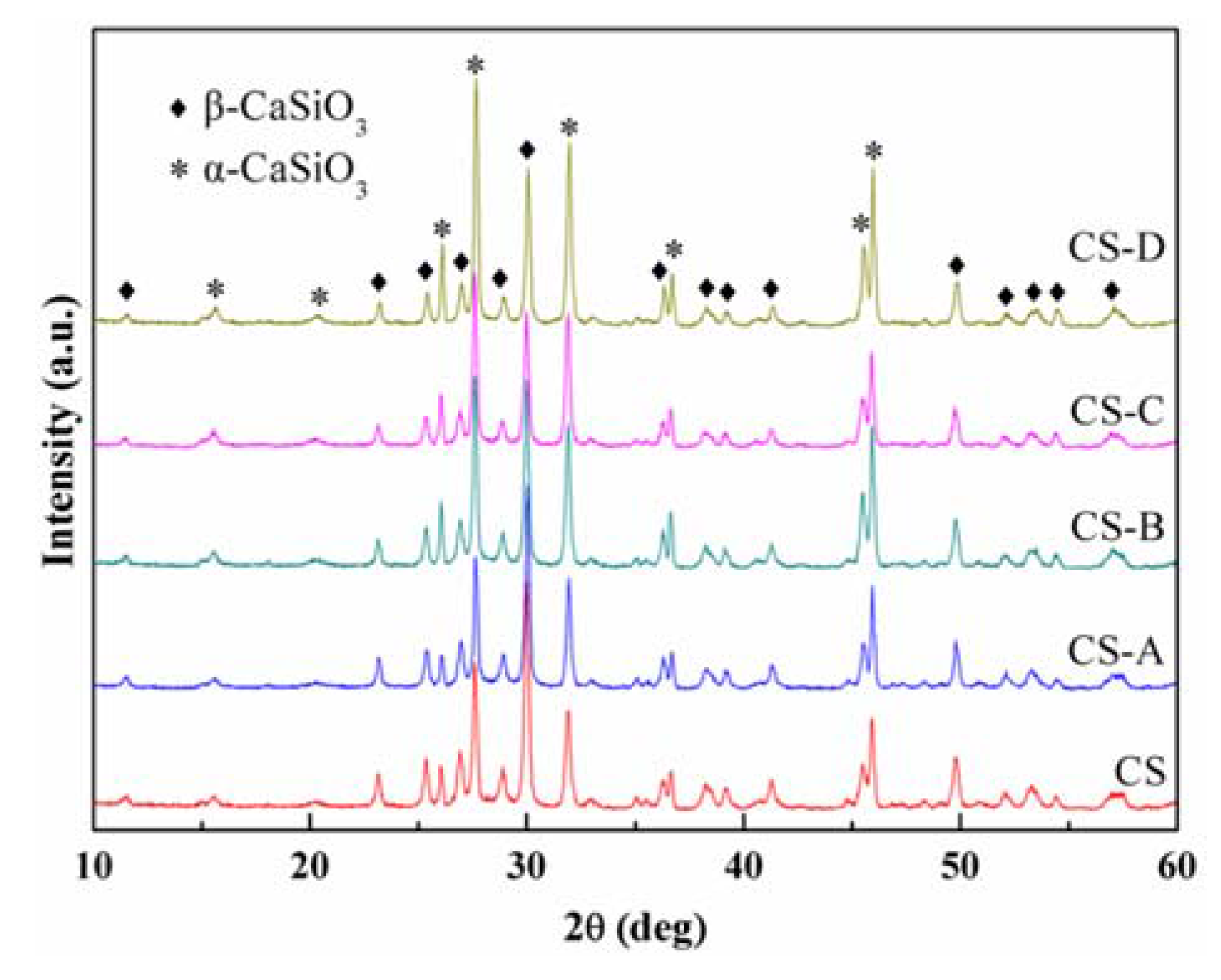
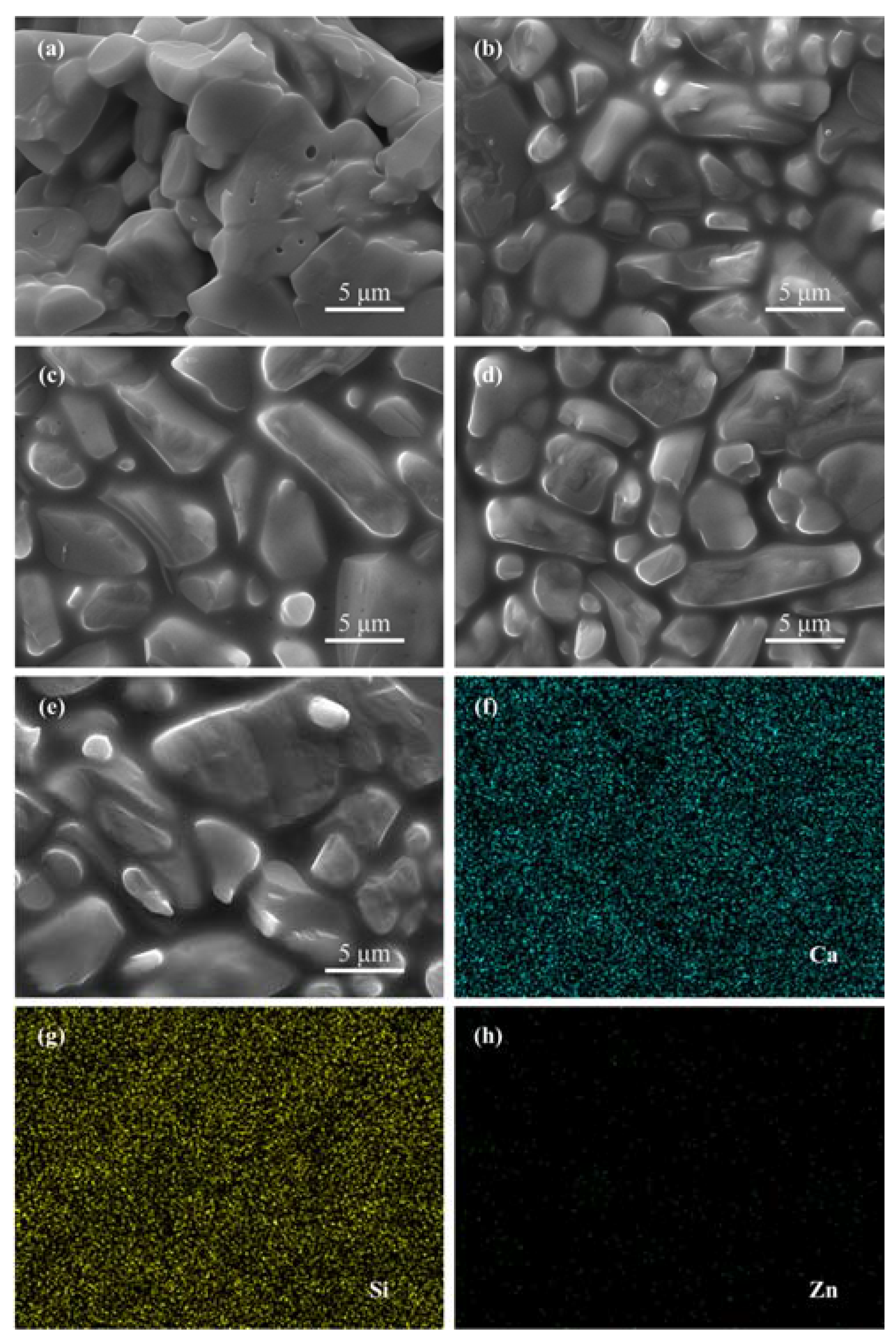
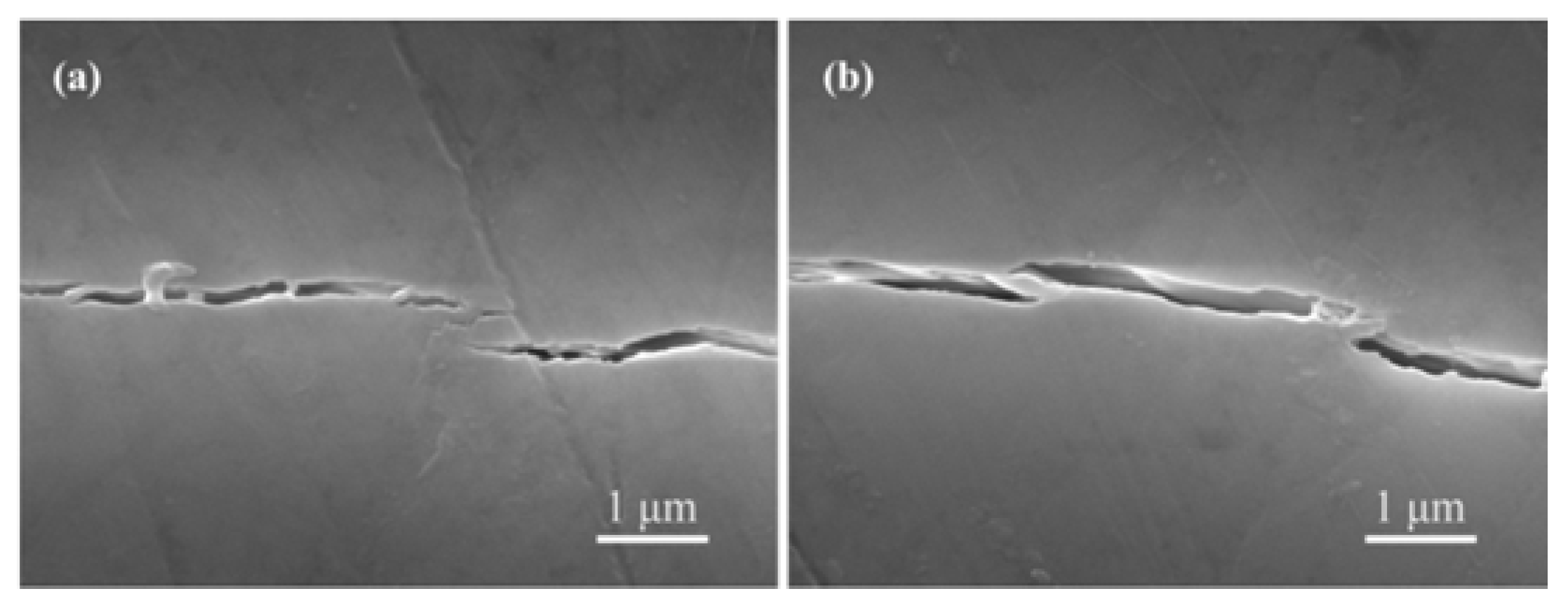
3.1. Sintering Behavior
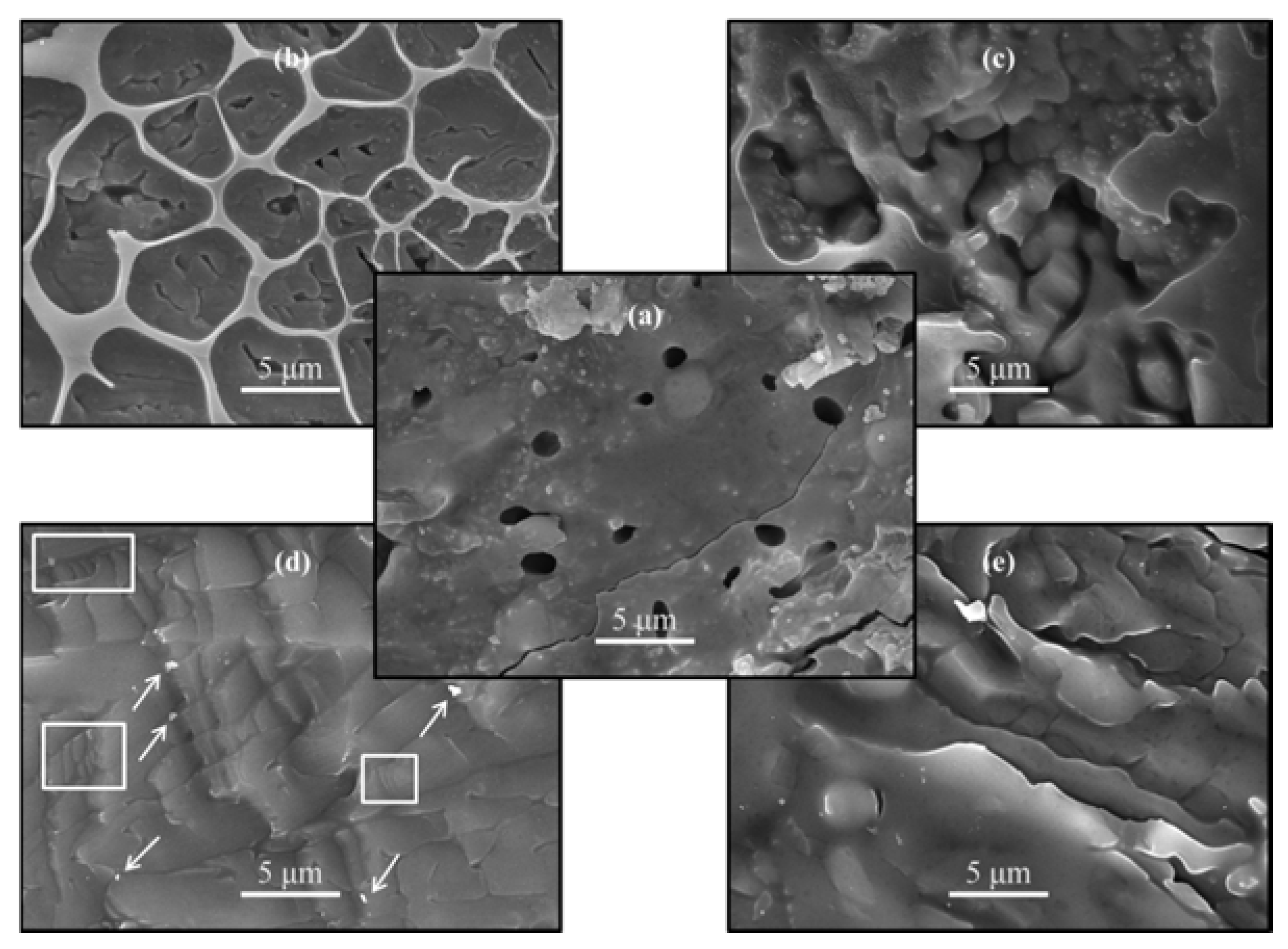
3.2. Mechanical Properties
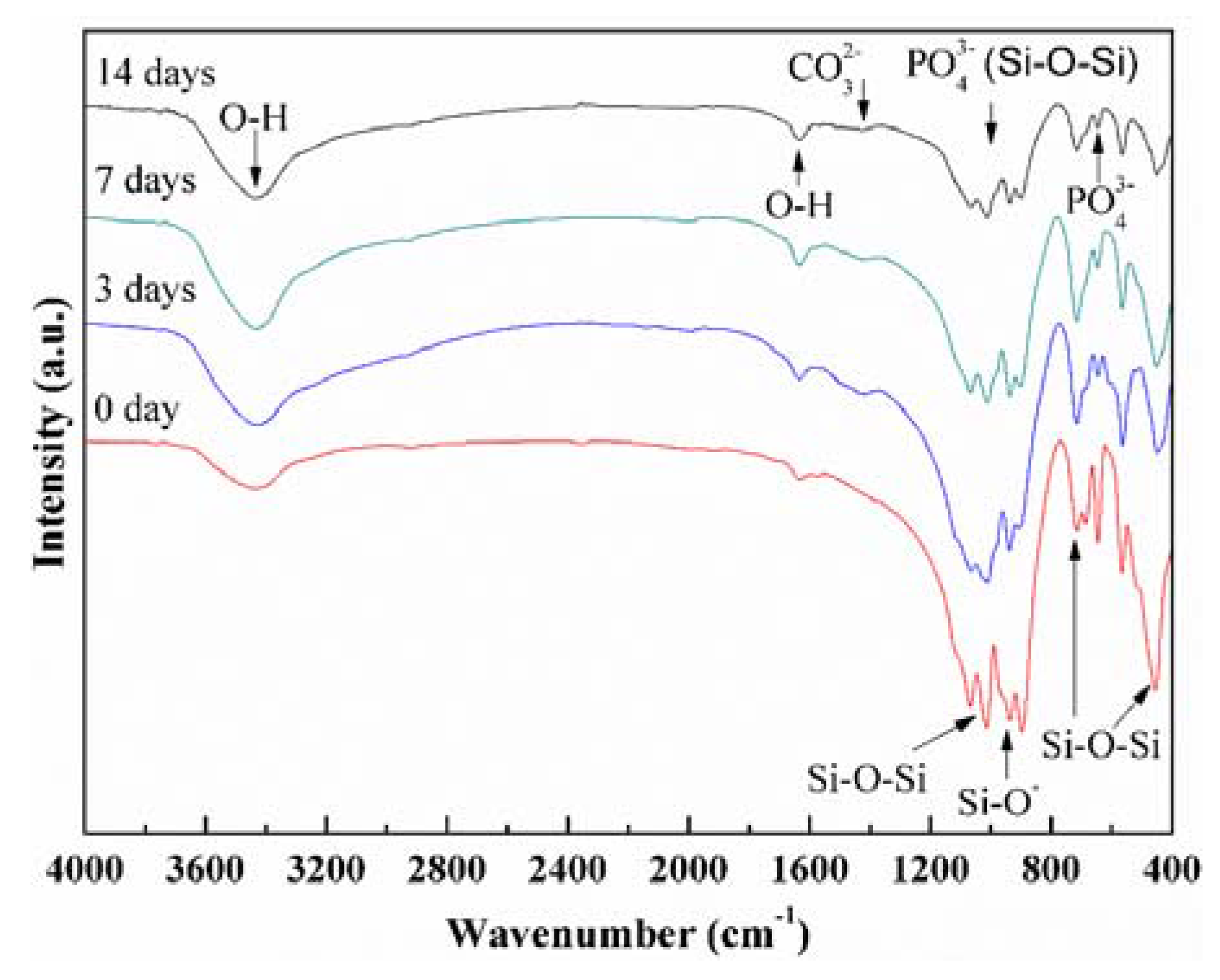
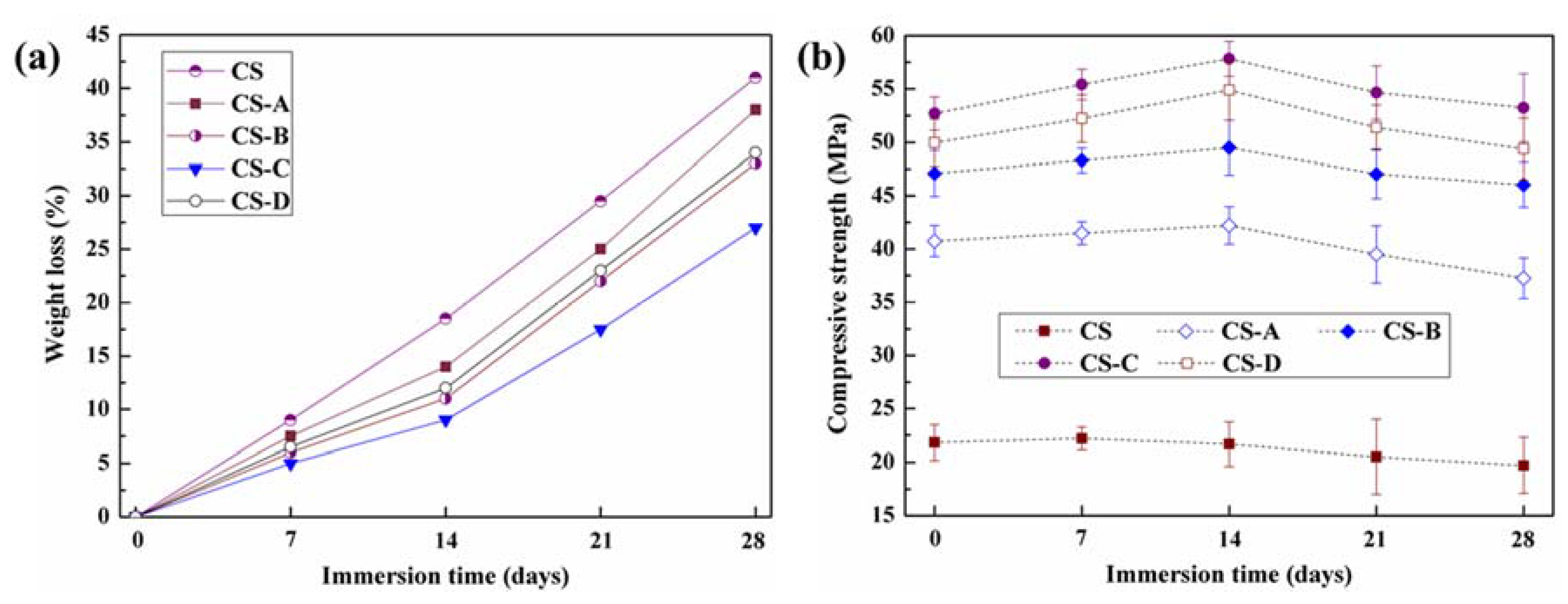
3.3. Bioactivity and Biodegradability
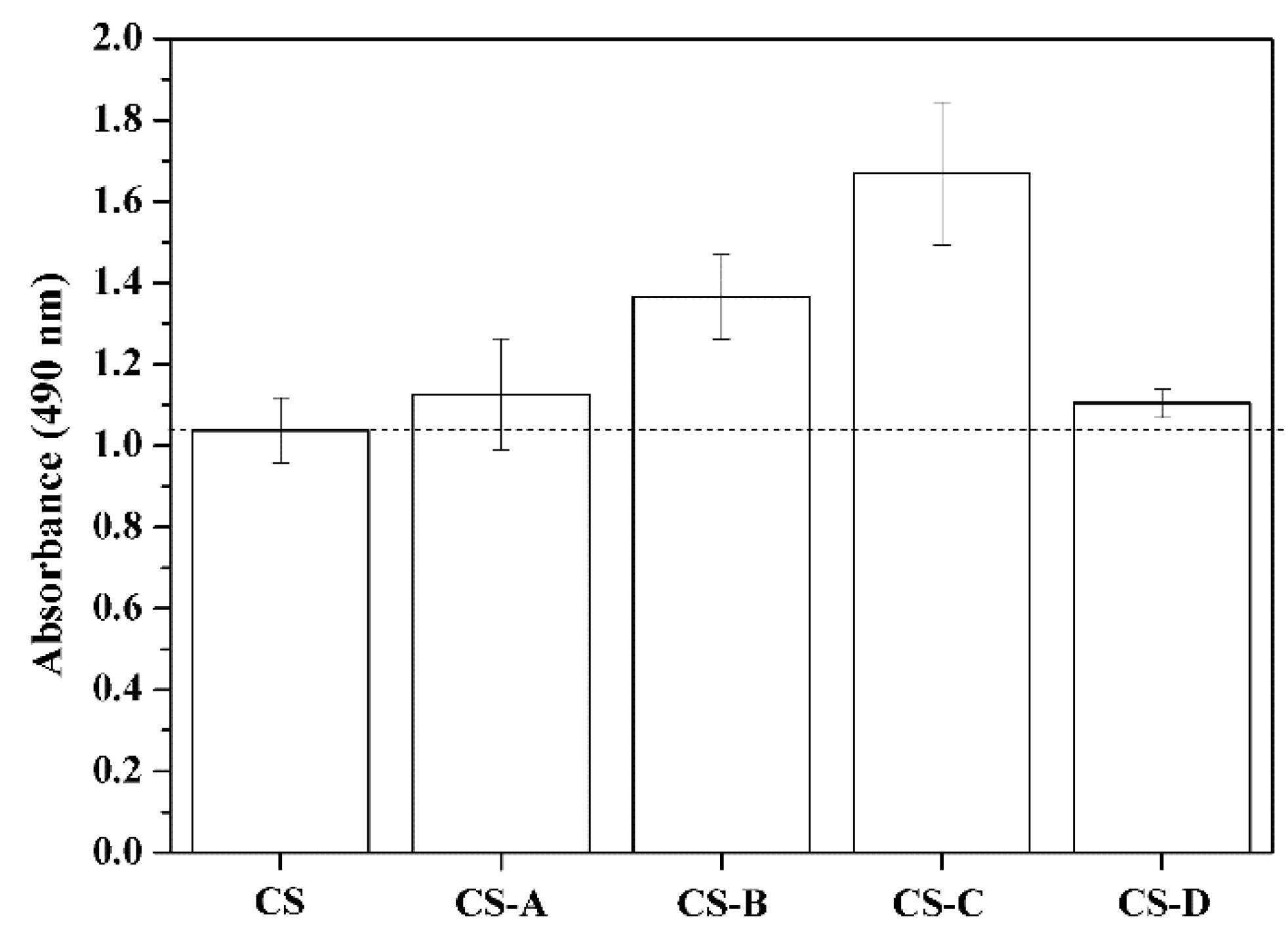
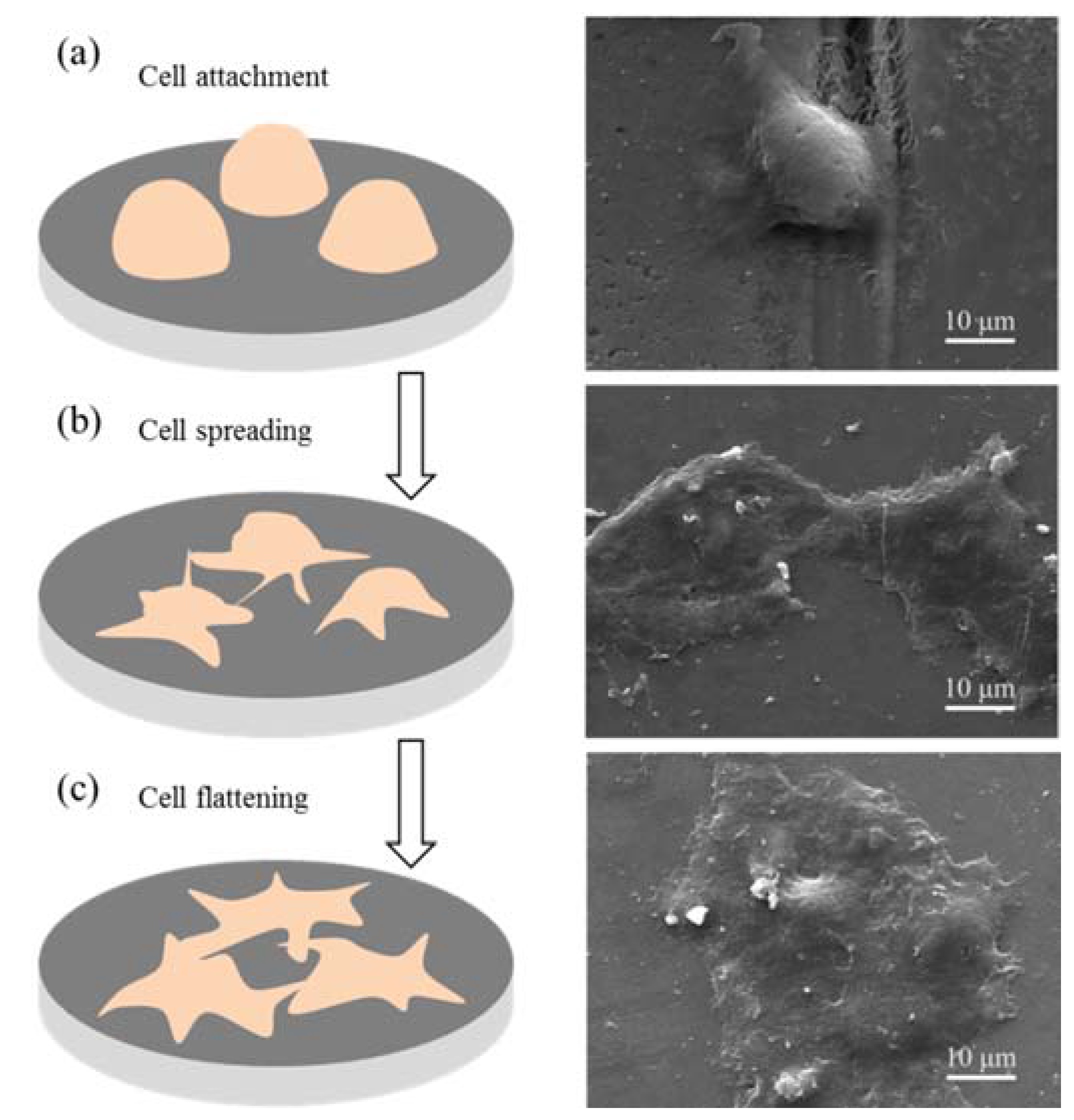
3.4. Cytocompatibility and Cell Viability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Salinas, A.J.; Vallet-Regí, M. Bioactive ceramics: From bone grafts to tissue engineering. RSC Adv. 2013, 3, 11116–11131. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Ciapetti, G.; Taddei, P.; Perut, F.; Tinti, A.; Cardoso, M.V.; Van, M.B.; Prati, C. Apatite formation on bioactive calcium-silicate cements for dentistry affects surface topography and human marrow stromal cells proliferation. Dent. Mater. 2010, 26, 974–992. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Chen, C.; Tsai, X.T. Visualizing the Knowledge Domain of Nanoparticle Drug Delivery Technologies: A Scientometric Review. Appl. Sci. 2016, 6, 11. [Google Scholar] [CrossRef]
- Ni, S.; Chang, J.; Chou, L. A novel bioactive porous CaSiO3 scaffold for bone tissue engineering. J. Biomed. Mater. Res. A 2006, 76, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Mehrali, M.; Shirazi, S.F.S.; Baradaran, S.; Mehrali, M.; Metselaar, H.S.C.; Kadri, N.A.B.; Osman, N.A.A. Facile synthesis of calcium silicate hydrate using sodium dodecyl sulfate as a surfactant assisted by ultrasonic irradiation. Ultrason. Sonochem. 2014, 21, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Mehrali, M.; Moghaddam, E.; Shirazi, S.F.S.; Baradaran, S.; Mehrali, M.; Latibari, S.T.; Metselaar, H.S.C.; Kadri, N.A.; Zandi, K.; Osman, N.A.A. Synthesis, mechanical properties, and in vitro biocompatibility with osteoblasts of calcium silicate-reduced graphene oxide composites. ACS Appl. Mater. Interfaces 2014, 6, 3947–3962. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wu, C.; Lin, K.; Chang, J. The effect of poly(lactic-co-glycolic acid) (PLGA) coating on the mechanical, biodegradable, bioactive properties and drug release of porous calcium silicate scaffolds. Biomed. Mater. Eng. 2012, 22, 289–300. [Google Scholar] [PubMed]
- Vladescu, A.; Birlik, I.; Braic, V.; Toparli, M.; Celik, E.; Azem, F.A. Enhancement of the mechanical properties of hydroxyapatite by SiC addition. J. Mech. Behav. Biomed. Mater. 2014, 40, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Kantana, W.; Jarupoom, P.; Pengpat, K.; Eitssayeam, S.; Tunkasiri, T.; Rujijanagul, G. Properties of hydroxyapatite/zirconium oxide nanocomposites. Ceram. Int. 2013, 39, S379–S382. [Google Scholar] [CrossRef]
- Shirazi, F.S.; Mehrali, M.; Oshkour, A.A.; Metselaar, H.S.C.; Kadri, N.A.; Osman, N.A.A. Mechanical and physical properties of calcium silicate/alumina composite for biomedical engineering applications. J. Mech. Behav. Biomed. Mater. 2014, 30, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Ali, A.A.; Mahmoud, D.A.R.; El-Fiqi, A.M. Preparation and characterization of antibacterial P2O5-CaO-Na2O-Ag2O glasses. J. Biomed. Mater. Res. A 2011, 98, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-zadeh, K.; Ou, J.Z.; Daeneke, T.; Mitchell, A.; Sasaki, T.; Fuhrer, M.S. Two dimensional and layered transition metal oxides. Appl. Mater. Today 2016, 5, 73–89. [Google Scholar] [CrossRef]
- Aly, I.H.M.; Mohammed, L.A.A.; Al-Meer, S.; Elsaid, K.; Barakat, N.A.M. Preparation and characterization of wollastonite/titanium oxide nanofiber bioceramic composite as a future implant material. Ceram. Int. 2016, 42, 11525–11534. [Google Scholar] [CrossRef]
- Bewerse, C.; Brinson, L.C.; Dunand, D.C. NiTi with 3D-interconnected microchannels produced by liquid phase sintering and electrochemical dissolution of steel tubes. J. Mater. Process. Technol. 2014, 214, 1895–1899. [Google Scholar] [CrossRef]
- Lin, K.; Chang, J.; Liu, Z.; Zeng, Y.; Shen, R. Fabrication and characterization of 45S5 bioglass reinforced macroporous calcium silicate bioceramics. J. Eur. Ceram. Soc. 2009, 29, 2937–2943. [Google Scholar] [CrossRef]
- Parkhomey, O.; Pinchuk, N.; Sych, O.; Tomila, T.; Kuda, O.; Tovstonoh, H.; Gorban’, V.; Kolesnichenko, V.; Yan, E. Effect of particle size of starting materials on the structure and properties of biogenic hydroxyapatite/glass composites. Process. Appl. Ceram. 2016, 10, 1–8. [Google Scholar] [CrossRef]
- Silva, M.J.D.; Bartolomé, J.F.; Aza, A.H.D.; Mello-Castanho, S. Glass ceramic sealants belonging to BAS (BaO-Al2O3-SiO2) ternary system modified with B2O3 addition: A different approach to access the SOFC seal issue. J. Eur. Ceram. Soc. 2016, 36, 631–644. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Ke, X.; Wang, J.; Yang, G.; Yang, X.; He, D.; Shao, H.; He, Y.; Fu, J. 45S5 Bioglass analogue reinforced akermanite ceramic favorable for additive manufacturing mechanically strong scaffolds. RSC Adv. 2015, 5, 102727–102735. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Chen, X.; Sun, X.; Yang, G.; Guo, X.; Yang, H.; Gao, C.; Gou, Z. Incorporation of B2O3 in CaO-SiO2-P2O5 bioactive glass system for improving strength of low-temperature co-fired porous glass ceramics. J. Non-Cryst. Solids 2012, 358, 1171–1179. [Google Scholar] [CrossRef]
- Dzondogadet, M.; Mayapnzietchueng, R.; Hess, K.; Nabet, P.; Belleville, F.; Dousset, B. Action of boron at the molecular level. Biol. Trace Elem. Res. 2002, 85, 23–33. [Google Scholar] [CrossRef]
- Hakki, S.S.; Bozkurt, B.S.; Hakki, E.E. Boron regulates mineralized tissue-associated proteins in osteoblasts (MC3T3-E1). J. Trace Elem. Med. Biol. 2010, 24, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Carbajal, L.; Serena, S.; Caballero, A.; Saínz, M.A.; Detsch, R.; Boccaccini, A.R. Role of ZnO additions on the β/α phase relation in TCP based materials: Phase stability, properties, dissolution and biological response. J. Eur. Ceram. Soc. 2014, 34, 1375–1385. [Google Scholar] [CrossRef]
- Ito, A.; Kawamura, H.; Otsuka, M.; Ikeuchi, M.; Ohgushi, H.; Ishikawa, K.; Onuma, K.; Kanzaki, N.; Yu, S.; Ichinose, N. Zinc-releasing calcium phosphate for stimulating bone formation. Mater. Sci. Eng. C 2002, 22, 21–25. [Google Scholar] [CrossRef]
- Pan, Y.J.; Lin, J.H.; Chiang, K.C. Biomedical Applications of Antibacterial Nanofiber Mats Made of Electrospinning with Wire Electrodes. Appl. Sci. 2016, 6, 46. [Google Scholar] [CrossRef]
- Minaev, V.S.; Petrova, V.Z.; Timoshenkov, S.P.; Khafizov, R.R.; Sharagov, V.A. Glass Formation and glass-forming ability of melts in AIIO-B2O3 binary systems (AIIO = BeO, MgO, CaO, SrO, BaO, ZnO, CdO). Glass Phys. Chem. 2004, 30, 215–225. [Google Scholar] [CrossRef]
- Shuai, C.; Gao, C.; Nie, Y.; Hu, H.; Qu, H.; Peng, S. Structural design and experimental analysis of a selective laser sintering system with nano-hydroxyapatite powder. J. Biomed. Nanotechnol. 2010, 6, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Feng, P.; Gao, C.; Xiao, T.; Yu, K.; Shuai, C.; Peng, S. Microstructure evolution and mechanical properties improvement in liquid-phase-sintered hydroxyapatite by laser sintering. Materials 2015, 8, 1162–1175. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; O′Brien, F.J. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adhes. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef]
- Fedorovich, N.E.; Alblas, J.; Hennink, W.E.; Öner, F.C.; Dhert, W.J.A. Organ printing: The future of bone regeneration? Trends Biotechnol. 2011, 29, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Ning, C.; Lin, K.; Chen, L. Preparation and characterization of PLLA/CaSiO3/apatite composite films. Int. J. Appl. Ceram. Technol. 2012, 9, 133–142. [Google Scholar] [CrossRef]
- Ni, S.; Lin, K.; Chang, J.; Chou, L. Beta-CaSiO3/beta-Ca3(PO4)2 composite materials for hard tissue repair: In vitro studies. J. Biomed. Mater. Res. A 2008, 85, 72–82. [Google Scholar] [CrossRef] [PubMed]
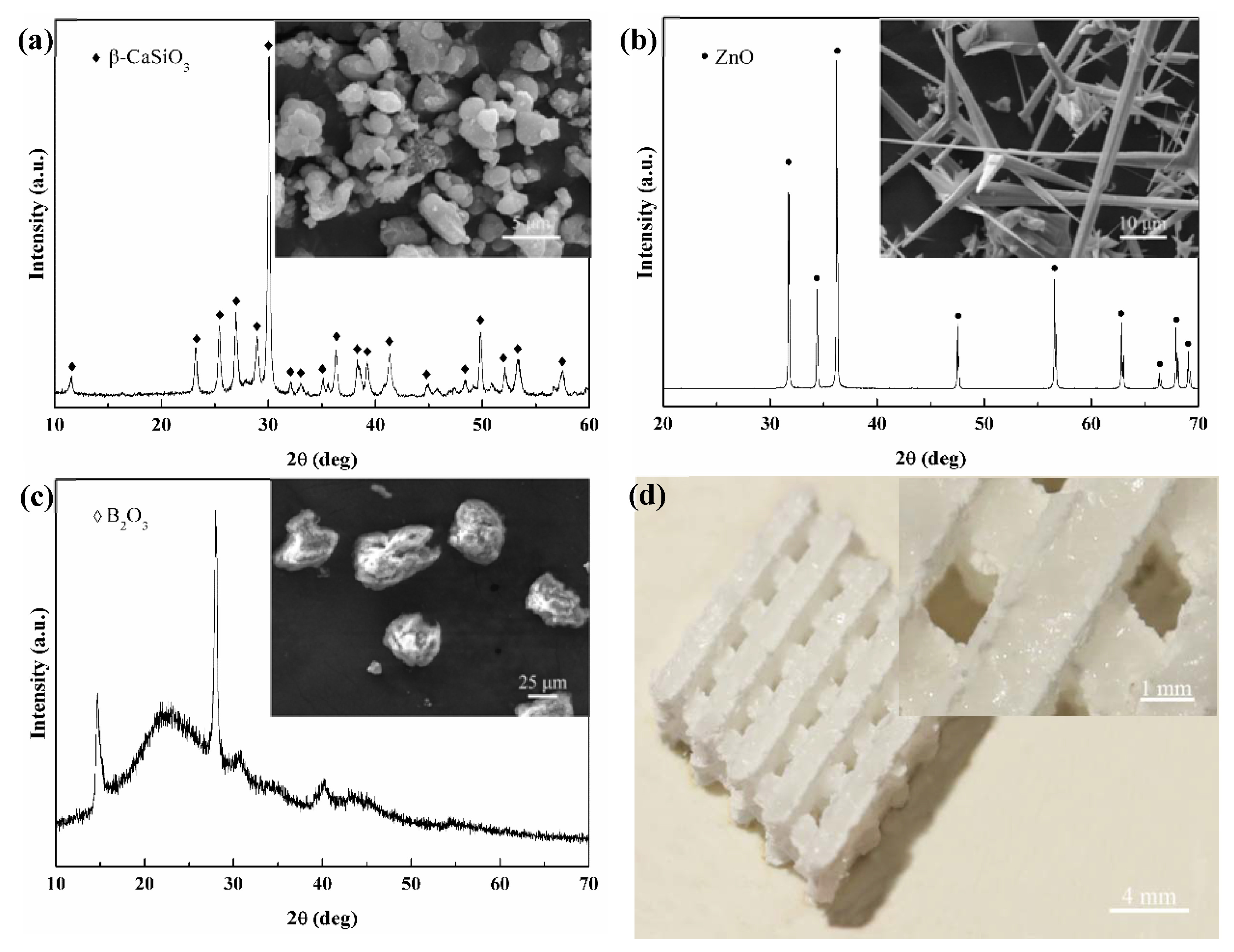

| Group | Compositions of Scaffolds | Compositions of Liquid Phase | ||
|---|---|---|---|---|
| CaSiO3 (wt %) | Liquid Phase (wt %) | B2O3 (wt %) | ZnO (wt %) | |
| CS | 100 | 0 | 0 | 0 |
| CS-A | 97 | 3 | 47 | 53 |
| CS-B | 97 | 3 | 40 | 60 |
| CS-C | 97 | 3 | 35 | 65 |
| CS-D | 97 | 3 | 25 | 75 |
| Group | CS | CS-A | CS-B | CS-C | CS-D |
|---|---|---|---|---|---|
| Hardness (GPa) | 4.95 ± 0.51 | 5.54 ± 0.46 | 6.03 ± 0.29 | 6.57 ± 0.39 | 6.71 ± 0.47 |
| Fracture toughness (MPa·m1/2) | 1.06 ± 0.12 | 1.214 ± 0.18 | 1.46 ± 0.14 | 1.57 ± 0.15 | 1.56 ± 0.13 |
| Compressive strength (MPa) | 21.82 ± 1.67 | 40.75 ± 1.46 | 47.09 ± 2.16 | 52.69 ± 1.54 | 49.97 ± 2.21 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuai, C.; Duan, S.; Gao, D.; Wu, P.; Gao, C.; Yang, Y.; Liu, L.; Yuan, F.; Yang, S.; Feng, P. Mechanically Strong CaSiO3 Scaffolds Incorporating B2O3-ZnO Liquid Phase. Appl. Sci. 2017, 7, 387. https://doi.org/10.3390/app7040387
Shuai C, Duan S, Gao D, Wu P, Gao C, Yang Y, Liu L, Yuan F, Yang S, Feng P. Mechanically Strong CaSiO3 Scaffolds Incorporating B2O3-ZnO Liquid Phase. Applied Sciences. 2017; 7(4):387. https://doi.org/10.3390/app7040387
Chicago/Turabian StyleShuai, Cijun, Songlin Duan, Dan Gao, Ping Wu, Chengde Gao, Youwen Yang, Long Liu, Fulai Yuan, Sheng Yang, and Pei Feng. 2017. "Mechanically Strong CaSiO3 Scaffolds Incorporating B2O3-ZnO Liquid Phase" Applied Sciences 7, no. 4: 387. https://doi.org/10.3390/app7040387






