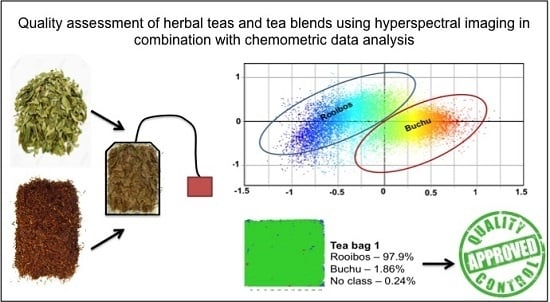
Hyperspectral Imaging as a Rapid Quality Control Method for Herbal Tea Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. SWIR Hyperspectral Imaging Analysis
2.2.1. Image Acquisition
2.2.2. Principal Component Analysis (PCA)
2.2.3. Partial Least Squares Discriminant Analysis
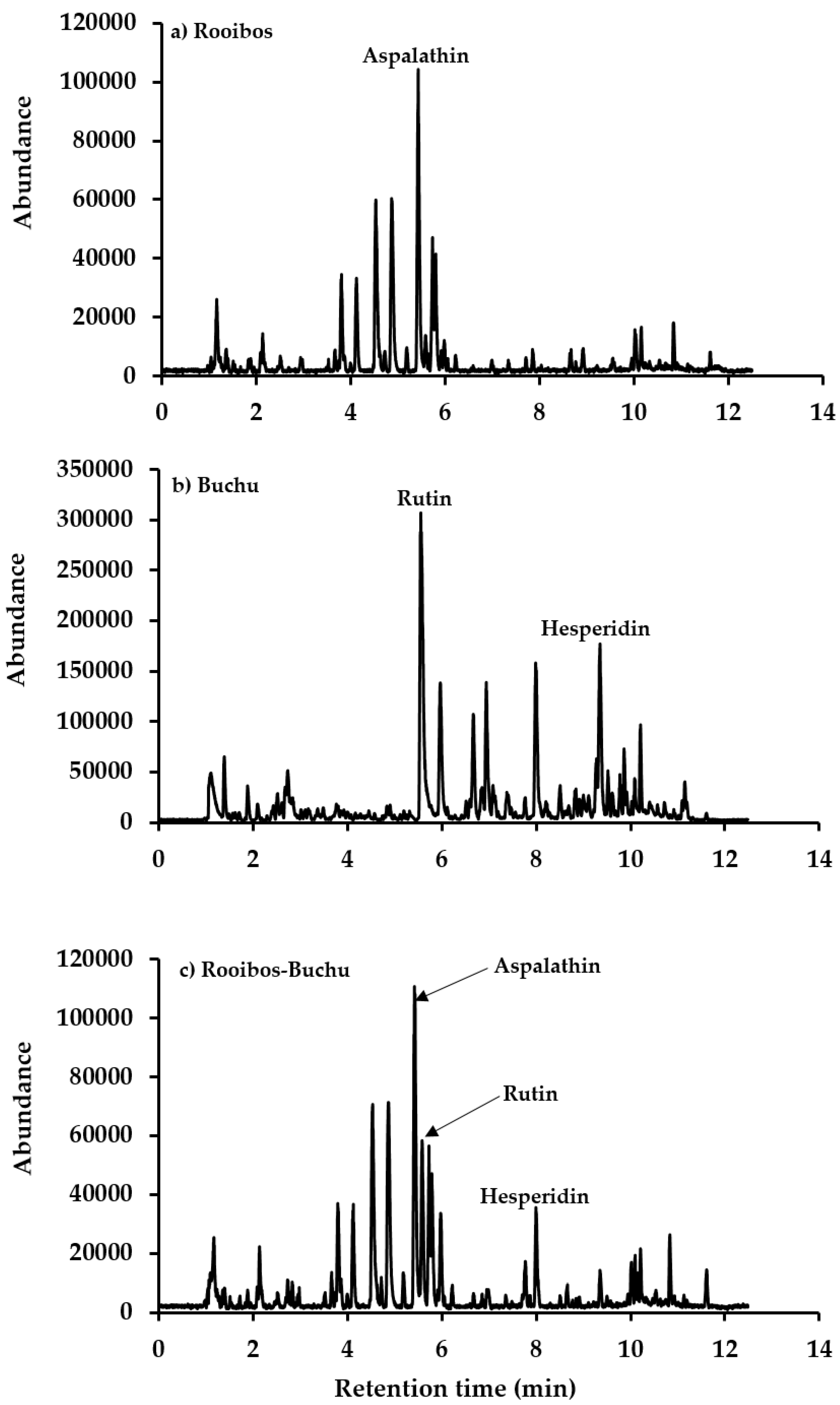
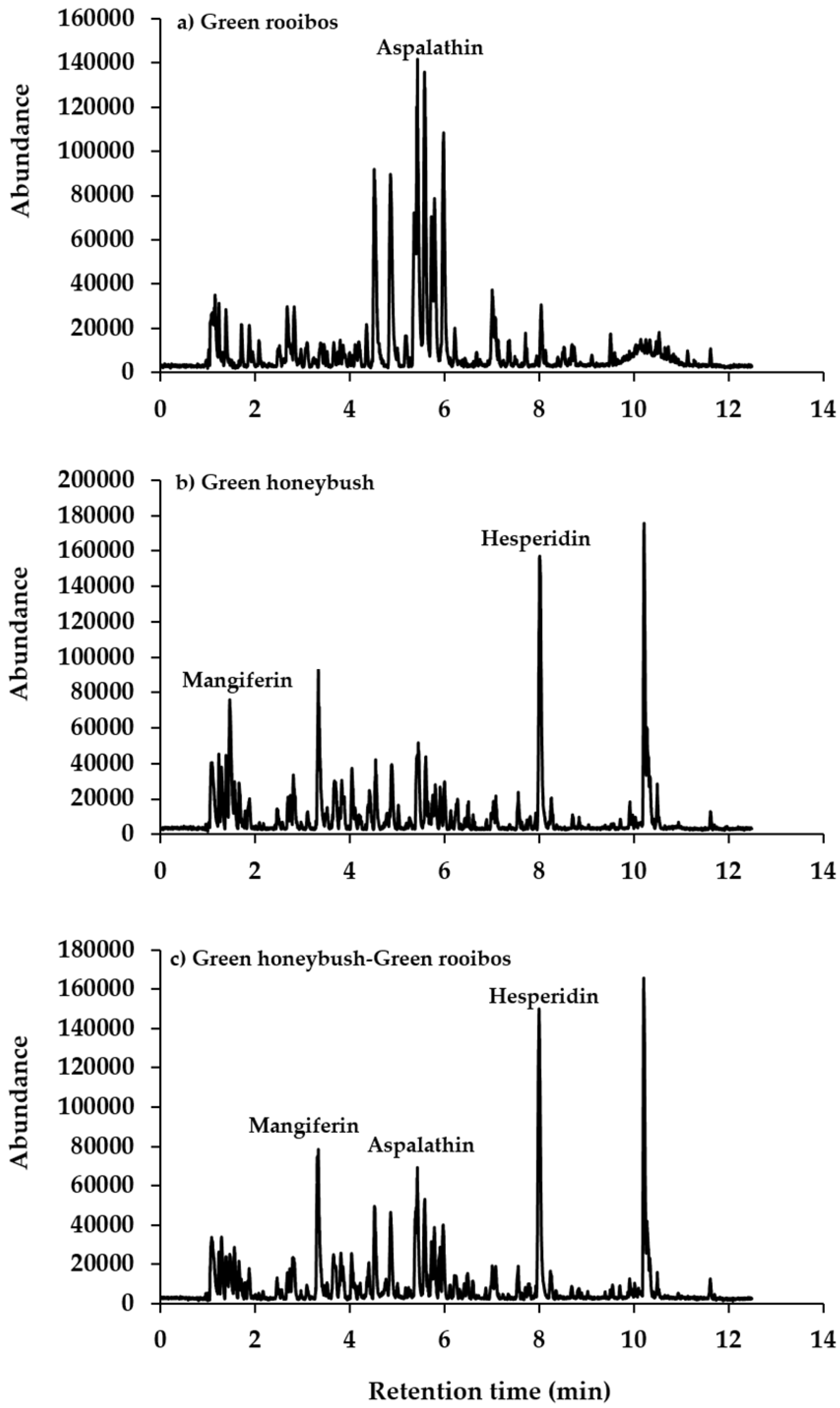
2.3. Ultra-High Performance Liquid Chromatography Mass Spectrometry (UHPLC-MS) Analysis
3. Results and Discussion
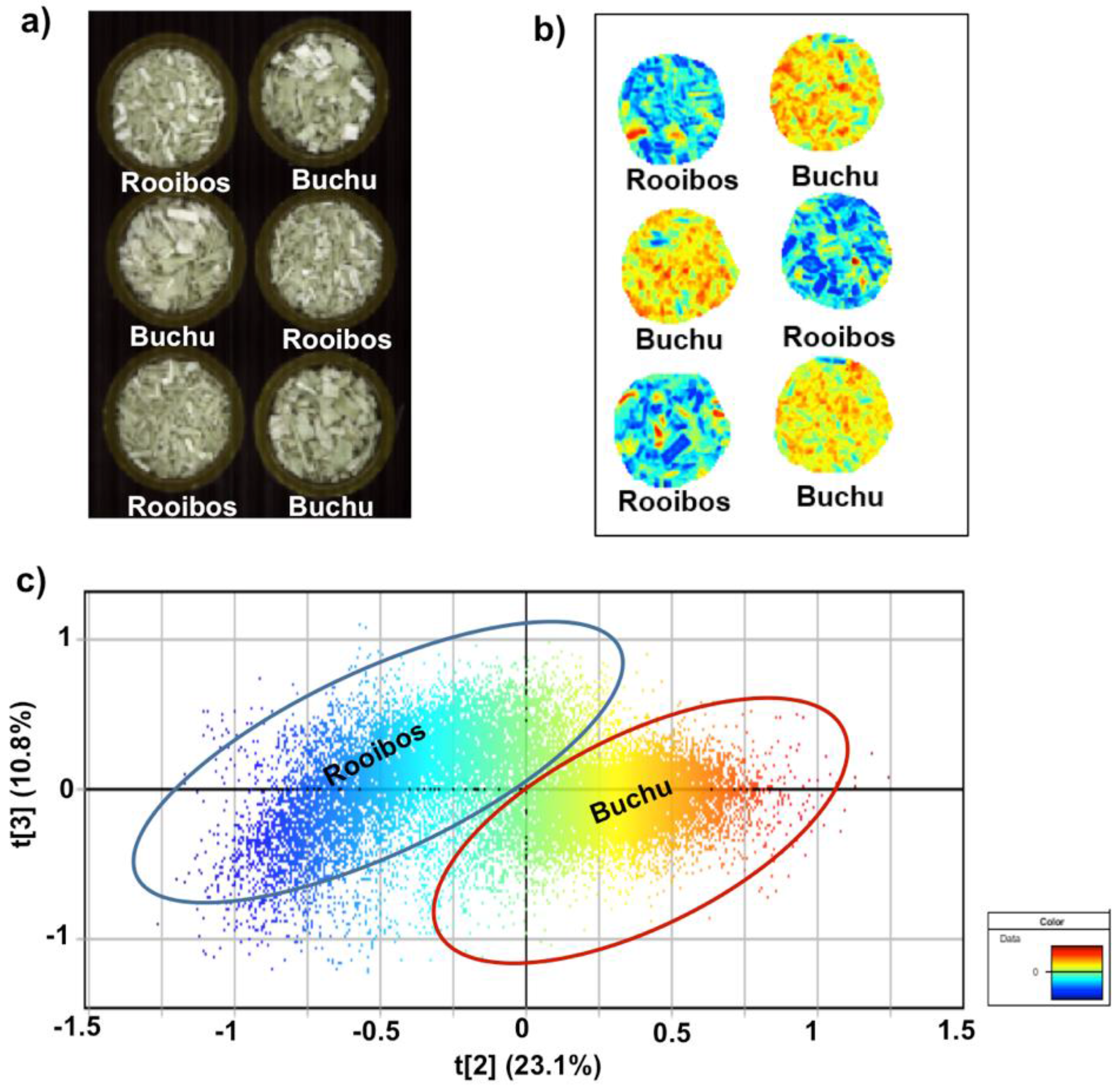
3.1. Rooibos and Buchu Blend
3.2. Green Rooibos and Green Honeybush Blend
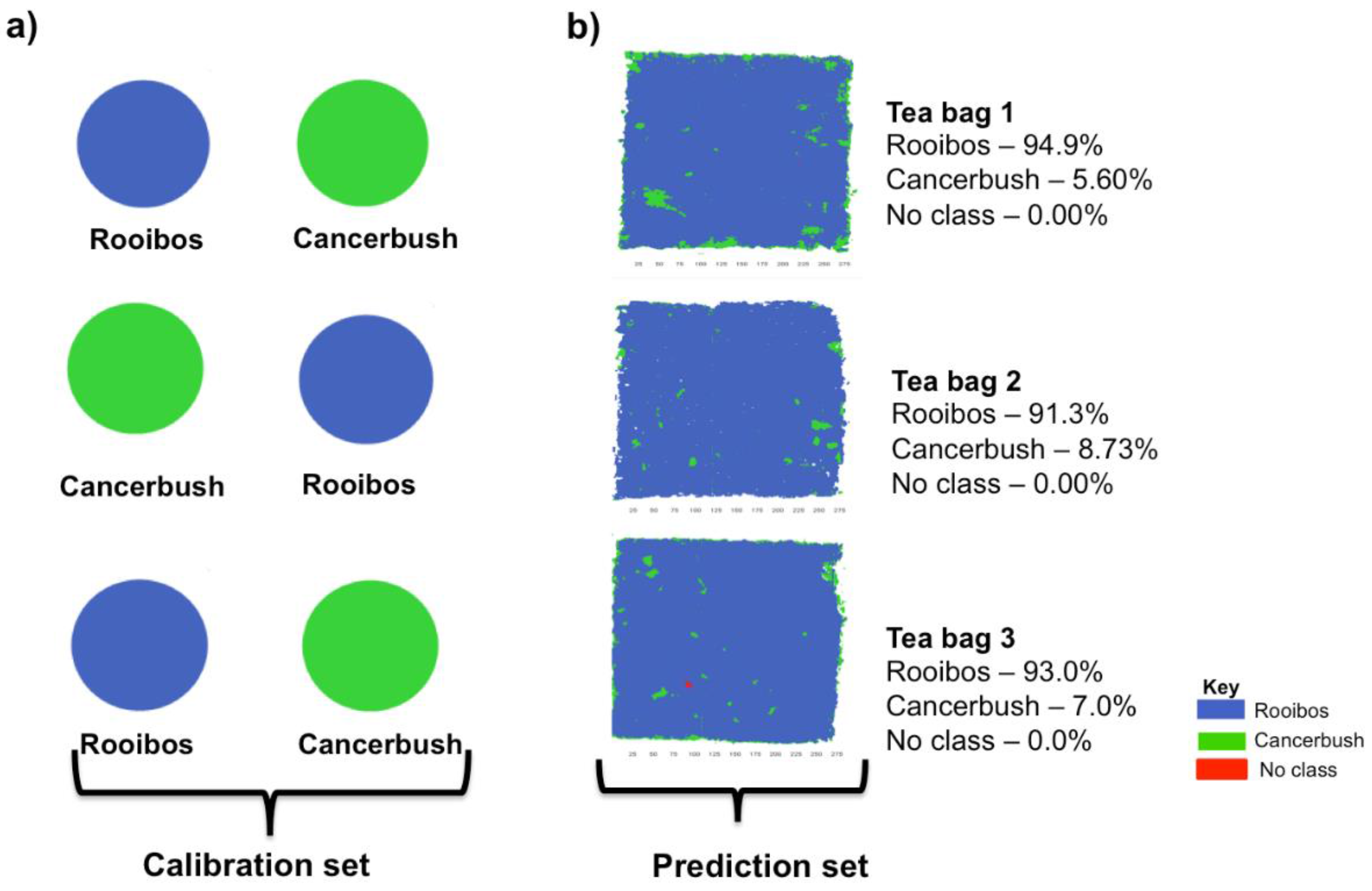
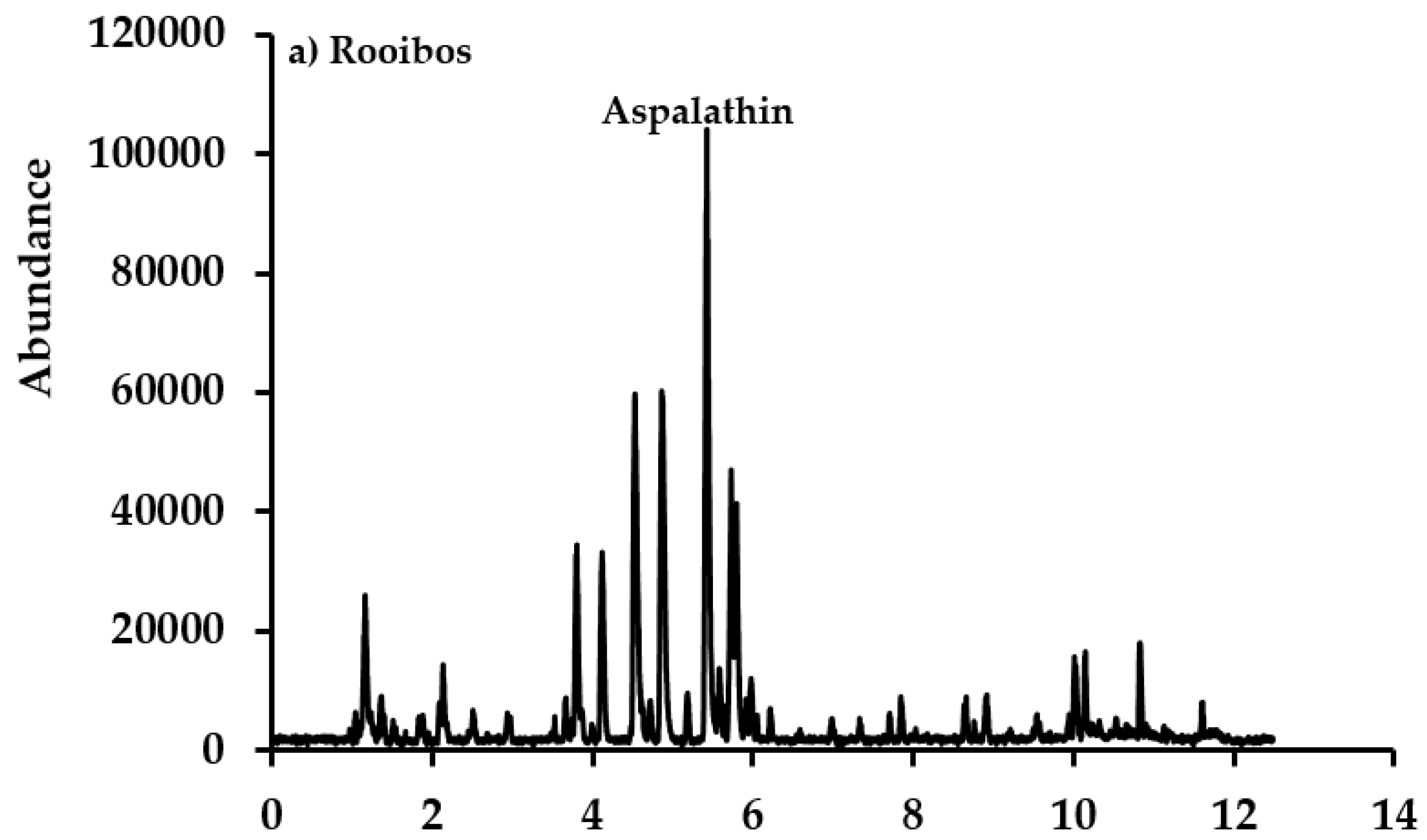
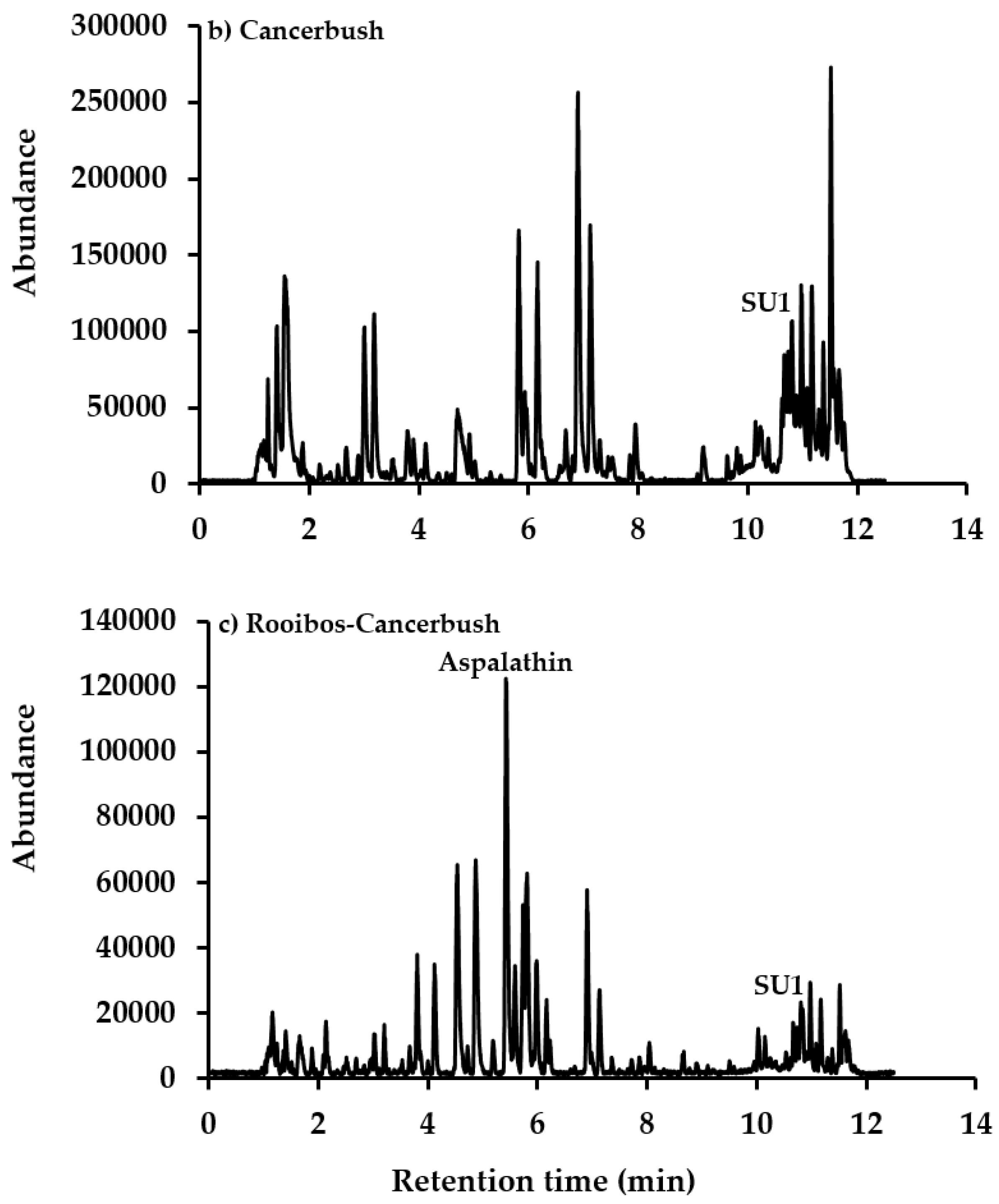
3.3. Rooibos and Cancer Bush Blend
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Keating, B.; Lindstrom, A.; Lynch, M.E.; Blumenthal, M. Sales of Tea & Herbal Tea Increase 5.9% in United States in 2013. HerbalEGram 2014, 11. Available online: http://cms.herbalgram.org/heg/volume11/11November/TeaMarketReport.html?ts=1415729734&signature=a7e7b30e947490d53d11df28f1d4bd61 (accessed on 26 June 2016). [Google Scholar]
- Ferdman, R.A. Where the World’s Biggest Tea Drinkers Are. 2014. Available online: http://qz.com/168690/where-the-worlds-biggest-tea-drinkers-are/ (accessed on 27 June 2016).
- Xanthopoulou, A.; Ganopoulos, L.; Kalivas, A.; Osathanunkul, M.; Chatzopoulou, P.; Tsaftaris, A.; Madesis, P. Multiplex HRM analysis as a tool for rapid molecular authentication of nine herbal teas. Food Control 2015, 60, 113–116. [Google Scholar] [CrossRef]
- Zegarac, J.P.; Samec, D.; Piljac, A. Herbal teas: A focus on antioxidant properties. In Tea in Health and Disease Prevention; Preedy, V.R., Ed.; E-Publishing Inc.: London, UK, 2013; pp. 129–140. [Google Scholar]
- Viljoen, A.M.; Vermaak, I. Special Issue—Quality control. S. Afr. J. Bot. 2012, 82, 1–3. [Google Scholar] [CrossRef]
- Cano, J.H.; Volpato, V. Herbal mixtures in the traditional medicine of Eastern Cuba. J. Ethnopharmacol. 2004, 90, 293–316. [Google Scholar] [CrossRef] [PubMed]
- Joubert, E.; De Beer, D. Rooibos (Aspalathus linearis) beyond the farm gate: From herbal tea to potential phytopharmaceutical. S. Afr. J. Bot. 2011, 77, 869–886. [Google Scholar] [CrossRef]
- Van Wyk, B.E.; Van Oudtshoorn, B.; Gericke, N. Medicinal Plants of South Africa, 1st ed.; Briza Publications: Pretoria, South Africa, 1997; p. 290. [Google Scholar]
- Watt, J.M.; Breyer-Brandwijk, M.G. The Medicinal and Poisonous Plants of Southern Africa, 1st ed.; E&S Livingstone: Edinburgh, UK, 1932; p. 70. [Google Scholar]
- He, X.; Li, J.; Zhao, W.; Liu, R.; Zhang, L.; Kong, X. Chemical fingerprint analysis for quality control and identification of Ziyang green tea by HPLC. Food Chem. 2015, 171, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, F.; Yang, F.; Fang, Y.; Xin, Z.; Zhao, L.; Hu, Q. Size effect of Se-enriched green tea particles on in vitro antioxidant and antitumor Activities. J. Agric. Food Chem. 2008, 56, 4529–4533. [Google Scholar] [CrossRef] [PubMed]
- Newmaster, S.G.; Grguric, M.; Shanmughanandhan, D.; Ramalingam, S.; Ragupathy, S. DNA barcoding detects contamination and substitution in North American herbal products. BMC Med. 2013, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Vermaak, I.; Viljoen, A.; Lindström, S.W. Hyperspectral imaging in the quality control of herbal medicines—The case of neurotoxic Japanese star anise. J. Pharm. Biomed. Anal. 2013, 75, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Yang, Y. Chemical fingerprint analysis for quality assessment and control of Bansha herbal tea using paper spray mass spectrometry. Anal. Chim. Acta 2013, 785, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.Z.; An, Y.Q.; Jiang, X.L.; Tang, D.Q.; Gao, Y.Y.; Zhao, H.T.; WU, X.W. Development of a novel method combining HPLC fingerprint and multi-ingredients quantitative analysis for quality evaluation of traditional chinese medicine preparation. Talanta 2011, 85, 885. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.T.; Xie, P.S.; Liu, H.P. Evaluation of traditional Chinese herbal medicine: Chaihu (Bupleuri Radix) by both high-performance liquid chromatographic and high-performance thin-layer chromatographic fingerprint and chemometric analysis. J. Chromatogr. A 2009, 1216, 2150–2155. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhao, J.; Chaitep, S.; Guo, Z. Simultaneous analysis of main catechins contents in green tea (Camellia sinensis (L.)) by Fourier transform near infrared reflectance (FT-NIR) spectroscopy. Food Chem. 2009, 113, 1272–1277. [Google Scholar] [CrossRef]
- Hall, M.N.; Robertson, A.; Scotter, C.N.G. Near-infrared reflectance prediction of quality, theaflavin content and moisture content of black tea. Food Chem. 1988, 27, 61–75. [Google Scholar] [CrossRef]
- Luypaert, J.; Zhang, M.H.; Massart, D.L. Feasibility study for the using near infrared spectroscopy in the qualitative and quantitative of green tea, Camellia sinensis (L.). Anal. Chim. Acta 2003, 487, 303–312. [Google Scholar] [CrossRef]
- Zhang, M.H.; Luypaert, J.; Xu, Q.S.; Massart, D.L. Determination of total antioxidant capacity in green tea by NIRS and multivariate calibration. Talanta 2004, 62, 25–35. [Google Scholar] [CrossRef]
- Budı́nová, G.; Vláčil, D.; Mestek, O.; Volka, K. Application of infrared spectroscopy to the assessment of authenticity of tea. Talanta 1998, 47, 255–260. [Google Scholar] [CrossRef]
- Sandasi, M.; Vermaak, I.; Chen, W.; Viljoen, A.M. Hyperspectral imaging and chemometric modelling of Echinacea—A novel approach in the quality control of herbal medicines. Molecules 2014, 19, 13104–13121. [Google Scholar] [CrossRef] [PubMed]
- Kelman, T.; Ren, J.; Marshall, S. Effective classification of Chinese tea samples in hyperspectral imaging. Artif. Intell. Res. 2013, 2, 87–96. [Google Scholar] [CrossRef]
- Qin, J.; Chao, K.; Kim, M.S.; Lu, R.; Burks, T.F. Hyperspectral and multispectral imaging for evaluating food safety and quality. J. Food Eng. 2013, 118, 157–171. [Google Scholar] [CrossRef]
- Yu, K.; Zhao, Y.; Li, X.; Shao, Y.; Zhu, F.; He, Y. Identification of crack features in fresh jujube using Vis/NIR hyperspectral imaging combined with image processing. Comput. Electron. Agric. 2014, 103, 1–10. [Google Scholar] [CrossRef]
- Shikanga, A.E.; Viljoen, A.M.; Vermaak, I.; Combrinck, S. A novel approach in herbal quality control using hyperspectral imaging: Discriminating between Sceletium tortuosum and Sceletium crassicaule. Phytochem. Anal. 2013, 24, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Manley, M.; Du Toit, G.; Geladi, P. Tracking diffusion of conditioning water in single wheat kernels of different hardness by near infrared hyperspectral imaging. Anal. Chim. Acta 2011, 686, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Gowen, A.A.; O’Donnell, C.P.; Cullen, P.J.; Downey, G.; Frias, J.M. Hyperspectral imaging—An emerging process analytical tool for food quality and safety control. Trends Food Sci. Technol. 2007, 18, 590–598. [Google Scholar] [CrossRef]
- Loranty, A.; Rembiałkowska, E.; Rosa, E.A.S.; Bennett, R.N. Identification, quantification and availability of carotenoids and chlorophylls in fruit, herb and medicinal teas. J. Food Compos. Anal. 2010, 23, 432–441. [Google Scholar] [CrossRef]
- Tankeu, S.; Vermaak, I.; Chen, W.; Sandasi, M.; Viljoen, A.M. Differentiation between two “fang ji” herbal medicines, Stephania tetrandra and the nephrotoxic Aristolochia fangchi, using hyperspectral imaging. Phytochemistry 2016, 122, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Sandasi, M.; Vermaak, I.; Chen, W.; Viljoen, A.M. Skullcap and Germander: Preventing potential toxicity through the application of hyperspectral imaging and multivariate image analysis as a novel quality control method. Planta Med. 2014, 80, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, Q.; Cai, J.; Ouyang, Q. Automated tea quality classification by hyperspectral imaging. Appl. Opt. 2009, 48, 3557–3564. [Google Scholar] [CrossRef] [PubMed]




| Tea Bag Blend | Raw Material Constituents | Company Formulation (%) | Ave | Std dev | Hyperspectral Imaging Percentage Pixel Abundance in Tea Bag Tea Bags | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |||||
| Rooibos and buchu | Rooibos | 97.00 | 97.09 | ±1.12 | 97.90 | 98.00 | 97.60 | 98.50 | 97.70 | 98.40 | 96.00 | 94.50 | 95.60 | 96.60 | 96.90 | 98.00 | 97.50 | 96.90 | 95.50 | 96.70 | 97.00 | 98.30 |
| Buchu | 3.00 | 2.68 | ±1.05 | 1.86 | 1.71 | 2.22 | 1.47 | 2.08 | 1.24 | 3.68 | 4.98 | 4.05 | 3.13 | 2.96 | 1.79 | 2.44 | 2.69 | 4.24 | 3.12 | 2.82 | 1.70 | |
| No class | - | 0.23 | ±0.14 | 0.23 | 0.27 | 0.15 | 0.08 | 0.21 | 0.38 | 0.35 | 0.57 | 0.36 | 0.26 | 0.16 | 0.20 | 0.09 | 0.41 | 0.23 | 0.01 | 0.23 | 0.04 | |
| Green rooibos and green honeybush | Green honeybush | 65.00 | 80.33 | ±17.52 | 62.30 | 53.60 | 54.80 | 75.10 | 63.10 | 58.30 | 82.50 | 58.70 | 94.10 | 91.50 | 88.80 | 76.60 | 88.80 | 81.20 | 66.70 | 73.30 | 83.70 | 71.40 |
| Green rooibos | 35.00 | 19.47 | ±17.32 | 37.70 | 43.60 | 45.20 | 24.80 | 36.90 | 41.70 | 17.50 | 41.30 | 5.89 | 8.51 | 11.20 | 23.40 | 11.20 | 18.80 | 33.30 | 26.70 | 16.30 | 28.60 | |
| No class | - | 0.06 | ±0.10 | 0.03 | 0.01 | 0.02 | 0.11 | 0.01 | 0.00 | 0.00 | 0.02 | 0.03 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.05 | 0.02 | |
| Rooibos and cancer bush | Rooibos | 96.00 | 94.40 | ±2.85 | 98.60 | 95.70 | 96.10 | 95.30 | 91.3 | 96.30 | 89.40 | 97.70 | 96.60 | 90.00 | 97. 00 | 94.40 | 91.30 | 92.10 | 93.40 | 92.90 | 98.20 | 92.90 |
| Cancer bush | 4.00 | 5.58 | ±2.86 | 1.40 | 4.24 | 3.83 | 4.65 | 8.70 | 3.59 | 10.60 | 2.34 | 3.39 | 9.96 | 2.99 | 5.60 | 8.73 | 7.92 | 6.63 | 7.15 | 1.75 | 7.12 | |
| No class | - | 0.02 | ±0.02 | 0.01 | 0.01 | 0.05 | 0.05 | 0.00 | 0.07 | 0.00 | 0.00 | 0.00 | 0.04 | 0.01 | 0.00 | 0.00 | 0.01 | 0.03 | 0.00 | 0.03 | 0.00 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djokam, M.; Sandasi, M.; Chen, W.; Viljoen, A.; Vermaak, I. Hyperspectral Imaging as a Rapid Quality Control Method for Herbal Tea Blends. Appl. Sci. 2017, 7, 268. https://doi.org/10.3390/app7030268
Djokam M, Sandasi M, Chen W, Viljoen A, Vermaak I. Hyperspectral Imaging as a Rapid Quality Control Method for Herbal Tea Blends. Applied Sciences. 2017; 7(3):268. https://doi.org/10.3390/app7030268
Chicago/Turabian StyleDjokam, Majolie, Maxleene Sandasi, Weiyang Chen, Alvaro Viljoen, and Ilze Vermaak. 2017. "Hyperspectral Imaging as a Rapid Quality Control Method for Herbal Tea Blends" Applied Sciences 7, no. 3: 268. https://doi.org/10.3390/app7030268