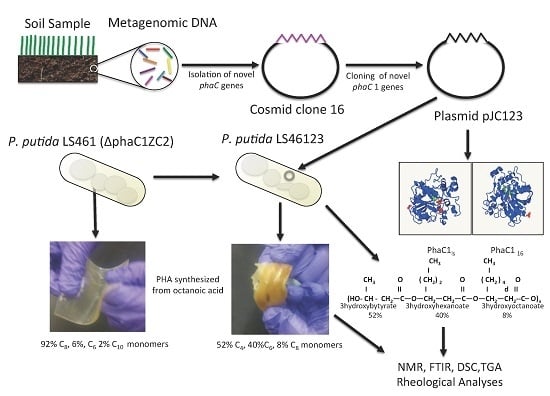
Synthesis and Physical Properties of Polyhydroxyalkanoate Polymers with Different Monomer Compositions by Recombinant Pseudomonas putida LS46 Expressing a Novel PHA SYNTHASE (PhaC116) Enzyme
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Plasmids
2.2. Construction of P. putida LS461 with Partial Deletion of the PHA Synthesis Gene Cluster
2.3. Construction and Transfer of pJC123 into P. putida LS461
2.4. PHA Synthesis
2.5. PHA Production by P. putida LS46123 in Medium with Different Carbon Sources
2.6. Production and Purification of PHAs
2.7. Physical Properties of Polymer Produced by P. putida LS46123
2.7.1. NMR and FTIR Spectra of PHA Polymers
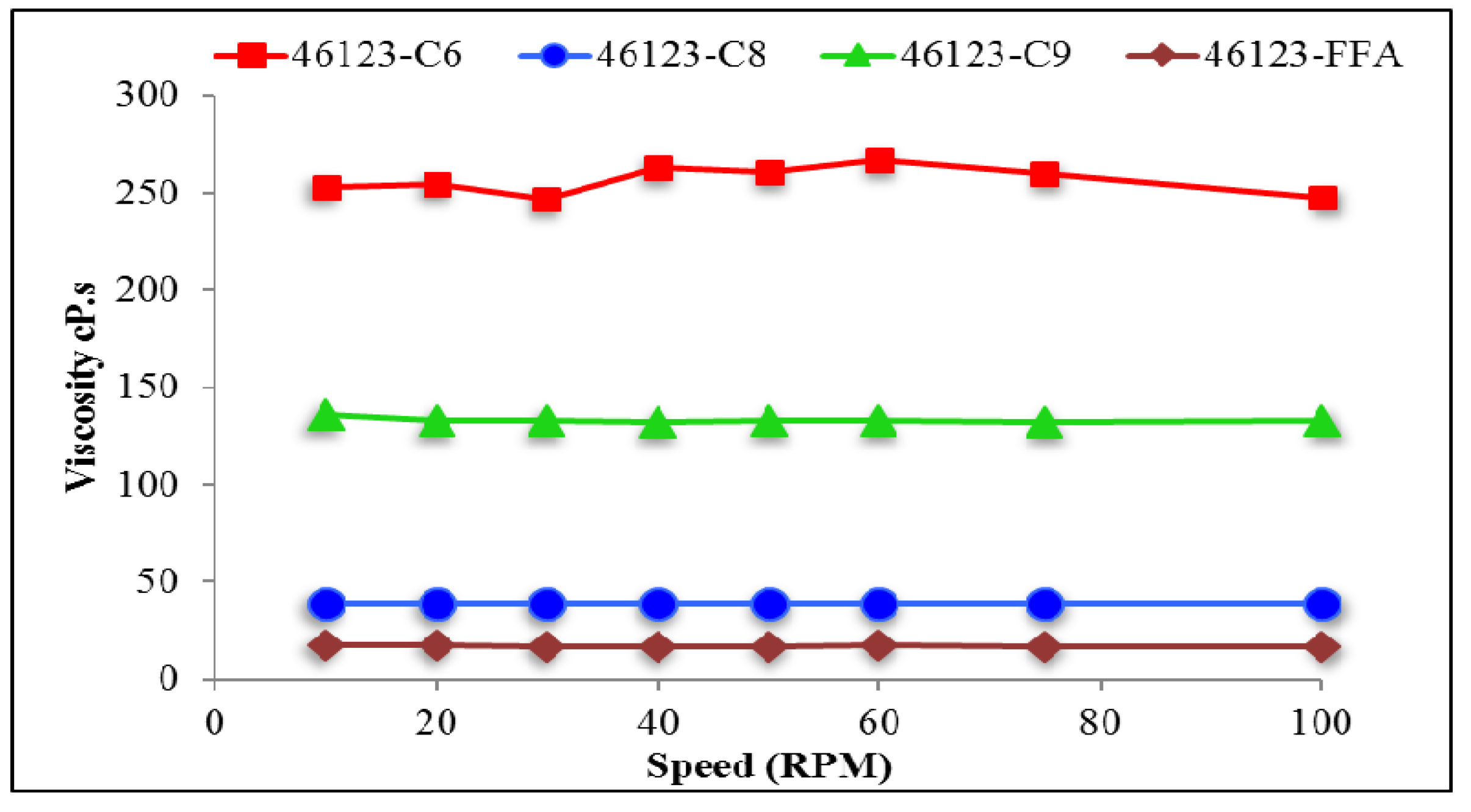
2.7.2. Viscosity of PHAs
2.7.3. Differential Scanning Calorimetric (DSC) Analysis
2.7.4. Thermal Gravimetric Analysis (TGA)
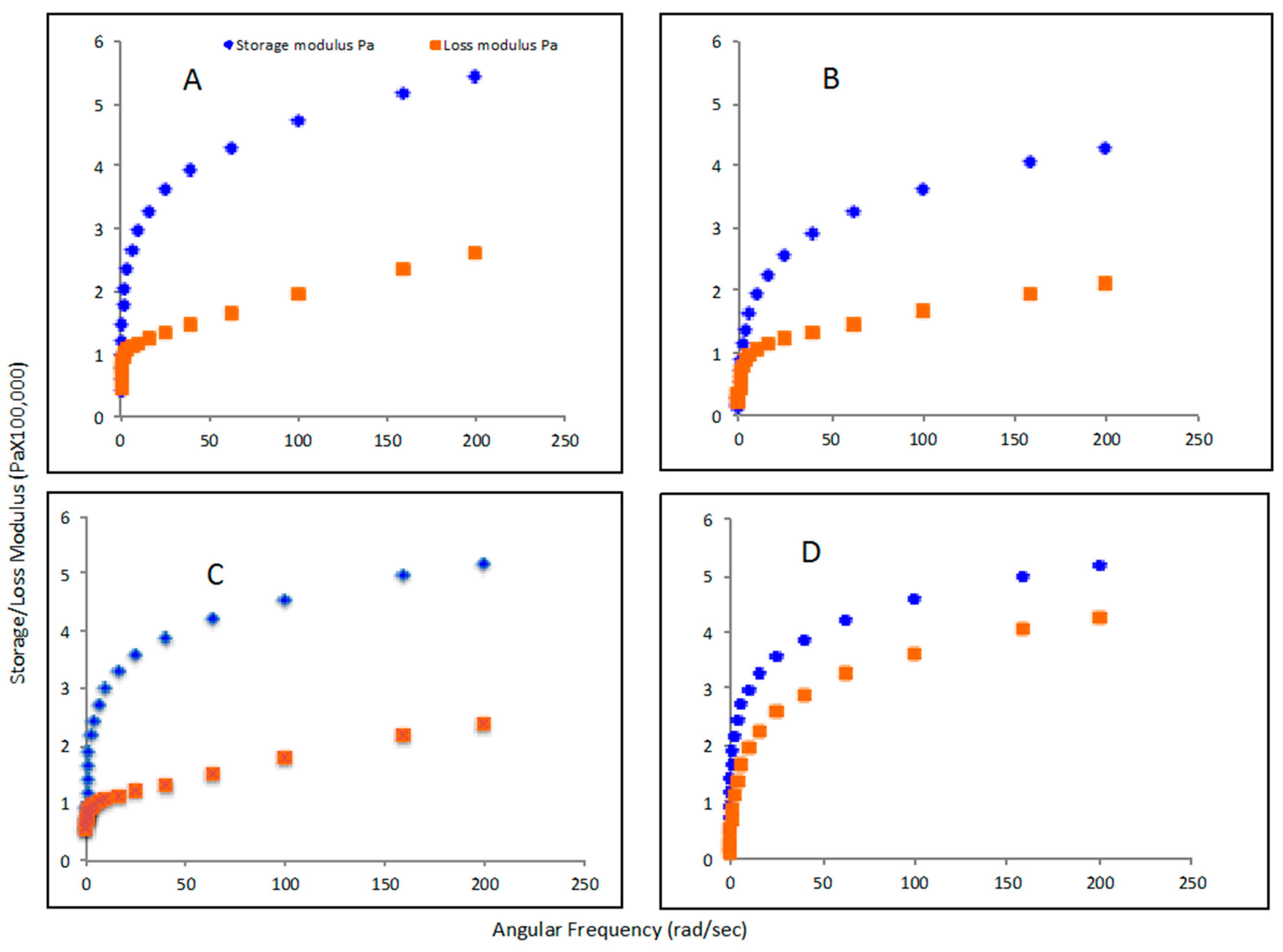
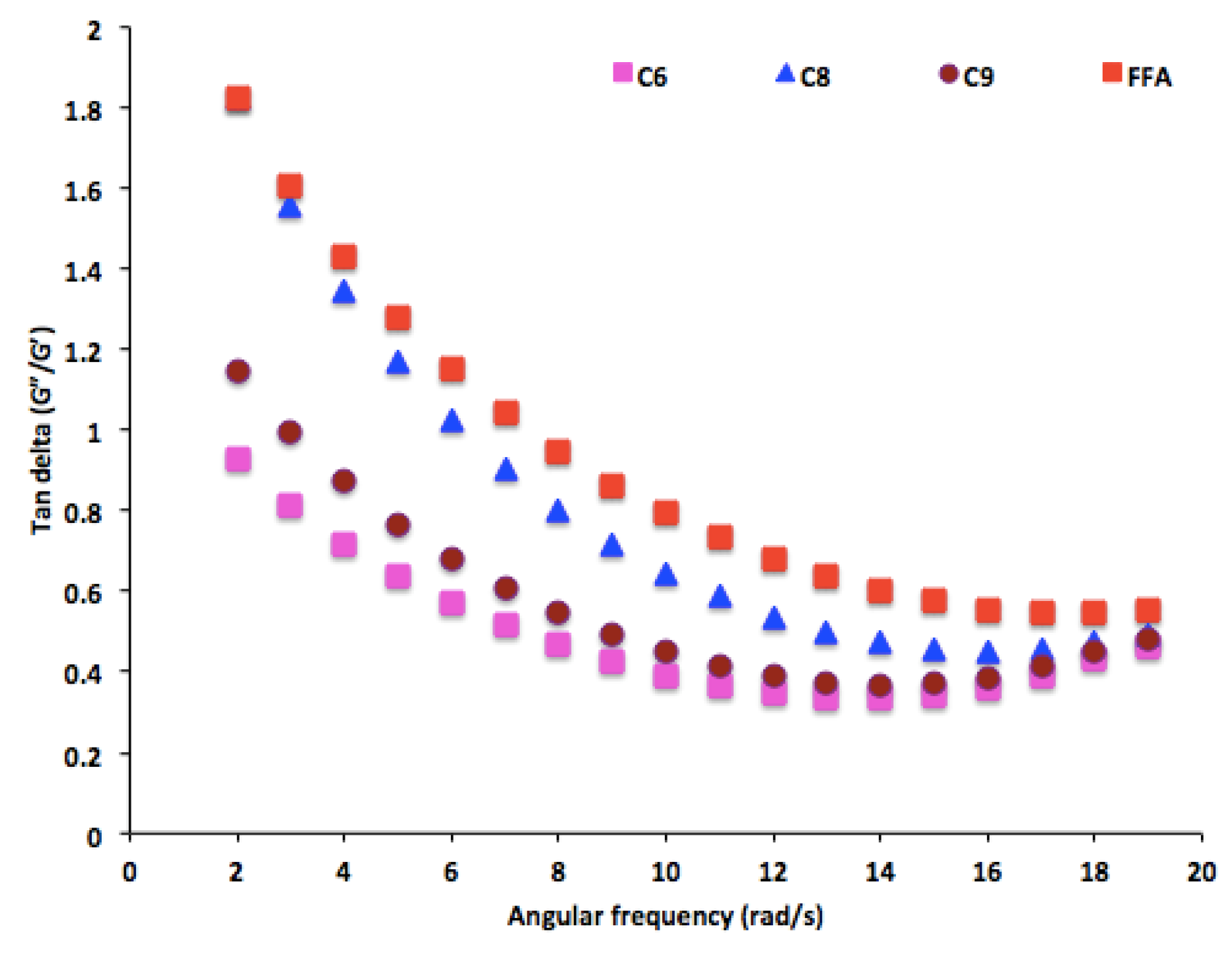
2.7.5. Rheological Properties of PHA Polymers
2.8. Comparison of P. putida LS46 and P. putida LS46123 PhaC116 Proteins
3. Results
3.1. Cell Biomass and PHAs Production
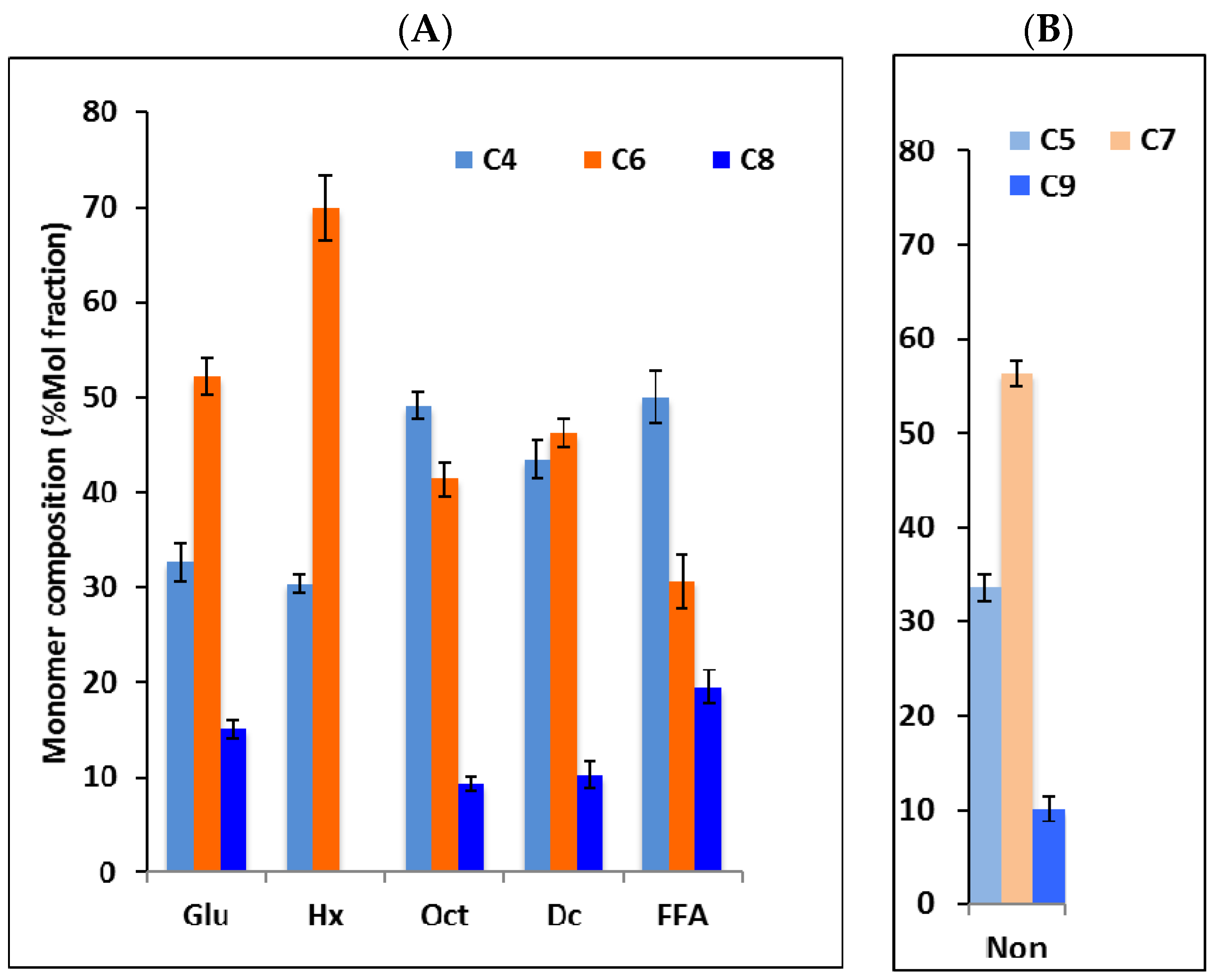
3.2. Monomer Composition of PHAs Produced by P. putida LS46123
3.3. Physical Properties of Polymer Produced by P. putida LS46123
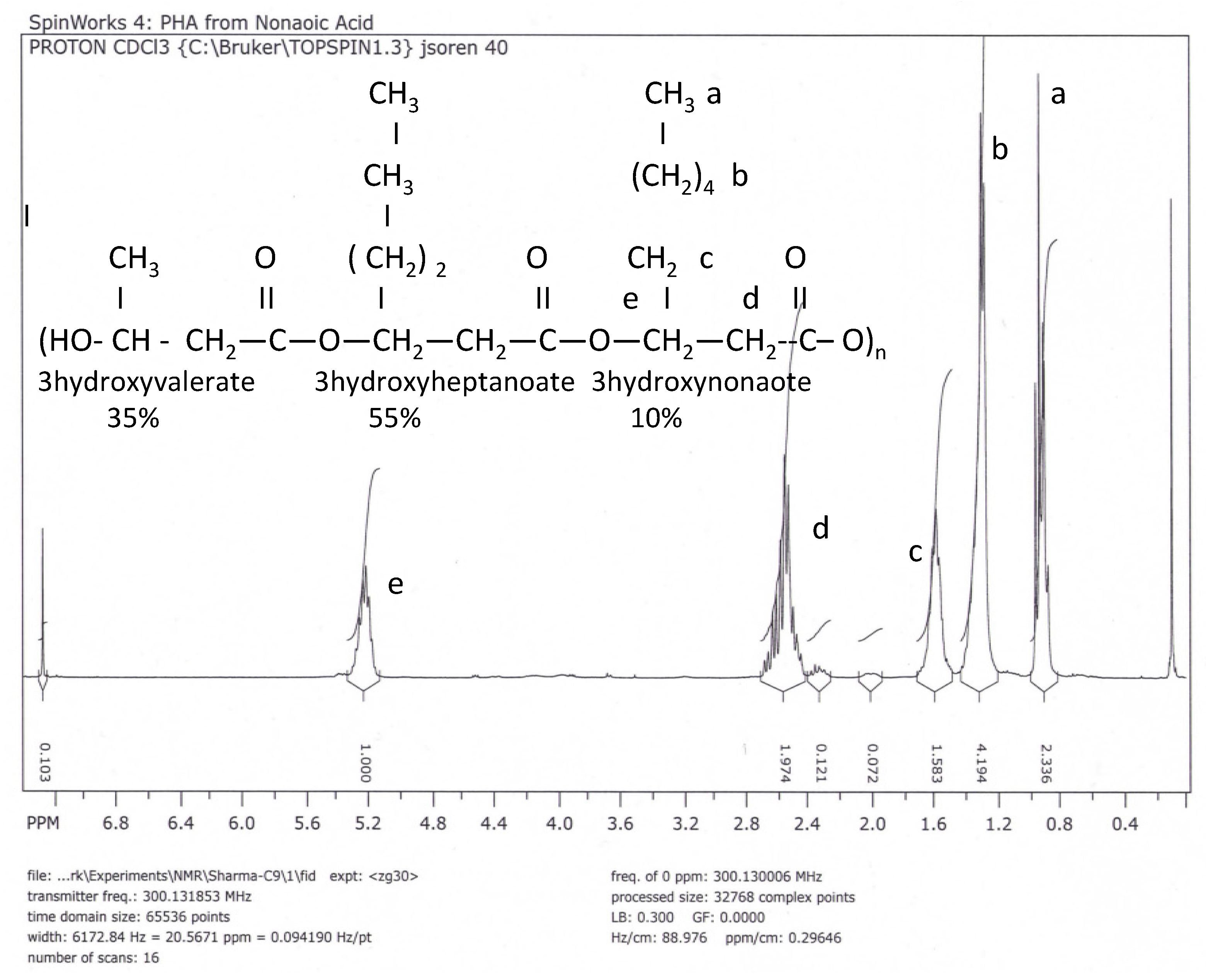
NMR and FITR Spectra
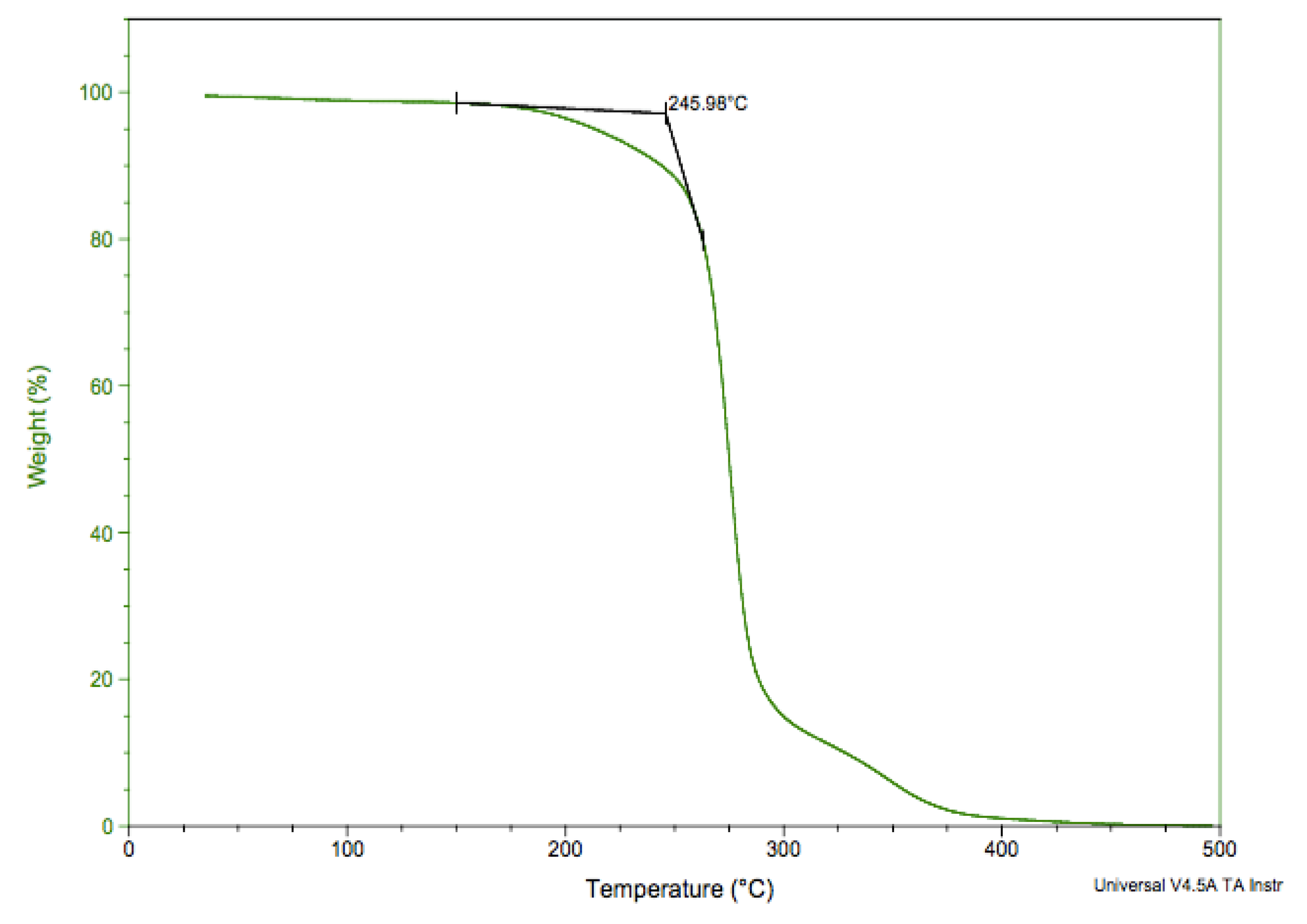
3.4. Glass Transition, Melting, and Thermal Degradation Temperatures
3.5. Rheological Properties of PHA Synthesized by P. putida LS46123
3.6. Comparison between PhaC1 Proteins of P. putida LS46 and P. putida LS46123
3.7. Predicted 3D Structure of PhaC1 Proteins
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Park, S.J.; Kim, T.W.; Kim, M.K.; Lee, S.Y.; Lim, S.C. Advanced bacterial polyhydroxyalkanoates: Towards a versatile and sustainable platform for unnatural tailor-made polyesters. Biotechnol. Adv. 2012, 30, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Madison, L.L.; Huisman, G.W. Metabolic engineering of poly(3-hydroxyalkanoates): From DNA to plastic. Microbiol. Mol. Biol. Rev. 1999, 63, 21–53. [Google Scholar] [PubMed]
- Hazer, B.; Steinbüchel, A. Increased diversification of polyhydroxyalkanoates by modification reactions for industrial and medical applications. Appl. Microbiol. Biotechnol. 2007, 74, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.; Zuber, M.; Zia, K.M.; Noreen, A.; Anjum, M.N.; Tabasum, S. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int. J. Biol. Macromol. 2016, 89, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Salerno, A.; Dias, M.; Reiterer, A.; Braunegg, G. Modern biotechnological polymer synthesis: A review. Food Technol. Biotechnol. 2010, 48, 255–269. [Google Scholar]
- Amache, R.; Sukan, A.; Safari, M.; Roy, I.; Keshavarz, T. Advances in PHAs production. Chem. Eng. Technol. 2009, 32, 931–936. [Google Scholar]
- Pachekoski, W.M.; Agnelli, J.A.M.; Belem, L.P. Thermal, mechanical and morphological properties of poly(hydroxybutyrate) and polypropylene blends after processing. Mater. Res. 2009, 12, 159–164. [Google Scholar] [CrossRef]
- Noda, I.; Green, P.R.; Satkowski, M.M.; Schechtman, L.A. Preparation and properties of a novel class of polyhydroxyalkanoate copolymers. Biomacromolecules 2005, 6, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Noda, I.; Blake Lindsey, S.; Caraway, D. Nodax™ Class PHA Copolymers: Their properties and applications. In Plastics from Bacteria: Natural Functions and Applications; Chen, G.-Q., Ed.; Microbiology Monographs; Springer: Berlin/Heidelberg, Germany, 2010; Volume 14, pp. 238–255. [Google Scholar]
- Kunioka, M.; Doi, Y. Thermal degradation of microbial copolyesters: Poly(3-hydroxybutyrate-co-3- hydroxyvalerate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Macromolecules 1990, 23, 1933–1936. [Google Scholar] [CrossRef]
- Avella, M.; Martuscelli, E.; Raimo, M. Properties of blends and composites based on poly(3-hydroxy) butyrate (PHB) and poly(3-hydroxybutyrate-hydroxyvalerate) (PHBV) copolymers. J. Mater. Sci. 2000, 35, 523–545. [Google Scholar] [CrossRef]
- Qiu, Y.-Z.; Han, J.; Guo, J.-J.; Chen, G.-Q. Production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from gluconate and glucose by recombinant Aeromonas hydrophila and Pseudomonas putida. Biotechnol. Lett. 2005, 27, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Fu, J.; Cicek, N.; Sparling, R.; Levin, D.B. Kinetics of medium-chain-length polyhydroxyalkanoate production by a novel isolate of Pseudomonas putida LS46. Can. J. Microbiol. 2012, 58, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Fu, J.; Zhang, X.; Fristensky, B.; Davenport, K.; Chain, P.S.G.; Sparling, R.; Levin, D.B. Draft genome sequence of medium chain length polyhydroxyalkanoate producing Pseudomonas putida LS46. Genome Announc. 2013, 18, e0015113. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Fu, J.; Zhang, X.; Fristensky, B.; Sparling, R.; Levin, D.B. Genome features of Pseudomonas putida LS46, a novel polyhydroxyalkanoate producer and its comparison with other P. putida strains. AMB Express 2014, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Sharma, P.; Spicer, V.; Krokhin, O.V.; Zhang, X.; Fristensky, B.; Cicek, N.; Sparling, R.; Levin, D.B. Quantitative ‘Omics analyses of medium chain length polyhydroxyalkanaote metabolism in Pseudomonas putida LS46 cultured with waste glycerol and waste fatty acids. PLoS ONE 2015, 10, e0142322. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Sharma, P.; Spicer, V.; Krokhin, O.V.; Zhang, X.; Fristensky, B.; Wilkin, J.A.; Cicek, N.; Sparling, R.; Levin, D.B. Effects of impurities in biodiesel-derived glycerol on growth and expression of heavy metal ion homeostasis genes and gene products in Pseudomonas putida LS46. Appl. Microbiol. Biotechnol. 2015, 99, 5583–5592. [Google Scholar] [CrossRef] [PubMed]
- Naimsiri, N.; Delamarre, S.G.; Kim, Y.R.; Batt, C.A. Engineering of chimeric class polyhydroxyalkanoate synthases. Appl. Envrion. Microbiol. 2004, 70, 6789–6799. [Google Scholar] [CrossRef] [PubMed]
- Matsusaki, H.; Abe, H.; Doi, Y. Biosynthesis and properties of poly(3Hydroxybutyrate-co- 3Hydroxyalkanoates) by recombinant strains of Pseudomonas sp. 61-3. Biomacromolecules 2000, 1, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Nakae, S.; Taguchi, K.; Matsusaki, H.; Seki, M.; Doi, Y. Biosynthesis of poly(3Hydroxybutyrate-co-3Hydroxyalkanoates) copolymer from sugars by recombinant Ralstonia eutropha harboring the phac1ps and the phagps genes of Pseudomonas sp. 61-3. Biomacromolecules 2001, 2, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Matsusaki, H.; Abe, H.; Taguchi, K.; Fukui, T.; Doi, Y. Biosynthesis of poly-(3Hydroxybutyrate-co- 3Hydroxyalkanoates) by recombinant bacteria expressing the PHA synthase gene phaC1 from Pseudomonas sp. 61-3. Appl. Microbiol. Biotechnol. 2000, 53, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Fukui, T.; Doi, Y. Cloning and analysis of the poly(3Hydroxybutyrate-co-3Hydroxyhexanoate) biosynthesis genes of Aeromonas caviae. J. Bacteriol. 1997, 179, 4821–4830. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, T.; Watanabe, S.; Sato, S.; Hiraishi, T.; Abe, H.; Doi, Y. Variation in copolymer composition and molecular weight of polyhydroxyalkanoate generated by saturation mutagenesis of Aeromonas caviae PHA synthase. Macromol. Biosci. 2007, 7, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Lee, S.Y. Biosynthesis of poly-(3Hydroxybutyrate-co-3Hydroxyalkanoates) by metabolically engineered Escherichia coli strains. Appl. Biochem. Biotechnol. 2004, 114, 335–346. [Google Scholar] [CrossRef]
- Gao, X.; Yuan, X.; Shi, Z.Y.; Guo, Y.Y.; Shen, X.W.; Chen, J.C.; Wu, Q.; Chen, G.Q. Production of copolyesters of 3Hydroxybutyrate and medium-chain-length 3Hydroxyalkanoates by E. coli containing an optimized PHA synthase gene. Microb. Cell Fact. 2012, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, R.S.; Bhat, S.G.; Chandrasekaran, M. Amplification and sequence analysis of phaC gene of polyhydroxybutyrate producing Vibrio azureus BTKB33 isolated from marine sediments. Ann. Microbiol. 2016, 66, 299–306. [Google Scholar] [CrossRef]
- Ling, S.C.; Tsuge, T.; Sudesh, K. Biosynthesis of novel polyhydroxyalkanoate containing 3Hydroxy-4- methylvalerate by Chromobacterium sp. USM2. J. Appl. Microbiol. 2011, 111, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.M.; Sudesh, K. Identification of a new polyhydroxyalkanoate (PHA) producer Aquitalea sp. USM4 (JCM 19919) and characterization of its PHA synthase. J. Biosci. Bioeng. 2016, 122, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Pinnell, L.; Engel, K.; Neufeld, J.D.; Charles, T.C. Versatile broad-host-range cosmids for construction of high quality metagenomic libraries. J. Microbiol. Methods 2014, 99, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Schallmey, M.; Ly, A.; Wang, C.; Meglei, G.; Voget, S.; Streit, W.R.; Driscoll, B.T.; Charles, T.C. Harvesting of novel polyhydroxyalkanaote (PHA) synthase encoding genes from a soil metagenome library using phenotypic screening. FEMS Microbiol. Lett. 2011, 321, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Charles, T.C. Novel polyhydroxyalkanoate copolymers produced in Pseudomonas putida by metagenomic polyhydroxyalkanoate synthases. Appl. Microbiol. Biotechnol. 2016, 100, 7611–7627. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, A.; Tauch, A.; Jsger, W.; Kalinowski, J.; Thierbachb, G.; Puhler, A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: Selection of defined deletions in the chromosome of Corynebacterium glutumicum. Gene 1994, 145, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Figurski, D.H.; Helinski, D.R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Nat. Acad. Sci. USA 1979, 76, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Priefer, U.; Pühler, A. A broad host-range mobilization system for in vivo genetic engineering: Transposon mutagenesis in gram-negative bacteria. Biotechnology 1983, 1, 784–791. [Google Scholar] [CrossRef]
- Huijberts, G.N.M.; Eggink, G.; De Waard, P.; Huisman, G.W.; Witholt, B. Pseudomonas putida KT2442 cultivated on glucose accumulates poly(3Hydroxyalkanoates) consisting of saturated and unsaturated monomers. Appl. Environ. Microbiol. 1992, 58, 536–544. [Google Scholar] [PubMed]
- Hong, K.; Sun, S.; Tian, W.; Chen, G.Q.; Huang, W. A rapid method for detecting bacterial polyhydroxyalkanoates in intact cells by Fourier transform infrared spectroscopy. Appl. Microbiol. Biotechnol. 1999, 51, 523526. [Google Scholar] [CrossRef]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Możejko-Ciesielska, J.; Kiewisz, R. Bacterial polyhydroxyalkanoates: Still fabulous? Microbiol. Res. 2016, 192, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.; Li, Z.J.; Ye, H.M.; Yuan, M.Q.; Chen, G.Q. Metabolic engineering for microbial production of polyhydroxyalkanoates consisting of high 3Hydroxyhexanoate content by recombinant Aeromonas hydrophila. Bioresour. Technol. 2010, 101, 6096–6102. [Google Scholar] [CrossRef] [PubMed]
- Sheu, D.; Lee, C.Y. Altering the substrate specificity of polyhydroxyalkanoate synthase 1derived from Pseudomonas putida Gpo1 by localized semi-random mutagenesis. J. Bacteriol. 2004, 186, 4177–4184. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Dong, C.L.; Wang, S.Y.; Ye, H.M.; Chen, G.Q. Microbial production of polyhydroxyalkanoate block copolymer by recombinant Pseudomonas putida. Appl. Microbiol. Biotechnol. 2011, 90, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Hein, S.; Paletta, J.R.J.; Steinbuchel, A. Cloning, characterization and comparison of the Pseudomonas mendocina polyhydroxyalkanoate synthases PhaC1 and PhaC2. Appl. Microbiol. Biotechnol. 2002, 58, 229–236. [Google Scholar] [PubMed]
- Matsusaki, H.; Manji, S.; Taguchi, K.; Kato, M.; Fukui, T.; Doi, Y. Cloning and molecular analysis of the Poly(3Hydroxybutyrate) and Poly(3Hydroxybutyrate-co-3Hydroxyalkanoate) biosynthesis genes in Pseudomonas sp. strain 61-3. J. Bacteriol. 1998, 180, 6459–6467. [Google Scholar] [PubMed]
- Hoffmann, N.; Rehm, B.H.A. Regulation of polyhydroxyalkanoate biosynthesis in Pseudomonas putida and Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2004, 237, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huisman, G.W.; Wonink, E.; DeKoning, G.J.M.; Preusting, H.; Witholt, B. Synthesis of poly (3Hydroxyalkanoates) by mutant and recombinant Pseudomonas strains. Appl. Microbiol. Biotechnol. 1992, 38, 1–5. [Google Scholar]
- Thirumala, M.; Reddy, S.R. Production of PHA by recombinant organisms. Int. J. Life Sci. Biotechnol. Pharma Res. 2012, 1, 40–62. [Google Scholar]
- Hong, K.; Chen, G.Q.; Yu, P.F.; Zhang, G.; Liu, Y.; Chua, H. Effect of C:N Molar ratio on monomer composition of polyhydroxyalkanoates produced by Pseudomonas mendocina 0806 and Pseudomonas pseudoalkaligenes YS1. Appl. Biochem. Biotechnol. 2000, 84–86, 971–980. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, W.; Jian, J.; Wu, Q.; Chen, G.Q. Molecular cloning and functional analysis of two polyhydroxyalkanoate synthases from two strains of Aeromonas hydrophila spp. FEMS Microbiol. Lett. 2005, 243, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Fukui, T.; Yokomizo, S.; Kobayashi, G.; Doi, Y. Co-expression of polyhydroxyalkanoate synthase and (R)-enoyl-CoA hydratase genes of Aeromonas caviae establishes copolyester biosynthesis pathway in Escherichia coli. FEMS Microbiol. Lett. 1999, 170, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Kichise, T.; Fukui, T.; Yoshida, Y.; Doi, Y. Biosynthesis of polyhydroxyalkanoates (PHA) by recombinant Ralstonia eutropha and effects of PHA synthase activity on in vivo PHA biosynthesis. Int. J. Biol. Macromol. 1999, 25, 69–77. [Google Scholar] [CrossRef]
- Nomura, C.T.; Tanaka, T.; Gan, Z.; Kuwabara, K.; Abe, H.; Takase, K.; Taguchi, K.; Doi, Y. Effective enhancement of short-chain-length-medium-chain-length polyhydroxyalkanoate copolymer production by co-expression of genetically engineered 3ketoacyl-acyl-carrier-protein synthase III (fabh) and polyhydroxyalkanoate synthesis genes. Biomacromolecules 2004, 5, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.H.; Jung, Y.K.; Kang, H.O.; Kim, T.W.; Park, S.J.; Lee, S.Y. Tailor-made type II Pseudomonas PHA synthases and their use for the biosynthesis of polylactic acid and its copolymer in recombinant Escherichia coli. Appl. Microbiol. Biotechnol. 2011, 90, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, T.; Saito, Y.; Kikkawa, Y.; Hiraishi, T.; Doi, Y. Biosynthesis and compositional regulation of poly[(3Hydroxybutyrate)-co-(3Hydroxyhexanoate)] in recombinant Ralstonia eutropha expressing mutated polyhydroxyalkanoate synthase genes. Macromol. Biosci. 2004, 4, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.W.; Shi, Z.Y.; Song, G.; Li, Z.J.; Chen, G.Q. Engineering of polyhydroxyalkanoate (PHA) synthase PhaC2Ps of Pseudomonas stutzeri via site-specific mutation for efficient production of PHA copolymers. Appl. Microbiol. Biotechnol. 2011, 91, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Takase, K.; Matsumoto, K.; Taguchi, S.; Doi, Y. Alteration of substrate chain-length specificity of type II synthase for polyhydroxyalkanoate biosynthesis by in vitro evolution: In vivo and in vitro enzyme assays. Biomacromolecules 2004, 5, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Aoki, E.; Takase, K.; Doi, Y.; Taguchi, S. In vivo and in vitro characterization of Ser477x mutations in polyhydroxyalkanoate (PHA) synthase 1 from Pseudomonas sp. 61-3: Effects of beneficial mutations on enzymatic activity, substrate specificity, and molecular weight of PHA. Biomacromolecules 2006, 7, 2436–2442. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Tsai, P.-C.; Hsu, C.-H.; Lee, C.-Y. Critical residues of class II PHA synthase for expanding the substrate specificity and enhancing the biosynthesis of polyhydroxyalkanoate. Enzym. Microb. Technol. 2014, 56, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.A.; Rehm, B.H.A. Replacement of the catalytic nucleophile cysteine-296 by serine in class II polyhydroxyalkanoate synthase from Pseudomonas aeruginosa-mediated synthesis of a new polyester: Identification of catalytic residues. Biochem. J. 2003, 374, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Shozui, F.; Matsumoto, K.; Sasaki, T.; Taguchi, S. Engineering of polyhydroxyalkanoate synthase by Ser477X/Gln481X saturation mutagenesis for efficient production of 3Hydroxybutyrate-based copolyesters. Appl. Microbiol. Biotechnol. 2010, 84, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Savenkova, L.; Gercberga, Z.; Bibers, I.; Kalnin, M. Effect of 3Hydroxyvalerate content on some physical and mechanical properties of polyhydroxyalkanoates produced by Azotobacter chroococcum. Process Biochem. 2000, 36, 445–450. [Google Scholar] [CrossRef]
- Kim, J.S.; Renekar, D.H. Mechanical properties of composites using ultrafine electrospun fibres. Polym. Compos. 1999, 20, 124–131. [Google Scholar] [CrossRef]
- Gerard, T.; Budtova, T. Morphology and molten-state rheology of polylactide and polyhydroxyalkanoate blends. Eur. Polym. J. 2012, 48, 1110–1117. [Google Scholar] [CrossRef]
- De Koning, D. Physical properties of bacterial poly((R)-3-hydroxyalkanoates). Can. J. Microbiol. 1995, 41, 303–309. [Google Scholar] [CrossRef]
- Ren, Q.; Sierro, N.; Kellerhals, M.; Kessler, B.; Witholt, B. Properties of engineered poly-3-hydroxyalkanoates produced in recombinant Escherichia coli strains. Appl. Environ. Microbiol. 2000, 66, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Corre, Y.M.; Bruzaud, S.; Audic, J.L.; Grohens, Y.; Koller, M.; Atlić, A.; Dias, M. Morphology and functional properties of commercial polyhydroxyalkanoates: A comprehensive and comparative study. Polym. Test. 2012, 31, 226–235. [Google Scholar] [CrossRef]





| Strain/Plasmid/Primer | Characteristics | References |
|---|---|---|
| P. putida LS46 | P. putida isolated from waste water | [13] |
| P. putida LS461 | phaC1-phaZ-phaC2 deletion mutant of P. putida LS46 | This study |
| P. putida LS46123 | P. putida LS461 carrying plasmid pJC123; TcR | This study |
| E. coli DH5α | ΔlacZ, ΔM15, Δ(lacZYA-argF), U169, recA1, endA1, hsdR17(rK-mK+), supE44, thi-1, gyrA96, relA1 | Qiagen, Hilden, Germany |
| pRK7813 | IncP oriT cos lacZα, TcR | [30] |
| pJC123 | Derivative of pRK7813 carrying phaC116 gene from clone 16; TcR | [31] |
| pK18mobsacB | Narrow host-range cloning vector; sacB, KmR | [32] |
| pRK2013 | Helper plasmid pRK290 derivative; KmR | [33] |
| pPHAC1C2 | pK18mobsacB carrying 840 bp from phaC1 and 857 bp from phaC2 for operon knockout | This study |
| Pphac1forXbal | 5′-CTAGTCTAGATGCGGTCAAACGCTTCTTCGAAAC-3′ | This study |
| Pphac1revHindIII | 5′-GATCAAGCTTGTCAGCGGGTTGCTCTTGAACATT-3′ | This study |
| Pphac2forHindIII | 5′-GATCAAGCTTAGACACGCCAGTGGATCGATGAAA-3′ | This study |
| Pphac2revNhel | 5′-CTAGGCTAGCTCTTGCCGAGCAGGTAGTTGTTGA-3′ | This study |
| Strains | Carbon Source | Cell Biomass (g/L) | % PHA/cdw |
|---|---|---|---|
| P. putida LS46123 | Glucose (2% w/v) | 2.2 ± 0.3 | 9.2 ± 3.2 |
| Hexanoate (20 mM) | 1.1 ± 0.2 | 16.2 ± 2.1 | |
| Octanoate (20 mM) | 1.1 ± 0.1 | 17.3 ± 1.5 | |
| Nonanoate (20 mM) | 1.4 ± 0.2 | 10.5 ± 0.8 | |
| Decanoate (20 mM) | 1.6 ± 0.1 | 14.1 ± 0.8 | |
| REG-FFA (2% v/v) | 2.1 ± 0.2 | 27.1 ± 1.7 | |
| P. putida LS46 | Glucose (2% w/v) | 2.7 ± 0.4 | 22.6 ± 0.8 |
| Hexanoate (20 mM) | 2.1 | 19.1 | |
| Octanoate (20 mM) | 2.5 | 48.9 | |
| Nonanoate (20 mM) | 2.4 | 28.1 | |
| Decanoate (20 mM) | 2.5 | 33.7 | |
| REG-FFA (2% v/v) | 4.6 | 40.3 |
| Substrate for PHA Production | Glass Transition Temperature (Tg °C) | Melting Temperature (Tm°C) | Degradation Temperature (Td °C) | Wave Number (cm−1) | |
|---|---|---|---|---|---|
| C=O | C–O | ||||
| Hexanoic Acid | −28.7 | 138 | 254.5 | 1732 | 1170 |
| Octanoic Acid | −32.6 | 162 | 258.3 | 1734 | 1167 |
| Nonanoic Acid | −30.2 | 158 | 254.5 | 1734 | 1165 |
| Decanoic Acid | −31.0 | 166 | ND# | ND | ND |
| Biodiesel fatty acid | −34.4 | 140 | 248.6 | 1734 | 1177 |
| Polyhydroxyalkanoates P(3HA) | −44.0 | 42 | 260 | 1742 | 1165 |
| * Homopolymer of PHB | 9.0 | 178 | 280 | 1728 | 1185 |
| * Copolymer P(3HB-co-3HV) | −6.0 to −10.0 | 137–170 | 275 | ND # | ND # |
| PhaC1 Enzyme | Mol % of 3HB | Amino Acid Substation in Conserved Regions of PhaC1 Proteins | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | |||||||||||||||||
| 228 | 230 | 231 | 292 | 293 | 295 | 297 | 377 | 379 | 399 | 401 | 403 | 404 | 481 | 482 | 483 | 484 | 520 | 521 | 523 | 524 | ||
| Wild 1 | 12 | F | V | F | M | L | A | S | N | W | N | T | R | L | Q | S | I | L | L | H | Q | T |
| pL1-6 | 60 | I | L | V | V | V | T | L | A | C | I | P | L | G | ||||||||
| pD7-47 | 7 | I | L | V | V | V | T | L | A | C | I | P | L | G | ||||||||
| pPS-A2 | 36 | Y | I | L | A | V | V | V | V | A | G | I | P | L | D | |||||||
| pPS-C2 | 50 | Y | I | L | A | M | V | V | V | M | A | G | V | P | L | G | ||||||
| pPS-E1 | 45 | I | L | T | V | V | T | A | C | V | V | P | D | L | E | |||||||
| LS46 2 | -- | F | V | F | M | L | A | S | N | W | N | T | R | L | Q | S | I | L | L | H | Q | S |
| PhaC1163 | Up to 50 | Y | V | L | F | K | L | A | N | W | S | S | R | L | Q | T | L | V | E | M | I | K |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, P.K.; Munir, R.I.; Blunt, W.; Dartiailh, C.; Cheng, J.; Charles, T.C.; Levin, D.B. Synthesis and Physical Properties of Polyhydroxyalkanoate Polymers with Different Monomer Compositions by Recombinant Pseudomonas putida LS46 Expressing a Novel PHA SYNTHASE (PhaC116) Enzyme. Appl. Sci. 2017, 7, 242. https://doi.org/10.3390/app7030242
Sharma PK, Munir RI, Blunt W, Dartiailh C, Cheng J, Charles TC, Levin DB. Synthesis and Physical Properties of Polyhydroxyalkanoate Polymers with Different Monomer Compositions by Recombinant Pseudomonas putida LS46 Expressing a Novel PHA SYNTHASE (PhaC116) Enzyme. Applied Sciences. 2017; 7(3):242. https://doi.org/10.3390/app7030242
Chicago/Turabian StyleSharma, Parveen K., Riffat I. Munir, Warren Blunt, Chris Dartiailh, Juijun Cheng, Trevor C. Charles, and David B. Levin. 2017. "Synthesis and Physical Properties of Polyhydroxyalkanoate Polymers with Different Monomer Compositions by Recombinant Pseudomonas putida LS46 Expressing a Novel PHA SYNTHASE (PhaC116) Enzyme" Applied Sciences 7, no. 3: 242. https://doi.org/10.3390/app7030242