Infrared Spectroscopy as Molecular Probe of the Macroscopic Metal-Liquid Interface
Abstract
:1. Introduction
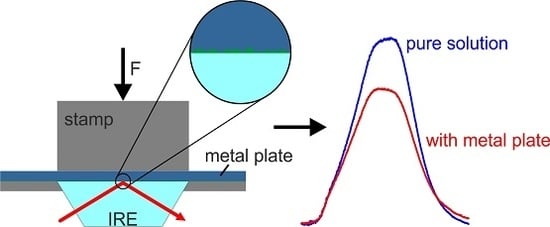
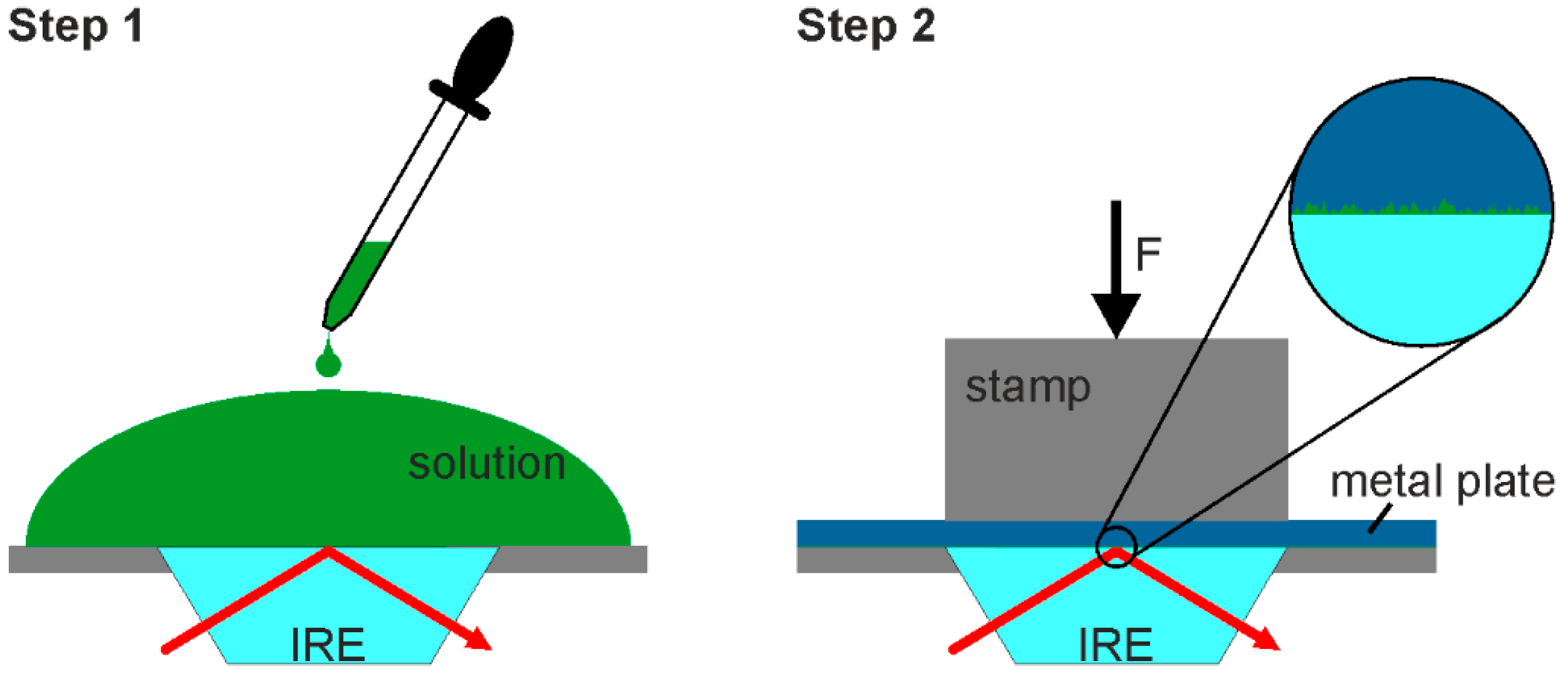
2. Materials and Methods
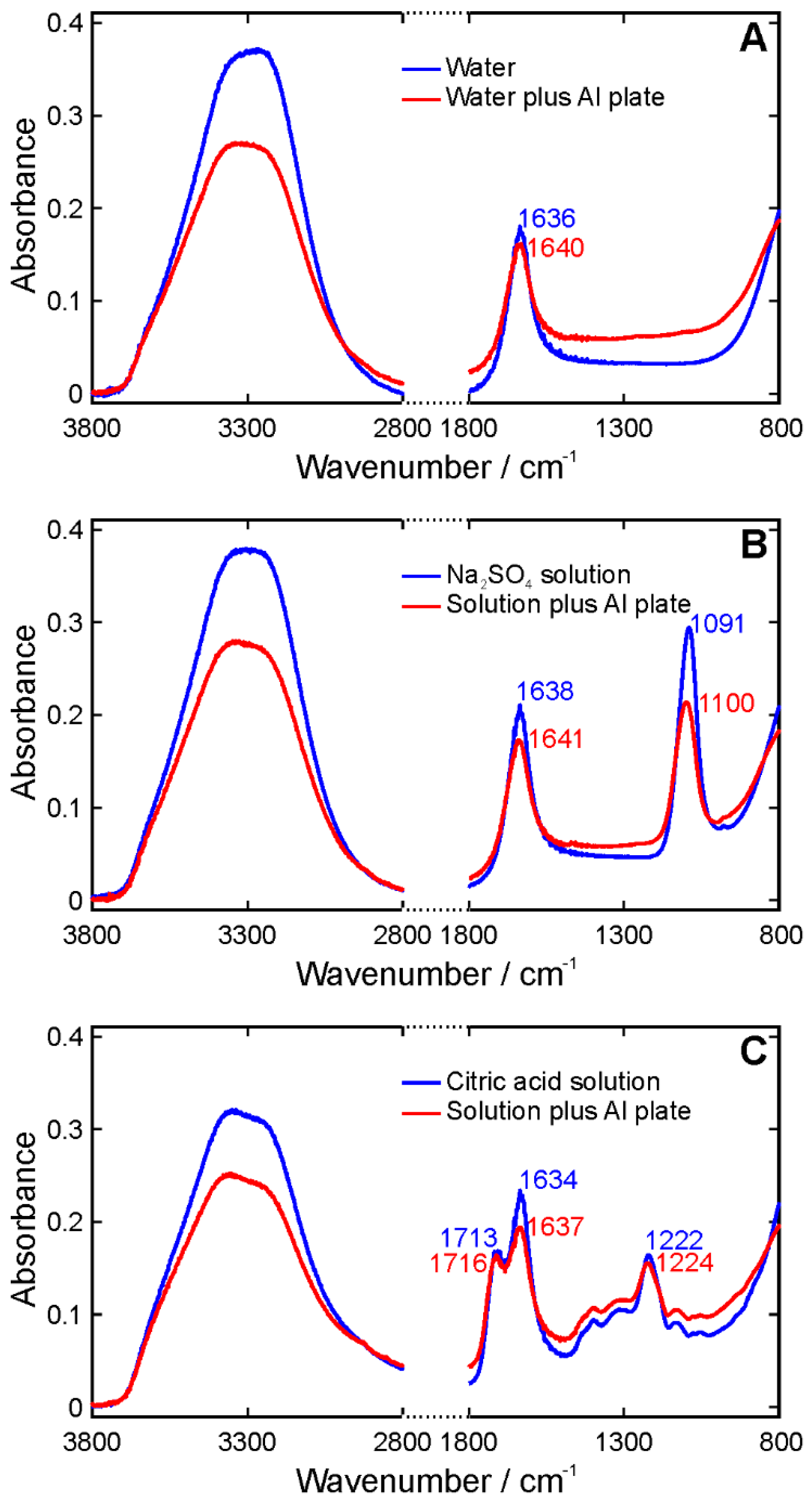
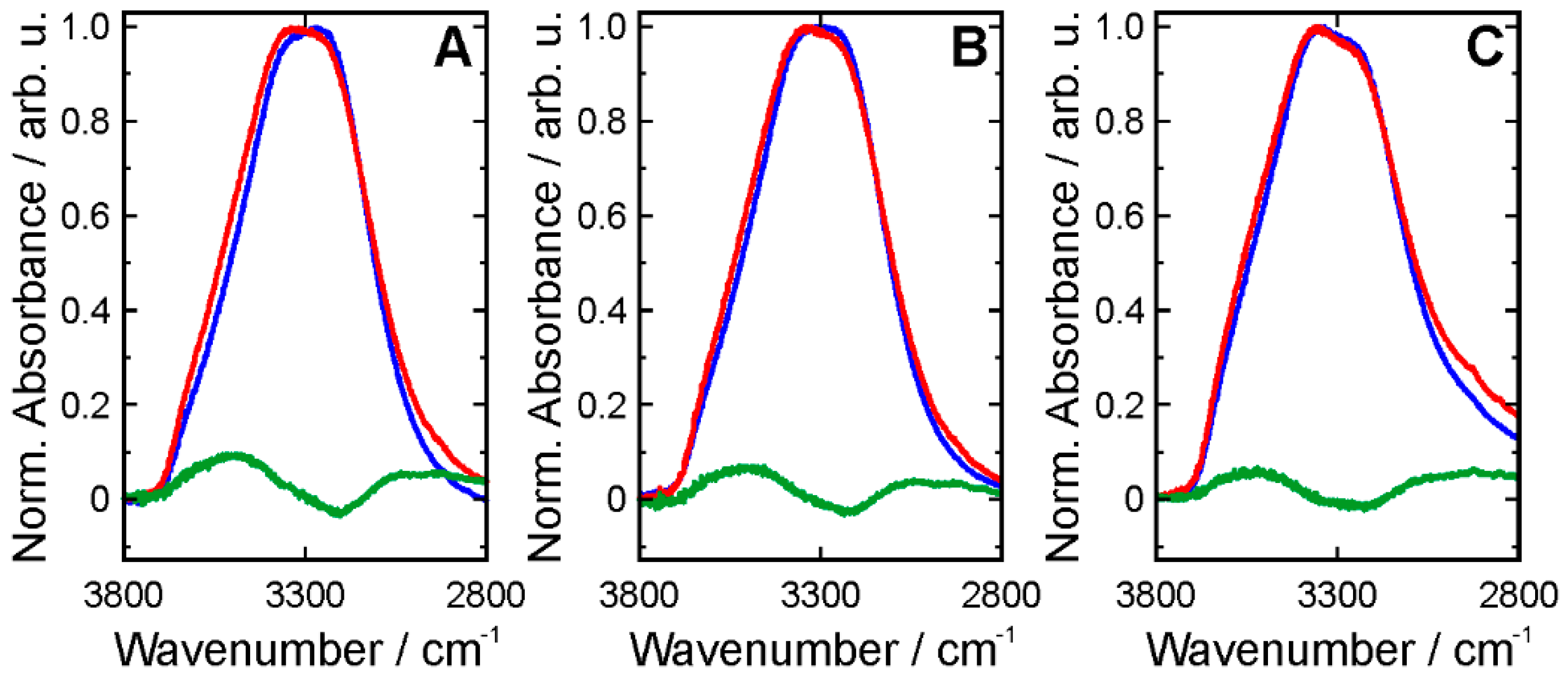
3. Results
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.B.; Norskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 335, 146. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, V.R.; Strmcnik, D.; Lopes, P.P.; Markovic, N.M. Energy and fuels from electrochemical interfaces. Nat. Mater. 2017, 16, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, Y.; Guo, L. Nature-Inspired Electrochemical Energy-Storage Materials and Devices. Adv. Energy Mater. 2017, 7, 1601709. [Google Scholar] [CrossRef]
- Cho, J.; Jeong, S.; Kim, Y. Commercial and research battery technologies for electrical energy storage applications. Prog. Energy Combust. Sci. 2015, 48, 84–101. [Google Scholar] [CrossRef]
- Manam, N.S.; Harun, W.S.W.; Shri, D.N.A.; Ghani, S.A.C.; Kurniawan, T.; Ismail, M.H.; Ibrahim, M.H.I. Study of corrosion in biocompatible metals for implants: A review. J. Alloys Compd. 2017, 701, 698–715. [Google Scholar] [CrossRef]
- Dwivedi, D.; Lepkova, K.; Becker, T. Carbon steel corrosion: A review of key surface properties and characterization methods. RSC Adv. 2017, 7, 4580–4610. [Google Scholar] [CrossRef]
- Zehentbauer, F.M.; Bain, E.J.; Kiefer, J. Multiple parameter monitoring in a direct methanol fuel cell. Meas. Sci. Technol. 2012, 23, 045602. [Google Scholar] [CrossRef]
- Salmeron, M.; Schlögl, R. Ambient pressure photoelectron spectroscopy: A new tool for surface science and nanotechnology. Surf. Sci. Rep. 2008, 63, 169–199. [Google Scholar] [CrossRef]
- Rasko, J.; Domok, A.; Baan, K.; Erdohelyi, A. FTIR and mass spectrometric study of the interaction of ethanol and ethanol-water with oxide-supported platinum catalysts. Appl. Catal. A 2006, 299, 202–211. [Google Scholar] [CrossRef]
- McCue, A.J.; Mutch, G.A.; McNab, A.I.; Campbell, S.; Anderson, J.A. Quantitative determination of surface species and adsorption sites using Infrared spectroscopy. Catal. Today 2016, 259, 19–26. [Google Scholar] [CrossRef]
- Kraack, J.P.; Hamm, P. Surface-sensitive and surface-specific ultrafast two-dimensional vibrational spectroscopy. Chem. Rev. 2017, 117, 10623–10664. [Google Scholar] [CrossRef] [PubMed]
- Vidal, F.; Tadjeddine, A. Sum-frequency generation spectroscopy of interfaces. Rep. Prog. Phys. 2005, 68, 1095–1127. [Google Scholar] [CrossRef]
- Kiefer, J. Recent advances in the characterization of gaseous and liquid fuels by vibrational spectroscopy. Energies 2015, 8, 3165–3197. [Google Scholar] [CrossRef] [Green Version]
- McNay, G.; Eustace, D.; Smith, W.E.; Faulds, K.; Graham, D. Surface-Enhanced Raman Scattering (SERS) and Surface-Enhanced Resonance Raman Scattering (SERRS): A Review of Applications. Appl. Spectrosc. 2011, 65, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.M.; Griffiths, P.R. Handbook of Vibrational Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Kiefer, J. Simultaneous acquisition of absorption and fluorescence spectra of strong absorbers utilizing an evanescent supercontinuum. Opt. Lett. 2016, 41, 5684–5687. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, J.; Frank, K.; Schuchmann, H.P. Attenuated total reflection infrared (ATR-IR) spectroscopy of a water-in-oil emulsion. Appl. Spectrosc. 2011, 65, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, J.; Grabow, J.; Kurland, H.-D.; Müller, F.A. Characterization of Nanoparticles by Solvent Infrared Spectroscopy. Anal. Chem. 2015, 87, 12313–12317. [Google Scholar] [CrossRef] [PubMed]
- Torregrosa-Coque, R.; Alvarez-Garcia, S.; Martin-Martinez, J.M. Migration of low molecular weight moiety at rubber-polyurethane interface: An ATR-IR spectroscopy study. Int. J. Adhes. Adhes. 2011, 31, 389–397. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.W.; Cai, W.B. Recent applications of in situ ATR-IR spectroscopy in interfacial electrochemistry. Curr. Opin. Electrochem. 2017, 1, 73–79. [Google Scholar] [CrossRef]
- Zandi, O.; Hamann, T.W. Determination of photoelectrochemical water oxidation intermediates on haematite electrode surfaces using operando infrared spectroscopy. Nat. Chem. 2016, 8, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Osawa, M. Dynamic process in electrochemical reactions studied by surface-enhanced infrared absorption spectroscopy (SEIRAS). Bull. Chem. Soc. Jpn. 1997, 70, 2861–2880. [Google Scholar] [CrossRef]
- Koichumanova, K.; Visan, A.; Geerdink, B.; Lammertink, R.G.H.; Mojet, B.L.; Seshan, K.; Lefferts, L. ATR-IR spectroscopic cell for in situ studies at solid-liquid interface at elevated temperatures and pressures. Catal. Today 2017, 283, 185–194. [Google Scholar] [CrossRef]
- Mudunkotuwa, I.A.; Al Minshid, A.; Grassian, V.H. ATR-FTIR spectroscopy as a tool to probe surface adsorption on nanoparticles at the liquid-solid interface in environmentally and biologically relevant media. Analyst 2014, 139, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Kraack, J.P.; Kaech, A.; Hamm, P. Surface Enhancement in Ultrafast 2D ATR IR Spectroscopy at the Metal-Liquid Interface. J. Phys. Chem. C 2016, 120, 3350–3359. [Google Scholar] [CrossRef]
- Kraack, J.P.; Kaech, A.; Hamm, P. Molecula-specific interactions of diatomic adsorbates at metal-liquid interfaces. Struct. Dyn. 2017, 4, 044009. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.; Kler, P.A.; Berli, C.L.A.; Collins, S.E. Design and operational limits of an ATR-FTIR spectroscopic microreactor for investigating reactions at liquid-solid interface. Chem. Eng. J. 2014, 243, 197–206. [Google Scholar] [CrossRef]
- Hind, A.R.; Bhargava, S.K.; McKinnon, A. At the solid/liquid interface: FTIR/ATR—The tool of choice. Adv. Colloid Interface Sci. 2001, 93, 91–114. [Google Scholar] [CrossRef]
- Schmidt, D.A.; Miki, K. Structural correlations in liquid water: A new interpretation of IR spectroscopy. J. Phys. Chem. A 2007, 111, 10119–10122. [Google Scholar] [CrossRef] [PubMed]
- Wallace, V.M.; Dhumal, N.R.; Zehentbauer, F.M.; Kim, H.J.; Kiefer, J. Revisiting the Aqueous Solutions of Dimethyl Sulfoxide by Spectroscopy in the Mid- and Near-Infrared: Experiments and Car-Parrinello Simulations. J. Phys. Chem. B 2015, 119, 14780–14789. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Jemmis, E.D. Red-, blue-, or no-shift in hydrogen bonds: A unified explanation. J. Am. Chem. Soc. 2007, 129, 4620–4632. [Google Scholar] [CrossRef] [PubMed]
- Dhumal, N.R.; Singh, M.P.; Anderson, J.A.; Kiefer, J.; Kim, H.J. Molecular interactions of a Cu-based metal-organic framework with a confined imidazolium-based ionic liquid: A combined density functional theory and experimental vibrational spectroscopy study. J. Phys. Chem. C 2016, 120, 3295–3304. [Google Scholar] [CrossRef]
- Averett, L.A.; Griffiths, P.R. Effective path length in attenuated total reflection spectroscopy. Anal. Chem. 2008, 80, 3045–3049. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiefer, J.; Zetterberg, J.; Ehn, A.; Evertsson, J.; Harlow, G.; Lundgren, E. Infrared Spectroscopy as Molecular Probe of the Macroscopic Metal-Liquid Interface. Appl. Sci. 2017, 7, 1229. https://doi.org/10.3390/app7121229
Kiefer J, Zetterberg J, Ehn A, Evertsson J, Harlow G, Lundgren E. Infrared Spectroscopy as Molecular Probe of the Macroscopic Metal-Liquid Interface. Applied Sciences. 2017; 7(12):1229. https://doi.org/10.3390/app7121229
Chicago/Turabian StyleKiefer, Johannes, Johan Zetterberg, Andreas Ehn, Jonas Evertsson, Gary Harlow, and Edvin Lundgren. 2017. "Infrared Spectroscopy as Molecular Probe of the Macroscopic Metal-Liquid Interface" Applied Sciences 7, no. 12: 1229. https://doi.org/10.3390/app7121229