Effect of Molybdenum on the Microstructures and Properties of Stainless Steel Coatings by Laser Cladding
Abstract
:1. Introduction
2. Materials and Methods
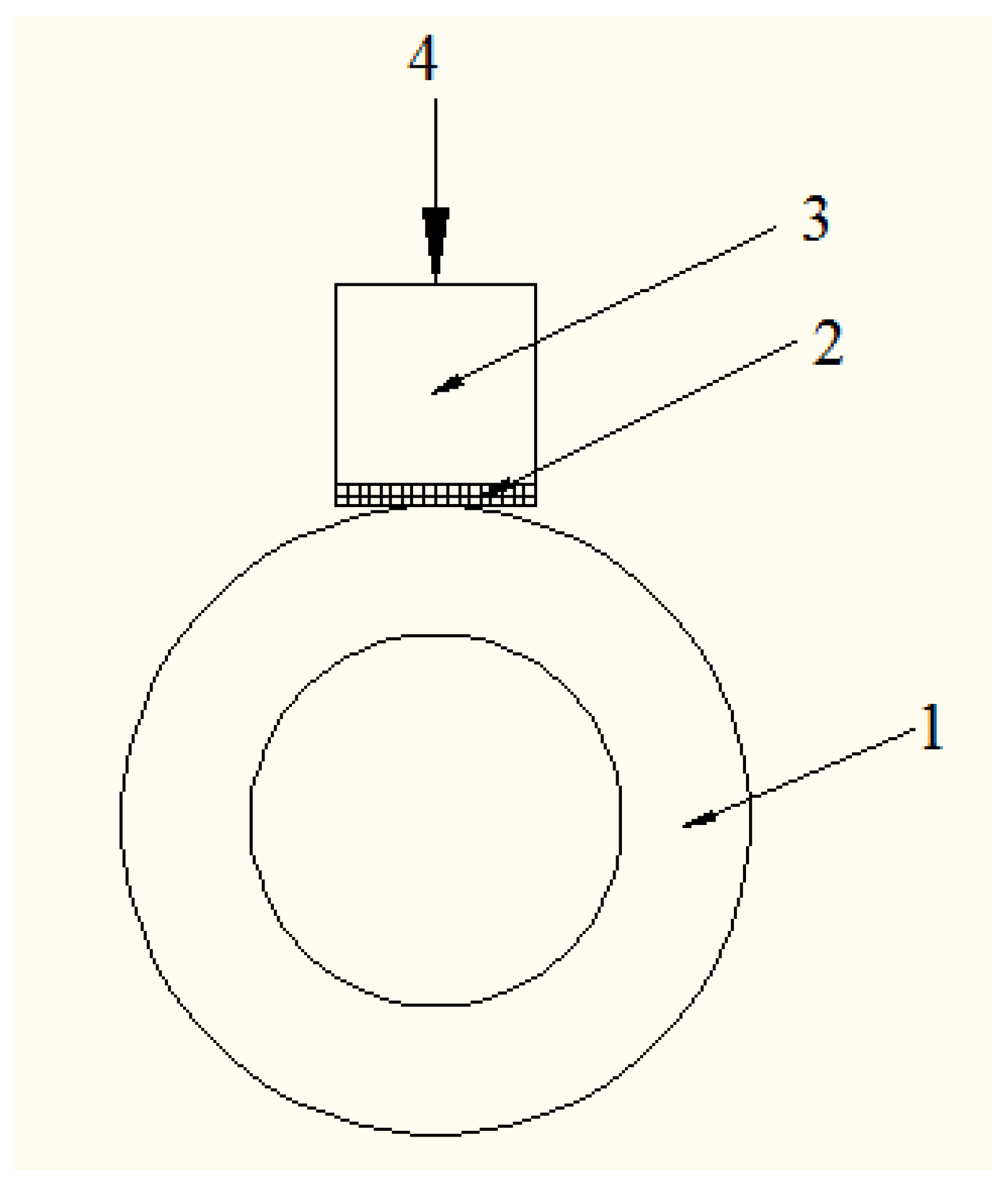
2.1. Specimen Preparation
2.2. Microstructure Observation
2.3. Hardness and Wear Resistance Test
2.4. Electrochemical Corrosion
3. Results and Discussion
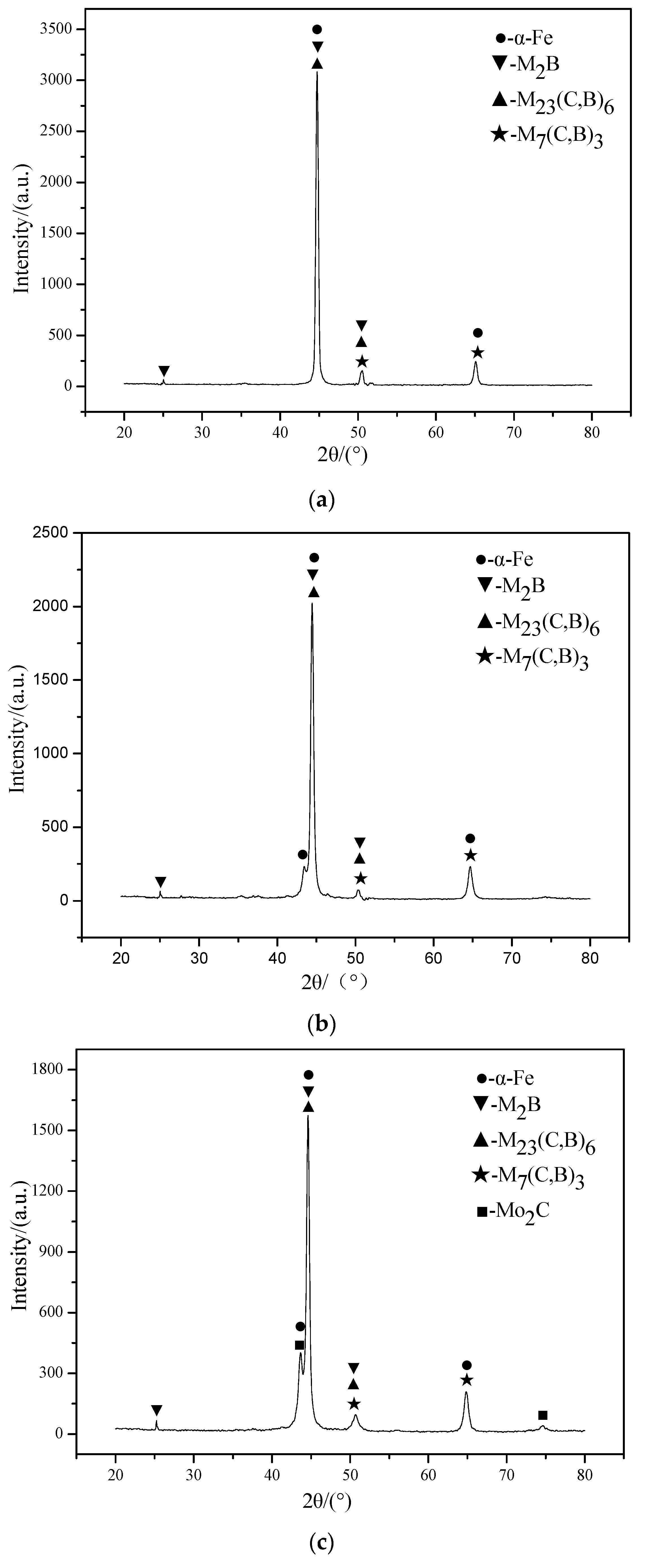
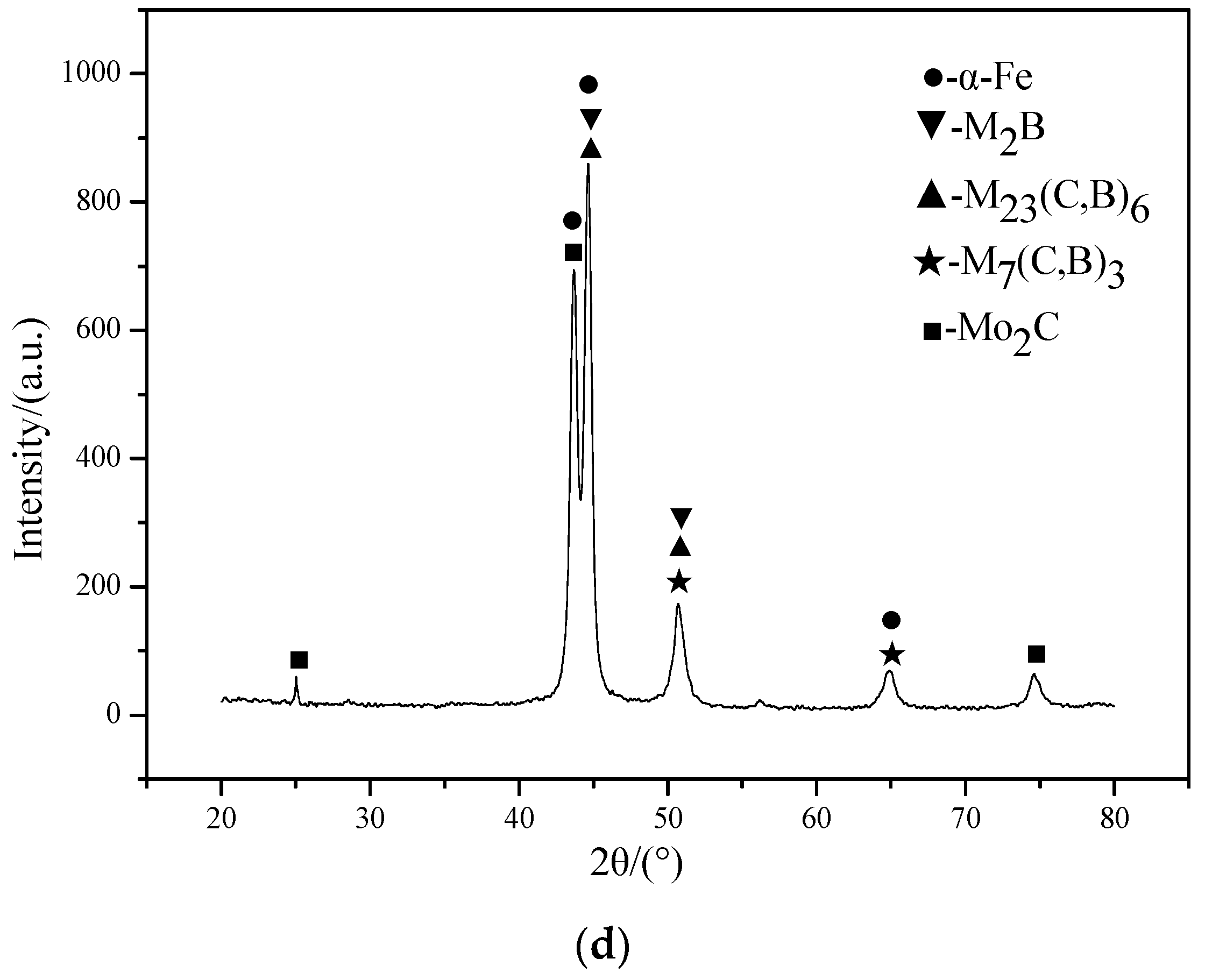
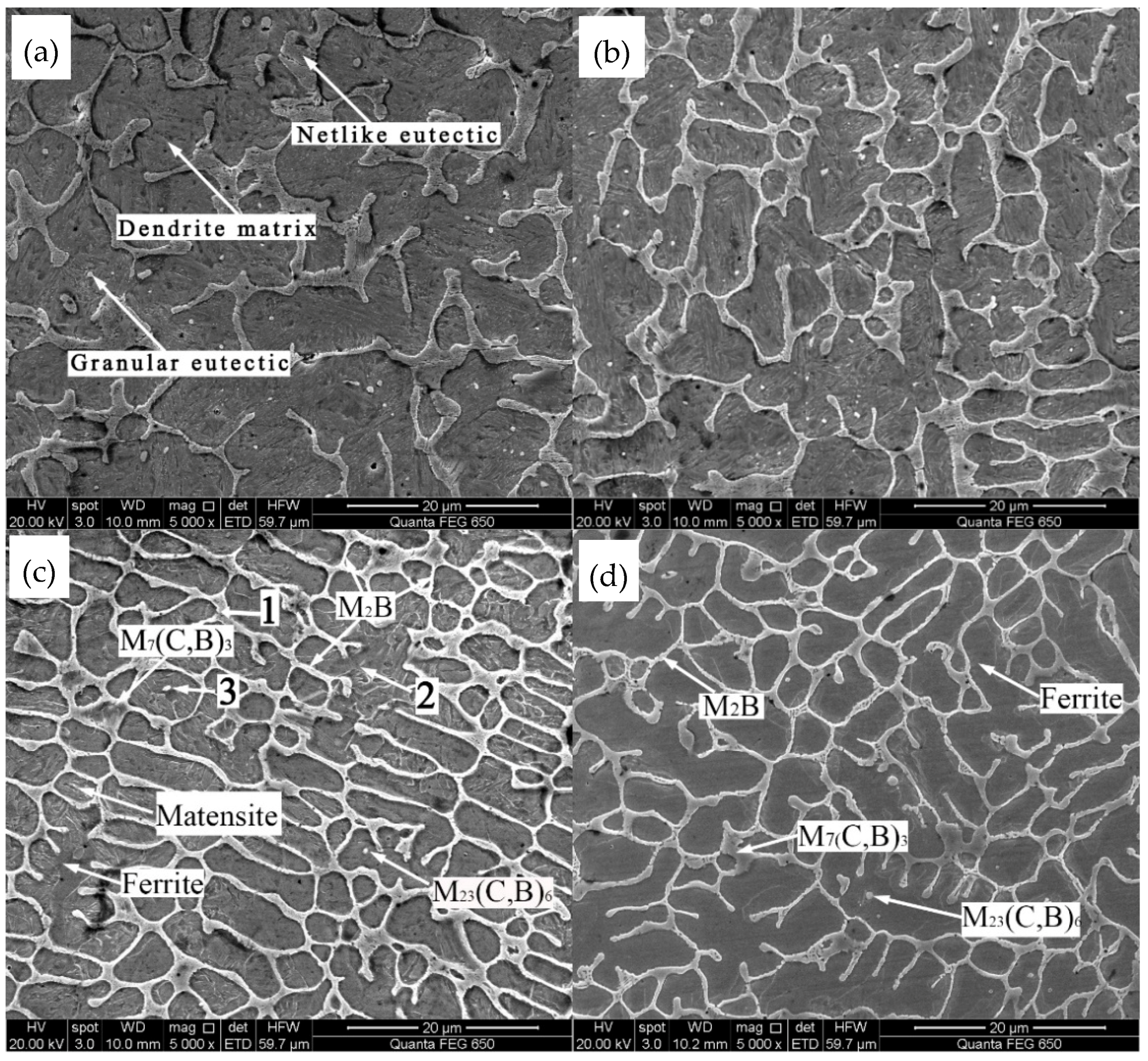
3.1. Microstructure Analysis
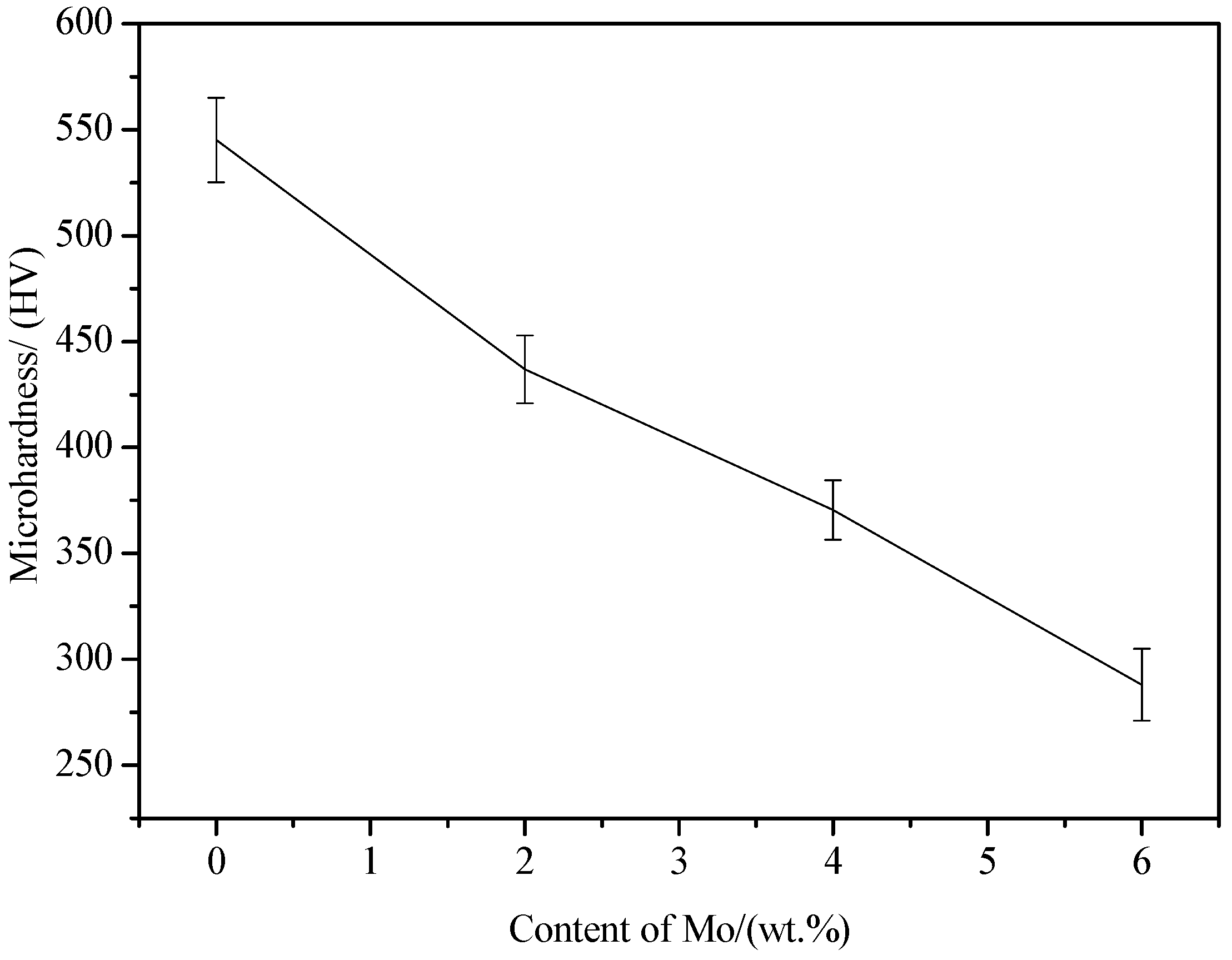
3.2. Hardness Analysis
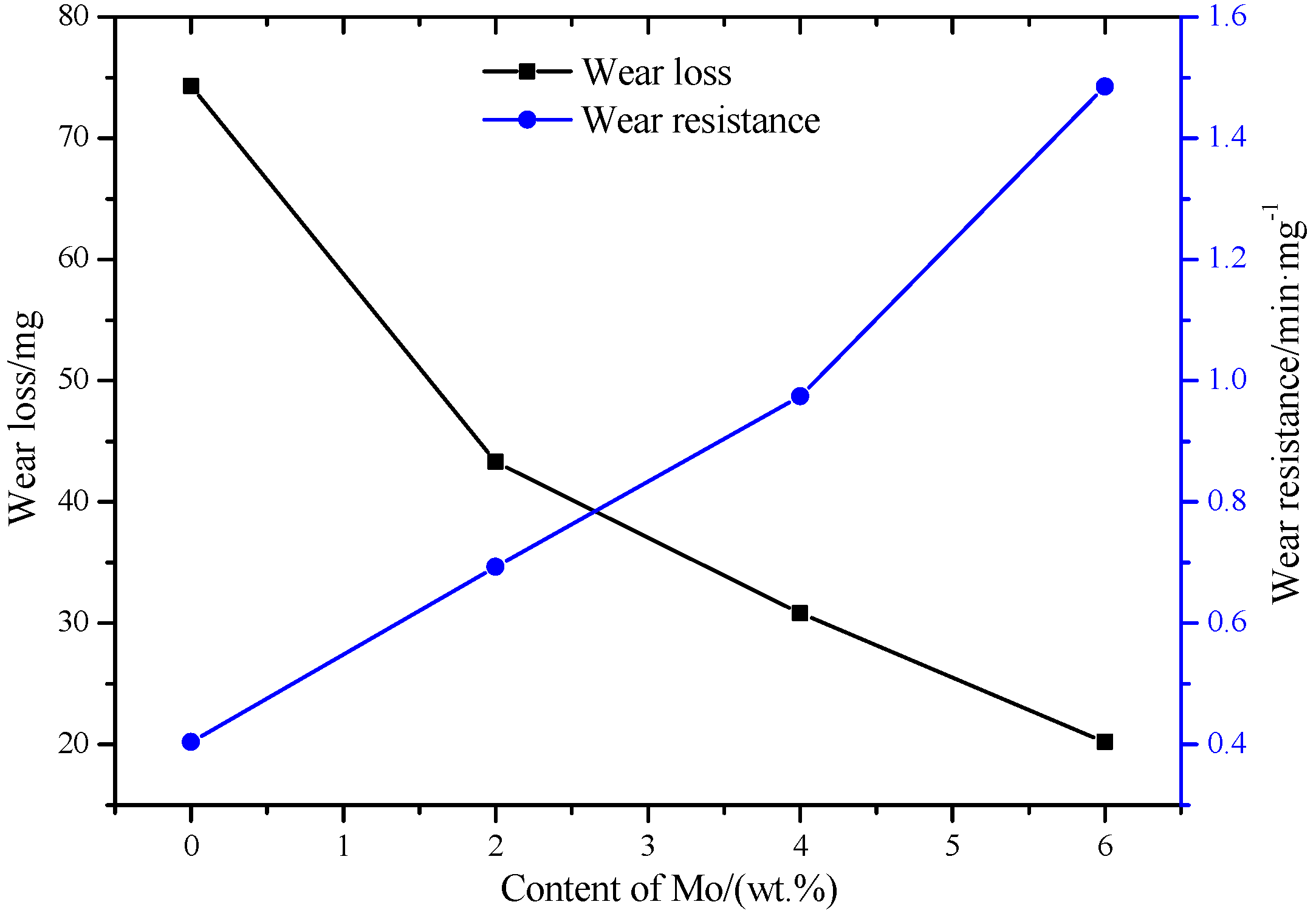
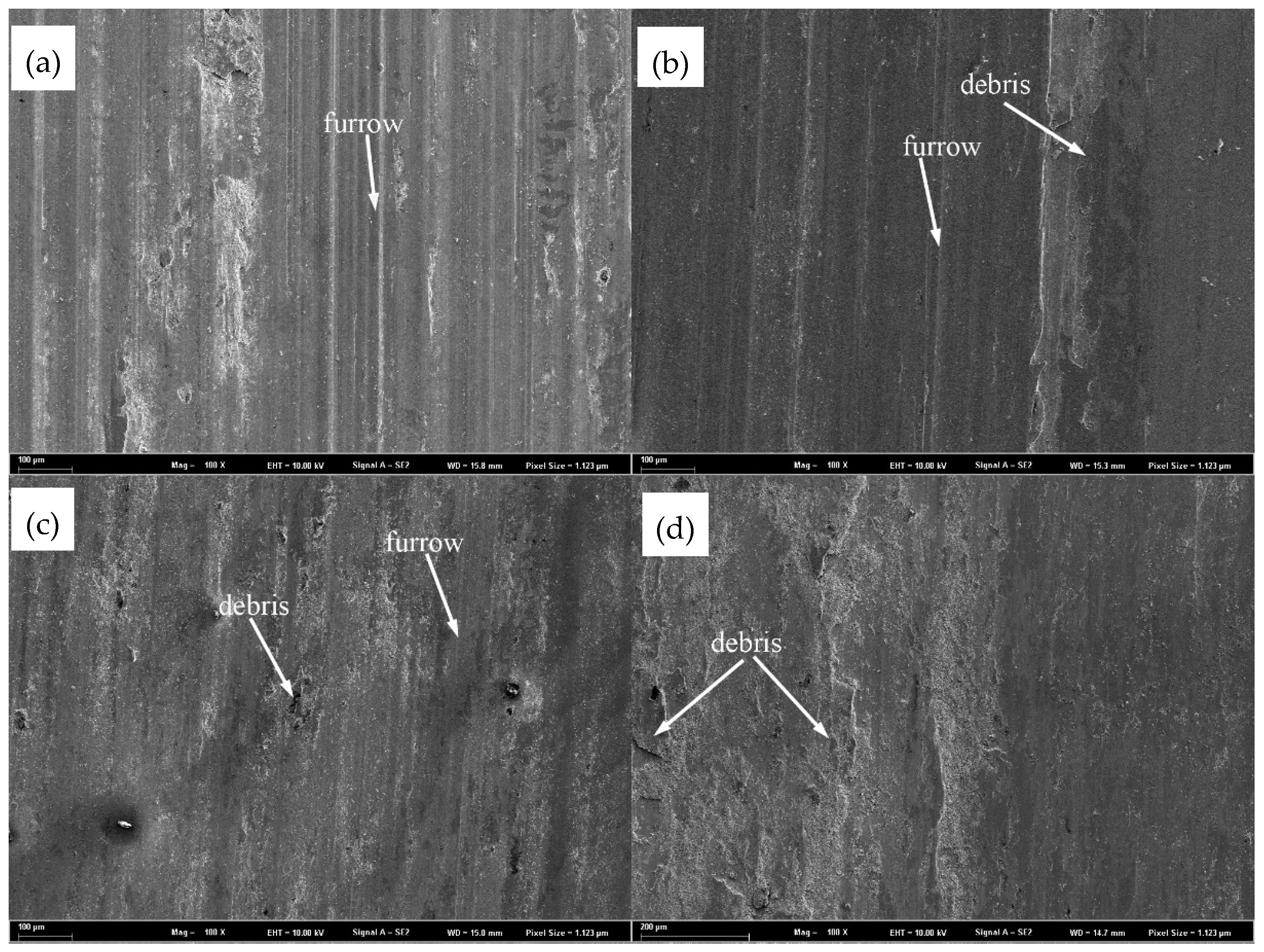
3.3. Wear Resistance
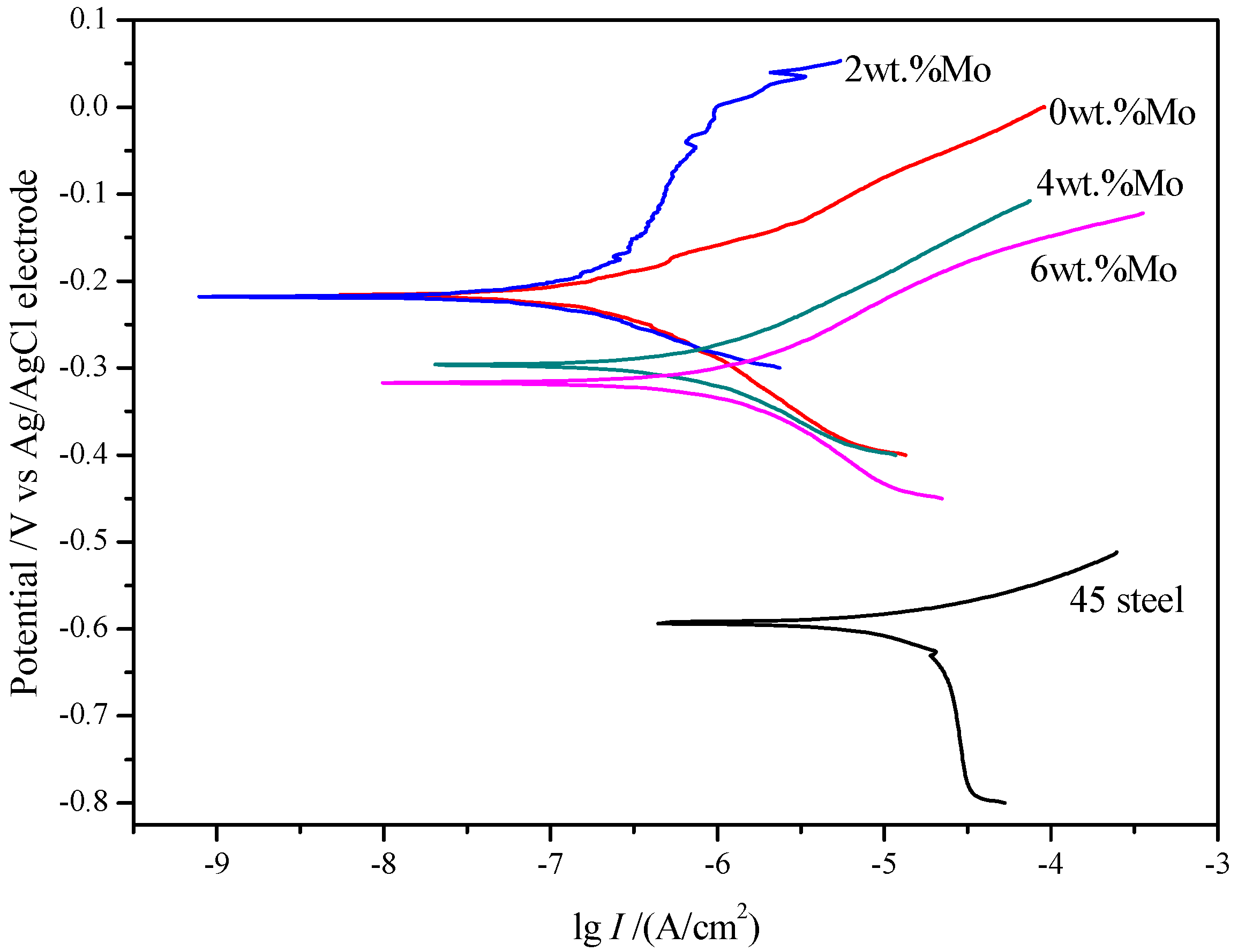
3.4. Electrochemical Test Analysis
4. Conclusions
- (1)
- With the increase of Mo content, the amount of M7(C, B)3 decreases gradually, while the amount of M2B, M23(C, B)6, and Mo2C gradually increases. When the Mo content is 4.0 wt. % and above, Mo2C phase appears in the coating.
- (2)
- The microstructure of the coatings is transformed from planar crystals to cell crystals, dendrites, and equiaxed crystals with the increase of distance from the fusion line. The amount of martensite decreases, while the amount of ferrite gradually increases with the increase of Mo content.
- (3)
- The microhardness decreases and the wear resistance of the coatings gradually increases with the increase of Mo content. The wear resistance of the 6.0 wt. % Mo coating is about 3.7 times that of the Mo-free coating.
- (4)
- The corrosion resistance of the 45 steel substrate is significantly improved by the laser cladding coating. With the increase of Mo content, the corrosion resistance of the coating first increases and then decreases. The coating with 2.0 wt. % Mo has the best corrosion resistance.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vera, R.; Rosales, B.M.; Tapia, C. Effect of the exposure angle in the corrosion rate of plain carbon steel in a marine atmosphere. Corros. Sci. 2003, 45, 321–337. [Google Scholar] [CrossRef]
- Storojeva, L.; Ponge, D.; Kaspar, R.; Raabe, D. Development of microstructure and texture of medium carbon steel during heavy warm deformation. Acta. Mater. 2004, 52, 2209–2220. [Google Scholar] [CrossRef]
- Yang, Y.W.; Fu, H.G.; Lei, Y.P.; Wang, K.M.; Zhu, L.L.; Jiang, L. Phase Diagram Calculation and Analyze on Cast High-Boron High-Speed Steel. J. Mater. Eng. Perform. 2015, 25, 409–420. [Google Scholar] [CrossRef]
- Kolman, D.G. A review of the potential environmentally assisted failure mechanisms of austenitic stainless steel storage containers housing stabilized radioactive compounds. Corros. Sci. 2001, 43, 99–125. [Google Scholar] [CrossRef]
- Ronkainen, H.; Varjus, S.; Holmberg, K. Friction and wear properties in dry, water-and oil-lubricated DLC against alumina and DLC against steel contacts. Wear 1998, 222, 120–128. [Google Scholar] [CrossRef]
- Gao, H.; Jiao, X.; Zhou, C.; Shen, Q.; Yu, Y. Study on Remote Control Underwater Welding Technology Applied in Nuclear Power Station. Proc. Eng. 2011, 15, 4988–4993. [Google Scholar] [CrossRef]
- Birger, E.M.; Moskvitin, G.V.; Polyakov, A.N.; Arkhipov, V.E. Industrial laser cladding: Current state and future. Weld. Int. 2011, 25, 234–243. [Google Scholar] [CrossRef]
- Simunovic, K.; Saric, T.; Simunovic, G. Different Approaches to the Investigation and Testing of the Ni-Based Self-Fluxing Alloy Coatings—A Review. Part 1: General Facts, Wear and Corrosion Investigations. Tribol. Trans. 2014, 57, 955–979. [Google Scholar] [CrossRef]
- Han, B.; Li, M.; Wang, Y. Microstructure and Wear Resistance of Laser Clad Fe-Cr3C2 Composite Coating on 35CrMo Steel. J. Mater. Eng. Perform. 2013, 22, 3749–3754. [Google Scholar] [CrossRef]
- Grum, J.; Žnidaršič, M. Microstructure, Microhardness, and Residual Stress Analysis of Laser Surface Cladding of Low-Carbon Steel. Mater. Manuf. Process. 2004, 19, 243–258. [Google Scholar] [CrossRef]
- Zhao, W.; Zha, G.C.; Kong, F.X.; Wu, M.L.; Feng, X.; Gao, S.Y. Strengthening Effect of Incremental Shear Deformation on Ti Alloy Clad Plate with a Ni-Based Alloy Laser-Clad Layer. J. Mater. Eng. Perform. 2017, 26, 2411–2416. [Google Scholar] [CrossRef]
- Paatsch, W. Energy turnaround—A challenge for surface technology. Trans. Inst. Met. Finish. 2016, 94, 228–230. [Google Scholar] [CrossRef]
- Tan, H.; Luo, Z.; Li, Y.; Yan, F.; Duan, R.; Huang, Y. Effect of strengthening particles on the dry sliding wear behavior of Al2O3–M7C3/Fe metal matrix composite coatings produced by laser cladding. Wear 2015, 324–325, 36–44. [Google Scholar] [CrossRef]
- Ray, A.; Arora, K.S.; Lester, S.; Shome, M. Laser cladding of continuous caster lateral rolls: Microstructure, wear and corrosion characterisation and on-field performance evaluation. J. Mater. Process. Technol. 2014, 214, 1566–1575. [Google Scholar] [CrossRef]
- Peng, H.; Li, R.; Yuan, T.; Wu, H.; Yan, H. Producing nanostructured Co–Cr–W alloy surface layer by laser cladding and friction stir processing. J. Mater. Res. 2015, 30, 717–726. [Google Scholar] [CrossRef]
- Xu, G.J.; Kutsuna, M. Cladding with Stellite 6 + WC using a YAG laser robot system. Surf. Eng. 2013, 22, 345–352. [Google Scholar] [CrossRef]
- Gao, W.; Zhao, S.; Liu, F.; Wang, Y.; Zhou, C.; Lin, X. Effect of defocus manner on laser cladding of Fe-based alloy powder. Surf. Coat. Technol. 2014, 248, 54–62. [Google Scholar] [CrossRef]
- Zeng, Q.; Sun, J.; Emori, W.; Jiang, S.L. Corrosion Behavior of Thermally Sprayed NiCrBSi Coating on 16MnR Low-Alloy Steel in KOH Solution. J. Mater. Eng. Perform. 2016, 25, 1773–1780. [Google Scholar] [CrossRef]
- Zhou, S.; Dai, X.; Zheng, H. Microstructure and wear resistance of Fe-based WC coating by multi-track overlapping laser induction hybrid rapid cladding. Opt. Laser. Technol. 2012, 44, 190–197. [Google Scholar] [CrossRef]
- Qu, S.; Wang, X.; Zhang, M.; Zou, Z. Microstructure and wear properties of Fe–TiC surface composite coating by laser cladding. J. Mater. Sci. 2008, 43, 1546–1551. [Google Scholar] [CrossRef]
- Zhou, S.; Dai, X.; Xiong, Z.; Wu, C.; Zhang, T.; Zhang, Z. Influence of Al addition on microstructure and properties of Cu–Fe-based coatings by laser induction hybrid rapid cladding. J. Mater. Res. 2014, 29, 865–873. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, S.; Gao, W.; Zhou, C.; Liu, F.; Lin, X. Microstructure and properties of laser cladding FeCrBSi composite powder coatings with higher Cr content. J. Mater. Process. Technol. 2014, 214, 899–905. [Google Scholar] [CrossRef]
- Lei, Y.; Sun, R.; Lei, J.; Tang, Y.; Niu, W. A new theoretical model for high power laser clad TiC/NiCrBSiC composite coatings on Ti6Al4V alloys. Opt. Lasers Eng. 2010, 48, 899–905. [Google Scholar] [CrossRef]
- Xu, J.S.; Zhang, X.C.; Xuan, F.Z.; Wang, Z.D.; Tu, S.T. Microstructure and Sliding Wear Resistance of Laser Cladded WC/Ni Composite Coatings with Different Contents of WC Particle. J. Mater. Eng. Perform. 2011, 21, 1904–1911. [Google Scholar] [CrossRef]
- Qu, K.L.; Wang, X.H.; Wang, Z.K.; Niu, W.Y. Effect of Mo on the VC–VB particles reinforced Fe-based composite coatings. Mater. Sci. Technol. 2016, 33, 333–339. [Google Scholar] [CrossRef]
- Ma, Q.S.; Li, Y.J.; Wang, J. Effects of Ti addition on microstructure homogenization and wear resistance of wide-band laser clad Ni60/WC composite coatings. Int. J. Refract. Met. Hard. Mater. 2017, 64, 225–233. [Google Scholar]
- Lin, Y.; Lei, Y.; Fu, H.; Lin, J. Mechanical properties and toughening mechanism of TiB2/NiTi reinforced titanium matrix composite coating by laser cladding. Mater. Des. 2015, 80, 82–88. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Wang, C.L.; Gao, Y. Mechanism of Rare Earth CeO2 on the Ni-Based Laser Cladding Layer of 6063 Al Surface. Rare Met. Mater. Eng. 2016, 45, 1002–1006. [Google Scholar]
- Weng, F.; Yu, H.; Chen, C.; Liu, J.; Zhao, L. Microstructures and properties of TiN reinforced Co-based composite coatings modified with Y2O3 by laser cladding on Ti–6Al–4V alloy. J. Alloys Compd. 2015, 650, 178–184. [Google Scholar] [CrossRef]
- Hou, Q.Y.; He, Y.Z.; Zhang, Q.A.; Gao, J.S. Influence of molybdenum on the microstructure and wear resistance of nickel-based alloy coating obtained by plasma transferred arc process. Mater. Des. 2007, 28, 1982–1987. [Google Scholar] [CrossRef]
- Wang, X.H.; Han, F.; Liu, X.M.; Qu, S.Y.; Zou, Z.D. Effect of molybdenum on the microstructure and wear resistance of Fe-based hardfacing coatings. Mater. Sci. Eng. A. 2008, 489, 193–200. [Google Scholar] [CrossRef]
- Ding, L.; Hu, S.; Quan, X.; Shen, J. Effect of Mo and nano-Nd2O3 on the microstructure and wear resistance of laser cladding Ni-based alloy coatings. Appl. Phys. A Mater. 2016, 122, 288. [Google Scholar] [CrossRef]
- Wang, K.M.; Fu, H.G.; Lei, Y.P.; Yang, Y.W.; Li, Q.T.; Su, Z.Q. Microstructure and property of Ni60A/WC composite coating fabricated by fiber laser cladding. Materialwiss. Werkstofftech. 2015, 46, 1177–1184. [Google Scholar] [CrossRef]
- Li, Q.; Lei, Y.; Fu, H. Growth mechanism, distribution characteristics and reinforcing behavior of (Ti, Nb)C particle in laser cladded Fe-based composite coating. Appl. Surf. Sci. 2014, 316, 610–616. [Google Scholar] [CrossRef]
- Kurz, W.; Giovanola, B.; Trivedi, R. Theory of microstructural development during rapid solidification. Acta. Metall. 1986, 34, 823–830. [Google Scholar] [CrossRef]
- Bansal, A.; Zafar, S.; Sharma, A.K. Microstructure and Abrasive Wear Performance of Ni-Wc Composite Microwave Clad. J. Mater. Eng. Perform. 2015, 24, 3708–3716. [Google Scholar] [CrossRef]
- Liu, Z.; Qi, H. Effects of substrate crystallographic orientations on crystal growth and microstructure formation in laser powder deposition of nickel-based superalloy. Acta Mater. 2015, 87, 248–258. [Google Scholar] [CrossRef]
- Li, B.; Jin, Z.; Ren, H.; Zhang, L.; Qu, W. Effect of molybdenum on microstructure and properties of low alloy high strength steel. Heat Treat. Met. 2016, 41, 145–147. [Google Scholar]
- Shin, J.C.; Doh, J.M.; Yoon, J.K.; Lee, D.Y.; Kim, J.S. Effect of molybdenum on the microstructure and wear resistance of cobalt-base Stellite hardfacing alloys. Surf. Coat. Technol. 2003, 166, 117–126. [Google Scholar] [CrossRef]
- Jarrett, R.N.; Tien, J.K. Effects of cobalt on structure, microchemistry and properties of a wrought nickel-base superalloy. Metall. Trans. A 1982, 13, 1021–1032. [Google Scholar] [CrossRef]
- Li, B.; Jin, Y.; Yao, J.; Li, Z.; Zhang, Q. Solid-state fabrication of WC p-reinforced Stellite-6 composite coatings with supersonic laser deposition. Surf. Coat. Technol. 2017, 321, 386–396. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, L. Microstructure of 10CrMnMo dual-phase steel under different intercritical quenching temperature. Heat Treat. Met. 2017, 42, 110–114. [Google Scholar]
- Min, T.; Gao, Y.; Li, Y.; Yang, Y.; Li, R.; Xie, X. First-Principles Calculations Study on the Electronic Structures, Hardness and Debye Temperatures of Chromium Carbides. Rare Met. Mater. Eng. 2012, 41, 271–275. [Google Scholar]
- Hou, Q.Y.; He, Y.Z.; Gao, J.S. Microstructure and wear resistance of Mo /Ni-based alloy coating produced by plasma cladding. Chin. J. Nonferrous Met. 2006, 16, 1595–1602. [Google Scholar]
- Xiong, Z.; Xu, Q.; He, Q.; Wang, Y. The effect of Mo content on the organization and the performance of the hypoeutectic high carbon, high chromium alloy. Electr. Weld. Mach. 2016, 46, 59–62. [Google Scholar]
- Liu, Z.J.; Liu, C.; Chen, H.; Li, Y.K.; Cheng, J.B.; Su, Y.H.; Liu, D. Impact wear-resistant hardfacing austenitic material. Trans. China Weld. Inst. 2005, 26, 9–12. [Google Scholar]
- Hu, Y.W.; Yin, Y.S. Analysis of affecting factors of wearing resistance of surfacing layer. J. Shenyang Univ. Technol. 2002, 24, 386–388. [Google Scholar]
- Li, D.; Zhou, F.; Yu, S. Microstructure and corrosion resistance of FeCrNiMnMoxB0.5 high entropy alloy coating prepared by laser cladding. High Power Laser Part. Beams 2016, 28, 1–6. [Google Scholar]

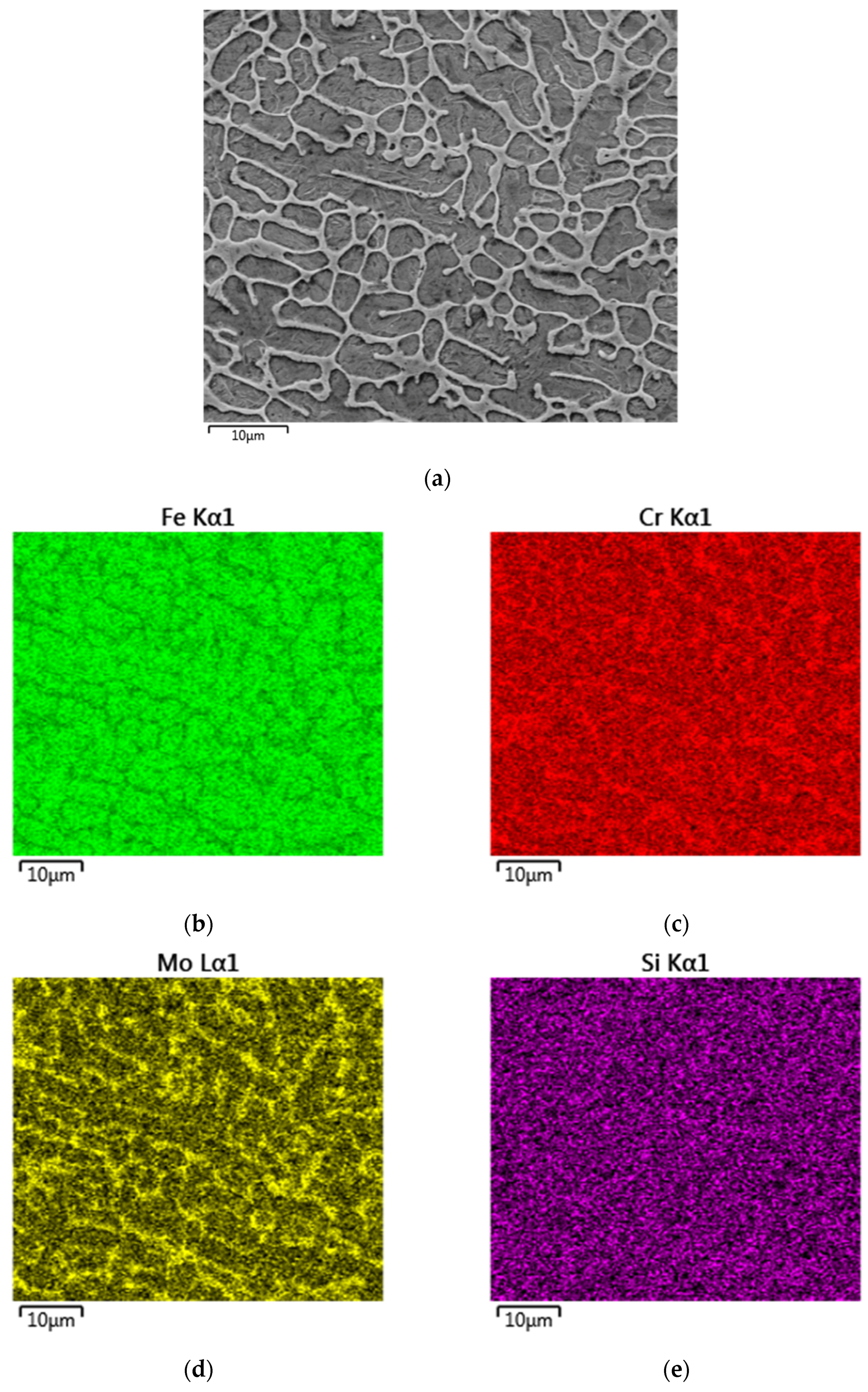
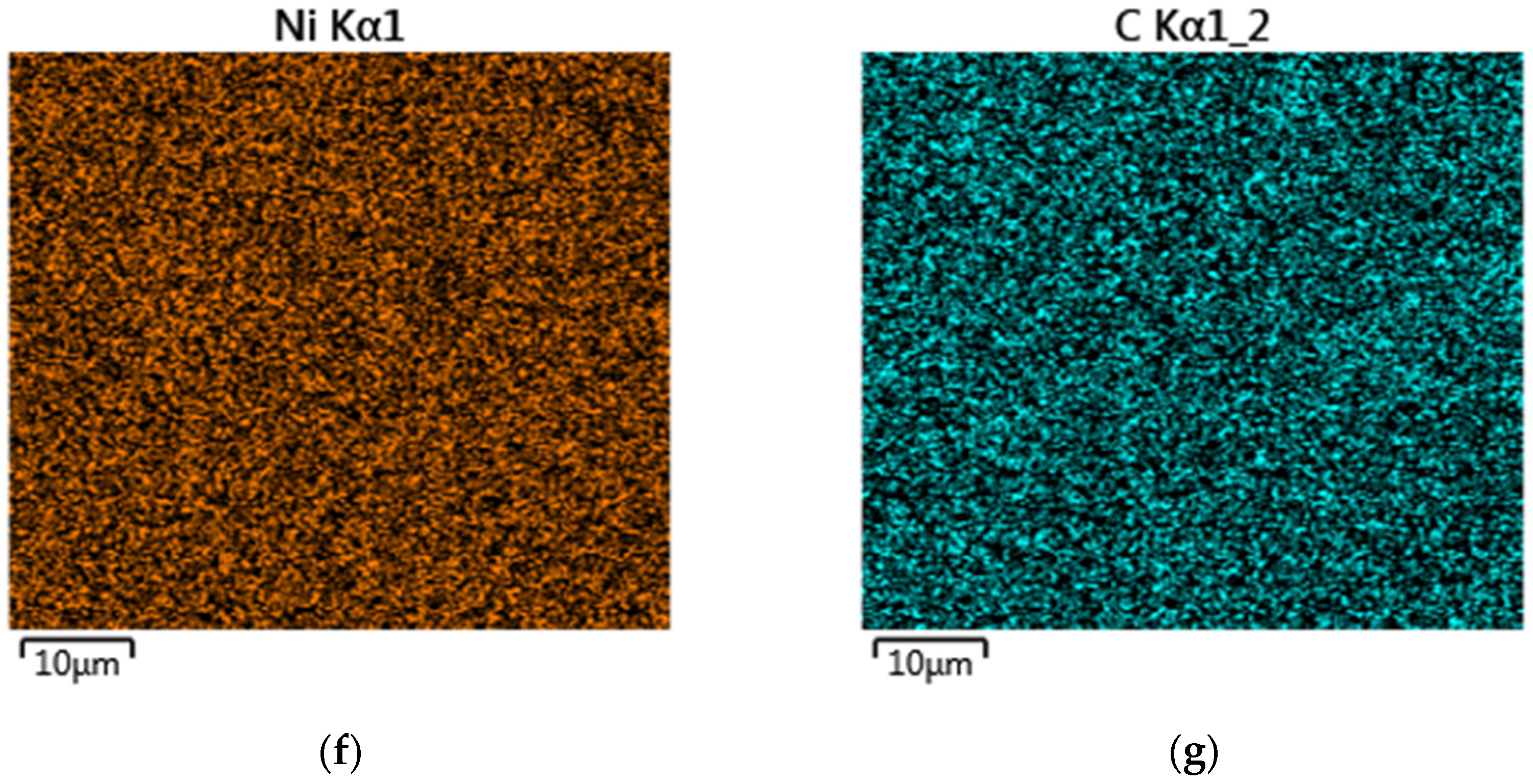
| Element | C | Cr | Ni | Si | Mn | B | Co | Fe |
|---|---|---|---|---|---|---|---|---|
| 45 steel | 0.44 | 0.15 | 0.15 | 0.24 | 0.69 | - | - | Bal. |
| 431 powder | 0.19 | 18.44 | 2.40 | 0.75 | 0.26 | 0.87 | 0.53 | Bal. |
| Point | Cr | C | B | Mo | Ni | Si | Fe |
|---|---|---|---|---|---|---|---|
| 1 | 19.978 | 4.877 | 4.789 | 4.674 | 1.910 | 0.428 | 63.344 |
| 2 | 15.336 | 2.606 | - | 1.801 | 1.937 | 0.783 | 77.537 |
| 3 | 14.224 | 11.034 | 0.139 | 1.780 | 1.697 | 0.779 | 70.347 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Chang, B.; Chen, J.; Fu, H.; Lin, Y.; Lei, Y. Effect of Molybdenum on the Microstructures and Properties of Stainless Steel Coatings by Laser Cladding. Appl. Sci. 2017, 7, 1065. https://doi.org/10.3390/app7101065
Wang K, Chang B, Chen J, Fu H, Lin Y, Lei Y. Effect of Molybdenum on the Microstructures and Properties of Stainless Steel Coatings by Laser Cladding. Applied Sciences. 2017; 7(10):1065. https://doi.org/10.3390/app7101065
Chicago/Turabian StyleWang, Kaiming, Baohua Chang, Jiongshen Chen, Hanguang Fu, Yinghua Lin, and Yongping Lei. 2017. "Effect of Molybdenum on the Microstructures and Properties of Stainless Steel Coatings by Laser Cladding" Applied Sciences 7, no. 10: 1065. https://doi.org/10.3390/app7101065





