Aligned Nanofiber Topography Directs the Tenogenic Differentiation of Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Scaffold Manufacturing and Characterization
2.2. Environmental Scanning Electron Microscopy
2.3. Isolation and Characterization of Primary Equine Mesenchymal Stem Cells
2.4. Cell Culture and Scaffold Seeding
2.5. Phase-Contrast and Time-Lapse Microscopy
2.6. Fluorescence Microscopy
2.7. RNA Isolation, Quantification, and Quantitative PCR
2.8. Biochemical Assay of Media Content
2.9. Statistical Analysis
3. Results
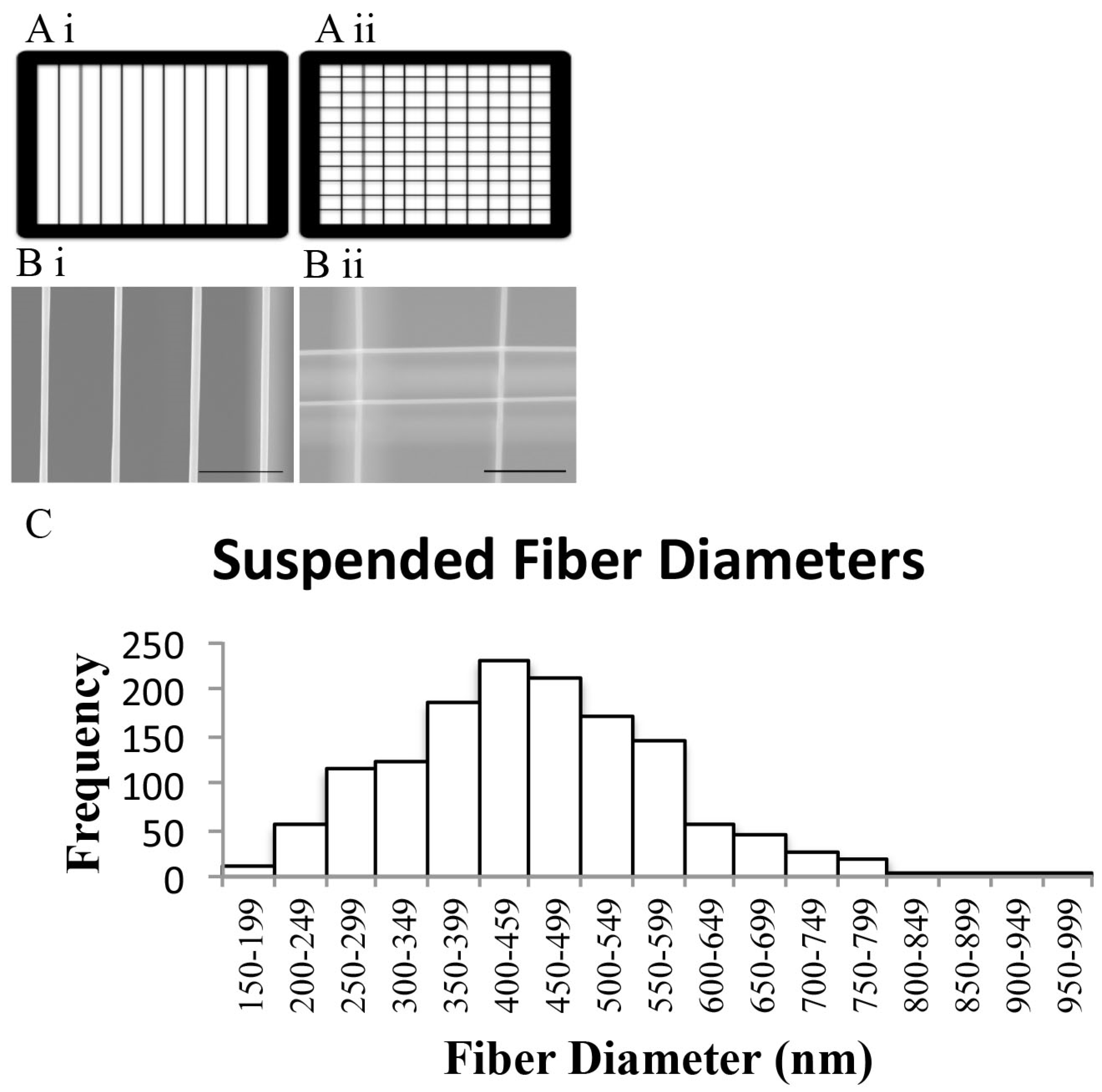
3.1. STEP-Manufactured Fibers Exhibit Fibril-Like Organization
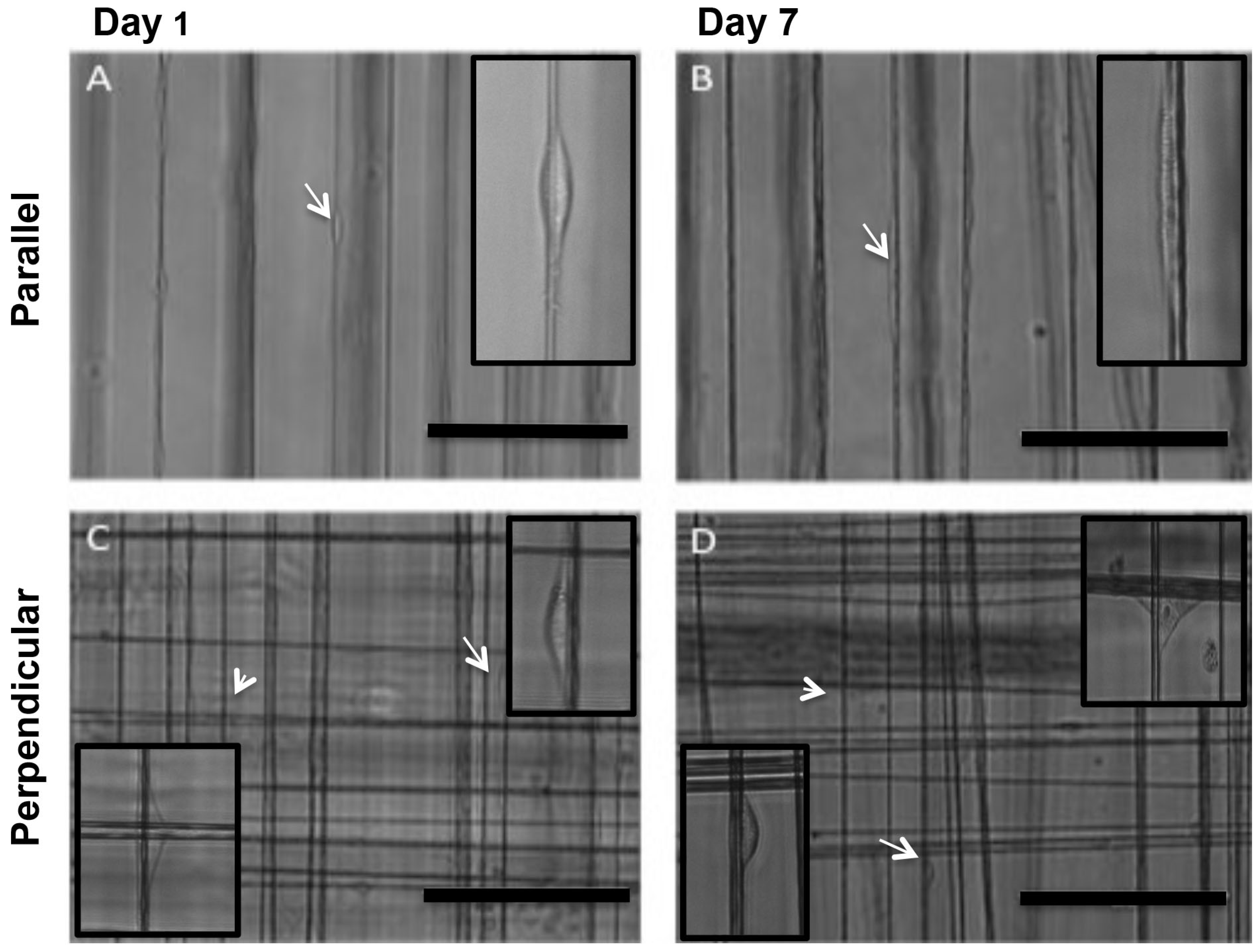
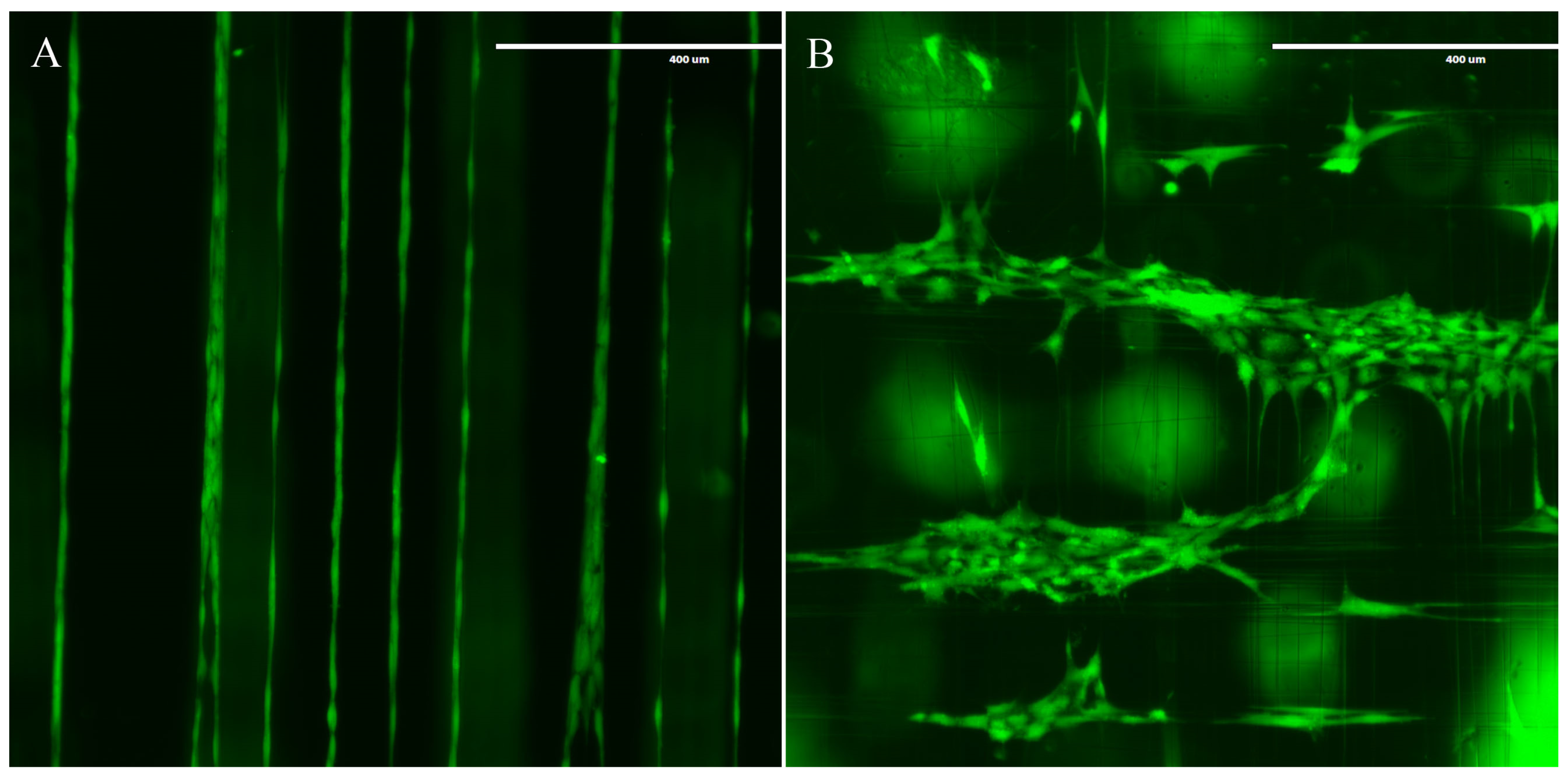
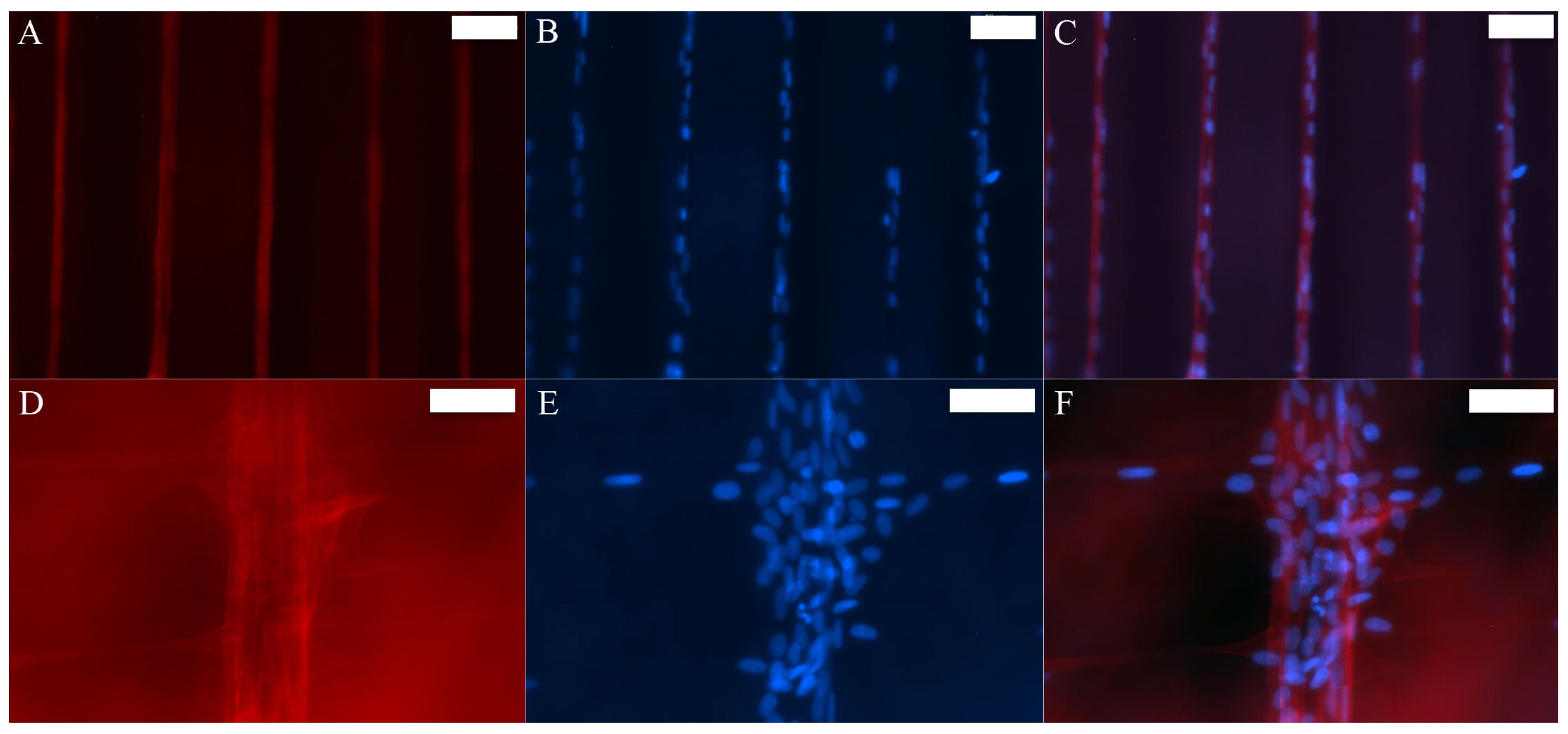
3.2. MSCs Exhibit Tenocyte-Like Morphology on Suspended Fibrous Scaffolds
3.3. Tendon-Associated Gene Expression Increased over Time
3.4. ECM Production Increased over Time
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Spaas, J.H.; Guest, D.J.; Van de Walle, G.R. Tendon regeneration in human and equine athletes: Ubi Sumus-Quo Vadimus (where are we and where are we going to)? Sports Med. 2012, 42, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Webb, W.R.; Dale, T.P.; Lomas, A.J.; Zeng, G.D.; Wimpenny, I.; El Haj, A.J.; Forsyth, N.R.; Chen, G.Q. The application of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds for tendon repair in the rat model. Biomaterials 2013, 34, 6683–6694. [Google Scholar] [CrossRef] [PubMed]
- Randelli, P.; Conforti, E.; Piccoli, M.; Ragone, V.; Creo, P.; Cirillo, F.; Masuzzo, P.; Tringali, C.; Cabitza, P.; Tettamanti, G.; et al. Isolation and characterization of 2 new human rotator cuff and long head of biceps tendon cells possessing stem cell-like self-renewal and multipotential differentiation capacity. Am. J. Sport Med. 2013, 41, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Mazzocca, A.D.; McCarthy, M.B.R.; Chowaniec, D.; Cote, M.P.; Judson, C.H.; Apostolakos, J.; Solovyova, O.; Beitzel, K.; Arciero, R.A. Bone marrow-derived mesenchymal stem cells obtained during arthroscopic rotator cuff repair surgery show potential for tendon cell differentiation after treatment with insulin. Arthroscopy 2011, 27, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Czaplewski, S.K.; Tsai, T.-L.; Duenwald-Kuehl, S.E.; Vanderby, R., Jr.; Li, W.-J. Tenogenic differentiation of human induced pluripotent stem cell-derived mesenchymal stem cells dictated by properties of braided submicron fibrous scaffolds. Biomaterials 2014, 35, 6907–6917. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.; Jana, S.; Tsao, C.T.; Zhang, M.Q. Tenogenic differentiation of human bone marrow stem cells via a combinatory effect of aligned chitosan-polycaprolactone nanofibers and TGF-beta 3. J. Mater. Chem. B 2013, 1, 6516–6524. [Google Scholar] [CrossRef]
- Jones, A.J.; Bee, J.A. Age-related and position-related heterogeneity of equine tendon extracellular-matrix composition. Res. Vet. Sci. 1990, 48, 357–364. [Google Scholar] [PubMed]
- Lin, Y.L.; Brama, P.A.J.; Kiers, G.H.; van Weeren, P.R.; DeGroot, J. Extracellular matrix compositon of the equine superficial digital flexor tendon: Relationship with age and anatomical site. J. Vet. Med. A 2005, 52, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.H.; Zhu, S.A.; Zhang, C.; Lu, P.; Hu, J.J.; Yin, Z.; Ma, Y.; Chen, X.; OuYang, H.W. Crucial transcription factors in tendon development and differentiation: Their potential for tendon regeneration. Cell Tissue Res. 2014, 356, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Murchison, N.D.; Price, B.A.; Conner, D.A.; Keene, D.R.; Olson, E.N.; Tabin, C.J.; Schweitzer, R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 2007, 134, 2697–2708. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.A.; Johnson, S.E. Expression of scleraxis and tenascin C in equine adipose and umbilical cord blood derived stem cells is dependent upon substrata and FGF supplementation. Cytotechnology 2014, 66, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Birch, H.L.; Bailey, J.V.B.; Bailey, A.J.; Goodship, A.E. Age-related changes to the molecular and cellular components of equine flexor tendons. Equine Vet. J. 1999, 31, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.N.; Sorushanova, A.; Lomas, A.J.; Mullen, A.M.; Pandit, A.; Zeugolis, D.I. Glycosaminoglycans in tendon physiology, pathophysiology, and therapy. Bioconj. Chem. 2015, 26, 1237–1251. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Jang, D.H.; Park, W.H.; Min, B.M. Fabrication and characterization of 3-dimensional PLGA nanofiber/microfiber composite scaffolds. Polymer 2010, 51, 1320–1327. [Google Scholar] [CrossRef]
- Orr, S.B.; Chainani, A.; Hippensteel, K.J.; Kishan, A.; Gilchrist, C.; Garrigues, N.W.; Ruch, D.S.; Guilak, F.; Little, D. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015, 24, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Chen, X.; Chen, J.L.; Shen, W.L.; Nguyen, T.M.H.; Gao, L.; Ouyang, H.W. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials 2010, 31, 2163–2175. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Nai, M.H.; Lim, C.T.; Le Visage, C.; Chan, J.K.Y.; Chew, S.Y. Polysaccharide nanofibers with variable compliance for directing cell fate. J. Biomed. Mater. Res. Part A 2015, 103, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Chainani, A.; Hippensteel, K.J.; Kishan, A.; Garrigues, N.W.; Ruch, D.S.; Guilak, F.; Little, D. Multilayered electrospun scaffolds for tendon tissue engineering. Tissue Eng. Part A 2013, 19, 2594–2604. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Huang, C.; Ke, Q.F.; He, C.L.; Wang, H.S.; Mo, X.M. Preparation and characterization of coaxial electrospun thermoplastic polyurethane/collagen compound nanofibers for tissue engineering applications. Colloid Surf. B 2010, 79, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Kazanci, M. Solvent and temperature effects on folding of electrospun collagen nanofibers. Mater. Lett. 2014, 130, 223–226. [Google Scholar] [CrossRef]
- Zhong, S.P.; Teo, W.E.; Zhu, X.; Beuerman, R.W.; Ramakrishna, S.; Yung, L.Y.L. An aligned nanofibrous collagen scaffold by electrospinning and its effects on in vitro fibroblast culture. J. Biomed. Mater. Res. Part A 2006, 79A, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, C.L.; Ruch, D.S.; Little, D.; Guilak, F. Micro-scale and meso-scale architectural cues cooperate and compete to direct aligned tissue formation. Biomaterials 2014, 35, 10015–10024. [Google Scholar] [CrossRef] [PubMed]
- Kishore, V.; Bullock, W.; Sun, X.H.; Van Dyke, W.S.; Akkus, O. Tenogenic differentiation of human MSCs induced by the topography of electrochemically aligned collagen threads. Biomaterials 2012, 33, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Nain, A.S.; Sitti, M.; Jacobson, A.; Kowalewski, T.; Amon, C. Dry spinning based spinneret based tunable engineered parameters (STEP) technique for controlled and aligned deposition of polymeric nanofibers. Macromol. Rapid Commun. 2009, 30, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Sheets, K.; Wang, J.; Meehan, S.; Sharma, P.; Ng, C.; Khan, M.; Koons, B.; Behkam, B.; Nain, A.S. Cell-fiber interactions on aligned and suspended nanofiber scaffolds. J. Biomater. Tissue Eng. 2013, 3, 355–368. [Google Scholar] [CrossRef]
- Wang, J.; Nain, A.S. Suspended micro/nanofiber hierarchical biological scaffolds fabricated using non-electrospinning STEP technique. Langmuir 2014, 30, 13641–13649. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Meth. 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.A.; Barrett, J.G.; Byron, C.R.; Yates, A.C.; Durgarn, S.S.; Evans, R.B.; Stewart, M.C. Comparison of equine tendon-, muscle-, and bone marrow-derived cells cultured on tendon matrix. Am. J. Vet. Res. 2009, 70, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Farndale, R.W.; Buttle, D.J.; Barrett, A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim. Biophys. Acta (BBA) Gen. Subj. 1986, 883, 173–177. [Google Scholar] [CrossRef]
- Voleti, P.B.; Buckley, M.R.; Soslowsky, L.J. Tendon healing: Repair and regeneration. Annu. Rev. Biomed. Eng. 2012, 14, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Youngstrom, D.W.; Barrett, J.G.; Jose, R.R.; Kaplan, D.L. Functional characterization of detergent-decellularized equine tendon extracellular matrix for tissue engineering applications. PLoS ONE 2013, 8, e64151. [Google Scholar] [CrossRef] [PubMed]
- Younesi, M.; Islam, A.; Kishore, V.; Anderson, J.M.; Akkus, O. Tenogenic induction of human MSCs by anisotropically aligned collagen biotextiles. Adv. Funct. Mater. 2014, 24, 5762–5770. [Google Scholar] [CrossRef] [PubMed]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef]
- Miyoshi, H.; Adachi, T. Topography design concept of a tissue engineering scaffold for controlling cell function and fate through actin cytoskeletal modulation. Tissue Eng. 2014, 20, 609–627. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Chen, X.; Song, H.-X.; Hu, J.-J.; Tang, Q.-M.; Zhu, T.; Shen, W.-L.; Chen, J.-L.; Liu, H.; Heng, B.C.; et al. Electrospun scaffolds for multiple tissues regeneration in vivo through topography dependent induction of lineage specific differentiation. Biomaterials 2015, 44, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Maharam, E.; Yaport, M.; Villanueva, N.L.; Akinyibi, T.; Laudier, D.; He, Z.; Leong, D.J.; Sun, H.B. Rho/Rock signal transduction pathway is required for MSC tenogenic differentiation. Bone Res. 2015, 3, 15015. [Google Scholar] [CrossRef] [PubMed]
- Subramony, S.D.; Dargis, B.R.; Castillo, M.; Azeloglu, E.U.; Tracey, M.S.; Su, A.; Lu, H.H. The guidance of stem cell differentiation by substrate alignment and mechanical stimulation. Biomaterials 2013, 34, 1942–1953. [Google Scholar] [CrossRef] [PubMed]
- Moffat, K.L.; Kwei, A.S.-P.; Spalazzi, J.P.; Doty, S.B.; Levine, W.N.; Lu, H.H. Novel nanofiber-based scaffold for rotator cuff repair and augmentation. Tissue Eng. Part A 2008, 15, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Franchi, M.; Torricelli, P.; Giavaresi, G.; Fini, M. Role of moderate exercising on Achilles tendon collagen crimping patterns and proteoglycans. Connect. Tissue Res. 2013, 54, 267–274. [Google Scholar] [CrossRef] [PubMed]



| Transcript | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) | Probe Sequence (5′-3′) |
|---|---|---|---|
| GAPDH | CAAGTTCCATGGCACAGTCAAG | GGCCTTTCCGTTGATGACAA | CCGAGCACGGGAAG |
| Scleraxis | CGCCCAGCCCAAACAG | TTGCTCAACTTTCTCTGGTTGCT | TCTGCACCTTCTGCC |
| Type I Collagen | GCCAAGAAGAAGGCCAAGAA | TGAGGCCGTCCTGTATGC | ACATCCCAGCAGTCACCT |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popielarczyk, T.L.; Nain, A.S.; Barrett, J.G. Aligned Nanofiber Topography Directs the Tenogenic Differentiation of Mesenchymal Stem Cells. Appl. Sci. 2017, 7, 59. https://doi.org/10.3390/app7010059
Popielarczyk TL, Nain AS, Barrett JG. Aligned Nanofiber Topography Directs the Tenogenic Differentiation of Mesenchymal Stem Cells. Applied Sciences. 2017; 7(1):59. https://doi.org/10.3390/app7010059
Chicago/Turabian StylePopielarczyk, Tracee L., Amrinder S. Nain, and Jennifer G. Barrett. 2017. "Aligned Nanofiber Topography Directs the Tenogenic Differentiation of Mesenchymal Stem Cells" Applied Sciences 7, no. 1: 59. https://doi.org/10.3390/app7010059





