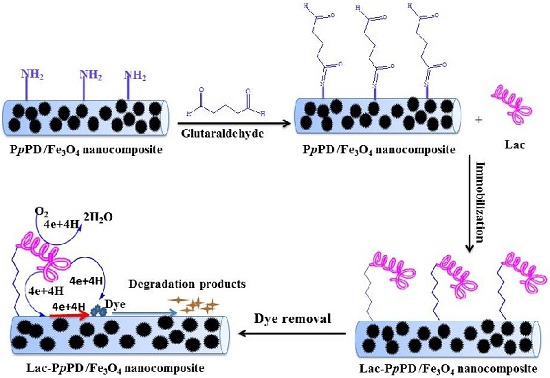
Laccase Immobilization on Poly(p-Phenylenediamine)/Fe3O4 Nanocomposite for Reactive Blue 19 Dye Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
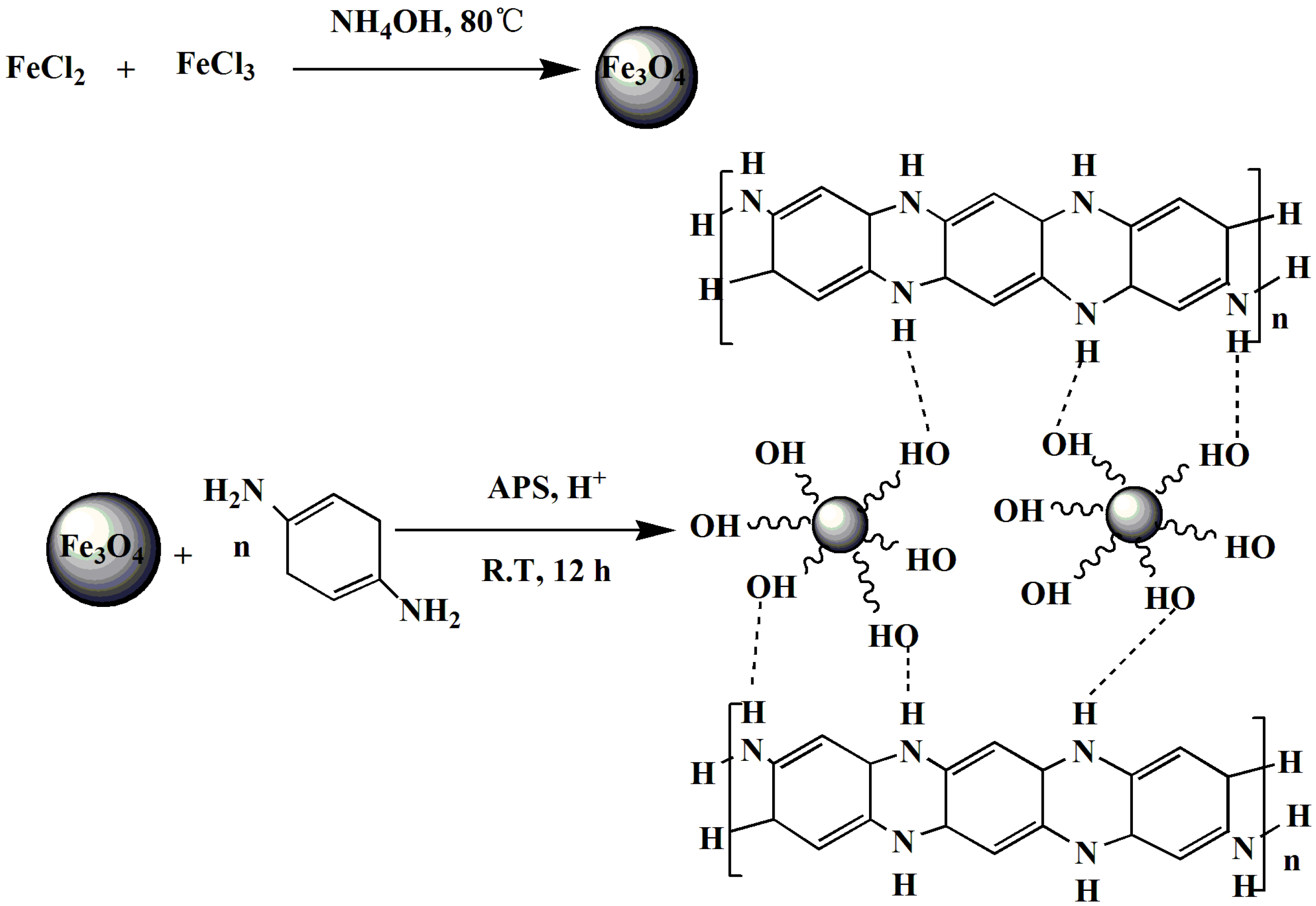
2.2. Synthesis of Magnetic Nanoparticles
2.3. Synthesis of PpPD/Fe3O4 Nanocomposite
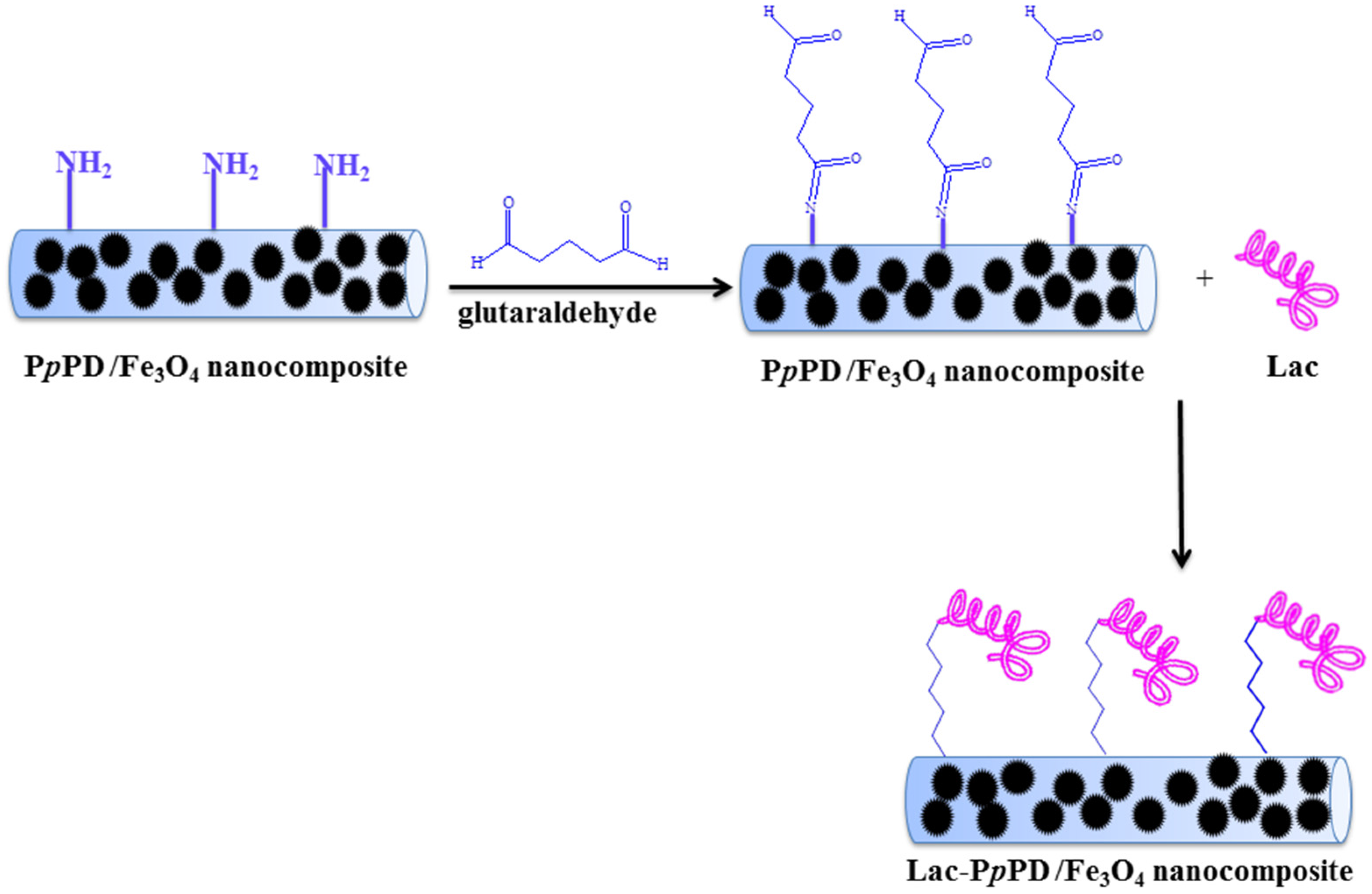
2.4. Immobilization of Lac on PpPD/Fe3O4 Nanocomposite
2.5. Measurement Activity of Free and Immobilized Lac
2.6. Evaluation of the Effects of pH and Temperature on Lac Activity and Stability
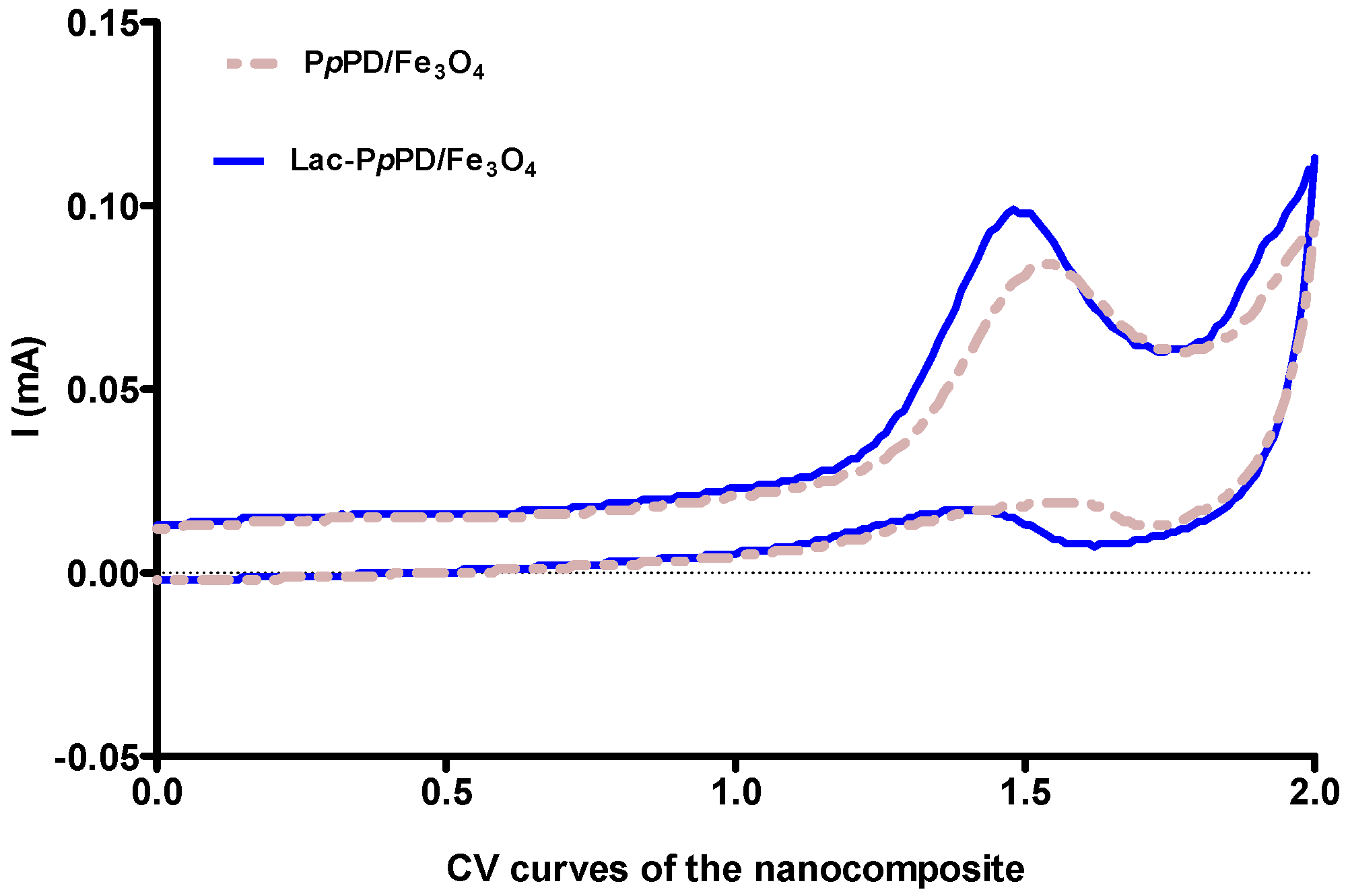
2.7. Electrochemical Analysis
2.8. Removal of RB-19 by Free and Immobilized Lac and Repeated Use
2.9. Measurements
3. Results and Discussion
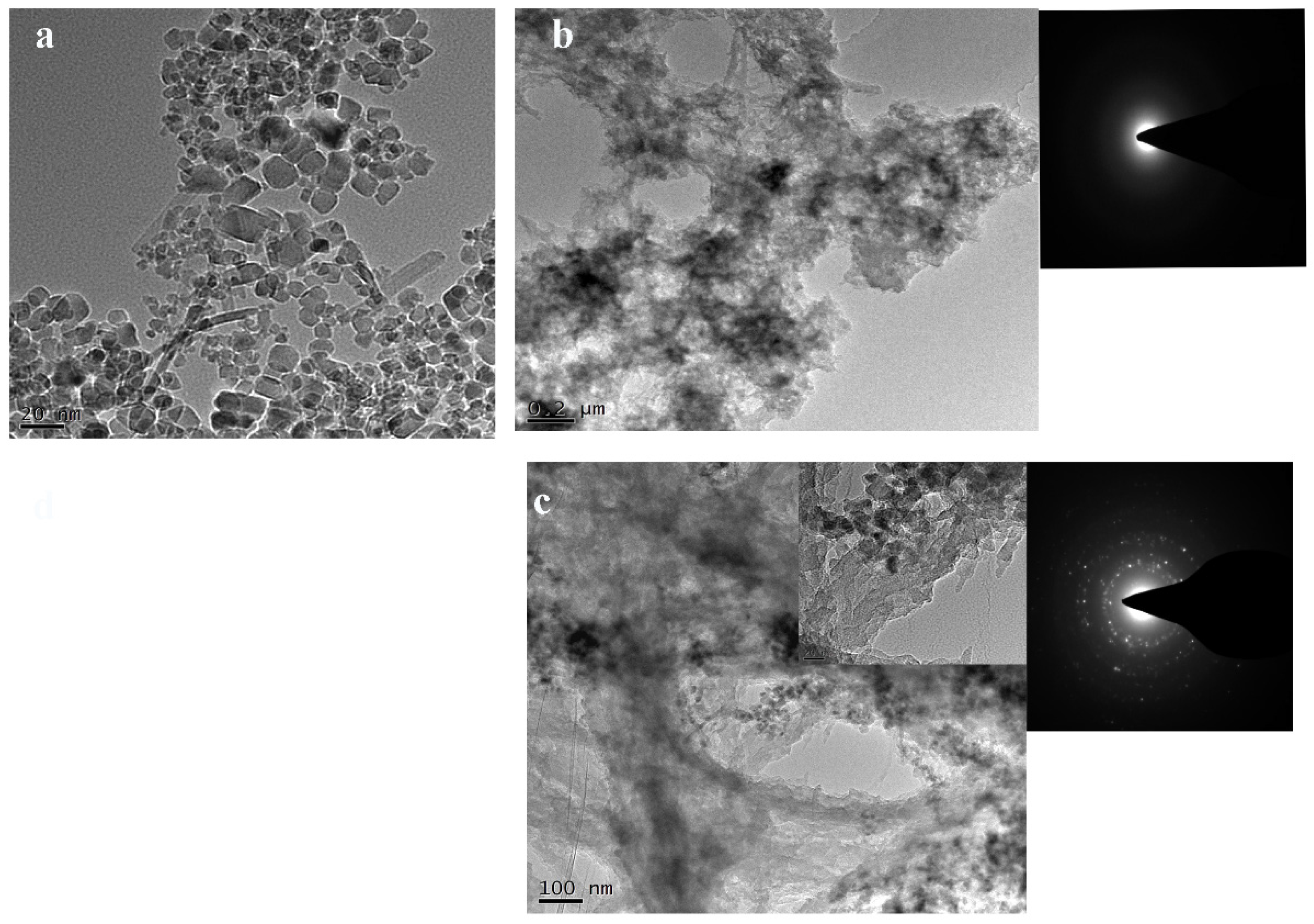
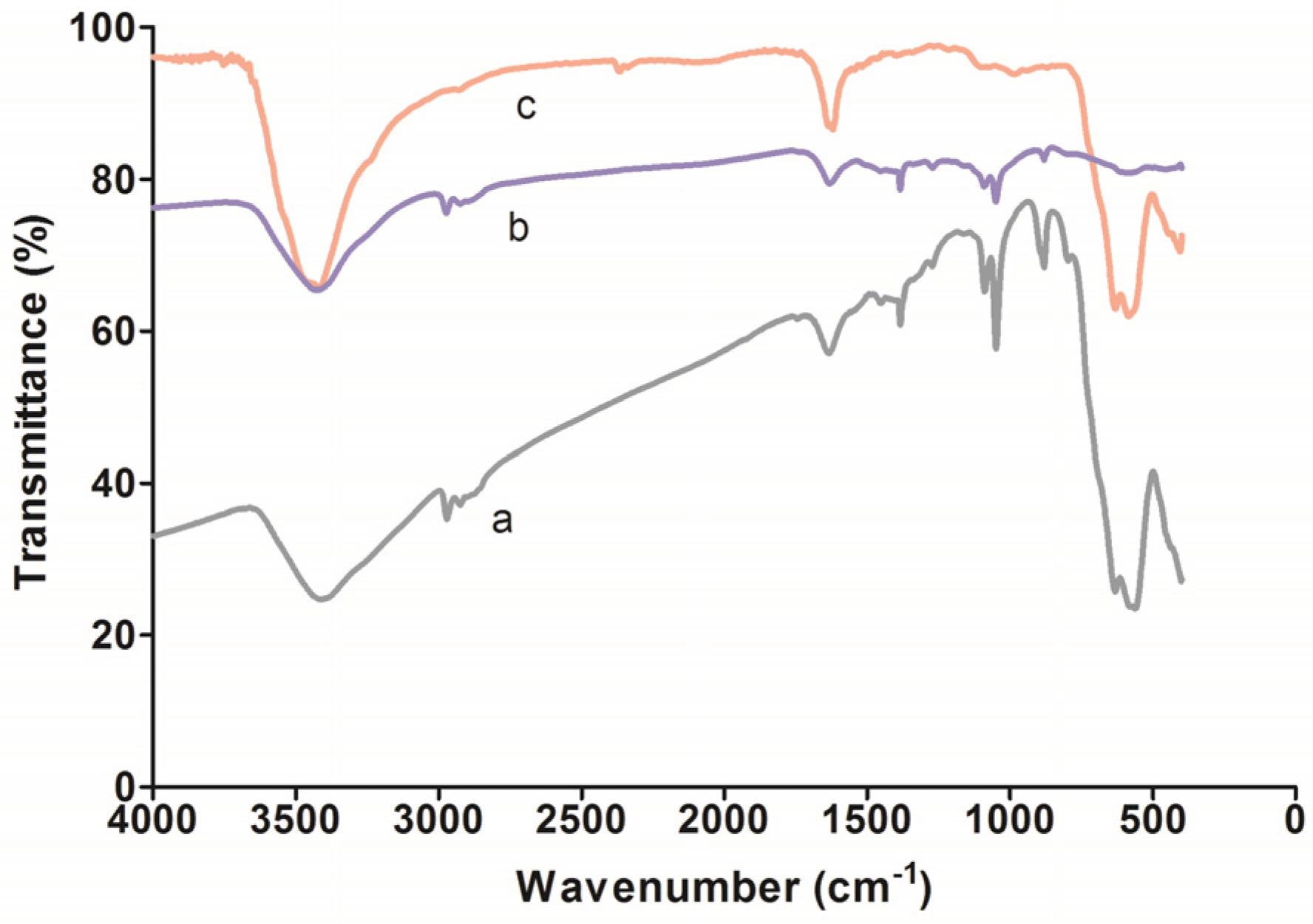
3.1. TEM Imaging and FT-IR Analysis
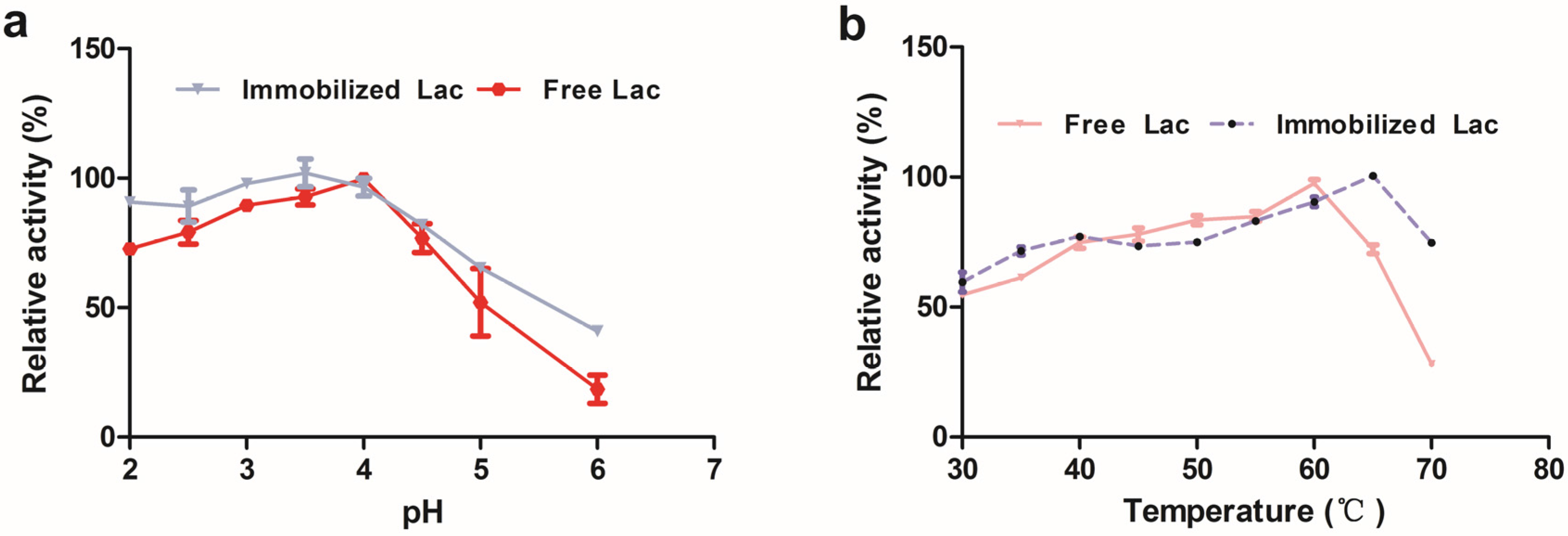
3.2. Effects of pH and Temperature on Lac Enzyme Activity
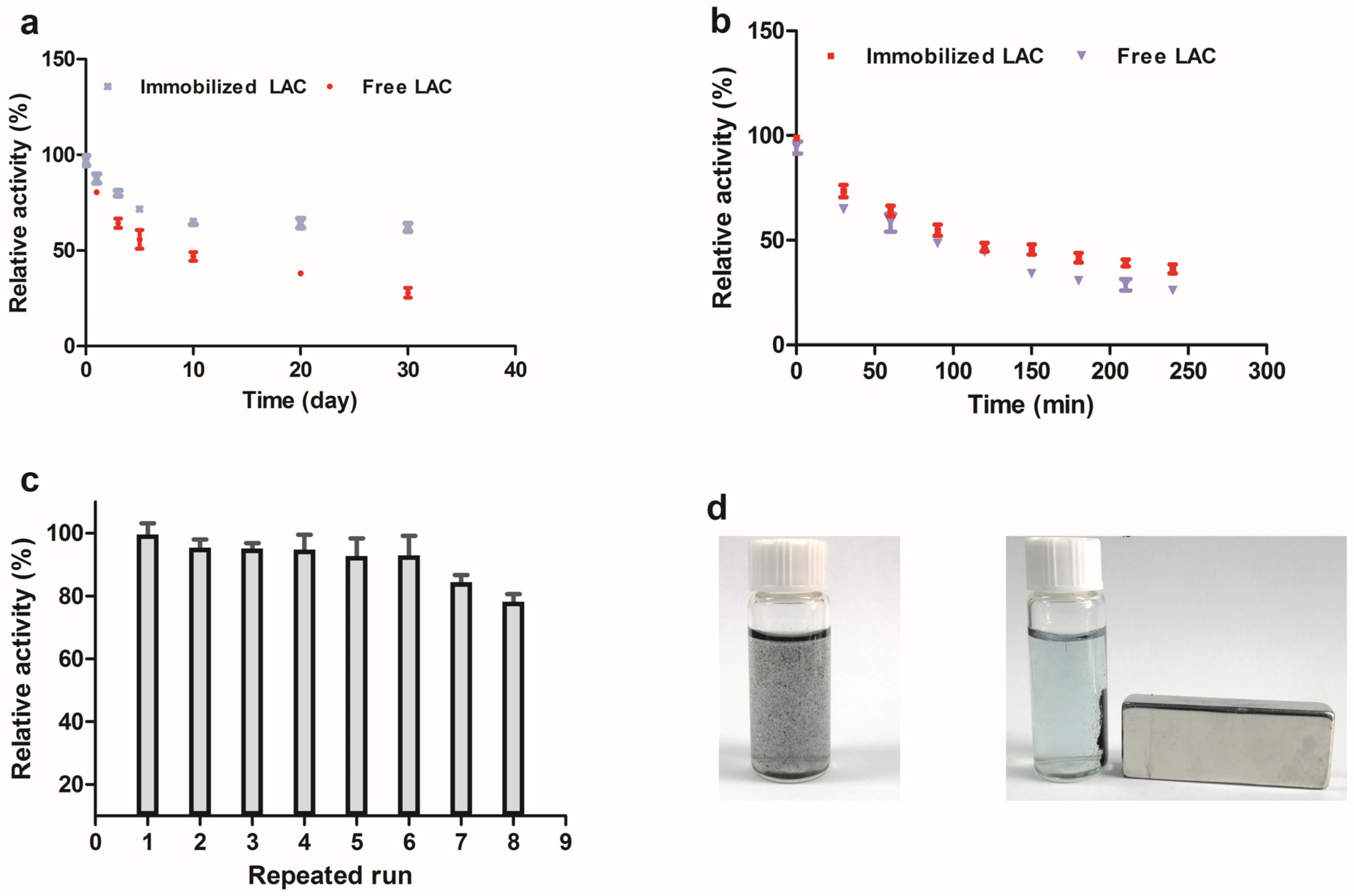
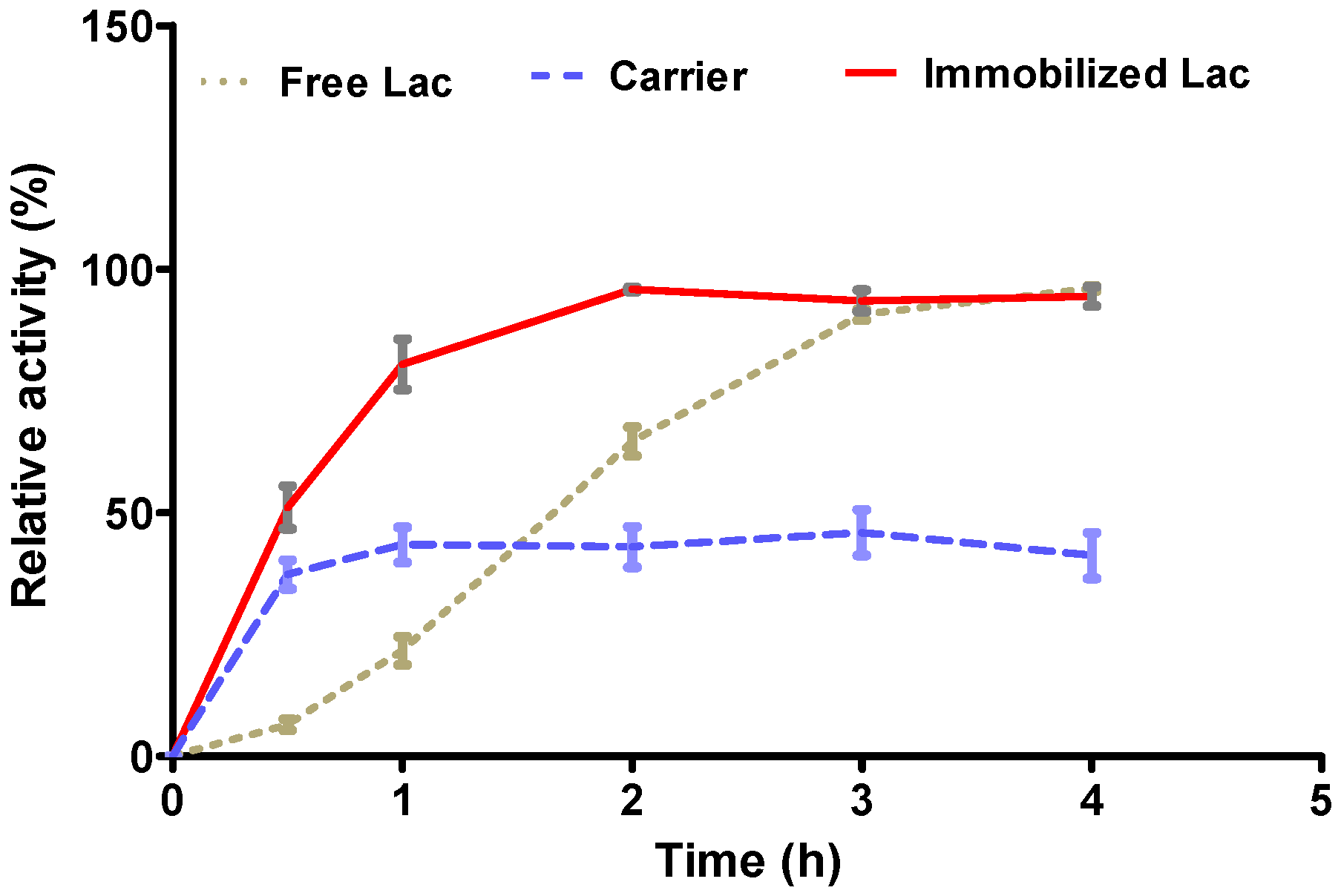
3.3. Stability Analysis
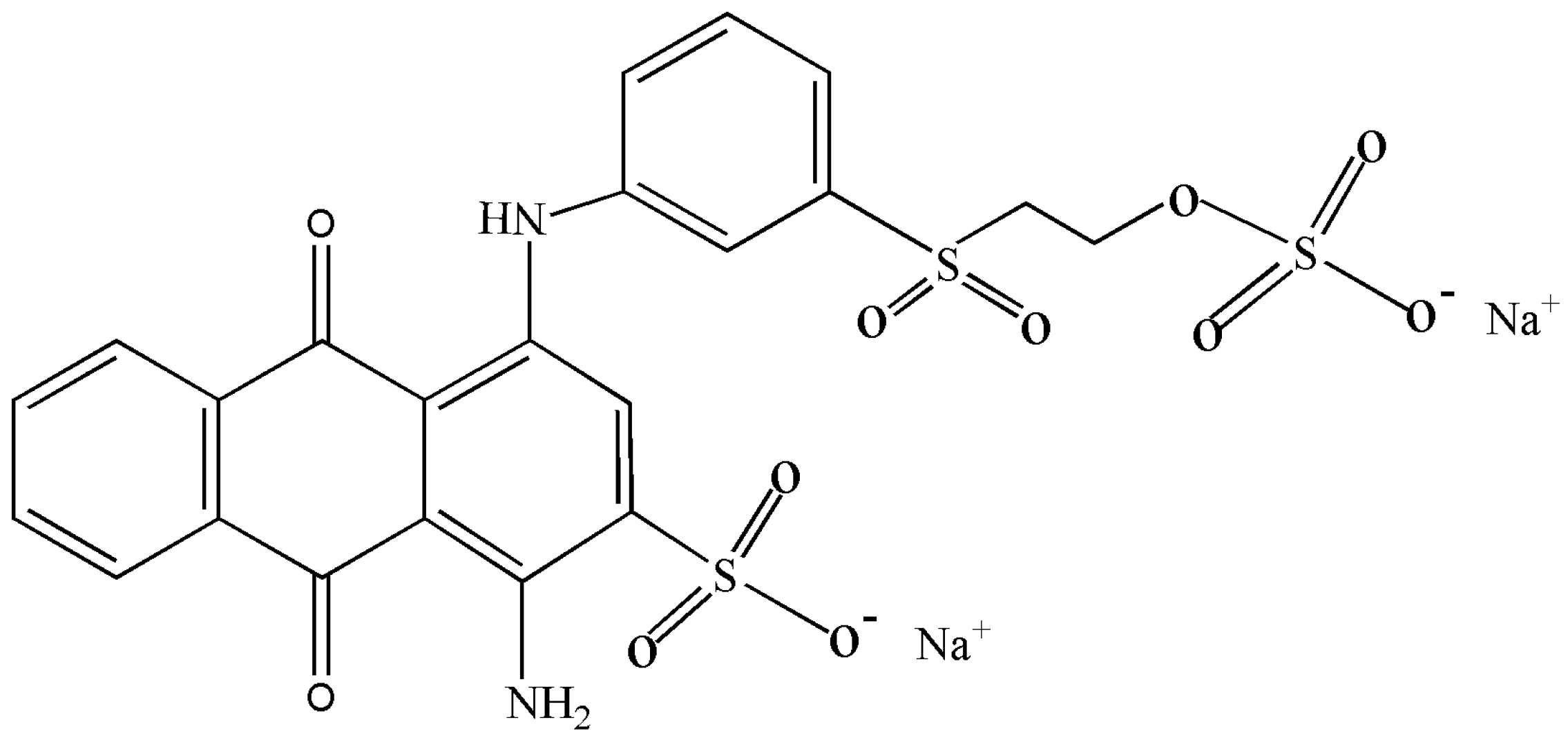
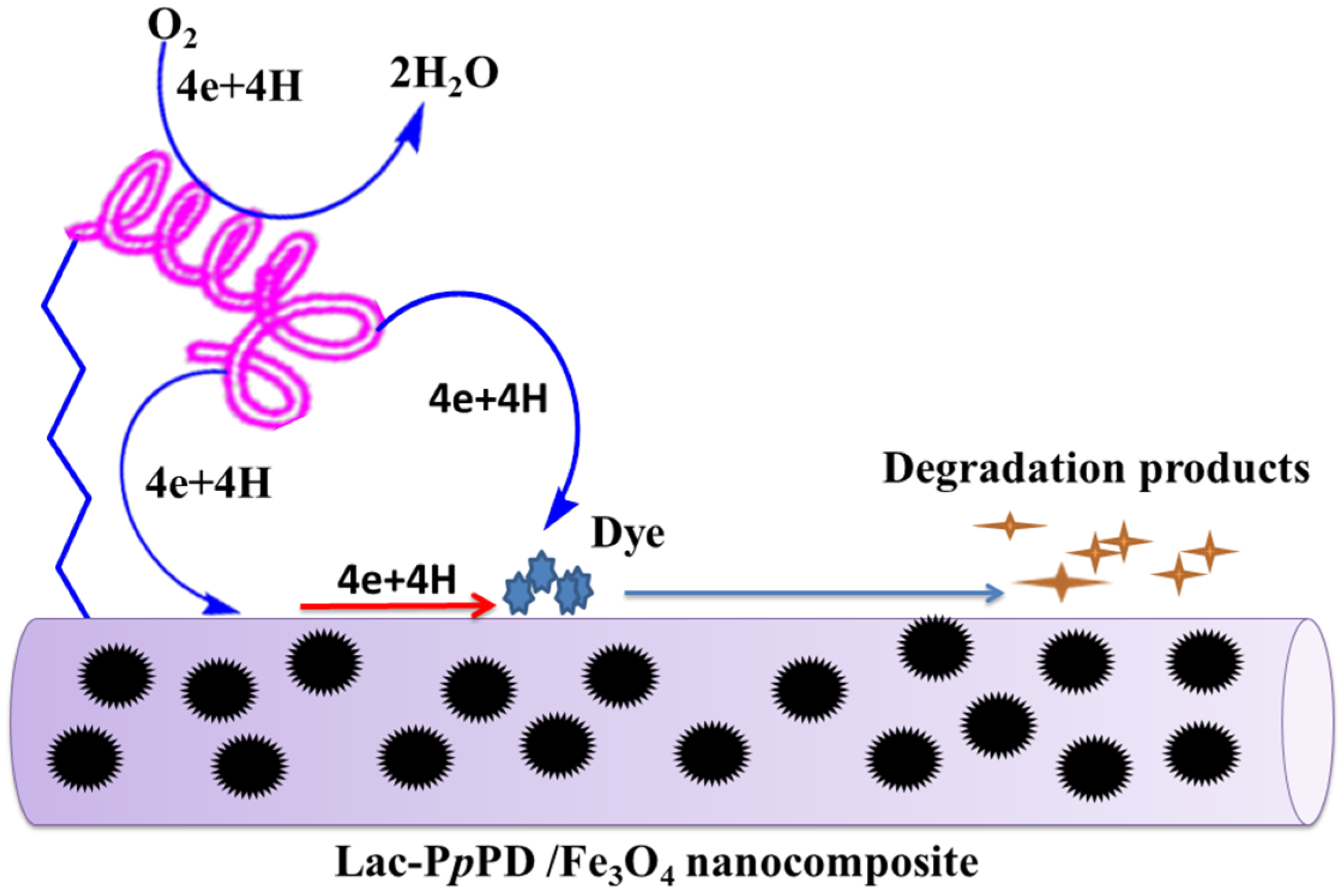
3.4. Removal of RB-19
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rivera-Hoyos, C.M.; Morales-Álvarez, E.D.; Poutou-Piñales, R.A.; Pedroza-Rodríguez, A.M.; Rodríguez-Vázquez, R.; Delgado-Boada, J.M. Fungal laccases. Fungal Biol. Rev. 2013, 27, 67–82. [Google Scholar] [CrossRef]
- Jones, S.M.; Solomon, E.I. Electron transfer and reaction mechanism of laccases. Cell. Mol. Life Sci. 2015, 72, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Senthivelan, T.; Kanagaraj, J.; Panda, R.C. Recent trends in fungal laccase for various industrial applications: An eco-friendly approach—A review. Biotechnol. Bioprocess Eng. 2016, 21, 19–38. [Google Scholar] [CrossRef]
- Mogharabi, M.; Faramarzi, M.A. Laccase and laccase-mediated systems in the synthesis of organic compounds. Adv. Synth. Catal. 2014, 356, 897–927. [Google Scholar] [CrossRef]
- Roth, S.; Spiess, A.C. Laccases for biorefinery applications: A critical review on challenges and perspectives. Bioprocess Biosyst. Eng. 2015, 38, 2285–2313. [Google Scholar] [CrossRef] [PubMed]
- Cañas, A.I.; Camarero, S. Laccases and their natural mediators: Biotechnological tools for sustainable eco-friendly processes. Biotechnol. Adv. 2010, 28, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Woodward, S.; Ellouz, M.; Dhouib, A. Decolourization and detoxification of textile industry wastewater by the laccase-mediator system. J. Hazard. Mater. 2009, 175, 802–808. [Google Scholar]
- Zucca, P.; Cocco, G.; Sollai, F.; Sanjust, E. Fungal laccases as tools for biodegradation of industrial dyes. Biocatalysis 2016, 1, 82–108. [Google Scholar] [CrossRef] [Green Version]
- Ursoiu, A.; Paul, C.; Kurtán, T.; Péter, F. Sol-gel entrapped Candida antarctica lipase B—A biocatalyst with excellent stability for kinetic resolution of secondary alcohols. Molecules 2012, 17, 13045–13061. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Husain, Q. Potential applications of enzymes immobilized on/in nano materials: A review. Biotechnol. Adv. 2012, 30, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Buthe, A.; Wu, S.; Ping, W. Nanoporous silica glass for the immobilization of interactive enzyme systems. Methods Mol. Biol. 2011, 679, 37–48. [Google Scholar] [PubMed]
- Hwang, E.T.; Gu, M.B. Enzyme stabilization by nano/microsized hybrid materials. Eng. Life Sci. 2013, 13, 49–61. [Google Scholar] [CrossRef]
- Wang, Z.-G.; Wan, L.-S.; Liu, Z.-M.; Huang, X.-J.; Xu, Z.-K. Enzyme immobilization on electrospun polymer nanofibers: An overview. J. Mol. Catal. B Enzym. 2009, 56, 189–195. [Google Scholar] [CrossRef]
- Ba, S.; Arsenault, A.; Hassani, T.; Jones, J.P.; Cabana, H. Laccase immobilization and insolubilization: From fundamentals to applications for the elimination of emerging contaminants in wastewater treatment. Crit. Rev. Biotechnol. 2012, 33, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Lalaoui, N.; Elouarzaki, K.; Le Goff, A.; Holzinger, M.; Cosnier, S. Efficient direct oxygen reduction by laccases attached and oriented on pyrene-functionalized polypyrrole/carbon nanotube electrodes. Chem. Commun. 2013, 49, 9281–9283. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.-L.; Hong, J.; Gao, Y.-F.; Yang, T.; Moosavi-Movahedi, A.A.; Ghourchian, H. Direct electron transfer of horseradish peroxidase on a functional nanocomplex modified glassy carbon electrode. Biomed. Mater. Eng. 2014, 24, 1079–1084. [Google Scholar] [PubMed]
- Patil, S.R.; Choudhary, A.S.; Sekar, N. Disperse styryl and azo dyes for polyester and nylon fibre: Synthesis, optical properties having the 1,2,4-triketo naphthoquinone skeleton. Fibers Polym. 2015, 16, 1068–1074. [Google Scholar] [CrossRef]
- Parmar, N.; Shukla, S.R. Microbial Decolorization of reactive dye solutions. CLEAN-Soil Air Water 2015, 43, 1426–1432. [Google Scholar] [CrossRef]
- Zhuo, R.; He, F.; Zhang, X.; Yang, Y. Characterization of a yeast recombinant laccase rLAC-EN3-1 and its application in decolorizing synthetic dye with the coexistence of metal ions and organic solvents. Biochem. Eng. J. 2015, 93, 63–72. [Google Scholar] [CrossRef]
- Chhabra, M.; Mishra, S.; Sreekrishnan, T.R. Immobilized laccase mediated dye decolorization and transformation pathway of azo dye acid red. J. Environ. Health Sci. Eng. 2015, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, D.; Liao, F.; Guo, T.; Wu, Z.; Zhang, T. Synthesis, characterization, morphology control of poly (p-phenylenediamine)-Fe3O4 magnetic micro-composite and their application for the removal of Cr2O72− from water. Synth. Met. 2012, 162, 2329–2336. [Google Scholar] [CrossRef]
- Yang, S.; Ye, C.; Song, X.; He, L.; Yan, S.; Liao, F. Theoretical calculations based synthesis of poly(p-phenylenediamine)-Fe3O4 composite: A magnetically recyclable photocatalyst with highly selectivity for acid dyes. RSC Adv. 2014, 4, 54810–54818. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; Yu, J.; Xia, J.; Zhang, C.; Yin, D.; Häfeli, U.O. Preparation and radiolabeling of surface-modified magnetic nanoparticles with rhenium-188 for magnetic targeted radiotherapy. J. Magn. Magn. Mater. 2004, 277, 165–174. [Google Scholar] [CrossRef]
- Zhang, Z.; Wan, M.; Wei, Y. Electromagnetic functionalized polyaniline nanostructures. Nanotechnology 2005, 16, 2827. [Google Scholar] [CrossRef]
- Lakouraj, M.M.; Zare, E.N.; Moghadam, P.N. Synthesis of novel conductive poly(p-phenylenediamine)/Fe3O4 nanocomposite via emulsion polymerization and investigation of antioxidant activity. Adv. Polym. Technol. 2014, 33, 509–516. [Google Scholar]
- Baghayeri, M.; Zare, E.N.; Lakouraj, M.M. A simple hydrogen peroxide biosensor based on a novel electro-magnetic poly(p-phenylenediamine)@Fe3O4 nanocomposite. Biosens. Bioelectron. 2014, 55, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Pradhan, L.; Devi, Y.P.; Meena, S.S.; Tewari, R.; Kumar, A.; Sharma, S.; Gajbhiye, N.S.; Vatsa, R.K.; Pandey, B.N. Induction heating studies of Fe3O4 magnetic nanoparticles capped with oleic acid and polyethylene glycol for hyperthermia. J. Mater. Chem. 2011, 21, 13388–13398. [Google Scholar] [CrossRef]
- Zhao, J.; Kwan, H.S. Characterization, molecular cloning, and differential expression analysis of laccase genes from the edible mushroomLentinula edodes. Appl. Environ. Microbiol. 1999, 65, 4908–4913. [Google Scholar] [PubMed]
- Baghayeri, M. Glucose sensing by a glassy carbon electrode modified with glucose oxidase and a magnetic polymeric nanocomposite. RSC Adv. 2015, 5, 18267–18274. [Google Scholar] [CrossRef]
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding enzyme immobilisation. Chem. Soc. Rev. 2009, 38, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Brady, D.; Jordaan, J. Advances in enzyme immobilisation. Biotechnol. Lett. 2009, 31, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Characteristic features and biotechnological applications of cross-linked enzyme aggregates (CLEAs). Appl. Microbiol. Biotechnol. 2011, 92, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Ma, N. Immobilized lipase on Fe3O4 nanoparticles as biocatalyst for biodiesel production. Energy Fuels 2009, 23, 1347–1353. [Google Scholar] [CrossRef]
- Guo, Z.; Bai, S.; Sun, Y. Preparation and characterization of immobilized lipase on magnetic hydrophobic microspheres. Enzyme Microb. Technol. 2003, 32, 776–782. [Google Scholar] [CrossRef]
- Huang, X.-J.; Yu, A.-G.; Xu, Z.-K. Covalent immobilization of lipase from Candida rugosa onto poly (acrylonitrile-co-2-hydroxyethyl methacrylate) electrospun fibrous membranes for potential bioreactor application. Bioresour. Technol. 2008, 99, 5459–5465. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Yin, L.; Niu, J. Laccase-carrying electrospun fibrous membranes for adsorption and degradation of PAHs in shoal soils. Environ. Sci. Technol. 2011, 45, 10611–10618. [Google Scholar] [CrossRef] [PubMed]
- Khoshhesab, Z.M.; Ahmadi, M. Removal of reactive blue 19 from aqueous solutions using NiO nanoparticles: Equilibrium and kinetic studies. Desalination Water Treat. 2016, 57, 1–12. [Google Scholar] [CrossRef]
- Zhao, Y.-D.; Zhang, W.-D.; Chen, H.; Luo, Q.-M.; Li, S.F.Y. Direct electrochemistry of horseradish peroxidase at carbon nanotube powder microelectrode. Sens. Actuators B Chem. 2002, 87, 168–172. [Google Scholar] [CrossRef]
- Feng, W.; Wu, Z.; Li, Y.; Feng, Y.; Yuan, X. The fabrication and electrochemical properties of electrospun nanofibers of a multiwalled carbon nanotube grafted by chitosan. Nanotechnology 2008, 19, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-G.; Ke, B.-B.; Xu, Z.-K. Covalent immobilization of redox enzyme on electrospun nonwoven poly (acrylonitrile-co-acrylic acid) nanofiber mesh filled with carbon nanotubes: A comprehensive study. Biotechnol. Bioeng. 2007, 97, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.-S.; Ke, B.-B.; Wu, J.; Xu, Z.-K. Catalase immobilization on electrospun nanofibers: Effects of porphyrin pendants and carbon nanotubes. J. Phys. Chem. C 2007, 111, 14091–14097. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Su, P.; Zhang, Y.; Shen, Y.; Han, D.; Ivaska, A.; Niu, L. Direct electron transfer of horseradish peroxidase and its electrocatalysis based on carbon nanotube/thionine/gold composites. Electrochem. Commun. 2008, 10, 306–310. [Google Scholar] [CrossRef]
- Liu, M.; Wen, Y.; Li, D.; He, H.; Xu, J.; Liu, C.; Yue, R.; Lu, B.; Liu, G. Electrochemical immobilization of ascorbate oxidase in poly(3,4-ethylenedioxythiophene)/multiwalled carbon nanotubes composite films. J. Appl. Polym. Sci. 2011, 122, 1142–1151. [Google Scholar] [CrossRef]
- Bourourou, M.; Elouarzaki, K.; Lalaoui, N.; Agnès, C.; Le Goff, A.; Holzinger, M.; Maaref, A.; Cosnier, S. Supramolecular immobilization of laccase on carbon nanotube electrodes functionalized with (methylpyrenylaminomethyl) anthraquinone for direct electron reduction of oxygen. Chem. Eur. J. 2013, 19, 9371–9375. [Google Scholar] [CrossRef] [PubMed]
- Karam, J.; Nicell, J.A. Potential applications of enzymes in waste treatment. J. Chem. Technol. Biotechnol. 1997, 69, 141–153. [Google Scholar] [CrossRef]
- Russo, M.E.; Giardina, P.; Marzocchella, A.; Salatino, P.; Sannia, G. Assessment of anthraquinone-dye conversion by free and immobilized crude laccase mixtures. Enzyme Microb. Technol. 2008, 42, 521–530. [Google Scholar] [CrossRef]
- Cabana, H.; Jones, J.P.; Agathos, S.N. Utilization of cross-linked laccase aggregates in a perfusion basket reactor for the continuous elimination of endocrine-disrupting chemicals. Biotechnol. Bioeng. 2009, 102, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Lloret, L.; Hollmann, F.; Eibes, G.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Immobilisation of laccase on Eupergit supports and its application for the removal of endocrine disrupting chemicals in a packed-bed reactor. Biodegradation 2012, 23, 373–386. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yan, M.; Geng, Y.; Huang, J. Laccase Immobilization on Poly(p-Phenylenediamine)/Fe3O4 Nanocomposite for Reactive Blue 19 Dye Removal. Appl. Sci. 2016, 6, 232. https://doi.org/10.3390/app6080232
Liu Y, Yan M, Geng Y, Huang J. Laccase Immobilization on Poly(p-Phenylenediamine)/Fe3O4 Nanocomposite for Reactive Blue 19 Dye Removal. Applied Sciences. 2016; 6(8):232. https://doi.org/10.3390/app6080232
Chicago/Turabian StyleLiu, Youxun, Mingyang Yan, Yuanyuan Geng, and Juan Huang. 2016. "Laccase Immobilization on Poly(p-Phenylenediamine)/Fe3O4 Nanocomposite for Reactive Blue 19 Dye Removal" Applied Sciences 6, no. 8: 232. https://doi.org/10.3390/app6080232