Hydration, Setting and Crack-Resistance Properties of Activated HVFACs at Early Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Mixtures and Test Procedures
2.1.1. Cementitious Materials
2.1.2. Mix Proportion Details
2.1.3. Test Variables
2.2. Instruments and Test Methods
2.2.1. Compressive Strength Test
2.2.2. Hydration Heat
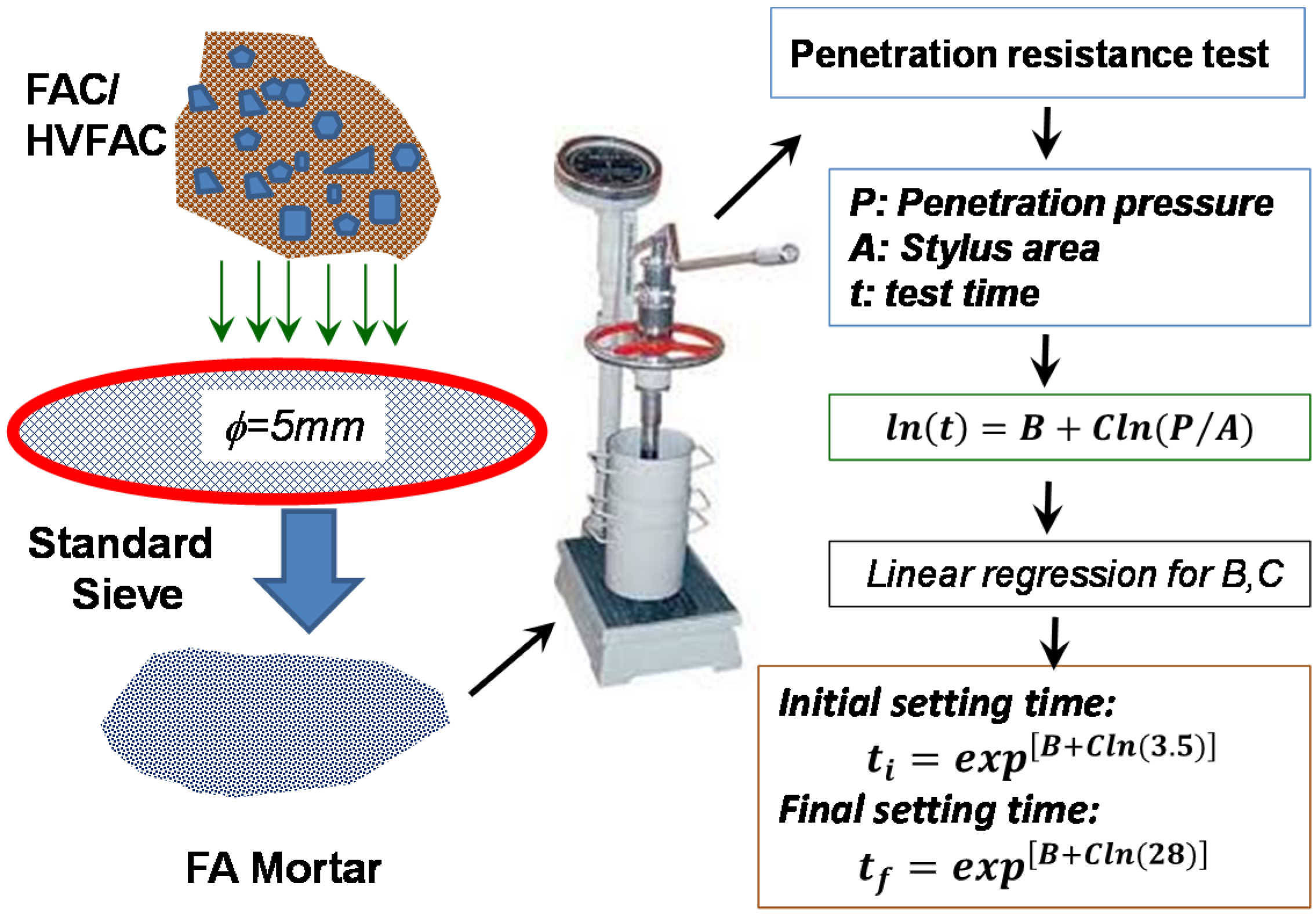
2.2.3. Setting Time
2.2.4. Crack-Resistance Test
3. Experimental Results and Observation
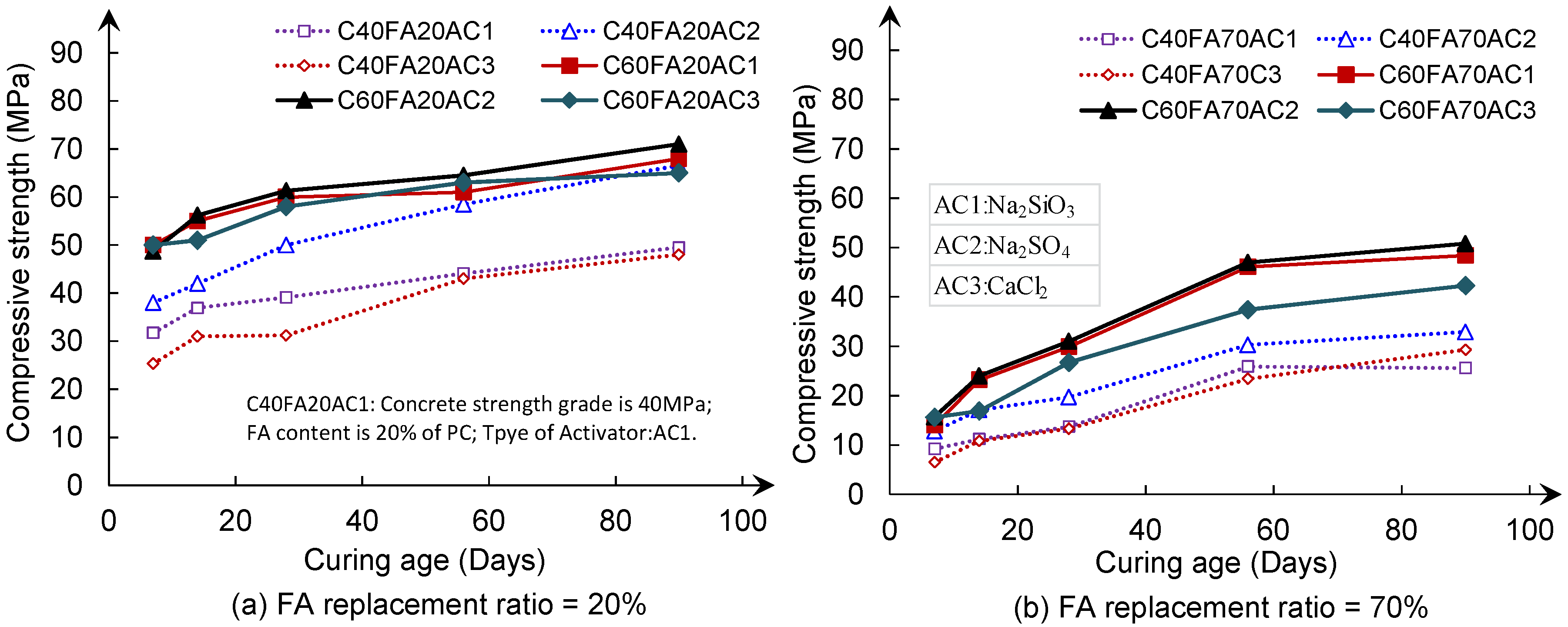
3.1. Compressive Strength
3.2. Setting Time
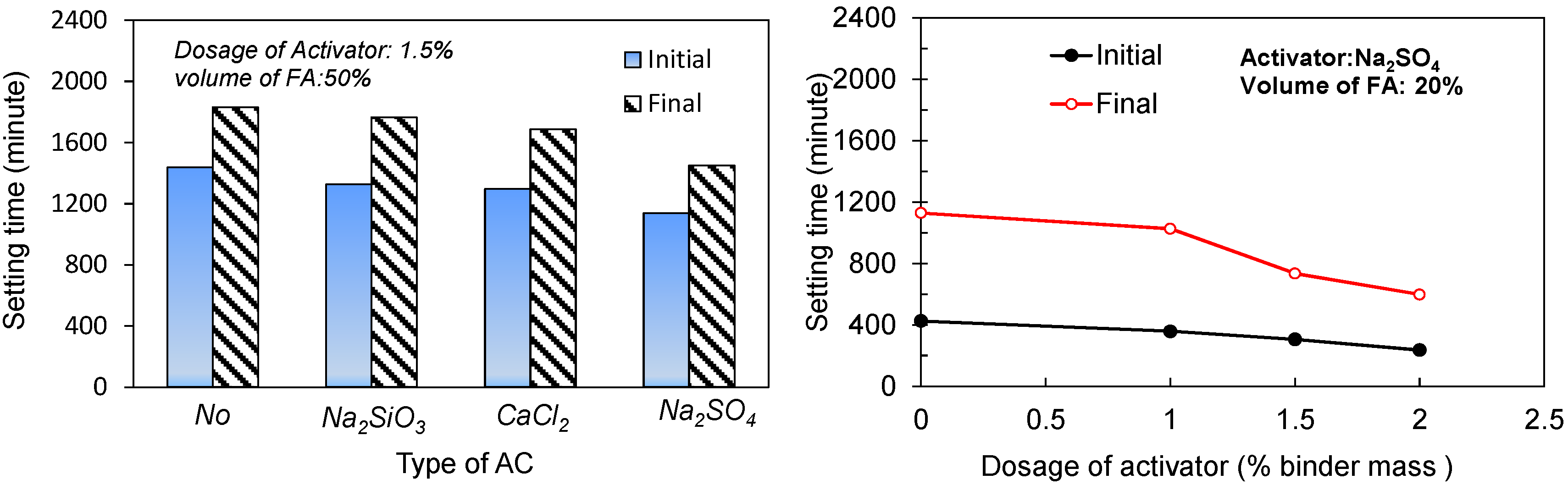
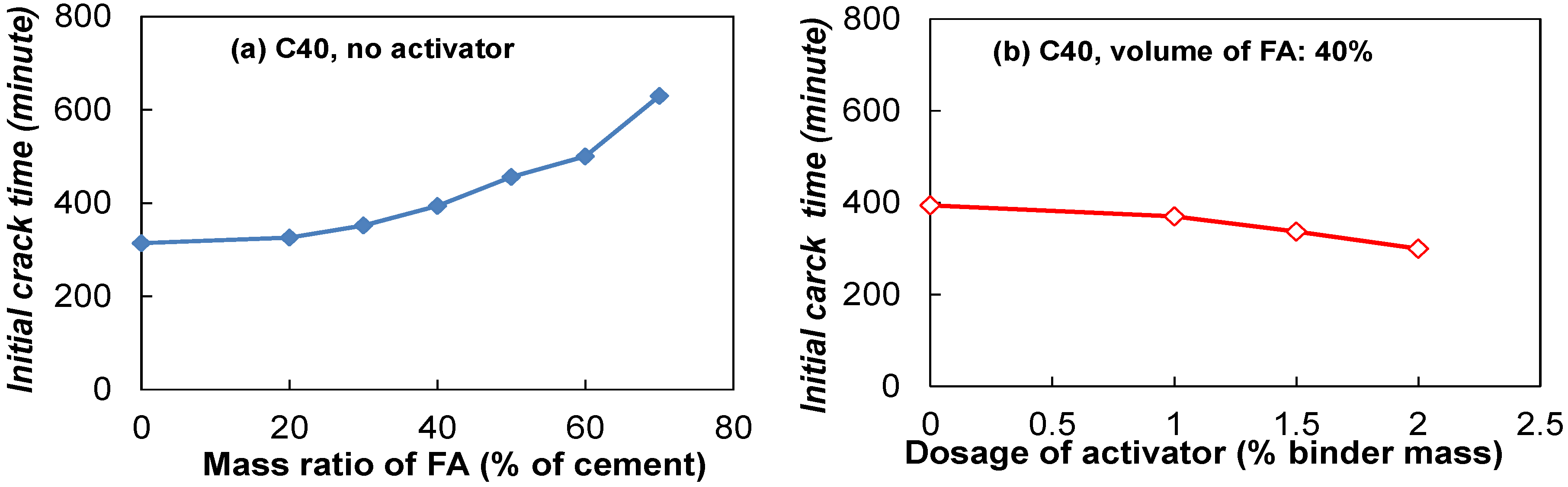
3.2.1. Effect of Fly Ash Dosage
3.2.2. Effect of the Type and Dosage of Activators
3.3. Hydration Heat
3.3.1. Effects of the Dosage of Fly Ash
3.3.2. Effect of Dosage of AC
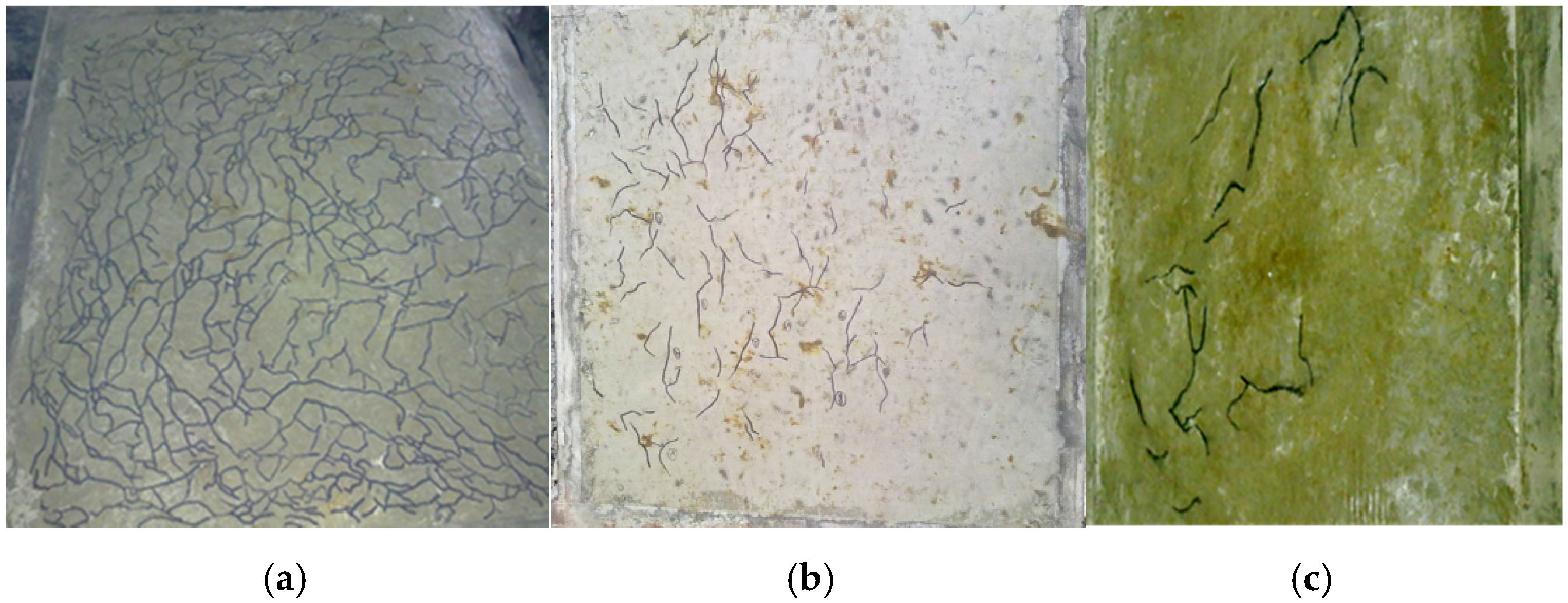
3.4. Initial Crack-Resistance Capacity of FAC/HVFAC
3.4.1. General Pattern
3.4.2. Time of Initial Cracks
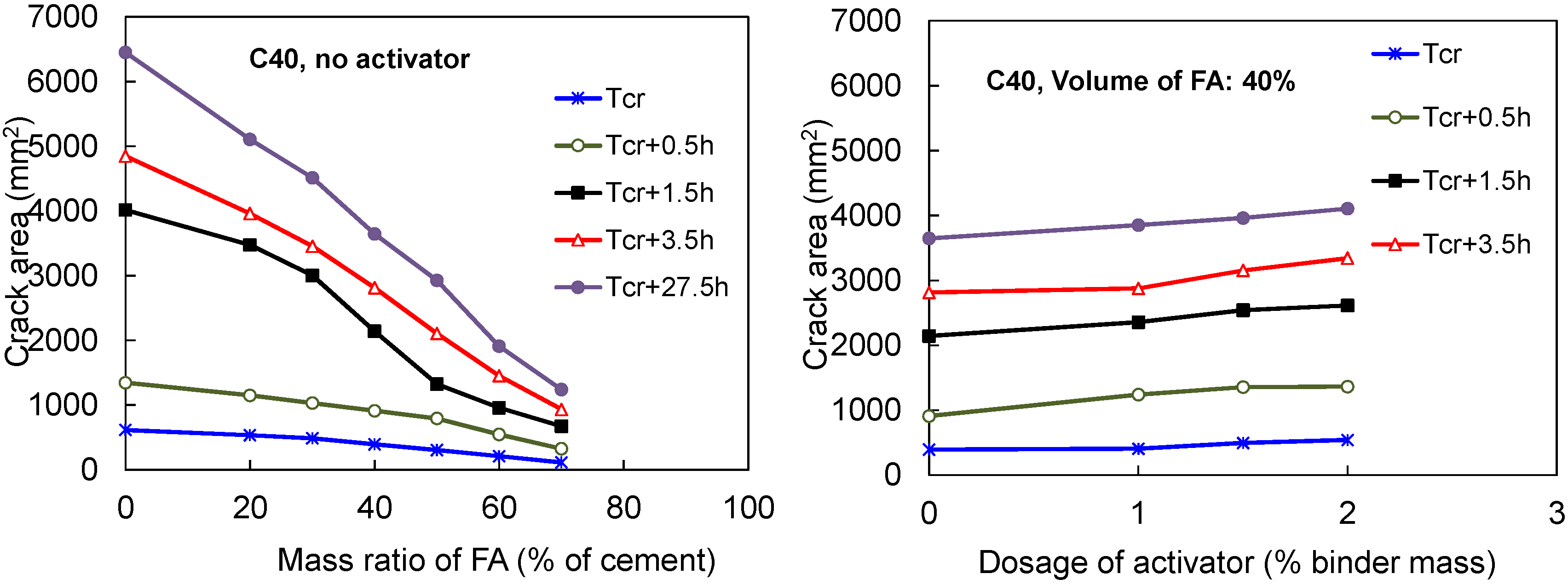
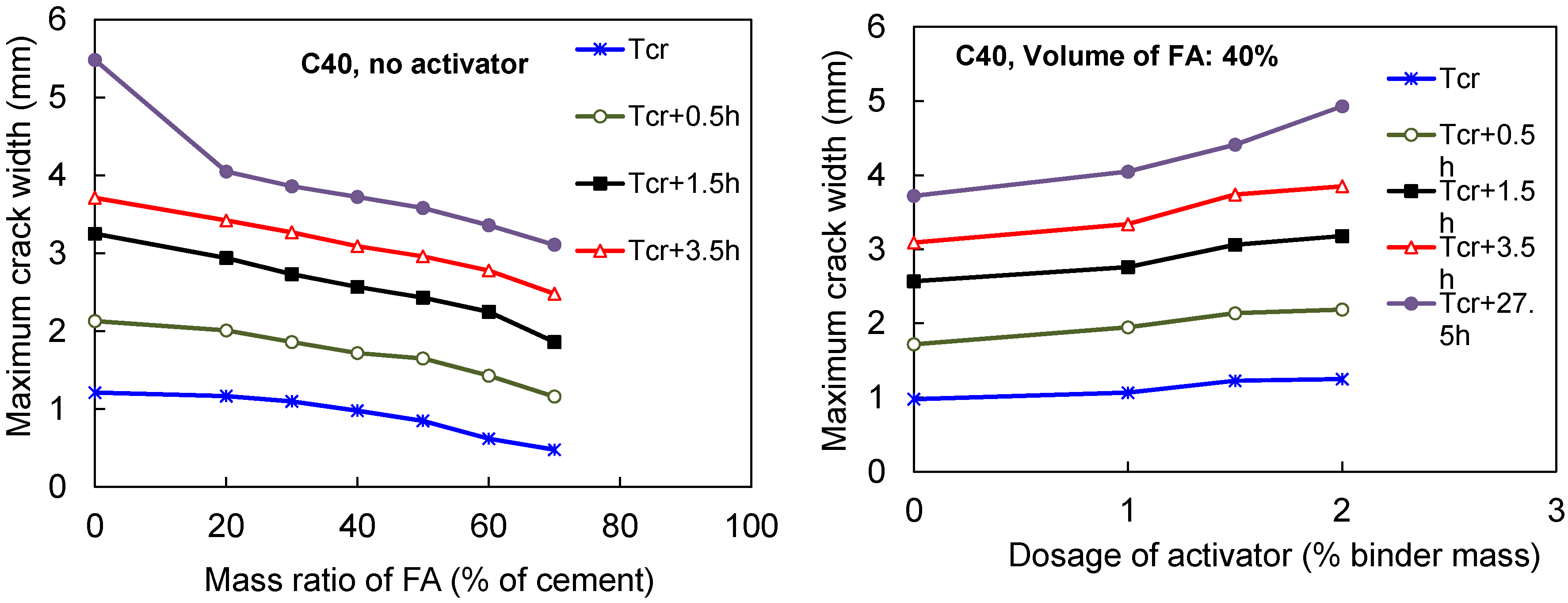
3.4.3. Total Cracked Area and Maximum Width of Cracks in FACs
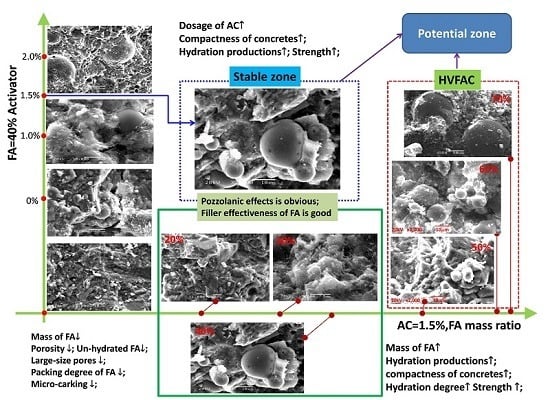
3.5. Microstructure Analyses
4. Models and Discussion
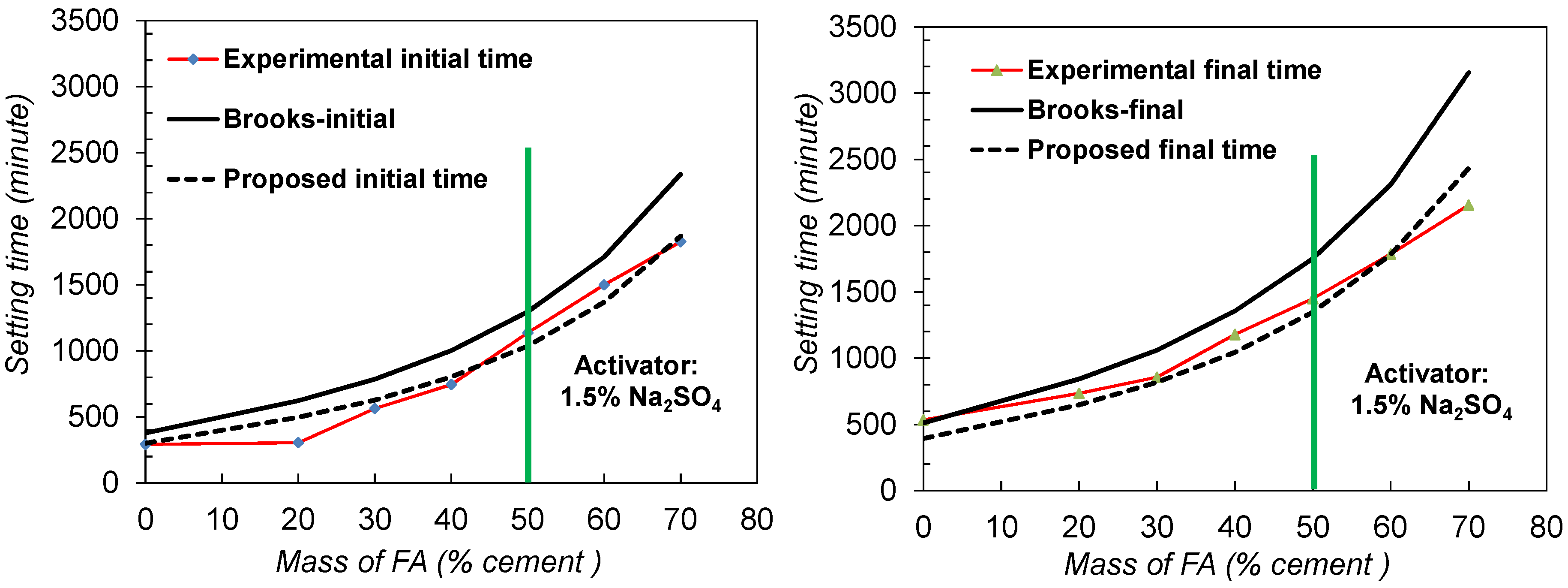
4.1. Existing Method for Predicting the Setting Time of FACs: Brooks Model
- RIS: relative initial setting time;
- RKT: relative rate coefficient taking into account the effect of temperature on setting;
- k: a rate coefficient that represents the rate of growth of the initial hydration;
- Sbc: the specific surface area of the blended cement;
- 1/γbc: the volume of blended cementitious materials per g;
- w/c: water/cement ratio;
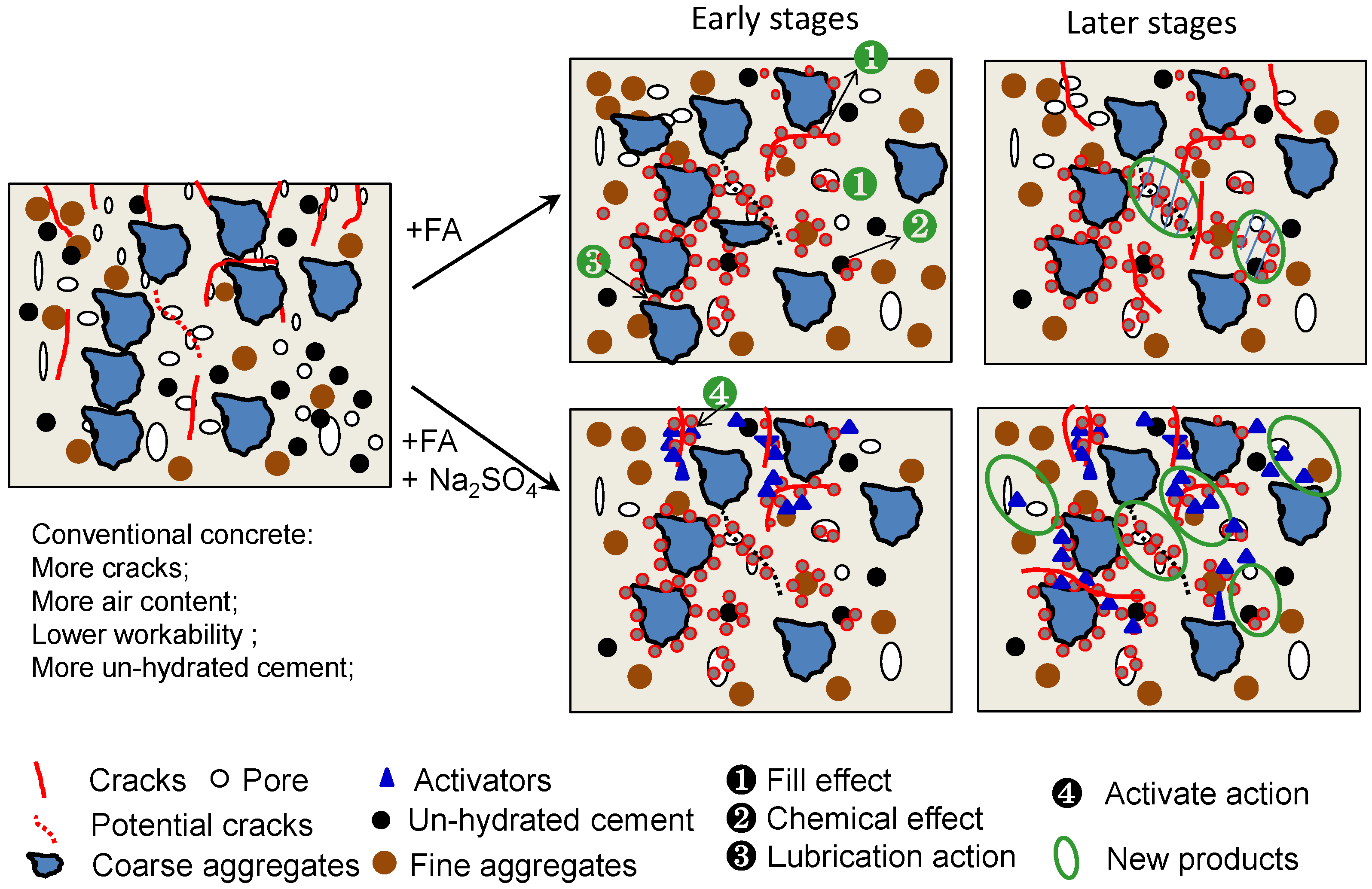
4.2. Mechanism Analysis of Crack-Resistance of FAC Using Na2SO4
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bilodeau, A.; Malhotra, V.M. High-volume fly ash system: concrete solution for sustainable development. ACI Mater. J. 2000, 97, 41–47. [Google Scholar]
- Sivasundaram, V. Thermal Crack Control of Mass Concrete. MSL Division Report MSL 86-93 (IR); Energy, Mines, and Resources Canada: Ottawa, ON, Canada, 1986. [Google Scholar]
- Giaccio, G.M.; Malhotra, V.M. Concrete incorporating high volumes of ASTM Class F fly ash. Cement. Concr. Aggregates 1988, 10, 88–95. [Google Scholar]
- Langley, W.S.; Carette, G.G.; Malhotra, V.M. Structural concrete incorporating high volumes of ASTM class fly ash. ACI Mater. J. 1989, 86, 507–514. [Google Scholar]
- Alasali, M.M.; Malhotra, V.M. Role of concrete incorporating high volumes of fly ash in controlling expansion due to alkali-aggregate reaction. ACI Mater. J. 1991, 88, 159–163. [Google Scholar]
- Sivasundaram, V.; Carette, G.G.; Malhotra, V.M. Mechanical properties, creep, and resistance to diffusion of chloride ions of concretes incorporating high volumes of ASTM Class F fly ashes from seven different sources. ACI Mater. J. 1991, 88, 407–416. [Google Scholar]
- Bilodeau, A.; Sivasundaram, V.; Painter, K.E.; Malhotra, V.M. Durability of concrete incorporating high volumes of fly ash from sources in the USA. ACI Mater. J. 1994, 91, 3–12. [Google Scholar]
- Bilodeau, A.; MalhotraI, V.M. Concretes Incorporating High Volumes of ASTM Class F Fly Ashes: Mechanical Properties and Resistance to De-icing Salt Scaling and to Chloride-Ion Penetration. ACI Spec. Publ. 1992, 132, 319–349. [Google Scholar]
- Carette, G.G.; Bilodeau, A.; Chevrier, R.L.; Malhotre, V.M. Mechanical properties of concrete incorporating high volumes of fly ash from sources in the US. ACI Mater. J. 1993, 90, 535–544. [Google Scholar]
- Bisaillon, A.; Rivest, M.; Malhotra, V.M. Performance of high-volume fly ash concrete in large experimental monoliths. ACI Mater. J. 1994, 91, 178–187. [Google Scholar]
- Bilodeau, A.; MalhotraI, V.M. Properties of high-volume fly ash concrete made with high early-strength ASTM Type III cement. ACI Spec. Publ. 1995, 153, 1–23. [Google Scholar]
- Sivasundaram, V.; Bilodeau, A.; Malhotra, V.M. Effect of Curing Conditions on High-Volume Fly Ash Concrete Made with ASTM Type I and III Cements and Silica Fume. ACI Spec. Publ. 1995, 154, 509–530. [Google Scholar]
- Li, D.; Chen, Y.; Shen, J.; Su, J.; Wu, X. The influence of alkalinity on activation and microstructure of fly ash. Cem. Concr. Res. 2000, 30, 881–886. [Google Scholar] [CrossRef]
- ACI Committee 232. Use of Fly Ash in Concrete; American Concrete Institute: Farmington Hills MI, USA, 2003. [Google Scholar]
- Malhotra, V.M. Super plasticized Fly Ash Concrete for Structural Applications. Concr. Int. 1986, 8, 28–31. [Google Scholar]
- Naik, T.R.; Ramme, B.W.; Tews, J.H. Pavement Construction with High-Volume Class C and Class F Fly Ash Concrete. ACI Mater. J. 1995, 92, 200–210. [Google Scholar]
- Poon, C.S.; Lam, L.; Wong, Y.L. A study on high strength concrete prepared with large volumes of low calcium fly ash. Cem. Concr. Res. 2000, 30, 447–455. [Google Scholar] [CrossRef]
- Atis, C.D. Heat evolution of high-volume fly ash concrete. Cem. Concr. Res. 2002, 32, 751–756. [Google Scholar] [CrossRef]
- Schwarz, N.; Neithalath, N. Influence of a fine glass powder on cement hydration: Comparison to fly ash and modeling the degree of hydration. Cem. Concr. Res. 2008, 38, 429–436. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Awal, A.A.; Shehu, I.A. Influence of high volume fly ash in controlling Heat of Hydration of Concrete. Int. J. Eng. Res. Appl. 2013, 3, 932–936. [Google Scholar]
- Sakai, E.; Miyahara, S.; Ohsawa, S.; Lee, S.H.; Daimon, M. Hydration of fly ash cement. Cem. Concr. Res. 2005, 35, 1135–1140. [Google Scholar] [CrossRef]
- Durán-Herrera, A.; Juarez, C.A.; Valdez, P.; Bentz, D.P. Evaluation of sustainable high-volume fly ash concretes. Cem. Concr. Compos. 2011, 33, 39–45. [Google Scholar] [CrossRef]
- Hardjito, D.; Wallah, S.E.; Sumajouw, D.J.; Rangan, B.V. On the development of fly ash-based geopolymer concrete. ACI Mater. J. 2004, 101, 467–472. [Google Scholar]
- Rangan, B.V. Mix design and production of fly ash based geopolymer concrete. Indian Concr. J. 2008, 82, 7–15. [Google Scholar]
- Barnes, P.; Bensted, J. Structure and Performance of Cements, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Shi, C.; Day, R.L. Acceleration of the reactivity of fly ash by chemical activation. Cem. Concr. Res. 1995, 25, 15–21. [Google Scholar] [CrossRef]
- Lee, C.Y.; Lee, H.K.; Lee, K.M. Strength and microstructural characteristics of chemically activated fly ash–cement systems. Cem. Concr. Res. 2003, 33, 425–431. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Sun, W.; Yan, H.D. Hydration of high-volume fly ash cement pastes. Cem. Concr. Compos. 2000, 22, 445–452. [Google Scholar] [CrossRef]
- Qian, J.; Shi, C.; Wang, Z. Activation of blended cements containing fly ash. Cem. Concr. Res. 2001, 31, 1121–1127. [Google Scholar] [CrossRef]
- Chinese Ministry of Construction. GB/T12959–2008 Test Methods for Heat of Hydration of Cement, China National Standard; China Standards Press: Beijing, China, 2008. (In Chinese)
- ASTM C186-15A. Standard Test Method for Heat of Hydration of Hydraulic Cement; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Chinese Ministry of Construction. GB/T50080–2002, Standard for Test Method of Performance on Ordinary Fresh Concrete; China Standards Press: Beijing, China, 2003. (In Chinese)
- Chinese Ministry of Construction. CECS 13–2009, Standard Test Method for Fiber Reinforced Concrete; China Standards Press: Beijing, China, 2009. (In Chinese)
- ASTM C1581/C1581M-09a. Standard Test Method for Determining Age at Cracking and Induced Tensile Stress Characteristics of Mortar and Concrete under Restrained Shrinkage; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar]
- ASTM C1579–13. Standard Test Method for Evaluating Plastic Shrinkage Cracking of Restrained Fiber Reinforced Concrete (Using a Steel Form Insert); ASTM International: West Conshohocken PA, USA, 2013. [Google Scholar]
- Yang, Z.; Weiss, W.J.; Olek, J. Interaction between Micro-Cracking, Cracking, and Reduced Durability of Concrete: Developing Methods for Considering Cumulative Damage in Life-Cycle Modeling; JTRP Technical Reports: West Lafayette, IN, USA, 2004. [Google Scholar]
- Brooks, J.J. Prediction of Setting Time of Fly Ash Concrete. ACI Mater. J. 2002, 99, 591–597. [Google Scholar]






| Physical Property (%) | Type | (+) 325 Mesh (+ 44 mm) | Water Demand | Water Content | Ignition Loss | Specific Gravity | Fineness m2/kg | |
| Fly ash II | 18 | 31 | 0.89 | 7.1 | 2.42 | - | ||
| PC | 9.5 | 26.2 | - | 1.9 | 3.14 | 350 | ||
| Chemical compositions (%) | Type | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Others |
| Fly ash II | 57.5 | 22.6 | 8.5 | 4.94 | 2.57 | 0.5 | 3.4 | |
| PC | 22.8 | 9.47 | 3.4 | 55.2 | 2.99 | 2.15 | 1.9 | |
| Groups | Series of Test No. 1 | w/b Ratio | Replacement Ratio of FA | Fly ash kg/m3 | Cement kg/m3 | Aggregates kg/m3 | Admixtures kg/m3 | Lime kg/m3 | Activator kg/m3 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Coarse | Fine | |||||||||
| Compressive + Setting time | C40-FAY-ACZ | 0.41 | Y = 0, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7 | 385Y | 385 (1-Y) | 1094 | 705 | 3.08 | 38.5 | 385Y |
| C60-FAY-ACZ | 0.30 | 530Y | 530 (1-Y) | 1113 | 562 | 7.95 | 38.5 | 386Y | ||
| Early crack | C40-FAY-ACZ | 0.41 | 385Y | 385 (1-Y) | 1094 | 705 | 3.08 | 38.5 | 385Y | |
| Groups | Test No. 1 | Cement (g) | Fly ash (g) | Water (ml) | Na2SO4 (g) | Curing Age (Days) |
|---|---|---|---|---|---|---|
| Hydration heat | FA00-AC00 | 40 | 0 | 100 | 0 | 1/3/7 |
| FA20-AC00 | 32 | 8 | ||||
| FA30-AC00 | 28 | 12 | ||||
| FA40-AC00 | 24 | 16 | ||||
| FA50-AC00 | 20 | 20 | ||||
| FA60-AC00 | 16 | 24 | - | |||
| FA70-AC00 | 12 | 28 | - | |||
| FA40-AC10 | 24 | 16 | 100 | 0.4 | 1/3/7 | |
| FA40-AC15 | 0.6 | |||||
| FA40-AC20 | 0.8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Cai, G.; Degée, H.; Huang, B.; Luo, Z. Hydration, Setting and Crack-Resistance Properties of Activated HVFACs at Early Stages. Appl. Sci. 2016, 6, 224. https://doi.org/10.3390/app6080224
Zhao J, Cai G, Degée H, Huang B, Luo Z. Hydration, Setting and Crack-Resistance Properties of Activated HVFACs at Early Stages. Applied Sciences. 2016; 6(8):224. https://doi.org/10.3390/app6080224
Chicago/Turabian StyleZhao, Jun, Gaochuang Cai, Hervé Degée, Bo Huang, and Zhongtao Luo. 2016. "Hydration, Setting and Crack-Resistance Properties of Activated HVFACs at Early Stages" Applied Sciences 6, no. 8: 224. https://doi.org/10.3390/app6080224