Recent Progress on Solution-Processed CdTe Nanocrystals Solar Cells
Abstract
:1. Introduction
2. Fabrication of CdTe NCs
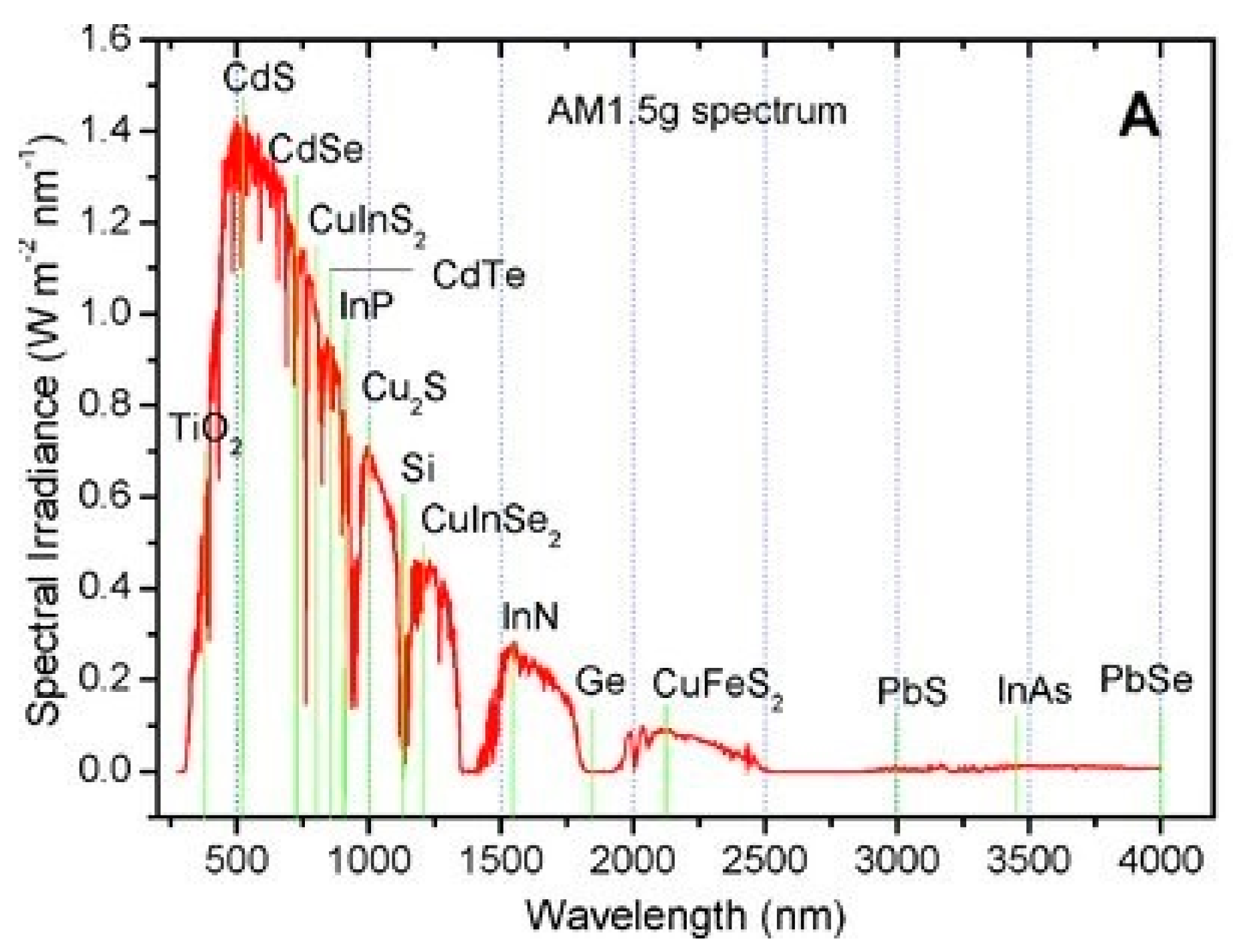
3. Optical Properties
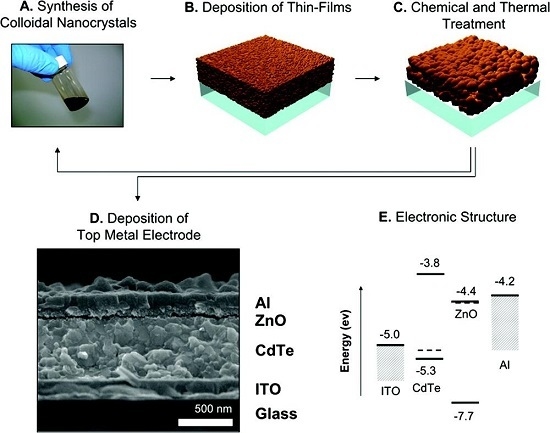
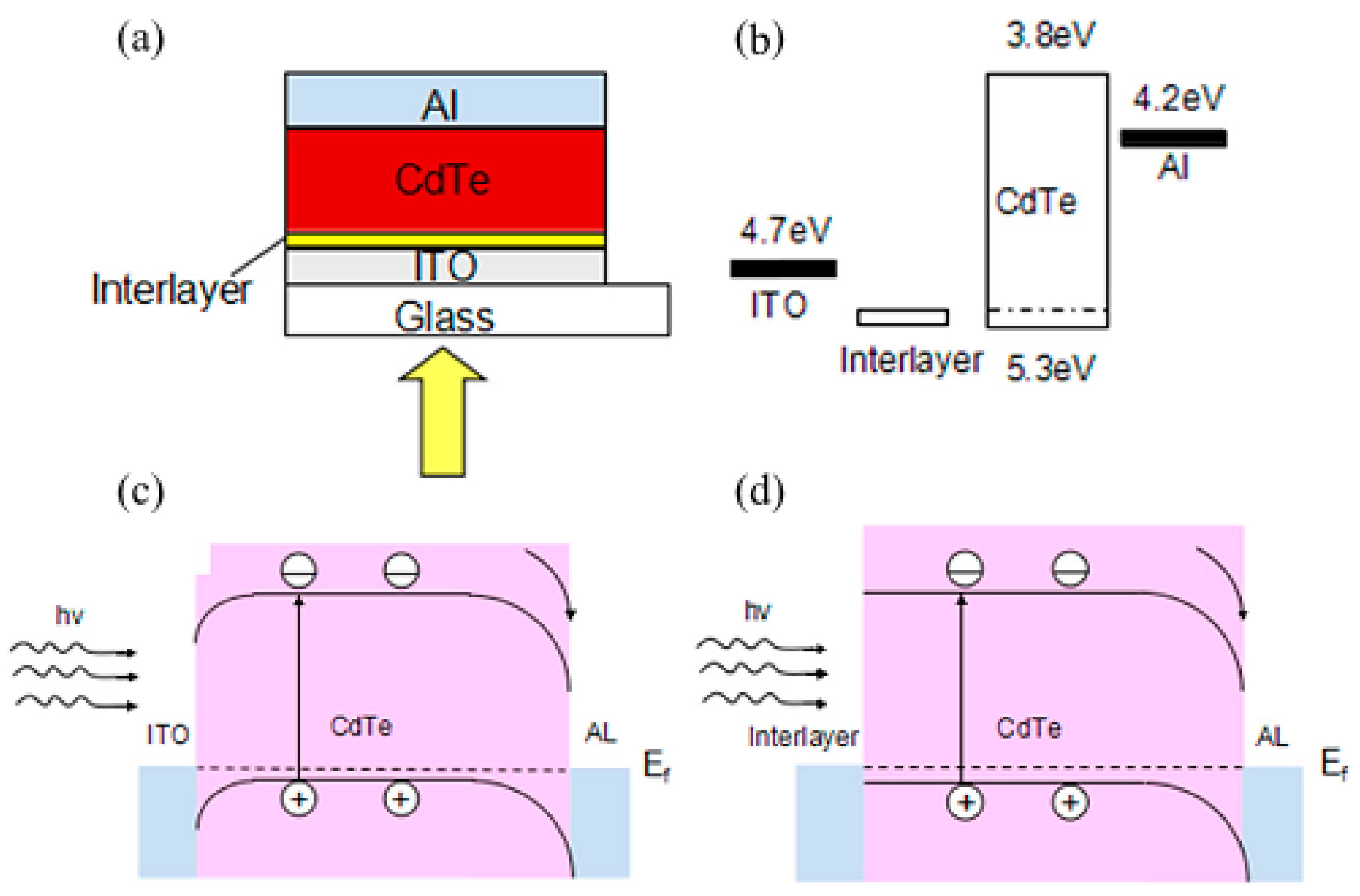
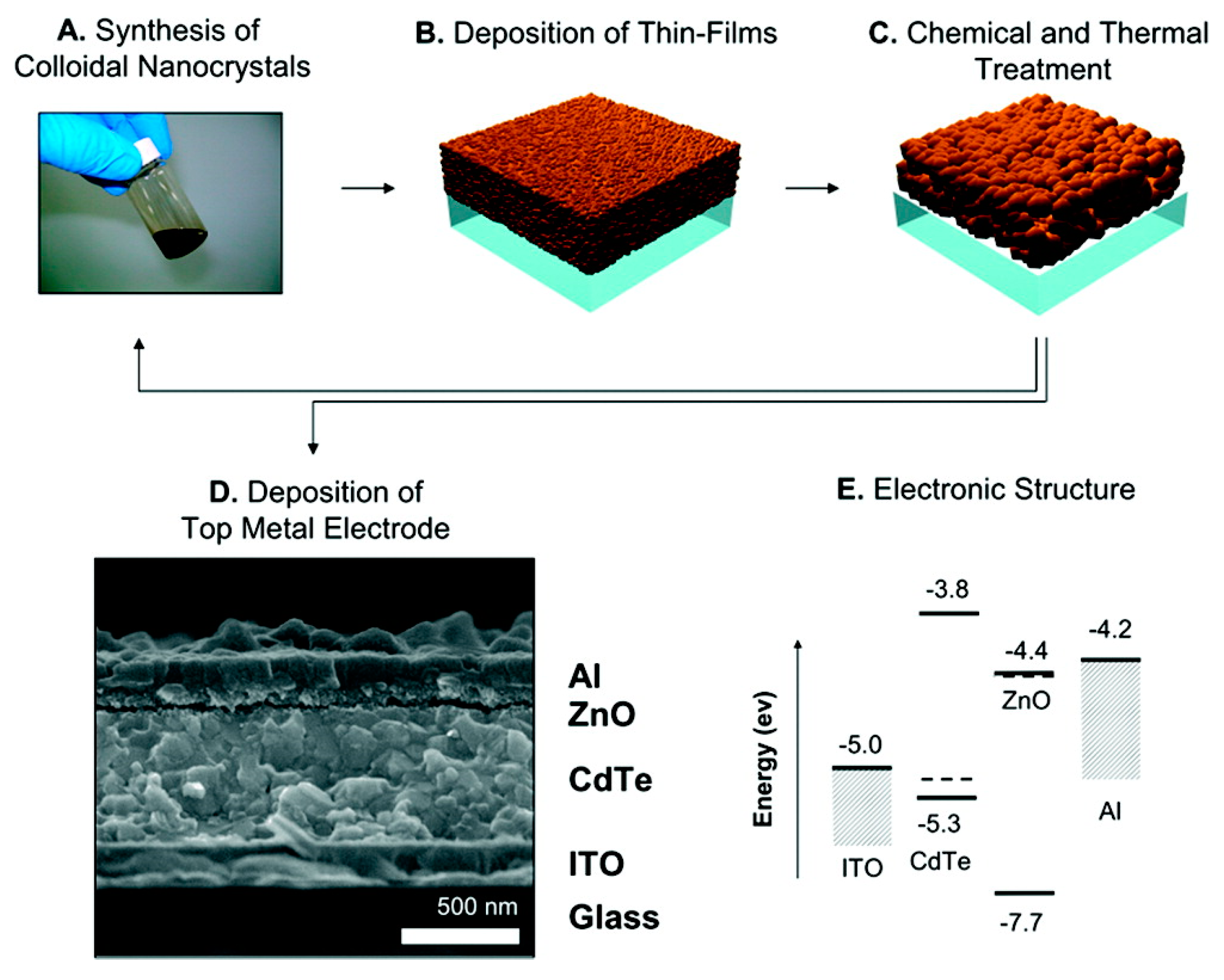
4. Schottky Junction Solar Cells
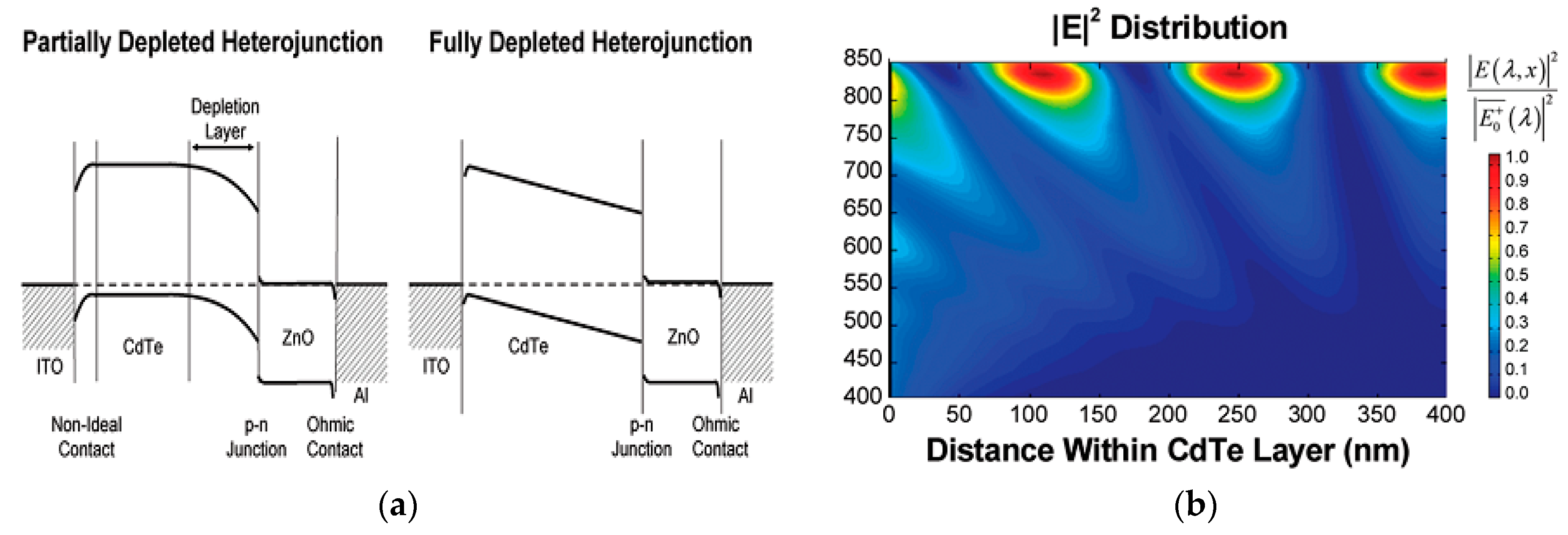
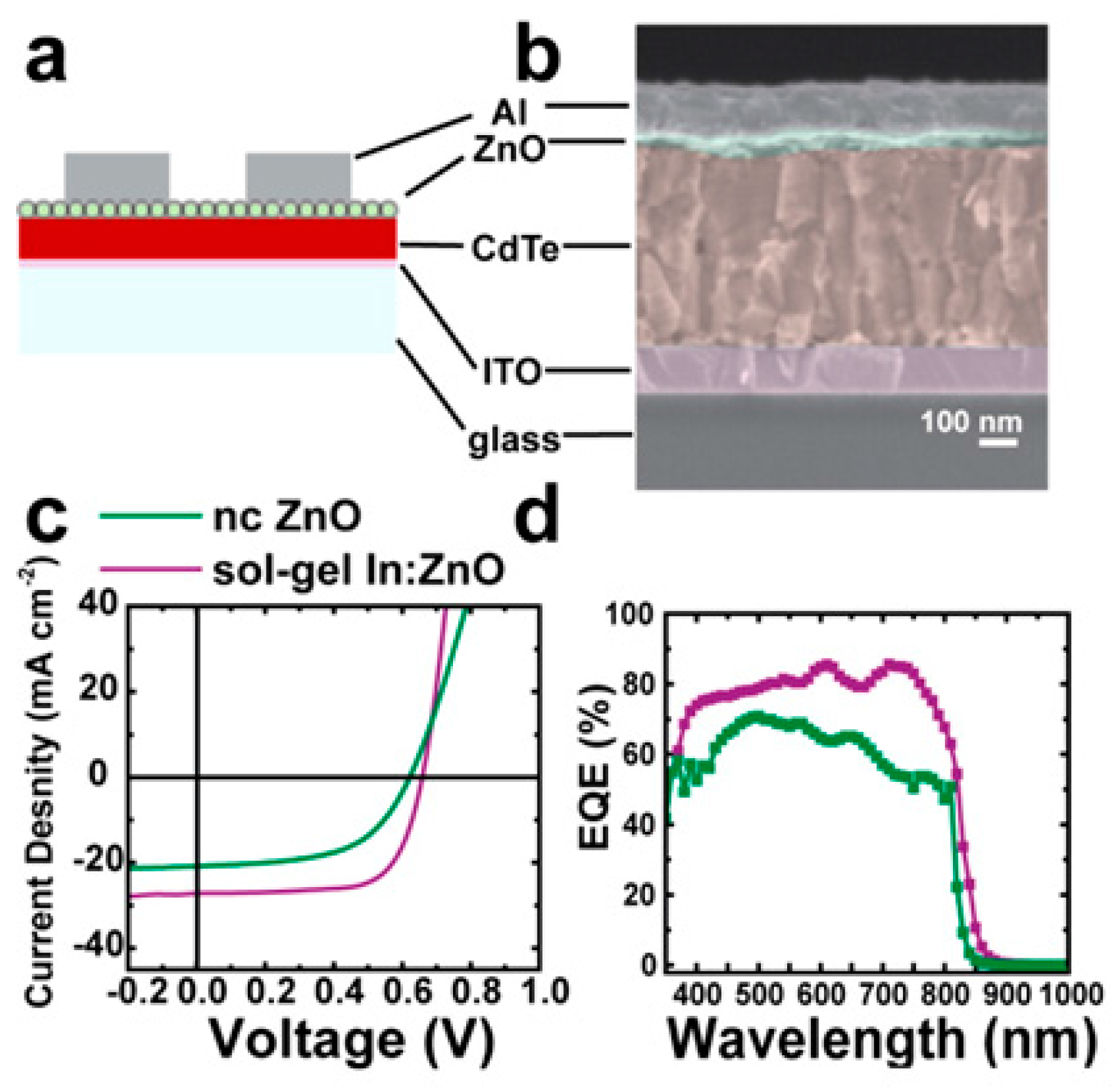
5. CdTe/ZnO Heterojunction Solar Cells
6. CdTe/CdSe NCs Heterojunction Solar Cells
7. Other NC Heterojunction Solar Cells
8. Hybrid Solar Cells
9. Perspectives and Conclusions
Acknowledgments
Conflicts of Interest
References
- Kim, G.; De García Arquer, F.P.; Yoon, Y.J.; Lan, X.; Liu, M.; Voznyy, O.; Yang, Z.; Fan, F.; Ip, A.H.; Kanjanaboos, P.; et al. High-efficiency colloidal quantum dot photovoltaics via robust self-assembled monolayers. Nano Lett. 2015, 15, 7691–7696. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, B.; Gao, Y.; Liu, H.; Tian, Y.; Qin, D.; Wu, H.; Hou, L.; Huang, W. Novel hybrid ligands for passivating PbS colloidal quantum dots to enhance the performance of solar cells. Nano-Micro Lett. 2015, 7, 325–331. [Google Scholar] [CrossRef]
- Chuang, C.M.; Brown, P.R.; Bulović, V.; Bawendi, M.G. Improved performance and stability in quantum dot solar cells through band alignment engineering. Nat. Mater. 2014, 13, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Ford, G.M.; Agrawal, R.; Hillhouse, H. Ink formulation and low-temperature incorporation of sodium to yield 12% efficient Cu(In,Ga)(S,Se)2 solar cells from sulfide nanocrystal inks. Prog. Photovolt.: Res. Appl. 2013, 21, 64–71. [Google Scholar] [CrossRef]
- Guo, Q.; Hillhouse, H.W.; Agrawal, R. Synthesis of Cu2ZnSnS4 nanocrystal ink and its use for solar cells. J. Am. Chem. Soc. 2009, 33, 11672–11673. [Google Scholar] [CrossRef] [PubMed]
- Gur, I.; Fromer, N.A.; Geier, M.L.; Alivisatos, A.P. Air-stable all inorganic nanocrystal solar cells processed from solution. Science 2005, 310, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.D.; Rodriguez, Y.W.; Yang, L.D.; Alers, G.B.; Carter, S.A. CdTe Schottky diodes from colloidal nanocrystals. Appl. Phys. Lett. 2010, 96, 242103. [Google Scholar] [CrossRef]
- Jasieniak, J.; MacDonald, B.I.; Watkins, S.E.; Mulvaney, P. Solution-processed sintered nanocrystal solar cells via Layer-by-Layer assembly. Nano Lett. 2011, 11, 2856–2864. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, B.I.; Martucci, A.; Rubanov, S.; Watkins, S.E.; Mulvaney, P.; Jasieniak, J.J. Layer-by-Layer assembly of sintered CdSexTe1–x nanocrystal solar cells. ACS Nano 2012, 6, 5995–6004. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, B.I.; Della Gaspera, E.; Watkins, S.E.; Mulvaney, P.; Jasieniak, J.J. Enhanced photovoltaic performance of nanocrystalline CdTe/ZnO solar cells using sol-gel ZnO and positive bias treatment. J. Appl. Phys. 2014, 115, 184501. [Google Scholar] [CrossRef]
- Wang, J.; Ling, T.; Qiao, S.; Du, X. Double open-circuit voltage of three-dimensional ZnO/CdTe solar cells by a balancing depletion layer. ACS Appl. Mater. Interfaces 2014, 6, 14718–14723. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Liu, H.; Gao, Y.; Qin, D.; Chen, J. Controlled synthesis of CdTe nanocrystals for high performanced Schottky thin film solar cells. J. Mater. Chem. 2012, 22, 19207–19212. [Google Scholar] [CrossRef]
- Tang, J.; Sargent, E.H. Infrared colloidal quantum dots for photovoltaics: Fundamentals and recent progress. Adv. Mater. 2011, 23, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Shokhovets, S.; Ambacher, O.; Gobsch, G. Conduction-band dispersion relation and electron effective mass in III-V and II-VI zinc-blende semiconductors. Phys. Rev. B. 2007, 76, 125203. [Google Scholar] [CrossRef]
- Peng, Z.A.; Peng, X. Formation of High-Quality CdTe, CdSe, and CdS nanocrystals using CdO as precursor. J. Am. Chem. Soc. 2001, 123, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.W.; Qu, L.; Guo, W.; Peng, X. Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem. Mater. 2003, 15, 2854–2860. [Google Scholar] [CrossRef]
- Yang, Y.A.; Wu, H.; Williams, K.R.; Cao, Y.C. Synthesis of CdSe and CdTe nanocrystals without precursor injection. Angew. Chem. 2005, 117, 6870–6873. [Google Scholar] [CrossRef]
- Liu, H.; Tao, H.; Yang, T.; Kong, L.; Qin, D.; Chen, J. A surfactant-free recipe for shape-controlled synthesis of CdSe nanocrystals. Nanotechnology 2011, 22, 045604. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.B.; Noris, D.J.; Bawendi, M.G. Synthesis and characterization of nearly monodisperse CdE (E = S, Se, Te) semiconductor nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Xie, R.; Rutherford, M.; Peng, X. Formation of High-Quality I−III−VI semiconductor nanocrystals by tuning relative reactivity of cationic precursors. J. Am. Chem. Soc. 2009, 131, 5691–5697. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Qin, D.; Jiang, X.; Liu, Y.; Wang, L.; Chen, J.; Cao, Y. Synthesis of high quality zinc-blende CdSe nanocrystals and their application in hybrid solar cells. Nanotechnology 2006, 17, 4736–4742. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Peng, Z.A.; Peng, X. Alternative routes toward high quality CdSe Nanocrystals. Nano Lett. 2001, 1, 333–337. [Google Scholar] [CrossRef]
- Peng, Z.A.; Peng, X. Nearly monodisperse and Shape-Controlled CdSe nanocrystals via alternative routes: Nucleation and growth. J. Am. Chem. Soc. 2002, 124, 3343–3353. [Google Scholar] [CrossRef] [PubMed]
- Sapra, S.; Poppe, J.; Eychmüller, A. CdSe nanorod synthesis: A new approach. Small 2007, 3, 1886–1888. [Google Scholar] [CrossRef] [PubMed]
- Loscutova, R.; Barron, A.R. Coating single-walled carbon nanotubes with cadmium chalcogenides. J. Mater. Chem. 2005, 15, 4346–4353. [Google Scholar] [CrossRef]
- Yu, W.W.; Wang, Y.A.; Peng, X. Formation and stability of size-, shape-, and Structure-Controlled CdTe nanocrystals: Ligand effects on monomers and nanocrystals. Chem. Mater. 2003, 15, 4300–4308. [Google Scholar] [CrossRef]
- Yu, W.W.; Peng, X. Formation of High-Quality CdS and other II−VI semiconductor Nanocrystals in noncoordinating solvents: Tunable reactivity of monomers. Angew. Chem. Int. Ed. 2002, 41, 2368–2371. [Google Scholar] [CrossRef]
- Li, Y.C.; Zhong, H.Z.; Li, R.; Zhou, Y.; Yang, C.H.; Li, Y.F. High-Yield fabrication and electrochemical characterization of tetrapodal CdSe, CdTe, and CdSexTe1–x nanocrystals. Adv. Funct. Mater. 2006, 16, 1705–1716. [Google Scholar] [CrossRef]
- Li, J.J.; Wang, Y.A.; Guo, W.; Keay, J.C.; Mishima, T.D.; Johnson, M.B.; Peng, X. Large-Scale synthesis of nearly monodisperse CdSe/CdS Core/Shell nanocrystals using Air-Stable reagents via successive ion layer adsorption and reaction. J. Am. Chem. Soc. 2003, 125, 12567–12575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, C.; Li, M.; Ji, X.; Zhang, J.; Yang, B. Fluorescent nanocrystal−polymer composites from aqueous nanocrystals: Methods without ligand exchange. Chem. Mater. 2005, 17, 4783–4788. [Google Scholar] [CrossRef]
- Hoppe, H.; Arnold, N.; Sariciftci, N.S.; Meissner, D. Modeling the optical absorption within conjugated polymer/fullerene-based bulk-heterojunction organic solar cells. Sol. Energy Mater. Sol. Cells 2003, 80, 105–113. [Google Scholar] [CrossRef]
- Yoon, W.; Foos, E.E.; Lumb, M.P. Solution processing of CdTe nanocrystals for thin-film solar cells. In Proceedings of the 2012 38th IEEE Photovoltaic Specialists Conference (PVSC), Austin, TX, USA, 3–8 June 2012; pp. 2621–2624.
- Gessert, T.A.; Wei, S.H.; Ma, J.; Albin, D.S.; Dhere, R.G.; Duenow, J.N.; Kuciauskas, D.; Kanevce, A.; Barnes, T.M.; Burst, J.M.; et al. Research strategies toward improving thin-film CdTe photovoltaic devices beyond 20% conversion efficiency. Sol. Energy Mater. Sol. Cells 2013, 119, 149–155. [Google Scholar] [CrossRef]
- Toušek, J.; Kindl, D.; Toušková, J.; Dolhov, S.; Poruba, A. Diffusion length in CdTe by measurement of photovoltage spectra in CdS/CdTe solar cells. J. Appl. Phys. 2001, 89, 460–465. [Google Scholar] [CrossRef]
- De Mello Donegá, C.; Liljeroth, P.; Vanmaekelbergh, D. Physicochemical evaluation of the Hot-Injection method, a synthesis route for monodisperse nanocrystals. Small 2005, 1, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yang, Y.; Gao, Y.; Qin, D.; Wu, H.; Hou, L.; Huang, W. Enhancement of open-circuit voltage and the fill factor in CdTe nanocrystal solar cells by using interface materials. Nanotechnology 2014, 25, 365203. [Google Scholar] [CrossRef] [PubMed]
- Lakhwani, G.; Roijmans, R.F.H.; Kronemeijer, A.J.; Gilot, J.; Janssen, R.A.J.; Meskers, S.C.J. Probing charge carrier density in a layer of photodoped ZnO nanoparticles by spectroscopic ellipsometry. J. Phys. Chem. C 2010, 114, 14804–14810. [Google Scholar] [CrossRef]
- Wood, A.; Giersig, M.; Hilgendorff, M.; Mulvancy, P. Size effects in ZnO: The cluster to quantum dot transition. Aust. J. Chem. 2003, 56, 1051–1057. [Google Scholar] [CrossRef]
- Panthani, M.G.; Kurley, J.M.; Crisp, R.W.; Dietz, T.C.; Ezzyat, T.; Luther, J.M.; Talapin, D.V. High efficiency solution processed sintered CdTe nanocrystal solar cells: The role of interfaces. Nano Lett. 2014, 14, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, M.; Kozuka, H.; Yoko, T. Sol-gel preparation of transparent and conductive Aluminum-Doped zinc oxide films with highly preferential crystal orientation. J. Am. Ceram. Soc. 1998, 81, 1622–1632. [Google Scholar] [CrossRef]
- MacDonald, B.I.; Gengenbach, T.R.; Watkins, S.E.; Mulvaney, P.; Jasieniak, J.J. Solution-processing of ultra-thin CdTe/ZnO nanocrystal solar cells. Thin Solid Films 2014, 558, 365–373. [Google Scholar] [CrossRef]
- Crisp, R.W.; Panthani, M.G.; Rance, W.L.; Duenow, J.N.; Parilla, P.A.; Callahan, R.; Dabney, M.S.; Berry, J.J.; Talapin, D.V.; Luther, J.M. Nanocrystal grain growth and device architectures for High-Efficiency CdTe Ink-Based photovoltaics. ACS Nano 2014, 8, 9063–9072. [Google Scholar] [CrossRef] [PubMed]
- Small, C.E.; Chen, S.; Subbiah, J.; Amb, C.M.; Tsang, S.W.; Reynolds, J.R.; So, F. High-efficiency inverted dithienogermole–thienopyrrolodione-based polymer solar cells. Nat. Photonics 2012, 6, 115–120. [Google Scholar] [CrossRef]
- Shockley, W.; Queisser, H.J. Detailed balance limit of efficiency of p-n junction solar cells. J. Appl. Phys. 1961, 32. [Google Scholar] [CrossRef]
- Chin, K.K. P-Doping limit and donor compensation in CdTe polycrystalline thin film solar cells. Sol. Energy Mater. Sol. Cells 2010, 94, 1627–1629. [Google Scholar] [CrossRef]
- Talapin, D.V.; Lee, J.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.R.; Lunt, R.R.; Zhao, N.; Osedach, T.P.; Wanger, D.D.; Chang, L.; Bawendi, M.G.; Bulović, V. Improved current extraction from ZnO/PbS quantum dot heterojunction photovoltaics using a MoO3 interfacial layer. Nano Lett. 2011, 11, 2955–2961. [Google Scholar] [CrossRef] [PubMed]
- Bezryadina, A.; France, C.; Graham, R.; Yang, L.; Carter, S.A.; Alers, G.B. Mid-gap trap states in CdTe nanoparticle solar cells. Appl. Phys. Lett. 2012, 100. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, J.J.; Lee, S.H.; Sohn, S.H. Study of CdTe/CdS heterostructure by CdCl2 heat treatment via in situ high temperature XRD. Sol. Energy Mater. Sol. Cell 2013, 109, 209–214. [Google Scholar] [CrossRef]
- Buurma, C.; Krishnamurthy, S.; Sivananthan, S. Shockley-Read-Hall lifetimes in CdTe. J. Appl. Phys. 2014, 116. [Google Scholar] [CrossRef]
- Chambers, B.A.; MacDonald, B.I.; Ionescu, M.; Deslandes, A.; Quinton, J.S.; Jasieniak, J.J.; Andersson, G.G. Examining the role of ultra-thin atomic layer deposited metal oxide barrier layers on CdTe/ITO interface stability during the fabrication of solution processed nanocrystalline solar cells. Sol. Energy Mater. Sol. Cell 2014, 125, 164–169. [Google Scholar] [CrossRef]
- Hsiao, K. Energy-band barrier to improve open-circuit voltage of CdTe solar cells. Sol. Energy Mater. Sol. Cell 2014, 120, 647–653. [Google Scholar] [CrossRef]
- Kosyachenko, L.; Toyama, T. Current–voltage characteristics and quantum efficiency spectra of efficient thin-film CdS/CdTe solar cells. Sol. Energy Mater. Sol. Cell 2014, 120, 512–520. [Google Scholar] [CrossRef]
- Gross, D.; Mora-Seró, I.; Dittrich, T.; Belaidi, A.; Mauser, C.; Houtepen, A.J.; Como, E.D.; Rogach, A.L.; Feldmann, J. Charge separation in type II tunneling multilayered structures of CdTe and CdSe nanocrystals directly proven by surface photovoltage spectroscopy. J. Am. Chem. Soc. 2010, 132, 5981–5983. [Google Scholar] [CrossRef] [PubMed]
- Ju, T.; Yang, L.L.; Carter, S. Thickness dependence study of inorganic CdTe/CdSe solar cells fabricated from colloidal nanoparticle solutions. J. Appl. Phys. 2010, 107, 104311. [Google Scholar] [CrossRef]
- Yoon, W.; Townsend, T.K.; Lumb, M.P.; Tischler, J.G.; Foos, E.E. Sintered CdTe nanocrystal thin films: Determination of optical constants and application in novel inverted heterojunction solar cells. IEEE Trans. Nanotechnol. 2014, 13, 551–556. [Google Scholar] [CrossRef]
- Townsend, T.K.; Foos, E.E. Fully solution processed all inorganic nanocrystal solar cells. Phys. Chem. Chem. Phys. 2014, 16, 16458–16464. [Google Scholar] [CrossRef] [PubMed]
- Townsend, T.K.; Heuer, W.B.; Foos, E.E.; Kowalski, E.; Yoon, W.; Tischler, J.G. Safer salts for CdTe nanocrystal solution processed solar cells: The dual roles of ligand exchange and grain growth. J. Mater. Chem. A. 2015, 3, 13057–13065. [Google Scholar] [CrossRef]
- Liu, H.; Tian, Y.Y.; Zhang, Y.J.; Gao, K.; Qin, D.H. Solution processed CdTe/CdSe nanocrystal solar cells with more than 5.5% efficiency by using an inverted device structure. J. Mater. Chem. C. 2015, 3, 4227–4234. [Google Scholar] [CrossRef]
- Katiyar, R.K.; Sahoo, S.; Gaur, A.P.S.; Singh, A.; Morell, G.; Katiyar, R.S. Studies of photovoltaic properties of nanocrystalline thin films of CdS–CdTe. J. Alloys Compd. 2011, 509, 10003–10006. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Y.; Lin, Y.; Gao, K.; Zhang, Y.; Liu, K.; Yang, Q.; Zhou, X.; Qin, D.; Wu, H.; et al. Solution-processed efficient CdTe nanocrystal/CBD-CdS hetero-junction solar cells with ZnO interlayer. J. Nanoparticle Res. 2013, 15. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, H.; Zeng, Q.; Wang, Y.; Xu, D.; Wang, L.; Wang, H.; Yang, B. In situ construction of nanoscale CdTe-CdS bulk heterojunctions for inorganic nanocrystal solar cells. Adv. Energy Mater. 2014, 4. [Google Scholar] [CrossRef]
- Chen, Z.; Zeng, Q.; Liu, F.; Jin, G.; Du, X.; Du, J.; Zhang, H.; Yang, B. Efficient inorganic solar cells from aqueous nanocrystals: The impact of composition on carrier dynamics. RSC Adv. 2015, 5, 74263–74269. [Google Scholar] [CrossRef]
- Jean, J.; Chang, S.; Brown, P.R.; Cheng, J.J.; Rekemeyer, P.H.; Bawendi, M.G.; Gradečak, S.; Bulović, V. ZnO nanowire arrays for enhanced photocurrent in PbS quantum dot solar cells. Adv. Mater. 2013, 25, 2790–2796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Jiang, S.; Lin, Y.; Ren, W.; Cai, H.; Wu, Y.; Zhang, Q.; Pan, N.; Luo, Y.; Wang, X. Improving the photovoltaic performance of solid-state ZnO/CdTe core–shell nanorod array solar cells using a thin CdS interfacial layer. J. Mater. Chem. A. 2014, 2, 5675–5681. [Google Scholar] [CrossRef]
- Huynh, W.U. Hybrid Nanorod-Polymer solar cells. Science 2002, 295, 2425–2427. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Nann, T. First solar cells based on CdTe nanoparticle/MEH-PPV composites. J. Mater. Res. 2004, 19, 1990–1994. [Google Scholar] [CrossRef]
- Hoppe, H.; Niggemann, M.; Winder, C.; Kraut, J.; Saricifitci, N.S. Nanoscale morphology of conjugated Polymer/Fullerene-based bulk-heterojunction solar cells. Adv. Funct. Mater. 2004, 14, 1005–1011. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, H.; Sun, H.; Yang, B. Preparation of polymer–nanocrystals hybrid solar cells through aqueous approaches. Nano Today 2012, 7, 316–326. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, H.; Du, X.; Cheng, X.; Chen, X.; Jiang, Y.; Yang, B. From planar-heterojunction to n–i structure: An efficient strategy to improve short-circuit current and power conversion efficiency of aqueous-solution-processed hybrid solar cells. Energy Environ. Sci. 2013, 6, 1597–1603. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, H.; Yu, W.; Li, Z.; Hou, J.; Wei, H.; Yang, B. Inverted Hybrid Solar Cells from Aqueous Materials with a PCE of 3.61%. Adv. Energy Mater. 2013, 3, 433–437. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Wei, H.; Zhang, H.; Chen, Q.; Xu, H.; Han, W.; Yang, B.; Sun, H. Unraveling charge separation and transport mechanisms in Aqueous-Processed Polymer/CdTe nanocrystal hybrid solar cells. Adv. Energy Mater. 2014, 4. [Google Scholar] [CrossRef]
- Zhou, Y.; Eck, M.; Krüger, M. Bulk-heterojunction hybrid solar cells based on colloidal nanocrystals and conjugated polymers. Energy Environ. Sci. 2010, 3, 1851–1864. [Google Scholar] [CrossRef]
- Du, X.; Chen, Z.; Li, Z.; Hao, H.; Zeng, Q.; Dong, C.; Yang, B. Dip-Coated gold nanoparticle electrodes for Aqueous-Solution-Processed Large-Area solar cells. Adv. Energy Mater. 2014, 4. [Google Scholar] [CrossRef]
- Wei, H.; Jin, G.; Wang, L.; Hao, L.; Na, T.; Wang, Y.; Tian, W.; Sun, H.; Zhang, H.; Wang, H.; et al. Synthesis of a Water-Soluble conjugated polymer based on thiophene for an Aqueous-Processed hybrid photovoltaic and photodetector device. Adv. Mater. 2014, 26, 3655–3661. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Chen, Z.; Li, F.; Xu, B.; Song, J.; Yan, L.; Jin, G.; Wen, S.; Wang, C.; Yang, B.; et al. High-Efficiency Aqueous-Solution-Processed hybrid solar cells based on P3HT dots and CdTe nanocrystals. ACS Appl. Mater. Interfaces 2015, 7, 7146–7152. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Wei, H.; Na, T.; Sun, H.; Zhang, H.; Yang, B. High-Efficiency Aqueous-Processed hybrid solar cells with an enormous herschel infrared contribution. ACS Appl. Mater. Interfaces 2014, 6, 8606–8612. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Chen, Z.; Zhao, Y.; Du, X.; Liu, F.; Jin, G.; Dong, F.; Zhang, H.; Yang, B. Aqueous-Processed inorganic thin-film solar cells based on CdSexTe1–x nanocrystals: The impact of composition on photovoltaic performance. ACS Appl. Mater. Interfaces 2015, 7, 23223–23230. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chen, Z.; Du, X.; Zeng, Q.; Ji, T.; Cheng, Z.; Jin, G.; Yang, B. High efficiency aqueous-processed MEH-PPV/CdTe hybrid solar cells with a PCE of 4.20%. J. Mater. Chem. A. 2016, 4, 1105–1111. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, F.; Zeng, Q.; Cheng, Z.; Du, X.; Jin, G.; Zhang, H.; Yang, B. Efficient aqueous-processed hybrid solar cells from a polymer with a wide bandgap. J. Mater. Chem. A. 2015, 3, 10969–10975. [Google Scholar] [CrossRef]
- Xie, Y.; Tan, Q.; Zhang, Z.; Lu, K.; Li, M.; Xu, W.; Qin, D.; Zhang, Y.; Hou, L.; Wu, H. Improving performance in CdTe/CdSe nanocrystals solar cells by using bulk nano-heterojunctions. J. Mater. Chem. C. 2016. [Google Scholar] [CrossRef]
- Dharmadasa, I.M.; Ojo, A.A.; Salim, H.I.; Dharmadasa, R. Next generation solar cells based on graded bandgap device structures utilising rod-type nano-materials. Energies 2015, 8, 5440–5458. [Google Scholar] [CrossRef]
- Ali, N.; Hussain, A.; Ahmed, R.; Wang, M.K.; Zhao, C.; UlHaq, B.; Fu, Y.Q. Advances in nanostructured thin film materials for solar cell applications. Renew. Sustain. Energy Rev. 2016, 59, 726–737. [Google Scholar] [CrossRef]
- Sehgal, P.; Narula, A.K. Quantum dot cosensitized solar cell based on PMOT@CdSe@ZnO core shell nanostructures with dual emission. J. Solid State Chem. 2016, 233, 428–437. [Google Scholar] [CrossRef]
- Unni, G.E.; Sasi, S.; Nair, A.S. Higher open-circuit voltage set by cobalt redox shuttle in SnO2 nanofibers-sensitized CdTe quantum dot solar cells. J. Energy Chem. 2016, 25, 481–488. [Google Scholar] [CrossRef]



| Structure | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) | Reference |
|---|---|---|---|---|---|
| ITO/PEDOT:PSS/PPV:CdTe/ZnO/Al | 0.50 | 10.7 | 40 | 2.14 | [69] |
| ITO/TiO2/CdTe/PPV:CdTe/MoO3/Au | 0.591 | 16.08 | 50.1 | 4.76 | [71] |
| ITO/TiO2/PNV:CdTe/MoO3/Au | 0.531 | 12.5 | 53.1 | 3.61 | [72] |
| ITO/TiO2/CdTe/PPV/MoO3-sol/Au-sol | 0.45 | 10.11 | 37 | 1.71 | [74] |
| ITO/TiO2/CdTe/PWTV:CdTe/MoO3/Au | 0.50 | 17.1 | 47.2 | 4.0 | [75] |
| ITO/TiO2/CdTe/P3HT:CdTe/MoO3/Au | 0.54 | 16.95 | 47.2 | 4.32 | [76] |
| ITO/TiO2/PPV:CdSexTe1−x/MoO3/Au | 0.552 | 13.94 | 43.5 | 3.35 | [78] |
| ITO/TiO2/MPPV:CdTe/MoO3/Au | 0.624 | 15.15 | 54.8 | 5.18 | [80] |
| Device Structure | PCE | Published Years | Reference |
|---|---|---|---|
| ITO/Au/CdTe/Al | 5.30% | 2014 | [37] |
| ITO/CdTe/ZnO/Al | 12.3% | 2014 | [40] |
| ITO/CdTe/CdSexTe1−x/ZnO/Al | 7.1% | 2012 | [9] |
| ITO/ZnO/CdSe/CdTe/Au | 5.81% | 2015 | [60] |
| ITO/TiO2/CdTe-CdS BHJ/Au | 5.73% | 2015 | [64] |
| ITO/TiO2/PPV:CdSexTe1−x/MoO3/Au | 3.35% | 2015 | [79] |
| ITO/TiO2/MPPV:CdTe/MoO3/Au | 5.18% | 2015 | [81] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, H.; Wu, R.; Xie, Y.; Tan, Q.; Qin, D.; Wu, H.; Huang, W. Recent Progress on Solution-Processed CdTe Nanocrystals Solar Cells. Appl. Sci. 2016, 6, 197. https://doi.org/10.3390/app6070197
Xue H, Wu R, Xie Y, Tan Q, Qin D, Wu H, Huang W. Recent Progress on Solution-Processed CdTe Nanocrystals Solar Cells. Applied Sciences. 2016; 6(7):197. https://doi.org/10.3390/app6070197
Chicago/Turabian StyleXue, Hao, Rongfang Wu, Ya Xie, Qiongxuan Tan, Donghuan Qin, Hongbin Wu, and Wenbo Huang. 2016. "Recent Progress on Solution-Processed CdTe Nanocrystals Solar Cells" Applied Sciences 6, no. 7: 197. https://doi.org/10.3390/app6070197