A Review of Double-Walled and Triple-Walled Carbon Nanotube Synthesis and Applications
Abstract
:1. Introduction and Motivation
1.1. Single-Walled Carbon Nanotubes
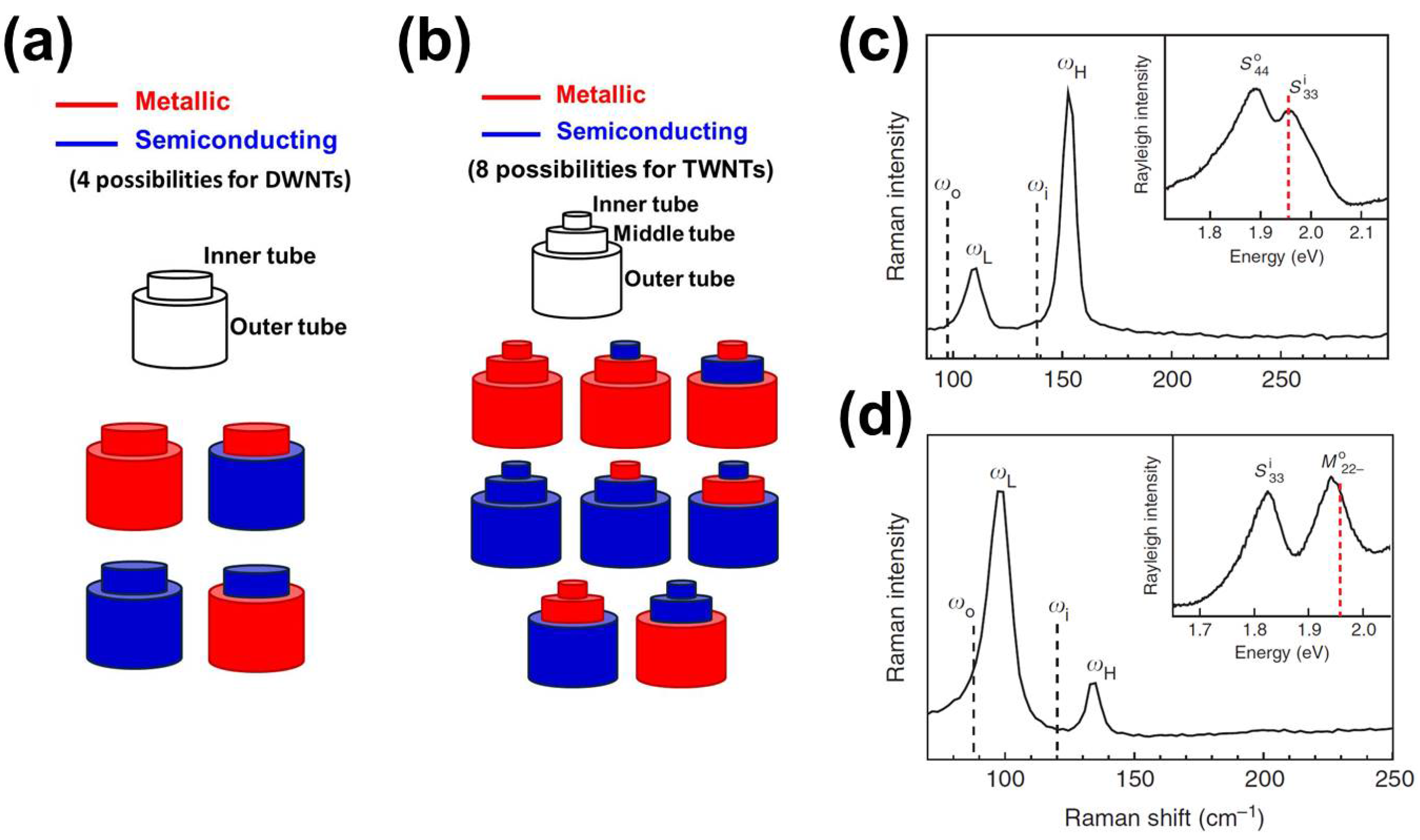
1.2. Double- and Triple-Walled Carbon Nanotubes
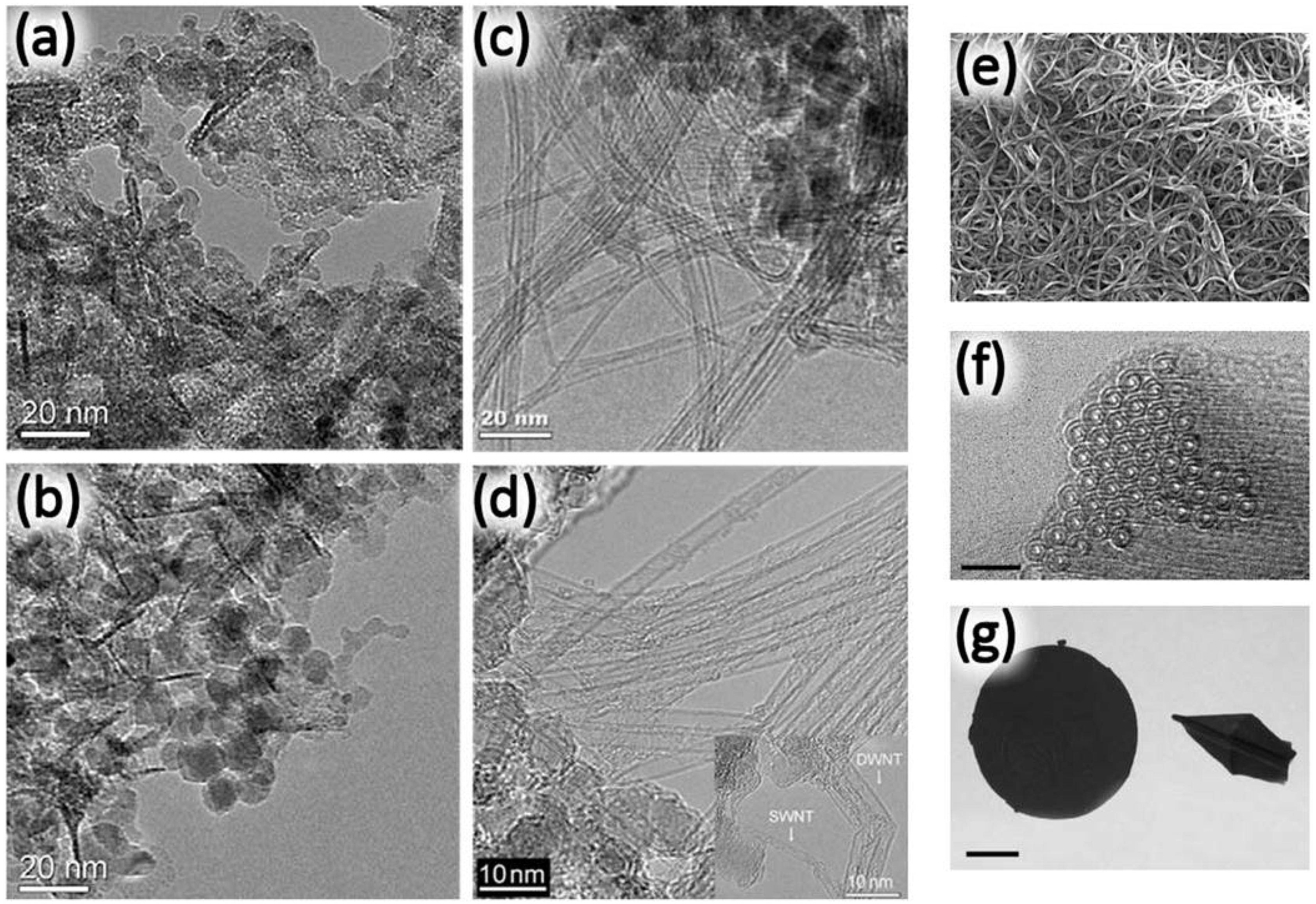
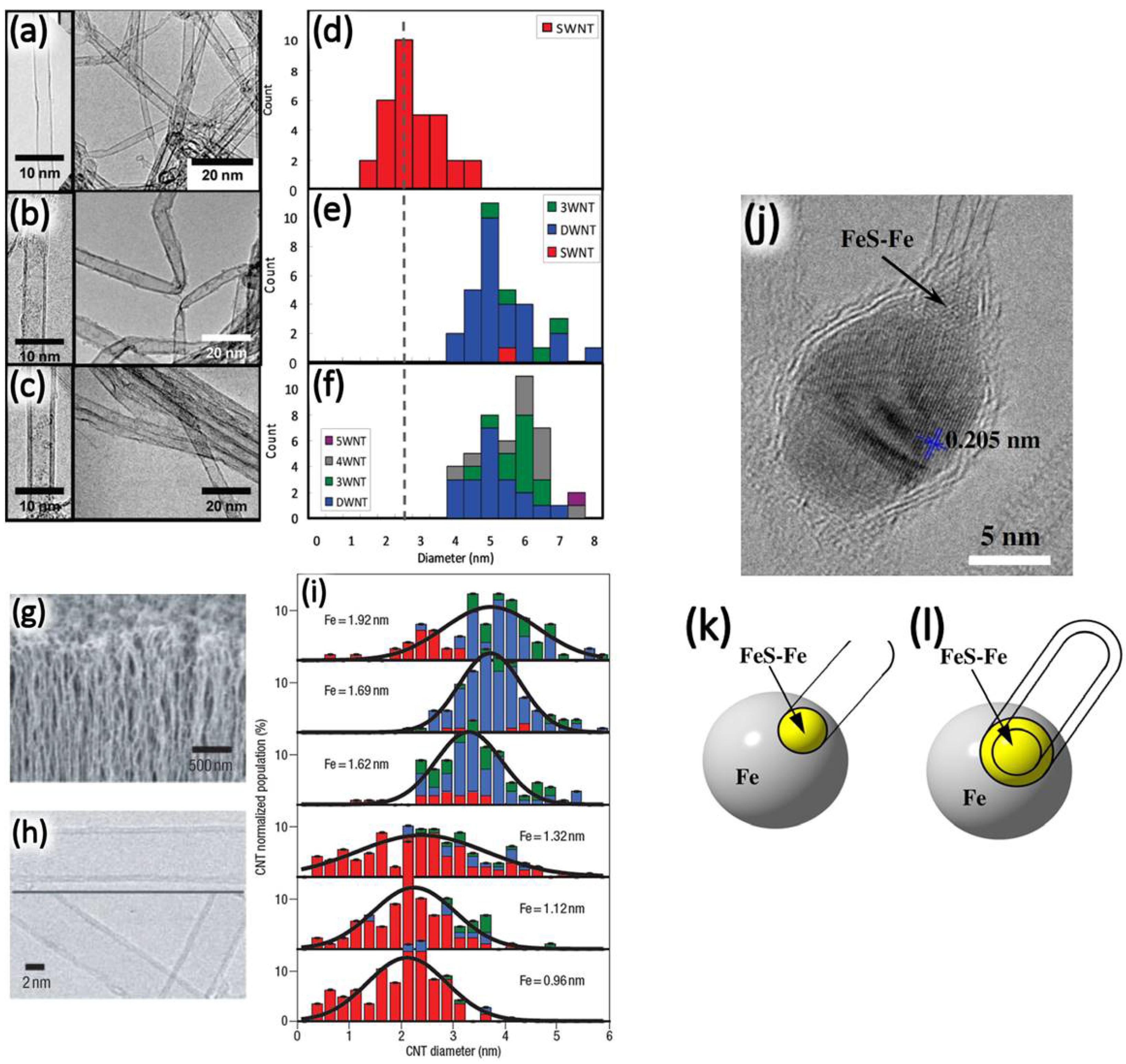
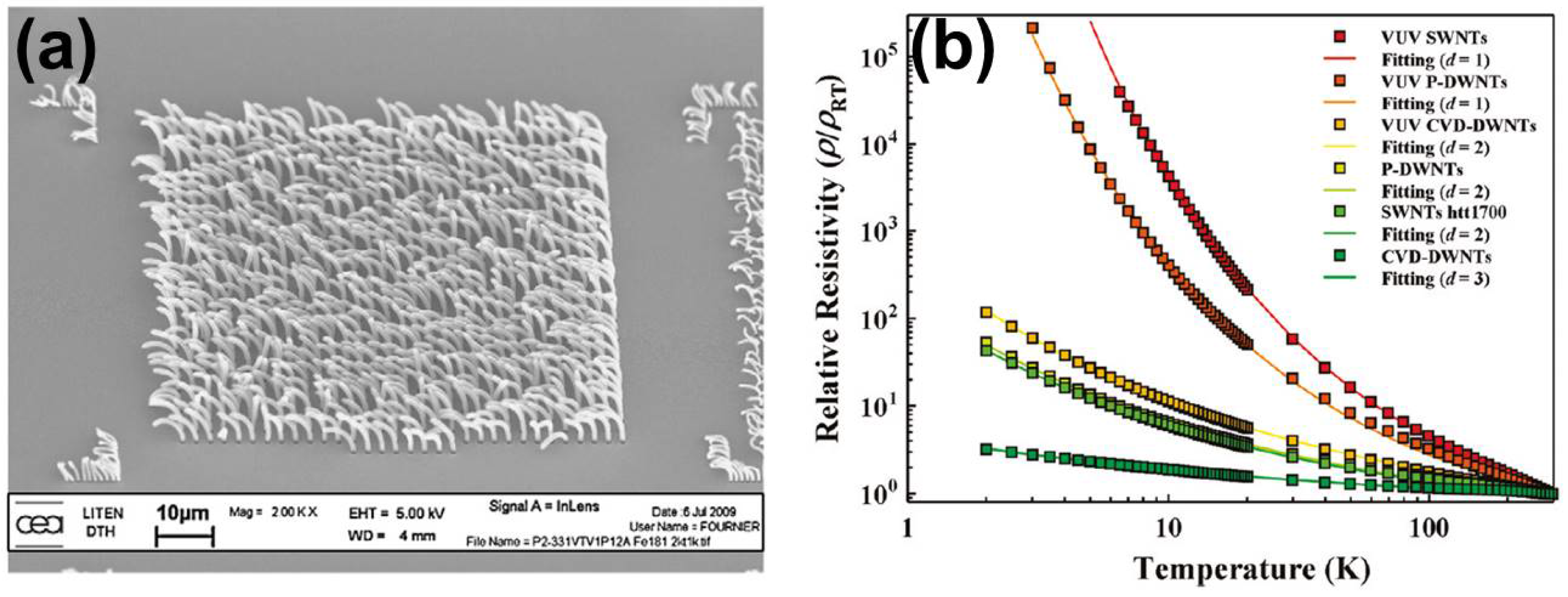
2. Synthesis and Characterization of DWNTs
2.1. The Arc Discharge Method
2.2. The Catalytic Chemical Vapor Deposition Method
2.2.1. The Supported Catalyst Method
Effect of Mo Addition
Effect of Water
2.2.2. The Floating Catalyst Method
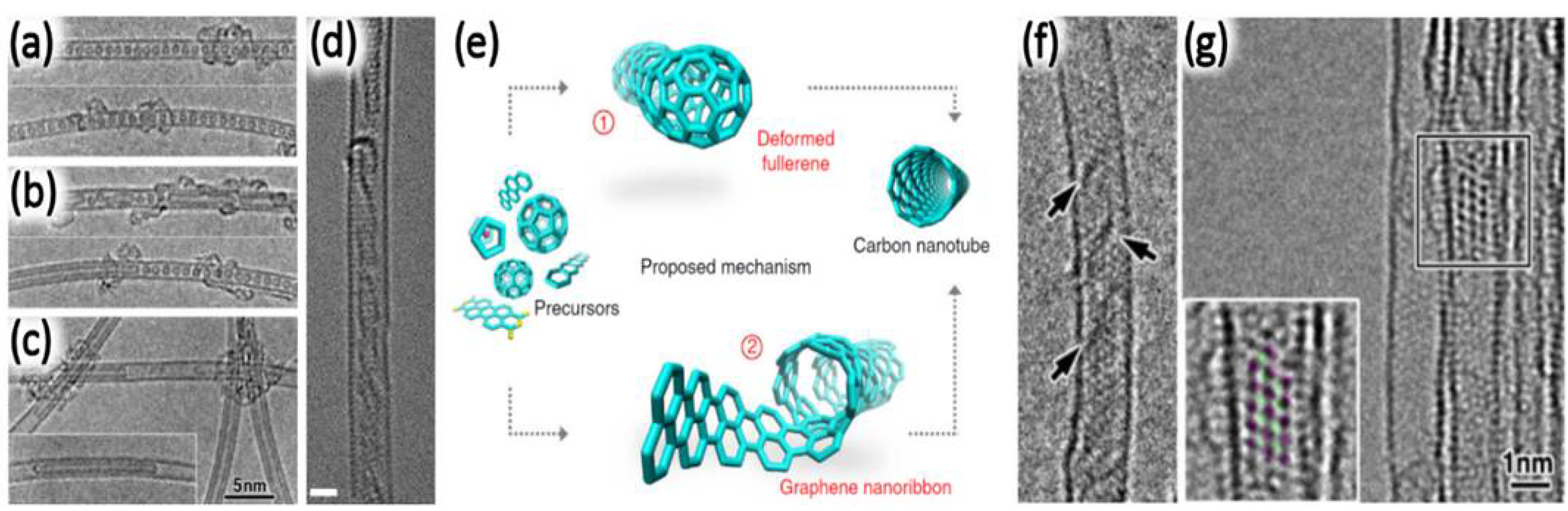
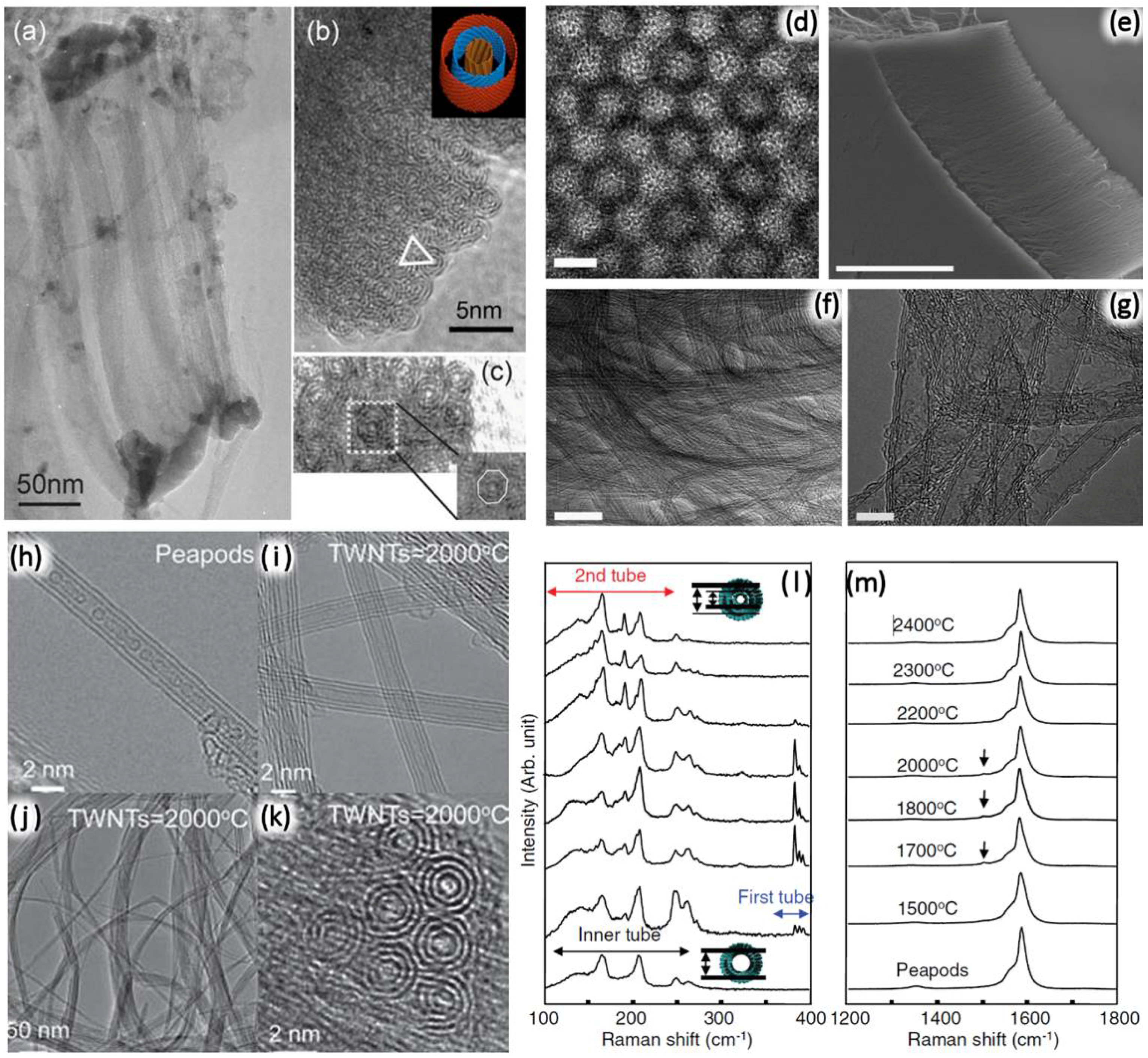
2.2.3. Annealing of Confined Molecules inside SWNTs
2.3. Brief Summary: Synthesis of DWNTs
3. Synthesis and Characterization of TWNTs
3.1. Annealing of Confined Molecules inside DWNTs
3.2. Brief Summary: Synthesis of TWNTs
4. Pea Pod-Derived Method: Advantages and Disadvantages
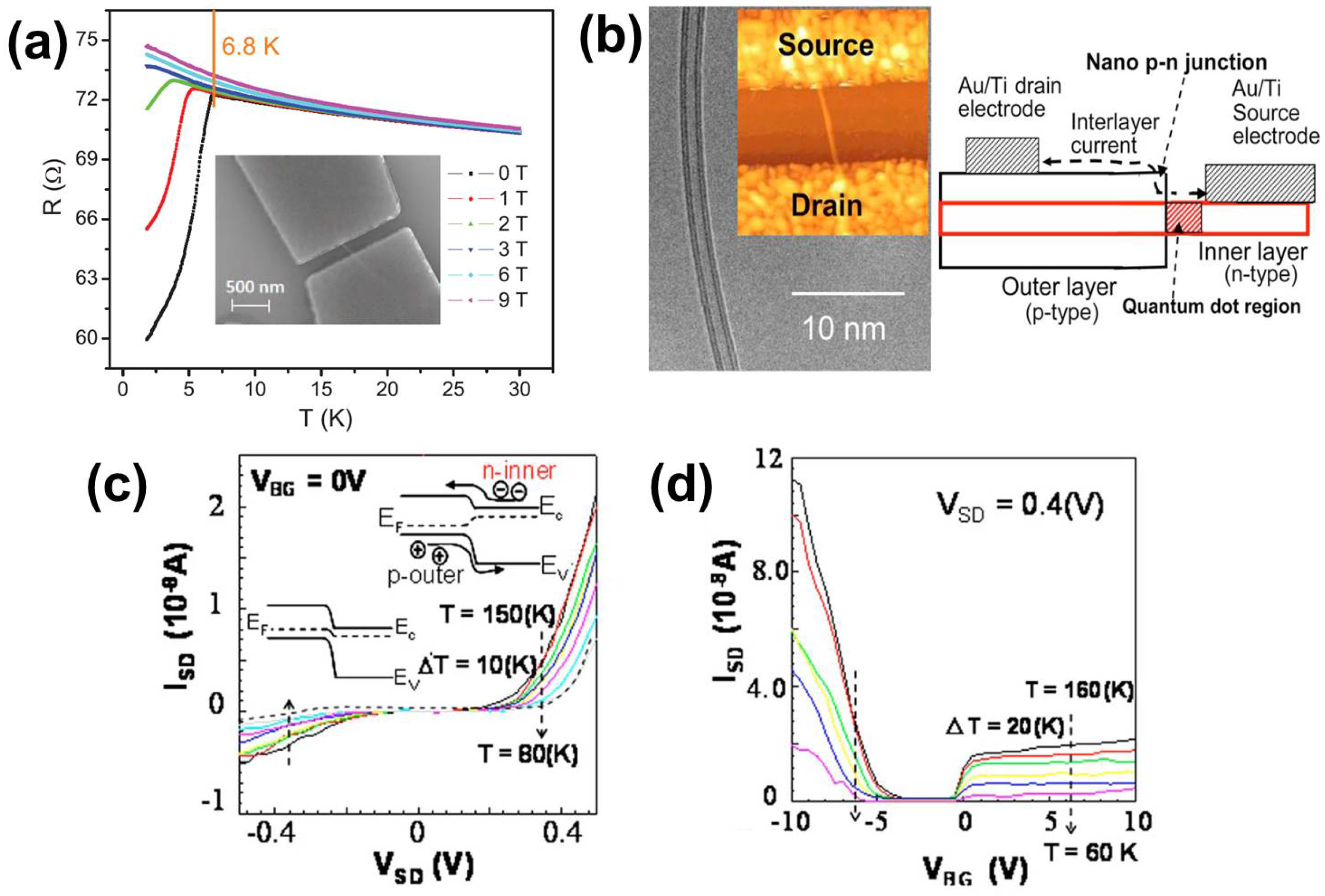
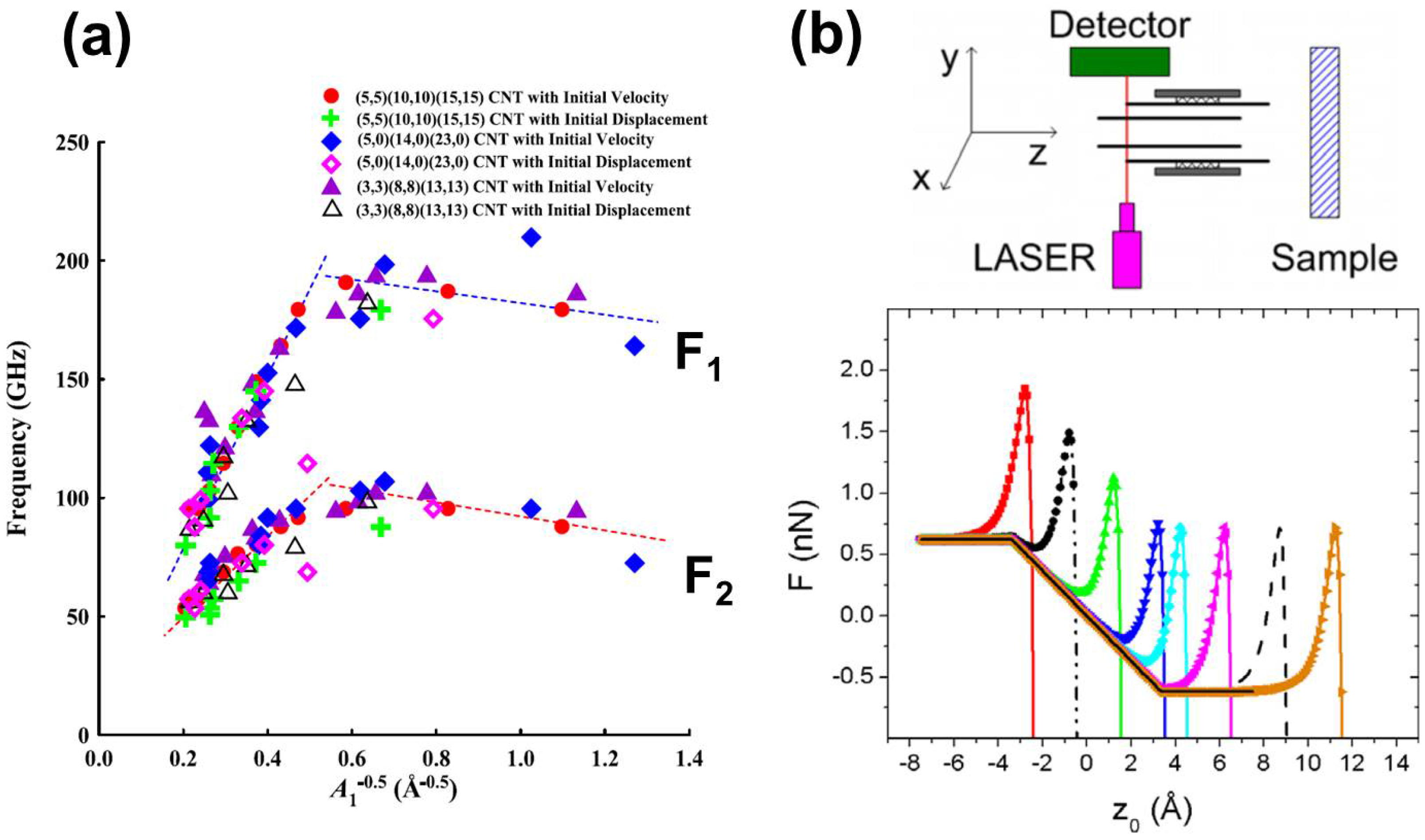
5. Applications Involving DWNTs and TWNTs
6. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Saito, R.; Hofmann, M.; Dresselhaus, G.; Jorio, A.; Dresselhaus, M.S. Raman spectroscopy of graphene and carbon nanotubes. Adv. Phys. 2011, 60, 413–550. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of graphene and carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Barros, E.B.; Jorio, A.; Samsonidze, G.G.; Capaz, R.B.; Souza Filho, A.G.; Mendes Filho, J.; Dresselhaus, G.; Dresselhaus, M.S. Review on the symmetry-related properties of carbon nanotubes. Phys. Rep. 2006, 431, 261–302. [Google Scholar] [CrossRef]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Kotov, V.N.; Uchoa, B.; Pereira, V.M.; Guinea, F.; Castro Neto, A.H. Electron-electron interactions in graphene: Current status and perspectives. Rev. Mod. Phys. 2012, 84, 1067–1125. [Google Scholar] [CrossRef]
- Araujo, P.T.; Pesce, P.B.C.; Dresselhaus, M.S.; Sato, K.; Saito, R.; Jorio, A. Resonance Raman spectroscopy of the radial breathing modes in carbon nanotubes. Physica E 2010, 42, 1251–1261. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Gomes, A.P.; Fantini, C.; Cancado, L.G.; Araujo, P.T.; Maciel, I.O.; Santos, A.P.; Furtado, C.A.; Peressinoto, V.S.T.; Plentz, F.; et al. Optical studies of carbon nanotubes and nanographites. Physica E 2007, 42, 88–92. [Google Scholar] [CrossRef]
- Mafra, D.L.; Araujo, P.T. Intra- and Interlayer Electron-Phonon Interactions in 12/12C and 12/13C BiLayer Graphene. Appl. Sci. 2014, 4, 207–239. [Google Scholar] [CrossRef]
- Saito, R.; Sato, K.; Araujo, P.T.; Mafra, D.L.; Dresselhaus, M.S. Gate modulated Raman spectroscopy of graphene and carbon nanotubes. Solid State Commun. 2013, 175, 18–34. [Google Scholar] [CrossRef]
- Kim, P.; Odom, T.W.; Huang, J.-L.; Lieber, C.M. Electronic density of states of atomically resolved single-walled carbon nanotubes: Van hove singularities and end states. Phys. Rev. Lett. 1999, 82, 1225–1228. [Google Scholar] [CrossRef]
- Misewich, J.A.; Martel, R.; Avouris, P.; Tsang, J.C.; Heinze, S.; Tersoff, J. Electrically induced optical emission from a carbon nanotube FET. Science 2003, 300, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Baughman, R.B.; Zakhidov, A.A.; de Heer, W.A. Carbon nanotubes—The route toward applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Bahena-Garrido, S.; Shimoi, N.; Abe, D.; Hojo, T.; Tanaka, Y.; Tohji, K. Plannar light source using a phosphor screen with single-walled carbon nanotubes as field emitters. Rev. Sci. Instrum. 2014, 85. [Google Scholar] [CrossRef] [PubMed]
- Gabor, N.M.; Zhong, Z.; Bosnick, K.; Park, J.; McEuen, P.L. Extremely efficient multiple electron-hole pair generation in carbon nanotube photodiodes. Science 2009, 325, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Gabor, N.M.; Zhong, Z.; Bosnick, K.; McEuen, P.L. Ultrafast photocurrent measurement of the escape time of electrons and holes from carbon nanotube p-i-n photodiodes. Phys. Rev. Lett. 2012, 108. [Google Scholar] [CrossRef] [PubMed]
- Kymakis, E.; Amaratunga, G.A.J. Single-wall carbon nanotube/conjugated polymer photovoltaic devices. Appl. Phys. Lett. 2002, 80, 112–114. [Google Scholar] [CrossRef]
- Ago, H.; Petritsch, K.; Shaffer, M.S.P.; Windle, A.H.; Friend, R.H. Composites of carbon nanotubes and conjugated polymers for photovoltaic devices. Adv. Mater. 1999, 11, 1281–1285. [Google Scholar] [CrossRef]
- Zhang, D.; Koungmin, R.; Liu, X.; Polikarpov, E.; Ly, J.; Tompson, M.E.; Zhou, C. Transparent, conductive, and flexible carbon nanotube films and their application in organic light-emitting diodes. Nano Lett. 2006, 6, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, L.; Wang, L.; Zhou, Y.; Grüner, G.; Marks, T.J. Organic light-emitting diodes having carbon nanotube anodes. Nano Lett. 2006, 11, 2472–2477. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Yang, K.S.; Muramatsu, H.; Hayashi, T.; Endo, M.; Terrones, M.; Dresselhaus, M.S. Double-wall carbon nanotubes: Synthesis, structural characterization, and application. Carbon Lett. 2014, 15, 77–88. [Google Scholar] [CrossRef]
- Kasperski, A.; Weibel, A.; Datas, L.; Grave, E.D.; Peigney, A.; Laurent, C. Large-diameter single-wall carbon nanotubes formed alongside small-diameter double-walled carbon nanotubes. J. Phys. Chem. C 2015, 119, 1524–1535. [Google Scholar] [CrossRef]
- Zhao, J.; Su, Y.; Yang, Z.; Wei, L.; Wang, Y.; Zhang, Y. Arc synthesis of double-walled carbon nanotubes in low pressure air and their superior field emission properties. Carbon 2013, 58, 92–98. [Google Scholar] [CrossRef]
- Su, Y.; Zhou, P.; Zhao, J.; Yang, Z.; Zhang, Y. Large-scale synthesis of few-walled carbon nanotubes by DC arc discharge in low-pressure flowing air. Mater. Res. Bull. 2013, 48, 3232–3235. [Google Scholar] [CrossRef]
- Taki, Y.; Shinohara, K.; Kikuchi, M.; Tanaka, A. Selective growth of vertically aligned single-, double-, and triple-walled carbon nanotubes by radiation-heated chemical vapor deposition. Jpn. J. Appl. Phys. 2008, 47, 721–724. [Google Scholar] [CrossRef]
- Kim, Y.A.; Muramatsu, H.; Hayashi, T.; Endo, M.; Terrones, M.; Dresselhaus, M.S. Thermal stability and structural changes of double-walled carbon nanotubes by heat treatment. Chem. Phys. Lett. 2004, 398, 87–92. [Google Scholar] [CrossRef]
- Qiu, H.; Shi, Z.; Gu, Z.; Qiu, J. Controllable preparation of triple-walled carbon nanotubes and their growth mechanism. Chem. Commun. 2007, 23, 1092–1094. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, Z.; Zhang, Q.; Zheng, Y.; Ieong, C.; He, M.; Lortz, R.; Cai, Y.; Wang, N.; Zhang, T.; et al. Superconductivity in bundles of double-wall carbon nanotubes. Sci. Rep. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, H.; Hayashi, T.; Kim, Y.A.; Shimamoto, D.; Endo, M.; Meunier, V.; Sumpter, B.G.; Terrones, M.; Dresselhaus, M.S. Bright photoluminescence from the inner tubes of peapod-derived double walled carbon nanotubes. Small 2009, 5, 2678–2682. [Google Scholar] [CrossRef] [PubMed]
- Charlier, J.-C.; Blase, X.; Roche, S. Electronic and transport properties of nanotubes. Rev. Mod. Phys. 2007, 79, 677–732. [Google Scholar] [CrossRef]
- Ho, Y.H.; Chang, C.P.; Shyu, F.L.; Chen, R.B.; Chen, S.C.; Lin, M.F. Electronic and optical properties of double-walled armchair carbon nanotubes. Carbon 2004, 42, 3159–3167. [Google Scholar] [CrossRef]
- Lambin, Ph.; Meunier, V.; Rubio, A. Electronic structure of polychiral carbon nanotubes. Phys. Rev. B 2000, 62, 5129–5135. [Google Scholar] [CrossRef]
- Wei, J.; Zhu, H.; Jiang, B.; Ci, L.; Dehai, W.E. Electronic properties of double-walled carbon nanotube films. Carbon 2003, 41, 2495–2500. [Google Scholar] [CrossRef]
- Tison, Y.; Giusca, C.E.; Stolojan, V.; Hayashi, Y.; Silva, R.P. The inner shell influence on the electronic structure of double-walled carbon nanotubes. Adv. Mater. 2008, 20, 189–194. [Google Scholar] [CrossRef]
- Soto, M.; Boyer, T.A.; Biradar, S.; Ge, L.; Vajtai, R.; Elías-Zúñiga, A.; Ajayan, P.M.; Barrera, E.V. Effect of interwall interaction on the electronic structure of double-walled carbon nanotubes. Nanotechnology 2015, 26. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Electronic structure of double-layer graphene tubules. J. Appl. Phys. 1993, 73, 494–500. [Google Scholar] [CrossRef]
- Charlier, J.-C.; Michenaud, J.-P. Energetics of multilayered carbon tubules. Phys. Rev. Lett. 1993, 70, 1858–1861. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Matsuo, R.; Kimura, T.; Dresselhaus, G.; Dresselhaus, M.S. Anomalous potential barrier of double-wall carbon nanotubes. Chem. Phys. Lett. 2001, 348, 187–193. [Google Scholar] [CrossRef]
- Park, H.J.; Oh, K.A.; Park, M.; Lee, H. Electrical properties and conductivity mapping of thin multilayered films containing different types of carbon nanotubes. J. Phys. Chem. C 2009, 113, 13070–13076. [Google Scholar] [CrossRef]
- Moon, S.; Song, W.; Lee, J.S.; Kim, N.; Kim, J.; Lee, S.-G.; Choi, M.-S. Eightfold shell filling in a double-wall carbon nanotube quantum dot. Phys. Rev. Lett. 2007, 99, 176804–176808. [Google Scholar] [CrossRef] [PubMed]
- Langer, L.; Bayot, V.; Grivei, E.; Issi, J.-P.; Heremans, J.P.; Olk, C.H.; Stockman, L.; van Haesendonck, C.; Bruynseraede, Y. Quantum transport in a multiwalled carbon nanotube. Phys. Rev. Lett. 1996, 76, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Martel, R.; Schmidt, T.; Shea, H.R.; Hertel, T.; Avouris, Ph. Single- and multi-wall carbon nanotube field-effect transistors. Appl. Phys. Lett. 1998, 73, 2447–2450. [Google Scholar] [CrossRef]
- Kim, P.; Shi, L.; Majumdar, A.; McEuen, P.L. Thermal Transport Measurements of Individual Multiwalled Nanotubes. Phys. Rev. Lett. 2001, 87, 215502–215506. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Lu, W.G.; Li, J.J.; Bai, X.D.; Gu, C.Z. Multichannel ballistic transport in multiwall carbon nanotubes. Phys. Rev. Lett. 2005, 95, 086601–086605. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-K.; Tomanek, D. Electronic and structural properties of multiwall carbon nanotubes. Phys. Rev. B 1998, 58. [Google Scholar] [CrossRef]
- Purcell, S.T.; Vincent, P.; Journet, C.; Binh, V.T. Hot nanotubes: Stable heating of individual multiwall carbon nanotubes to 2000 K induced by the field-emission current. Phys. Rev. Lett. 2002, 88, 105502–105506. [Google Scholar] [CrossRef] [PubMed]
- Cumings, J.; Zettl, A. Low-friction nanoscale linear bearing realized from multiwall carbon nanotubes. Science 2000, 289, 602–604. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.H.; Guduru, P.R.; Curtin, W.A. Enhancing mechanical properties of multiwall carbon nanotubes via sp3 interwall bridging. Phys. Rev. Lett. 2007, 98, 245501–245506. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.-W.; Oh, S.; Ihm, J.; Han, S. Field emission properties of double-wall carbon nanotubes. Nanotechnology 2004, 16. [Google Scholar] [CrossRef]
- Shimada, T.; Sugai, T.; Ohno, Y.; Kishimoto, S.; Mizutani, T.; Yoshida, H.; Okazaki, T.; Shinohara, H. Double-wall carbon nanotube field-effect transistors: Ambipolar transport characteristics. Appl. Phys. Lett. 2004, 84, 2412–2415. [Google Scholar] [CrossRef]
- Pantano, A.; Parks, D.M.; Boyce, M.C. Mechanics of deformation of single- and multi-wall carbon nanotubes. J. Mech. Phys. Solids 2004, 52, 789–821. [Google Scholar] [CrossRef]
- Liu, T.; Phang, I.Y.; Shen, L.; Chow, S.Y.; Zhang, W.-D. Morphology and mechanical properties of multiwalled carbon nanotubes reinforced nylon-6 composites. Macromolecules 2004, 37, 7214–7222. [Google Scholar] [CrossRef]
- Pumera, M. Electrochemical properties of double wall carbon nanotube electrodes. Nanoscale Res. Lett. 2007, 2, 87–93. [Google Scholar] [CrossRef]
- Koshino, M.; Moon, P.; Som, Y.-W. Incommensurate double-walled carbon nanotubes as one-dimensional moiré crystals. Phys. Rev. B 2015, 91. [Google Scholar] [CrossRef]
- Liu, K.; Jin, C.; Hong, X.; Kim, J.; Zettl, A.; Wang, E.; Wang, F. Van der Waals-coupled electronic states in incommensurate double-walled carbon nanotubes. Nat. Phys. 2014, 10, 737–742. [Google Scholar] [CrossRef]
- Liu, K.; Hong, X.; Wu, M.; Xiao, F.; Wang, W.; Bai, X.; Ager, J.W.; Aloni, S.; Zettl, A.; Wang, E.; et al. Quantum-coupled radial-breathing oscillations in double-walled carbon nanotubes. Nat. Commun. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Hirschmann, T.C.; Dresselhaus, M.S.; Muramatsu, H.; Seifert, M.; Wurstbauer, U.; Parzinger, E.; Nielsch, K.; Kim, Y.A.; Araujo, P.T. G′ band in double-and triple-walled carbon nanotubes: A Raman study. Phys. Rev. B 2015, 91. [Google Scholar] [CrossRef]
- Hirschmann, T.C.; Araujo, P.T.; Muramatsu, H.; Rodriguez-Nieva, J.F.; Seifert, M.; Nielsch, K.; Kim, Y.A.; Dresselhaus, M.S. Role of intertube interactions in double-and triple-walled carbon nanotubes. ACS Nano 2014, 8, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Hirschmann, T.C.; Araujo, P.T.; Muramatsu, H.; Zhang, X.; Nielsch, K.; Kim, Y.A.; Dresselhaus, M.S. Characterization of bundled and individual triple-walled carbon nanotubes by resonant Raman spectroscopy. ACS Nano 2013, 7, 2381–2387. [Google Scholar] [CrossRef] [PubMed]
- Noffsinger, J.; Cohen, M.L. Electron-phonon coupling and superconductivity in double-walled carbon nanotubes. Phys. Rev. B 2011, 83. [Google Scholar] [CrossRef]
- Murata, N.; Haruyama, J.; Ueda, Y.; Matsudaira, M.; Karino, H.; Yagi, Y.; Einarsson, E.; Chiashi, S.; Maruyama, S.; Sugai, T.; et al. Meissner effect in honeycomb arrays of multiwalled carbon nanotubes. Phys. Rev. B 2007, 76. [Google Scholar] [CrossRef]
- Takesue, I.; Haruyama, J.; Kobayashi, N.; Chiashi, S.; Maruyama, S.; Sugai, T.; Shinohara, H. Superconductivity in entirely end-bonded multiwalled carbon nanotubes. Phys. Rev. Lett. 2006, 96. [Google Scholar] [CrossRef] [PubMed]
- Barzola-Quiquia, J.; Esquinazi, P.; Lindel, M.; Spemann, D.; Muallem, M.; Nessim, G.D. Magnetic order and superconductivity observed in bundles of double-wall carbon nanotubes. Carbon 2015, 88, 16–25. [Google Scholar] [CrossRef]
- Latgé, A.; Grimm, D.; Ferreira, M.S. Magnetic field effects of double-walled carbon nanotubes. Braz. J. Phys. 2006, 36, 898–901. [Google Scholar]
- Postupna, O.; Long, R.; Prezhdo, O.V. Time-domain Ab initio simulation of energy transfer in double-walled carbon nanotubes. J. Phys. Chem. C 2015, 119, 12088–12094. [Google Scholar] [CrossRef]
- Bacsa, R.R.; Laurent, Ch.; Peigney, A.; Puech, P.; Hubel, H.; Dunstan, D.; Bacsa, W.S. Structural and mechanical properties of double wall carbon nanotubes. NSTI-Nanotech 2004 2004, 3, 214–217. [Google Scholar]
- Natsuki, T.; Hayashi, T.; Endo, M. Mechanical properties of single and double-walled carbon nanotubes under hydrostatic pressure. Appl. Phys. A 2006, 83, 13–17. [Google Scholar] [CrossRef]
- Huang, J.Y.; Chen, S.; Ren, Z.F.; Wang, Z.; Kmpa, K.; Naughton, M.J.; Chen, G.; Dresselhaus, M.S. Enhanced ductile behavior of tensile-elongated individual double-walled and triple-walled carbon nanotubes at high temperatures. Phys. Rev. Lett. 2007, 98. [Google Scholar] [CrossRef] [PubMed]
- Motta, M.; Moisala, A.; Kinloch, I.A.; Windle, A.H. High performance fibres from ‘dog bone’ carbon nanotubes. Adv. Mater. 2007, 19, 3721–3726. [Google Scholar] [CrossRef]
- Li, Y.-L.; Kinloch, I.A.; Windle, A.H. Direct spinning of carbon nanotube fibers from chemical vapor deposition synthesis. Science 2004, 304, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Kinloch, I.A.; Young, R.J.; Noé, L.; Monthioux, M. The Effect of stress transfer within double-walled carbon nanotubes upon their ability to reinforce composites. Adv. Mater. 2009, 21, 3591–3595. [Google Scholar] [CrossRef]
- Alencar, R.S.; Aguiar, A.L.; Paschoal, A.R.; Freire, P.T.C.; Kim, Y.A.; Muramatsu, H.; Endo, M.; Terrones, H.; Terrones, M.; San-Miguel, A.; et al. Pressure-induced selectivity for probing inner tubes in double- and triple-walled carbon nanotubes: A resonance raman study. J. Phys. Chem. C 2014, 118, 8153–8158. [Google Scholar] [CrossRef]
- Liu, Q.; Ren, W.; Li, F.; Cong, H.; Cheng, H.M.J. Synthesis and high thermal stability of double-wall carbon nanotubes using nickel formate dihydrate as catalyst precursor. Phys. Chem. C 2007, 111, 5006–5013. [Google Scholar] [CrossRef]
- Huang, H.; Kajiura, H.; Tsutsui, S.; Murakami, Y.; Ata, M. High-quality double-walled carbon nanotube super bundles grown in a hydrogen-free atmosphere. J. Phys. Chem. B 2003, 107, 8794–8798. [Google Scholar] [CrossRef]
- Chen, Z.-G.; Li, F.; Ren, W.-C.; Cong, H.; Liu, C.; Lu, G.Q.; Cheng, H.-M. Double-walled carbon nanotubes synthesized using carbon black as the dot carbon source. Nanotechnology 2006, 17, 3100–3104. [Google Scholar] [CrossRef]
- Li, L.; Li, F.; Liu, C.; Cheng, H.-M. Synthesis and characterization of double-walled carbon nanotubes from multi-walled carbon nanotubes by hydrogen-arc discharge. Carbon 2005, 43, 623–629. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, G.; Li, Z.; Zhao, Z. Preparation of double-walled carbon nanotubes from fullerene waste soot by arc-discharge. Carbon 2010, 48, 1312–1315. [Google Scholar] [CrossRef]
- Motta, M.; Moisala, A.; Kinloch, I.A.; Windle, A.H. The role of sulphur in the synthesis of carbon nanotubes by chemical vapour deposition at high temperatures. J. Nanosci. Nanotechnol. 2008, 8, 2442–2449. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, G.G.; Bernardo, C.A.; Gorkiewicz, D.W.; Alig, R.L. Role of sulfur in the production of carbon fibers in the vapor phase. Carbon 1994, 32, 569–576. [Google Scholar] [CrossRef]
- Hutchison, J.L.; Kiselev, N.A.; Krinichnaya, E.P.; Krestinin, A.V.; Loutfy, R.O.; Morawsky, A.P.; Muradyan, V.E.; Obraztsova, E.D.; Sloan, J.; Terekhov, S.V.; et al. Double-walled carbon nanotubes fabricated by a hydrogen arc discharge method. Carbon 2001, 39, 761–770. [Google Scholar] [CrossRef]
- Saito, Y.; Nakahira, T.; Uemura, S.J. Growth conditions of double-walled carbon nanotubes in arc discharge. Phys. Chem. B 2003, 107, 931–934. [Google Scholar] [CrossRef]
- Qiu, H.; Shi, Z.; Guan, L.; You, L.; Gao, M.; Zhang, S.; Qiu, J.; Gu, Z. High-efficient synthesis of double-wall carbon nanotubes by arc discharge method using chloride as a promoter. Carbon 2006, 44, 516–521. [Google Scholar] [CrossRef]
- Wang, S.-D.; Chang, M.-H.; Lan, K.M.-D.; Wu, C.-C.; Cheng, J.-J.; Chang, H.-K. Synthesis of carbon nanotubes by arc discharge in sodium chloride solution. Carbon 2005, 43, 1778–1814. [Google Scholar] [CrossRef]
- Liu, B.C.; Yu, B.; Zhang, M.X. Catalytic CVD synthesis of double-walled carbon nanotubes with a narrow distribution of diameters over Fe–Co/MgO catalyst. Chem. Phys. Lett. 2005, 407, 232–235. [Google Scholar] [CrossRef]
- Zhang, Q.; Qian, W.; Wen, Q.; Liu, Y.; Wang, D.; Wei, F. The effect of phase separation in Fe/Mg/Al/O catalysts on the synthesis of DWCNTs from methane. Carbon 2007, 45, 1645–1650. [Google Scholar] [CrossRef]
- Li, W.Z.; Wen, J.G.; Sennett, M.; Ren, Z.F. Clean double-walled carbon nanotubes synthesized by CVD. Chem. Phys. Lett. 2003, 368, 299–306. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Jia, Y. Synthesis of high-quality double-walled carbon nanotubes using porous MgO nanowire supported iron oxide as catalyst. Mater. Lett. 2013, 107, 46–49. [Google Scholar] [CrossRef]
- Ning, G.; Wei, F.; Wen, Q.; Luo, G.; Wang, Y.; Jin, Y. Improvement of Fe/MgO catalysts by calcination for the growth of single-and double-walled carbon nanotubes. Phys. Chem. B 2006, 110, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Coquay, P.; Peigney, A.; de Grave, E.; Flahaut, E.; Vandenberghe, R.E.; Laurent, C.J. Fe/Co alloys for the catalytic chemical vapor deposition synthesis of single- and double-walled carbon nanotubes (CNTs). 1. the CNT-Fe/Co-MgO system. Phys. Chem. B 2005, 109, 17813–17824. [Google Scholar] [CrossRef] [PubMed]
- Flahaut, E.; Peigney, A.; Laurent, C.; Rousset, A. Synthesis of single-walled carbon nanotube–Co–MgO composite powders and extraction of the nanotubes. J. Mater. Chem. 2000, 10, 249–252. [Google Scholar] [CrossRef]
- Zhu, J.; Yudasaka, M.; Iijima, S. A catalytic chemical vapor deposition synthesis of double-walled carbon nanotubes over metal catalysts supported on a mesoporous material. Chem. Phys. Lett. 2003, 380, 496–502. [Google Scholar] [CrossRef]
- Flahaut, E.; Bacsa, R.; Peigney, A.; Laurent, C. Gram-scale CCVD synthesis of double-walled carbon nanotubes. Chem. Commun. 2003, 1442–1443. [Google Scholar] [CrossRef] [Green Version]
- Cumings, J.; Mickelson, W.; Zettl, A. Simplified synthesis of double-wall carbon nanotubes. Solid State Commun. 2003, 126, 359–362. [Google Scholar] [CrossRef]
- Gruneis, A.; Rummeli, M.H.; Kramberger, C.; Barreiro, A.; Pichler, T.; Pfeiffer, R.; Kuzmany, H.; Gemming, T.; Buchner, B. High quality double wall carbon nanotubes with a defined diameter distribution by chemical vapor deposition from alcohol. Carbon 2006, 44, 3177–3182. [Google Scholar] [CrossRef]
- Ning, G.; Liu, Y.; Wei, F.; Wen, Q.; Luo, G.J. Porous and lamella-like Fe/MgO catalysts prepared under hydrothermal conditions for high-yield synthesis of double-walled carbon nanotubes. Phys. Chem. C 2007, 111, 1969–1975. [Google Scholar] [CrossRef]
- Flahaut, E.; Laurent, C.; Peigney, A. Catalytic CVD synthesis of double and triple-walled carbon nanotubes by the control of the catalyst preparation. Carbon 2005, 43, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, P.; Okazaki, T.; Sugai, T.; Kimura, J.; Kishi, N.; Sato, K.; Ozeki, Y.; Shinohara, H. Purification and characterization of double-wall carbon nanotubes synthesized by catalytic chemical vapor deposition on mesoporous silica. Chem. Phys. Lett. 2006, 418, 408–412. [Google Scholar] [CrossRef]
- Hiraoka, T.; Kawakubo, T.; Kimura, J.; Taniguchi, R.; Okamoto, A.; Okazaki, T.; Sugai, T.; Ozeki, Y.; Yoshikawa, M.; Shinohara, H. Selective synthesis of double-wall carbon nanotubes by CCVD of acetylene using zeolite supports. Chem. Phys. Lett. 2003, 382, 679–685. [Google Scholar] [CrossRef]
- Ramesh, P.; Okazaki, T.; Taniguchi, R.; Kimura, J.; Sugai, T.; Sato, K.; Ozeki, Y.; Shinohara, H. Selective chemical vapor deposition synthesis of double-wall carbon nanotubes on mesoporous silica. J. Phys. Chem. B 2005, 109, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Ago, H.; Nakamura, K.; Imamura, S.; Tsuji, M. Growth of double-wall carbon nanotubes with diameter-controlled iron oxide nanoparticles supported on MgO. Chem. Phys. Lett. 2004, 391, 308–313. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, W.Z.; Zhang, Q.; Ning, G.Q.; Luo, G.H.; Wang, Y.; Wang, D.Z.; Wei, F. Synthesis of high-quality, double-walled carbon nanotubes in a fluidized bed reactor. Chem. Eng. Technol. 2009, 32, 73–79. [Google Scholar] [CrossRef]
- Qi, H.; Qian, C.; Liu, J. Synthesis of uniform double-walled carbon nanotubes using iron disilicide as catalyst. Nano Lett. 2007, 7, 2417–2421. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.C.; Liu, B.C.; Lee, S.H.; Park, C.Y.; Kang, H.K.; Yang, C.-W.; Lee, C.J. Large-scale synthesis of high-quality double-walled carbon nanotubes by catalytic decomposition of n-hexane. J. Phys. Chem. B 2004, 108, 2192–2194. [Google Scholar] [CrossRef]
- Endo, M.; Muramatsu, H.; Takuya, H.; Kim, Y.A.; Terrones, M.; Dresselhaus, M.S. Nanotechnology: “Buckypaper” from coaxial nanotubes. Nature 2005, 433. [Google Scholar] [CrossRef] [PubMed]
- Biris, A.R.; Lupu, D.; Gruneis, A.; Ayala, P.; Rümmeli, M.H.; Pichler, T.; Li, Z.; Xu, Y.; Misan, I.; Dervishi, E.; et al. High-quality double-walled carbon nanotubes grown by a cold-walled radio frequency chemical vapor deposition process. Chem. Mater. 2008, 20, 3466–3472. [Google Scholar] [CrossRef]
- Matsumoto, K.; Murakami, T.; Isshiki, T.; Kisoda, K.; Harima, H. Synthesis and Raman study of double-walled carbon nanotubes. Diam. Relat. Mater. 2007, 16, 1188–1191. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Gong, K.; Dai, L.J. Structural evaluation along the nanotube length for super-long vertically aligned double-walled carbon nanotube arrays. Phys. Chem. C 2008, 112, 8136–8139. [Google Scholar] [CrossRef]
- Lamura, G.; Andreone, A.; Yang, Y.; Barbara, P.; Vigolo, B.; Herold, C.; Mareche, J.F.; Lagrange, P.; Cazayous, M.; Sacuto, A.; et al. High-crystalline single-and double-walled carbon nanotube mats grown by chemical vapor deposition. J. Phys. Chem. C 2007, 111, 15154–15159. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Zhang, L.-L.; Yu, W.-J.; Li, S.-S.; Hou, P.-X.; Cong, H.-T.; Liu, C.; Cheng, H.-M. Growth of double-walled carbon nanotubes from silicon oxide nanoparticles. Carbon 2013, 56, 167–172. [Google Scholar] [CrossRef]
- Gunjishima, I.; Inoue, T.; Yamamuro, S.; Sumiyama, K.; Okamoto, A. Synthesis of vertically aligned, double-walled carbon nanotubes from highly active Fe–V–O nanoparticles. Carbon 2007, 45, 1193–1199. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, S.; Bielecki, J.; Matic, A.; Wang, T.; Ye, L.-L.; Liu, J. Selective growth of double-walled carbon nanotubes on gold films. Mater. Lett. 2012, 72, 78–80. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Nagao, H.; Taniguchi, M.; Amano, H.; Ando, Y.; Hori, M. High-rate growth of films of dense, aligned double-walled carbon nanotubes using microwave plasma-enhanced chemical vapor deposition. Jpn. J. Appl. Phys. 2005, 44, L693–L695. [Google Scholar] [CrossRef]
- Kondo, D.; Sato, S.; Kawabata, A.; Awano, Y. Selective growth of vertically aligned double-and single-walled carbon nanotubes on a substrate at 590°C. Nanotechnology 2008, 19, 435601. [Google Scholar] [CrossRef] [PubMed]
- Baliyan, A.; Uchida, T.; Fukuda, T.; Nakajima, Y.; Hanajiri, T.; Maekawa, T. Synthesis of a forest of double/triple walled CNTs of uniform diameters by plasma enhanced CVD using monodisperse iron oxide nanoparticles. J. Mater. Chem. 2012, 22, 5277–5280. [Google Scholar] [CrossRef]
- Futaba, D.N.; Goto, J.; Yasuda, S.; Yamada, T.; Yumura, M.; Hata, K. A background level of oxygen-containing aromatics for synthetic control of carbon nanotube structure. J. Amer. Chem. Soc. 2009, 131, 15992–15993. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Namai, T.; Hata, K.; Futaba, D.N.; Mizuno, K.; Fan, J.; Yudasaka, M.; Yumura, M.; Iijima, S. Size-selective growth of double-walled carbon nanotube forests from engineered iron catalysts. Nat. Nanotechnol. 2006, 1, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Ci, L.; Rao, Z.; Zhou, Z.; Tang, D.; Yan, X.; Liang, Y.; Liu, D.; Yuan, H.; Zhou, W.; Wang, G.; et al. Double wall carbon nanotubes promoted by sulfur in a floating iron catalyst CVD system. Chem. Phys. Lett. 2002, 359, 63–67. [Google Scholar] [CrossRef]
- Zhou, Z.; Ci, L.; Chen, X.; Tang, D.; Yan, X.; Liu, D.; Liang, Y.; Yuan, H.; Zhou, W.; Wang, G.; et al. Controllable growth of double wall carbon nanotubes in a floating catalytic system. Carbon 2003, 41, 337–342. [Google Scholar] [CrossRef]
- Wei, J.; Jiang, B.; Wu, D.; Wei, B. Large-scale synthesis of long double-walled carbon nanotubes. J. Phys. Chem. B 2004, 108, 8844–8847. [Google Scholar] [CrossRef]
- Wei, J.; Zhu, H.; Jia, Y.; Shu, Q.; Li, C.; Wang, K.; Wei, B.; Zhu, Y.; Wang, Z.; Luo, J.; et al. The effect of sulfur on the number of layers in a carbon nanotube. Carbon 2007, 45, 2152–2158. [Google Scholar] [CrossRef]
- Zhong, X.H.; Li, Y.L.; Liu, Y.K.; Qiao, X.H.; Feng, Y.; Liang, J.; Jin, J.; Zhu, L.; Hou, F.; Li, J.Y. Continuous multilayered carbon nanotube yarns. Adv. Mater. 2010, 22, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.H.; Li, Y.L.; Feng, J.M.; Kang, Y.R.; Han, S.S. Fabrication of a multifunctional carbon nanotube “cotton” yarn by the direct chemical vapor deposition spinning process. Nanoscale 2012, 4, 5614–5618. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.-M.; Wang, R.; Li, Y.-L.; Zhong, X.-H.; Cui, L.; Guo, Q.-J.; Hou, F. One-step fabrication of high quality double-walled carbon nanotube thin films by a chemical vapor deposition process. Carbon 2010, 48, 3817–3824. [Google Scholar] [CrossRef]
- Ren, W.; Cheng, H.M. Aligned double-walled carbon nanotube long ropes with a narrow diameter distribution. J. Phys. Chem. B 2005, 109, 7169–7173. [Google Scholar] [CrossRef] [PubMed]
- Ago, H.; Nakamura, K.; Uehara, N.; Tsuji, M. Roles of metal-support interaction in growth of single-and double-walled carbon nanotubes studied with diameter-controlled iron particles supported on MgO. J. Phys. Chem. B 2004, 108, 18908–18915. [Google Scholar] [CrossRef]
- Ago, H.; Uehara, N.; Yoshihara, N.; Tsuji, M.; Yumura, M.; Tomonaga, N.; Setoguchi, T. Gas analysis of the CVD process for high yield growth of carbon nanotubes over metal-supported catalysts. Carbon 2006, 44, 2912–2918. [Google Scholar] [CrossRef]
- Hata, K.; Futaba, D.N.; Mizuno, K.; Namai, T.; Yumura, M.; Iijima, S. Water-assisted highly efficient synthesis of impurity-free single-walled carbon nanotubes. Science 2004, 306, 1362–1364. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Maigne, A.; Yudasaka, M.; Mizuno, K.; Futaba, D.N.; Yumura, M.; Iijima, S.; Hata, K. Revealing the secret of water-assisted carbon nanotube synthesis by microscopic observation of the interaction of water on the catalysts. Nano Lett. 2008, 8, 4288–4292. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-L.; Kinloch, I.A.; Windle, A.H. Direct spinning of carbon nanotube fibers from chemical vapor deposition synthesis. Science 2004, 304, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.W.; Monthioux, M.; Luzzi, D.E. Encapsulated C60 in carbon nanotubes. Nature 1998, 396, 323–324. [Google Scholar] [CrossRef]
- Bandow, S.; Hiraoka, T.; Yumura, T.; Hirahara, K.; Shinohara, H.; Iijima, S. Raman scattering study on fullerene derived intermediates formed within single-wall carbon nanotube: From pea pod to double-wall carbon nanotube. Chem. Phys. Lett. 2004, 384, 320–325. [Google Scholar] [CrossRef]
- Bandow, S.; Takizawa, M.; Hirahara, K.; Yudasaka, M.; Iijima, S. Raman scattering study of double-wall carbon nanotubes derived from the chains of fullerenes in single-wall carbon nanotubes. Chem. Phys. Lett. 2001, 337, 48–54. [Google Scholar] [CrossRef]
- Hernandez, E.; Meunier, V.; Smith, B.W.; Rurali, R.; Terrones, H.; Buongiorno Nardelli, M.; Terrones, M.; Luzzi, D.E.; Charlier, J.C. Fullerene coalescence in nanopea pods: A path to novel tubular carbon. Nano Lett. 2003, 3, 1037–1042. [Google Scholar] [CrossRef]
- Kalbac, M.; Kavan, L.; Juha, L.; Civis, S.; Zukalova, M.; Bittner, M.; Kubat, P.; Vorlicek, V.; Dunsch, L. Transformation of fullerene pea pods to double-walled carbon nanotubes induced by UV radiation. Carbon 2005, 43, 1610–1616. [Google Scholar] [CrossRef]
- Botka, B.; Fustos, M.E.; Klupp, G.; Kocsis, D.; Szekely, E.; Utczas, M.; Simandi, B.; Botos, A.; Hackl, R.; Kamaras, K. Low-temperature encapsulation of coronene in carbon nanotubes. Phys. Status Solidi B 2012, 249, 2432–2435. [Google Scholar] [CrossRef]
- Pfeiffer, R.; Pichler, T.; Kim, Y.A.; Kuzmany, H. Double-wall carbon nanotubes. In Springer Series on Topics in Appl. Phys., vol. 111; Jorio, A., Dresselhaus, M.S., Dresselhaus, G., Eds.; Springer-Verlag: Berlin, Germany, 2008; pp. 495–530. [Google Scholar]
- Lim, H.E.; Miyata, Y.; Kitaura, R.; Nishimura, Y.; Nishimoto, Y.; Irle, S.; Warner, J.H.; Kataura, H.; Shinohara, H. Growth of carbon nanotubes via twisted graphene nanoribbons. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Bandow, S.; Iijima, S. Formation of small-diameter carbon nanotubes from PTCDA arranged inside the single-wall carbon nanotubes. Chem. Phys. Lett. 2005, 413, 410–414. [Google Scholar] [CrossRef]
- Shiozawa, H.; Pichler, T.; Gruneis, A.; Pfeiffer, R.; Kuzmany, H.; Liu, Z.; Suenaga, K.; Kataura, H. A catalytic reaction inside a single-walled carbon nanotube. Adv. Mater. 2008, 20, 1443–1449. [Google Scholar] [CrossRef]
- Shiozawa, H.; Pichler, T.; Pfeiffer, R.; Kuzmany, H.; Kataura, H. Ferrocene encapsulated in single-wall carbon nanotubes: A precursor to secondary tubes. Phys. Status Solidi B 2007, 244, 4102–4105. [Google Scholar] [CrossRef]
- Guan, L.; Shi, Z.; Li, M.; Gu, Z. Ferrocene-filled single-walled carbon nanotubes. Carbon 2005, 43, 2780–2785. [Google Scholar] [CrossRef]
- Chamberlain, T.W.; Champness, N.R.; Schroder, M.; Khlobystov, A.N. A piggyback ride for transition metals: Encapsulation of exohedral metallofullerenes in carbon nanotubes. Chem. Eur. J. 2011, 17, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Brozena, A.H.; Wang, Y. Double-walled carbon nanotubes: Challenges and opportunities. Nanoscale 2011, 3, 503–518. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, Q.; Luo, G.; Wei, F. Rings of triple-walled carbon nanotube bundles. Appl. Phys. Lett. 2006, 89. [Google Scholar] [CrossRef]
- Wen, Q.; Qian, W.; Nie, J.; Cao, A.; Ning, G.; Wang, Y.; Hu, L.; Zhang, Q.; Huang, J.; Wei, F. 100 mm long, semiconducting triple-walled carbon nanotubes. Adv. Mater. 2010, 22, 1867–1871. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.-H.; Futaba, D.N.; Yumura, M.; Hata, K. Direct wall number control of carbon nanotube forests from engineered iron catalysts. J. Nanosci. Nanotechnol. 2013, 13, 2745–2751. [Google Scholar] [CrossRef] [PubMed]
- Baliyan, A.; Nakajima, Y.; Fukuda, T.; Uchida, T.; Hanajiri, T.; Maekawa, T. Synthesis of an ultradense forest of vertically aligned triple-walled carbon nanotubes of uniform diameter and length using hollow catalytic nanoparticles. J. Amer. Chem. Soc. 2014, 136, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Taki, Y.; Shinohara, K.; Kikuchi, M.; Tanaka, A. Selective growth of single-, double-, and triple-walled carbon nanotubes through precise control of catalyst diameter by radiation-heated chemical vapor deposition. Jpn. J. Appl. Phys. 2008, 47, 725–729. [Google Scholar] [CrossRef]
- Muramatsu, H.; Shimamoto, D.; Hayashi, T.; Kim, Y.A.; Terrones, M.; Endo, M.; Dresselhaus, M.S. Bulk synthesis of narrow diameter and highly crystalline triple-walled carbon nanotubes by coalescing fullerene pea pods. Adv. Mater. 2011, 23, 1761–1764. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, H.; Hayashi, T.; Fujisawa, K.; Tojo, T.; Ko, Y.-I.; Morelos-Gomez, A.; Yang, K.-S.; Kim, Y.A.; Endo, M.; Terrones, M.; et al. Boron-assisted coalescence of parallel multi-walled carbon nanotubes. RSC Advances 2013, 3, 26266–26270. [Google Scholar] [CrossRef]
- Araujo, P.T.; Jorio, A. The role of environmental effects on the optical transition energies and radial breathing mode frequency of single wall carbon nanotubes. Phys. Status Solidi B 2008, 245, 2201–2204. [Google Scholar] [CrossRef]
- Araujo, P.T.; Fantini, C.; Lucchese, M.M.; Dresselhaus, M.S.; Jorio, A. The effect of environment on the radial breathing mode of supergrowth single wall carbon nanotubes. Appl. Phys. Lett. 2009, 95. [Google Scholar] [CrossRef]
- Araujo, P.T.; Maciel, I.O.; Pesce, P.B.C.; Pimenta, M.A.; Doorn, S.K.; Qian, H.; Hartschuh, A.; Steiner, M.; Grigorian, L.; Hata, K.; et al. Nature of the constant factor in the relation between radial breathing mode frequency and tube diameter for single-wall carbon nanotubes. Phys. Rev. B 2008, 77. [Google Scholar] [CrossRef]
- Green, A.A.; Duch, M.C.; Hersam, M.C. Isolation of Single-walled carbon nanotube enantiomers by density differentiation. Nano Res. 2009, 2, 69–77. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, Y.; Wei, L.; Goh, K.; Yu, D.; Chen, Y. Catalysts for chirality selective synthesis of single-walled carbon nanotubes. Carbon 2015, 81, 1–19. [Google Scholar] [CrossRef]
- Yang, F.; Wang, X.; Zhang, D.; Yang, J.; Luo, D.; Xu, Z.; Wei, J.; Wang, J.-Q.; Xu, Z.; Peng, F.; et al. Chirality-specific growth of single-wall carbon nanotubes on solid alloy catalysts. Nature 2014, 510, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Dijon, J.; Fournier, A.; Szkutnik, P.D.; Okuno, H.; Jayet, C.; Fayolle, M. Carbon nanotubes for interconnects in future integrated circuits: The challenge of the density. Diam. Relat. Mater. 2010, 19, 382–388. [Google Scholar] [CrossRef]
- Fujisawa, K.; Komiyama, K.; Muramatsu, H.; Shimamoto, D.; Tojo, T.; Kim, Y.A.; Hayashi, T.; Endo, M.; Oshida, K.; Terrones, M.; et al. Chirality-dependent transport in double-walled carbon nanotube assemblies: The role of inner tubes. ACS Nano 2011, 5, 7547–7554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ning, Z.; Zhang, Y.; Zheng, Q.; Chen, Q.; Xie, H.; Zhang, Q.; Qian, W.; Wei, F. Superlubricity in centimetres-long double-walled carbon nanotubes under ambient conditions. Nat. Nanotechnol. 2013, 8, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Li, T.S.; Lee, C.H.; Lin, M.F. Transport properties of double-walled carbon nanotubes in a transverse electric field. J. Phys. Soc. Jpn. 2007, 76. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zhao, Y.; Adhikari, S.; Feng, Y.T. Vibration of axially strained triple-wall carbon nanotubes. J. Comput. Theor. Nanosci. 2010, 7, 1–10. [Google Scholar] [CrossRef]
- Jung, Y.C.; Shimamoto, D.; Muramatsu, H.; Kim, Y.A.; Hayashi, T.; Terrones, M.; Morinobu, E. Robust, conducting, and transparent polymer composites using surface-modified and individualized double-walled carbon nanotubes. Adv. Mater. 2008, 20, 4509–4512. [Google Scholar] [CrossRef]
- Shimizu, T.; Haruyama, J.; Nozawa, K.; Sugai, T.; Shinohara, H. Possible formation of interlayer nano-p-n junctions and quantum dot in double-walled carbon nanotube with electrode contacts to different layers. Appl. Phys. Lett. 2009, 94. [Google Scholar] [CrossRef]
- Chen, G.; Bandow, S.; Margine, E.R.; Nisoli, C.; Kolmogorov, A.N.; Crespi, V.H.; Gupta, R.; Sumanasekera, G.U.; Iijima, S.; Eklund, P.C. Chemically doped double-walled carbon nanotubes: Cylindrical molecular capacitors. Phys. Rev. Lett. 2003, 90. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, R.; Ku, D.C.; Kassakian, J.G.; Schindall, J.E. Electrochemical double-layer capacitors using carbon nanotube electrode structures. Proc. IEEE 2009, 97, 1837–1847. [Google Scholar] [CrossRef]
- Cooper, L.; Amano, H.; Hiraide, M.; Houkyou, S.; Jang, I.Y.; Kim, Y.J.; Muramatsu, H.; Kim, J.H.; Hayashi, T.; Kim, Y.A.; et al. Freestanding, bendable thin film for supercapacitors using DNA-dispersed double walled carbon nanotubes. Appl. Phys. Lett. 2009, 95. [Google Scholar] [CrossRef]
- Ma, C.C.; Zhao, Y.; Yam, C.Y.; Chen, G.; Jiang, Q. A tribological study of double-walled and triple-walled carbon nanotube oscillators. Nanotechnology 2005, 16, 1253–1264. [Google Scholar] [CrossRef]
- Kang, J.W.; Lee, J.H. Frequency characteristics of triple-walled carbon nanotube gigahertz devices. Nanotechnology 2008, 19, 285704–285710. [Google Scholar] [CrossRef] [PubMed]
- Popescu, A.; Woods, L.M.; Bondarev, I.V. A carbon nanotube oscillator as a surface profiling device. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- Baowan, D.; Hill, J.M. Force distribution for double-walled carbon nanotubes and gigahertz oscillators. Z. Angew. Math. Phys. 2007, 58, 857–875. [Google Scholar] [CrossRef]
- Pentaras, D.; Elishakoff, I. Free vibration of triple-walled carbon nanotubes. Acta Mech. 2011, 221, 239–249. [Google Scholar] [CrossRef]
- Zeng, Y.-H.; Jiang, W.-G.; Qin, Q.H. Oscillators based on double-walled armchair@zigzag carbon nanotubes containing inner tubes with different helical rises. Nanotechnology 2016, 27. [Google Scholar] [CrossRef] [PubMed]
- Joselevich, E.; Dai, H.; Liu, J.; Hata, K. Windle. In A Carbon Nanotubes: Advanced Topics in the Synthesis, Structure, Properties and Applications; Jorio, A., Dresselhaus, M.S., Dresselhaus, G., Eds.; Springer: Berlin, Germany, 2008; pp. 101–163. [Google Scholar]
- Wei, X.L.; Chen, Q.; Peng, L.M.; Cui, R.L.; Li, Y. Tensile loading of double-walled and triple-walled carbon nanotubes and their mechanical properties. J. Phys. Chem. C 2009, 113, 17002–17005. [Google Scholar] [CrossRef]
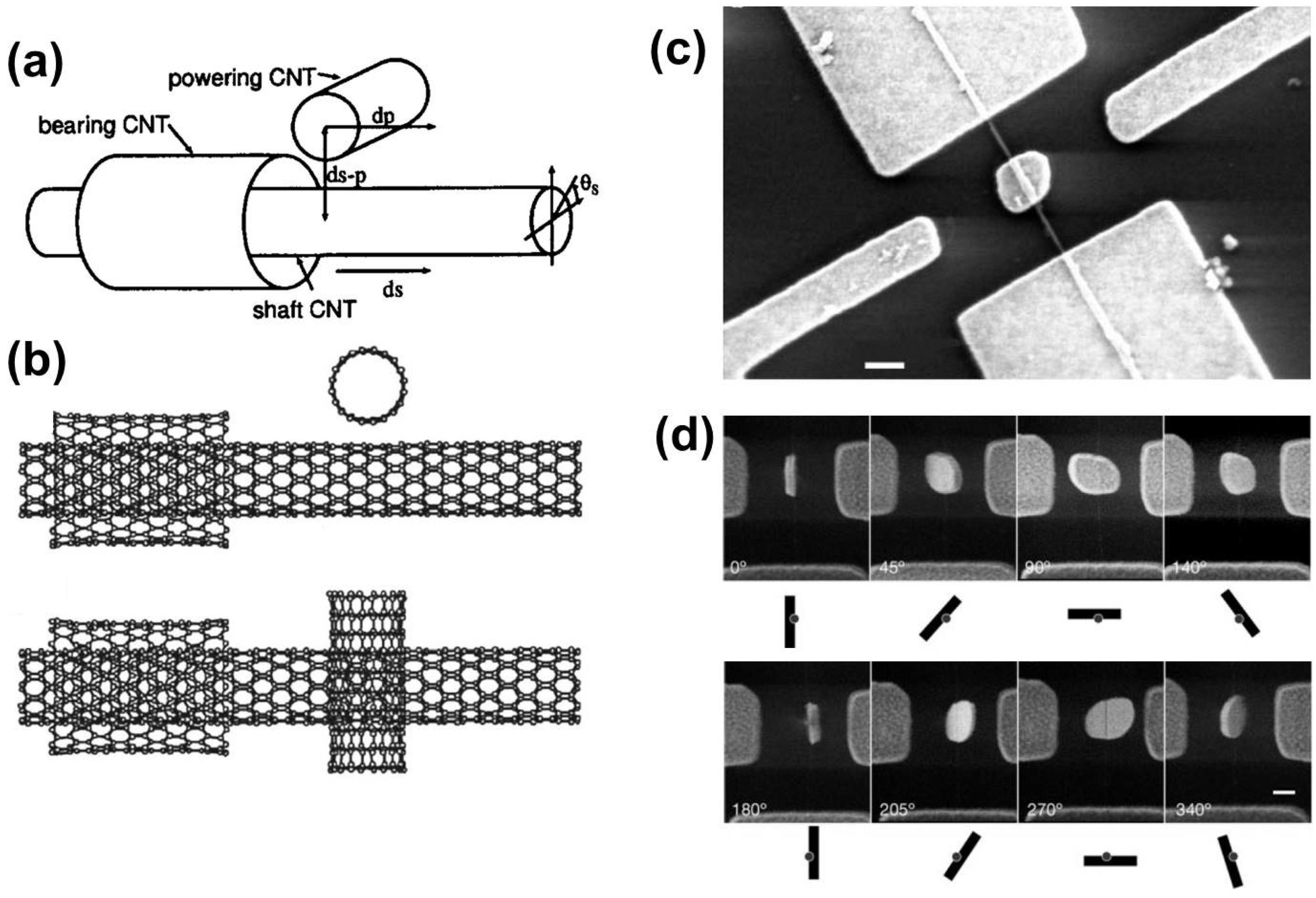
- Bourlon, B.; Glattli, D.C.; Miko, C.; Forró, L.; Bachtold, A. Carbon nanotube based bearing for rotational motions. Nano Lett. 2004, 4, 709–712. [Google Scholar] [CrossRef]
- Takagi, Y.; Uda, T.; Ohno, T. Carbon nanotube motors driven by carbon nanotube. J. Chem. Phys. 2008, 128. [Google Scholar] [CrossRef] [PubMed]
- Fennimore, A.M.; Yuzvinsky, T.D.; Han, W.-Q.; Fuhrer, M.S.; Cumings, J.; Zettl, A. Rotational actuators based on carbon nanotubes. Nature 2003, 424, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z. Coaxial stability of nano-bearings constructed by double-walled carbon nanotubes. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Hamaguchi, K.; Uemura, S.; Uchida, K.; Tasaka, Y.; Ikazaki, I.; Yumura, M.; Kasuya, A.; Nishina, Y. Field emission from multi-walled carbon nanotubes and its application to electron tubes. Appl. Phys. A 1998, 67, 95–100. [Google Scholar] [CrossRef]
- Lee, H.; Yoon, W.S.; Kim, E.J.; Jeunghee, P. In-Situ growth of copper sulfide nanocrystals on multiwalled carbon nanotubes and their application as novel solar cell and amperometric glucose sensor materials. Nano Lett. 2007, 7, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.Q.; Jiang, S.P.; Liang, Y.M.; Shen, P.K. Synthesis and characterization of platinum catalysts on multiwall carbon nanotubes by intermittent microwave irradiation for fuel cell applications. J. Phys. Chem. B 2006, 110, 5343–5350. [Google Scholar] [CrossRef] [PubMed]
- Li, T.S.; Lee, C.H.; Lim, M.F. Electron transport in double-walled carbon nanotubes. Eur. Phys. J. B 2007, 60, 45–50. [Google Scholar] [CrossRef]
- van de Lagemaat, J.; Barnes, T.M.; Rumbles, G.; Shaheen, S.E.; Coutts, T.J.; Weeks, C.; Levitsky, I.; Peltola, J.; Glatkowski, P. Organic solar cells with carbon nanotubes replacing In2O3:SnIn2O3:Sn as the transparent electrode. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Du Pasquier, A.; Unalan, H.E.; Kanwal, A.; Miller, S.; Chhowalla, M. Conducting and transparent single-wall carbon nanotube electrodes for polymer-fullerene solar cells. Appl. Phys. Lett. 2005, 87. [Google Scholar] [CrossRef]
- Rowel, M.W.; Topinka, M.A.; McGehee, M.D.; Prall, H.-J.; Dennler, G.; Sariciftci, N.S.; Hu, L.; Gruner, G. Organic solar cells with carbon nanotube network electrodes. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Jia, Y.; Wei, J.; Wang, K.; Cao, A.; Shu, Q.; Gui, X.; Zhu, Y.; Zhuang, D.; Zhang, G.; Ma, B.; et al. Nanotube–silicon heterojunction solar cells. Adv. Mater. 2008, 20, 4594–4598. [Google Scholar] [CrossRef]
- Wei, J.; Jia, Y.; Shu, Q.; Gu, Z.; Wang, K.; Zhuang, D.; Zhang, G.; Wang, Z.; Luo, J.; Cao, A.; et al. Double-walled carbon nanotube solar cells. Nano Lett. 2007, 7, 2317–2321. [Google Scholar] [CrossRef] [PubMed]
- Shu, Q.; Wei, J.; Wang, K.; Zhu, H.; Li, Y.J.; Gui, X.; Guo, N.; Li, X.; Ma, C.; Wu, D. Hybrid heterojunction and photoelectrochemistry solar cell based on silicon nanowires and double-walled carbon nanotubes. Nano Lett. 2009, 9, 4338–4342. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhao, F. Electrochemical sensor for chloramphenicol based on novel multiwalled carbon nanotubes@molecularly imprinted polymer. Biosens. Bioelectron. 2015, 64, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Lee, P.; Chou, J.B.; Xu, R.; Zhao, R.; Hart, A.J.; Kim, S.-G. Extremely elastic wearable carbon nanotube fiber strain sensor for monitoring of human motion. ACS Nano 2015, 9, 5929–5936. [Google Scholar] [CrossRef] [PubMed]
- Ansari, R.; Ajori, S.; Ameri, A. Stability characteristics and structural properties of single- anddouble-walled boron-nitride nanotubes under physical adsorption ofFlavin mononucleotide (FMN) in aqueous environment usingmolecular dynamics simulations. Appl. Surf. Sci. 2016, 366, 233–244. [Google Scholar] [CrossRef]
- Kiani, K. Nanomechanical sensors based on elastically supported double-walled carbon nanotubes. Appl. Math. Comput. 2015, 270, 216–241. [Google Scholar] [CrossRef]
- Yang, N.; Chen, X.; Ren, T.; Zhang, P.; Yang, D. Carbon nanotube based bio sensors. Sens. Actuators 2015, 207, 690–715. [Google Scholar] [CrossRef]
- Li, S.; Yin, G.; Wu, X.; Liu, C.; Luo, J. Supramolecular imprinted sensor for carbofuran detection based on a functionalized multiwalled carbon nanotube-supported Pd-Ir composite and methylene blue as catalyst. Electrochim. Acta 2016, 188, 294–300. [Google Scholar] [CrossRef]
- Jensen, K.; Kim, K.; Zettl, A. An atomic-resolution nanomechanical mass sensor. Nat. Nanotechnol. 2008, 3, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.; Schulz, M.J.; Kim, J.H.; Shanov, V.; Shi, D. A carbon nanotube strain sensor for structural health monitoring. Smart Mater. Struct. 2006, 15. [Google Scholar] [CrossRef]
- Gheibi, S.; Hassan, K.-M.; Khalilzadeh, M.A.; Bagheri, H. A new voltammetric sensor for electrocatalytic determination of vitamin C in fruit juices and fresh vegetable juice using modified multi-wall carbon nanotubes paste electrode. J. Food Sci. Technol. 2015, 52, 276–284. [Google Scholar] [CrossRef]
- Miyamoto, J.; Hattori, Y.; Noguchi, D.; Tanaka, H.; Ohba, T.; Utsmi, S.; Kanoh, H.; Kim, Y.A.; Muramatsu, H.; Hayashi, T.; et al. Efficient H2 adsorption by nanopores of high-purity double-walled carbon nanotubes. J. Am. Chem. Soc. 2006, 128, 12636–12637. [Google Scholar] [CrossRef] [PubMed]
- Piscanec, S.; Lazzeri, M.; Mauri, F.; Ferrari, A.C.; Robertson, J. Kohn anomalies and electron-phonon interactions in graphite. Phys. Rev. Lett. 2004, 93. [Google Scholar] [CrossRef] [PubMed]
- Araujo, P.T.; Mafra, D.L.; Sato, K.; Saito, R.; Kong, J.; Dresselhaus, M.S. Phonon self-energy corrections to nonzero wave-vector phonon modes in single-layer graphene. Phys. Rev. Lett. 2012, 109. [Google Scholar] [CrossRef] [PubMed]
- Mafra, D.L.; Kong, J.; Sato, K.; Saito, R.; Dresselhaus, M.S.; Araujo, P.T. Using gate-modulated Raman scattering and electron-phonon interactions to probe single-layer graphene: A different approach to assign phonon combination modes. Phys. Rev. B 2012, 86. [Google Scholar] [CrossRef]
- Araujo, P.T.; Mafra, D.L.; Sato, K.; Saito, R.; Kong, J.; Dresselhaus, M.S. Unraveling the interlayer-related phonon self-energy renormalization in bilayer graphene. Sci. Rep. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Araujo, P.T.; Frank, O.; Mafra, D.L.; Fang, W.; Kong, J.; Dresselhaus, M.S.; Kalbac, M. Mass-related inversion symmetry breaking and phonon self-energy renormalization in isotopically labeled AB-stacked bilayer graphene. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Lui, C.H.; Li, Z.; Mak, K.F.; Cappelluti, E.; Heinz, T.F. Observation of an electrically tunable band gap in trilayer graphene. Nat. Phys. 2011, 7, 944–947. [Google Scholar] [CrossRef]
- Lui, C.H.; Malard, L.M.; Kim, S.; Lantz, G.; Laverge, F.E.; Saito, R.; Heinz, T.F. Observation of out-of-plane vibrations in few-layer graphene. Nano Lett. 2012, 12, 5539–5544. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cassell, A.M.; Hongjie, D. Carbon nanotubes as AFM tips: Measuring DNA molecules at the liquid/solid interface. Surf. Interface Anal. 1999, 28, 8–11. [Google Scholar] [CrossRef]
- Hafner, J.H.; Cheung, C.L.; Woolley, A.T.; Lieber, C.M. Structural and functional imaging with carbon nanotube AFM probes. Prog. Biophys. Mol. Biol. 2001, 77, 73–110. [Google Scholar] [CrossRef]




| Catalyst/Precursor/Promoter | Carbon Source/Carrier/Atmosphere | Inner Tube (nm) | Outer Tube (nm) | Selectivity | Method |
|---|---|---|---|---|---|
| Fe-Co/MgO [84] | CH/N | 0.6–1.2 | 1.3–2.0 | - | Supporting (powder) |
| Fe-Mg-Al-O [85] | CH/H/Ar | - | 1.7–3.0 | - | Supporting (powder) |
| Co/MgO [86] | CH/H/N | - | 2.0–4.0 | - | Supporting (powder) |
| Fe/MgO-wire [87] | CH/H/Ar | - | - | - | Supporting (powder) |
| MgFeO/MgO [88] | CH/H | - | - | - | Supporting (powder) |
| MgFeCoO [89] | CH/H | 2.2 | - | 41% | Supporting (powder) |
| MgCoO [90] | CH/H | - | 3 | 45% | Supporting (powder) |
| Fe-Co/Mesoporous Silica [91] | CH/H | - | 4.3 | >50% | Supporting (powder) |
| MgCoO [92] | CH/H | 1.7 | 2.7 | 51% | Supporting (powder) |
| Fe/AlO [93] | CH | - | 1.0–4.0 | 55% | Supporting (powder) |
| Co-Mo/MgO [94] | EtOH | 0.6–1.3 | 1.3–2.0 | 70% | Supporting (powder) |
| Fe/MgO [95] | CH/Ar | - | 2.0–3.0 | >70% | Supporting (powder) |
| MgCoMoO [96] | CH/H | 1.43 | 2.01 | 77% | Supporting (powder) |
| Fe-Co/Mesoporous Silica [97] | EtOH/Ar | - | >2.0 | 70%–80% | Supporting (powder) |
| Co-Fe/Zeolite [98] | CH/Ar | - | 2.0–6.0 | 80% | Supporting (powder) |
| Fe-Co/Mesoporous Silica [99] | EtOH/Ar | - | 1.5–5.4 | 80% | Supporting (powder) |
| Fe (4 nm)/MgO [100] | CH/Ar | 0.91 | 1.68 | 82% | Supporting (powder) |
| Fe (10 nm)/MgO [100] | CH/Ar | 1.42 | 2.19 | 85% | Supporting (powder) |
| Fe/MgO [101] | CH/H/Ar | - | 2.05 | 87% | Supporting (powder) |
| FeSi/Si [102] | CH/CH/H/Ar | - | 4.5 | 90% | Supporting (powder) |
| Fe-Mo/MgO [103] | n-Hexane/H/Ar | 0.75–1.8 | 1.5–2.6 | >90% | Supporting (powder) |
| Mo/AlO, Fe/MgO [104] | CH/Ar | 0.9 | 1.6 | 95% | Supporting (powder) |
| Fe-Mo/MgO [105] | CH/H/Ar | - | 2.0 | 95% | Supporting (powder) |
| Co-Fe-Mo/MgO [106] | n-Hexane/Ar | 0.6–1.5 | 1.2–2.2 | 100% | Supporting (powder) |
| Fe (1 nm)/AlO (10 nm)/SiO/Si [107] | CH/H/He | - | 4.0 | - | Supporting (substrate) |
| Fe-Mo/AlO (10 nm)/SiO/Si [108] | CH/H | 1.0–2.0 | 2.0–3.0 | - | Supporting (substrate) |
| SiO (30 nm)/Si [109] | CH/H/Ar | - | 3.0–5.0 | 70% | Supporting (substrate) |
| Fe-V-O (3.1 nm)/Si [110] | CH/H/He | - | 3.7 | 74% | Supporting (substrate) |
| Fe (1 nm)/AlO (10 nm)/Au (1 μm)/Si [111] | CH/H/He | - | - | 79% | Supporting (substrate) |
| Co/TiN/Si [112] | CH/H | - | 4.5 | 80% | Supporting (substrate) |
| Fe (0.4 nm)/AlO (5 nm)/Si [113] | CH/H/Ar | - | 3.73 | 81% | Supporting (substrate) |
| FeO (3.8 nm)/Si [114] | CH/H | 3.6 | - | 81% | Supporting (substrate) |
| Fe (1 nm)/AlO (40 nm)/Si [115] | CH/H/He | - | 5.4 | 84% | Supporting (substrate) |
| Fe (1.69 nm)/AlO (30 nm)/SiO/Si [116] | HO/CH/H/He | - | 3.75 | 85% | Supporting (substrate) |
| Ferrocene-Sulfur [117] | CH | 0.40–2.19 | 1.05–2.89 | - | Floating |
| Ferrocene-Sulfur [118] | CH/Ar | - | - | - | Floating |
| Ferrocene-Sulfur [119] | Xylene/H/Ar | 2.0 | - | 80% | Floating |
| Ferrocene-Sulfur [120] | Xylene/H/Ar | - | 1.4–2.8 | 80% | Floating |
| Ferrocene-Thiophene [69] | EtOH, n-Hexane/H | - | - | - | Floating |
| Ferrocene-Thiophene [121] | Acetone/EtOH/H | - | - | - | Floating |
| Ferrocene-Thiophene [122] | EtOH/H | - | - | - | Floating |
| Ferrocene-Thiophene [123] | Acetone/Ar | 2.1 | 2.8 | 90% | Floating |
| Ferrocene-Thiophene [124] | CH/H | 1.0–1.3 | 1.7–2.0 | >90% | Floating |
| Catalyst/Precursor/Promoter | Carbon Source/Carrier/Atmosphere | Inner Tube (nm) | Middle Tube | Outer Tube (nm) | Selectivity | Method |
|---|---|---|---|---|---|---|
| Fe/Mo/Quartz [25] | EtOH/Ar/H | - | - | 3.80–4.80 | 62% | Supporting (substrate) |
| C@DWNT (pea pod) [26] | Ar | 0.60 | 1.20 | 2.00 | 45% | Supporting (substrate) |
| FeO/Si [147] | CH/H | 4.86 | - | - | 83% | Supporting (substrate) |
| FeCl/Si [145] | CH/H | 1.36–3.78 | 2.12–4.46 | 2.87–5.19 | 90% | Supporting (substrate) |
| Fe/AlO [146] | HO/CH/H | - | - | 8.00 | 59% | Supporting (substrate) |
| Co/Mo/Quartz [148] | EtOH/Ar/H | - | - | 4.00 | 76% | Supporting (substrate) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujisawa, K.; Kim, H.J.; Go, S.H.; Muramatsu, H.; Hayashi, T.; Endo, M.; Hirschmann, T.C.; Dresselhaus, M.S.; Kim, Y.A.; Araujo, P.T. A Review of Double-Walled and Triple-Walled Carbon Nanotube Synthesis and Applications. Appl. Sci. 2016, 6, 109. https://doi.org/10.3390/app6040109
Fujisawa K, Kim HJ, Go SH, Muramatsu H, Hayashi T, Endo M, Hirschmann TC, Dresselhaus MS, Kim YA, Araujo PT. A Review of Double-Walled and Triple-Walled Carbon Nanotube Synthesis and Applications. Applied Sciences. 2016; 6(4):109. https://doi.org/10.3390/app6040109
Chicago/Turabian StyleFujisawa, Kazunori, Hee Jou Kim, Su Hyeon Go, Hiroyuki Muramatsu, Takuya Hayashi, Morinobu Endo, Thomas Ch. Hirschmann, Mildred S. Dresselhaus, Yoong Ahm Kim, and Paulo T. Araujo. 2016. "A Review of Double-Walled and Triple-Walled Carbon Nanotube Synthesis and Applications" Applied Sciences 6, no. 4: 109. https://doi.org/10.3390/app6040109






