Biodegradable Nanoparticles Made of Amino-Acid-Based Ester Polymers: Preparation, Characterization, and In Vitro Biocompatibility Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the NPs (General Procedure)
2.2. NPs Size, Zeta-Potential and Morphology
2.3. Cytotoxicity (MTT) Assay
3. Results
3.1. Selection of the Polymers
3.2. Selection of the Organic Solvent
3.3. Fabrication of the NPs
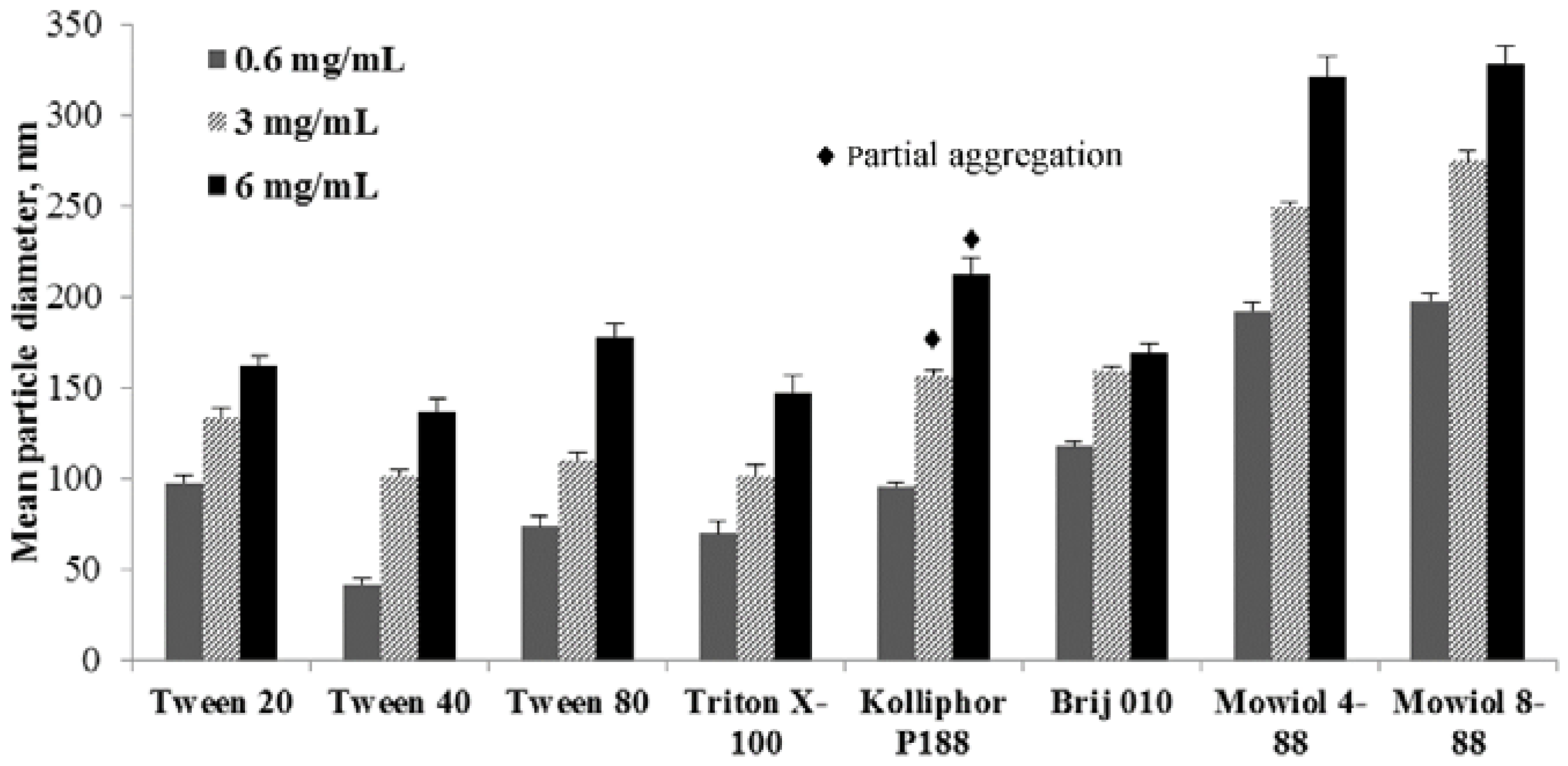
3.3.1. The Influence of a Polymer Concentration in the Organic Phase in the Presence of Various Surfactants in the Water Phase
| Parameters of the Process | |
| Invariable | Variable |
|
|
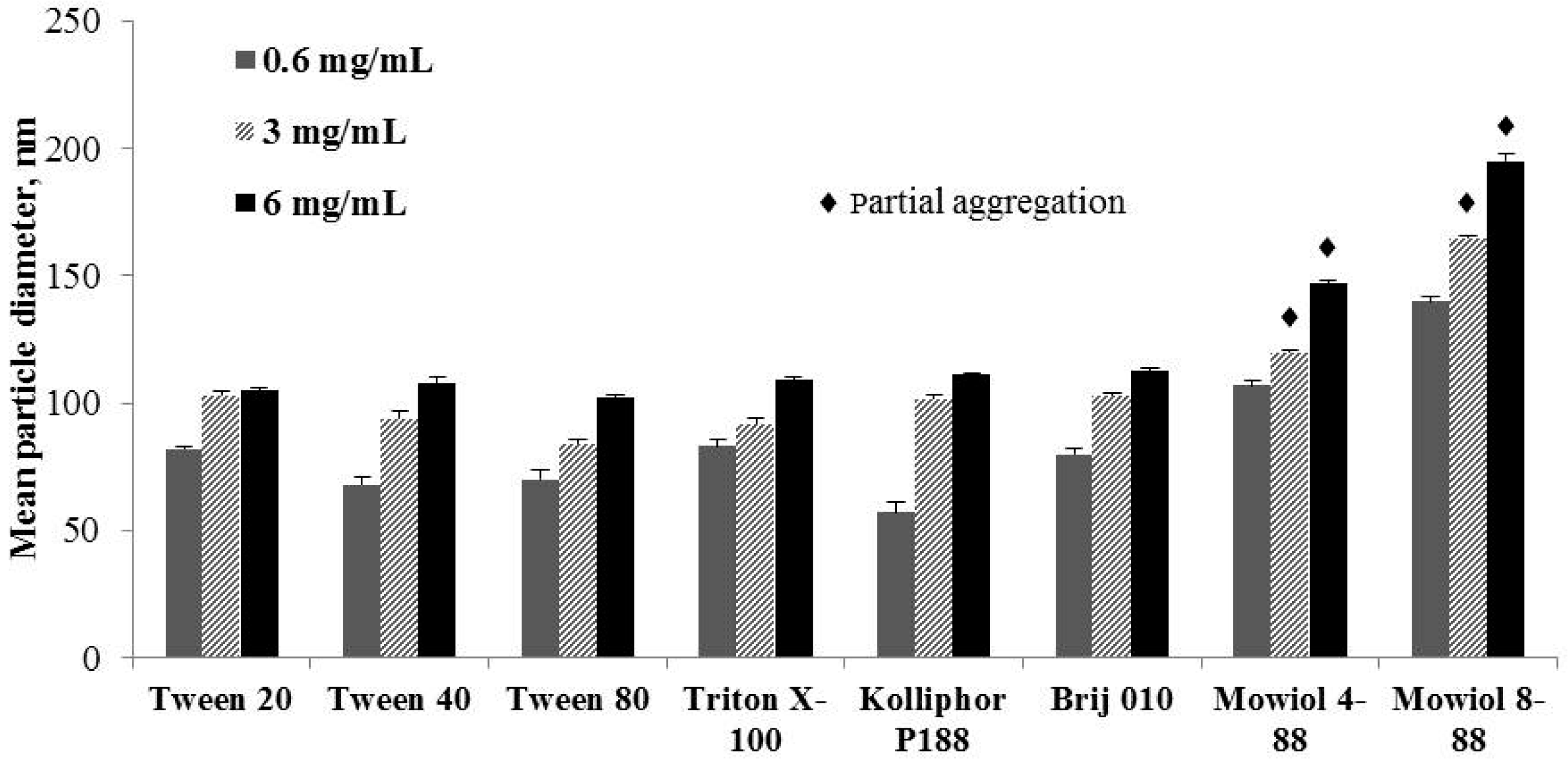
3.3.2. The Influence of a Polymer’s Nature
| Parameters of the Process | |
| Invariable | Variable |
|
|
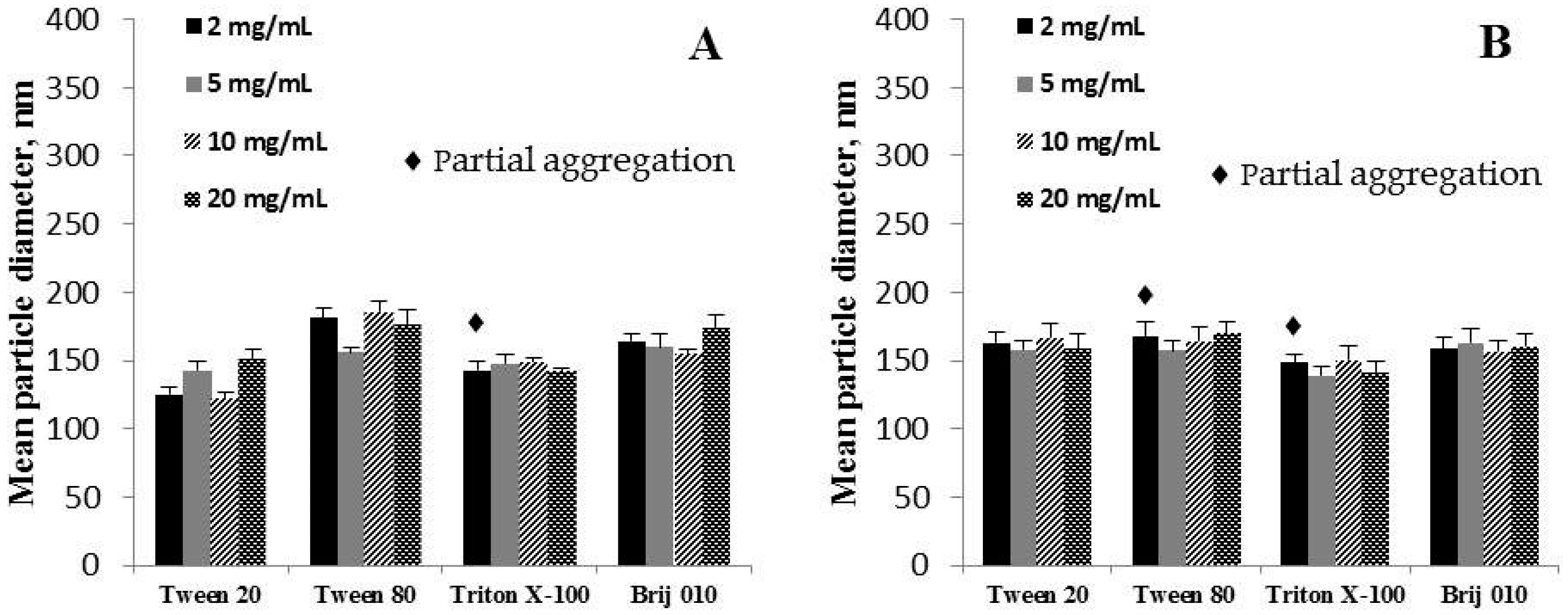
3.3.3. The Influence of a Surfactant’s Concentration in the Water Phase
| Parameters of the Process | |
| Invariable | Variable |
|
|
3.3.4. The Influence of an Organic Solvent
| Parameters of the Process | |
| Invariable | Variable |
|
|
3.3.5. The Influence of the O/W Ratio
| Parameters of the Process | |
| Invariable | Variable |
|
|
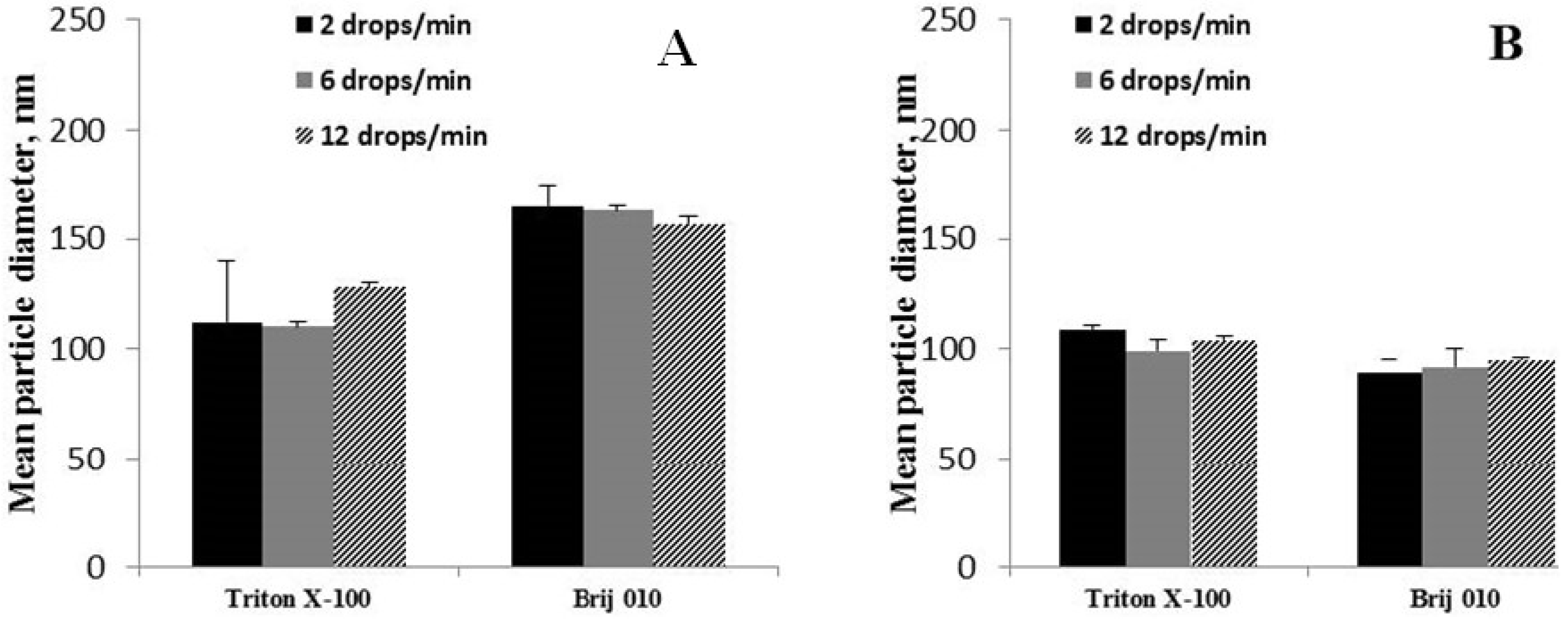
3.3.6. The Influence of the Organic Phase Dropping Rate to the Water Phase
| Parameters of the Process | |
| Invariable | Variable |
|
|
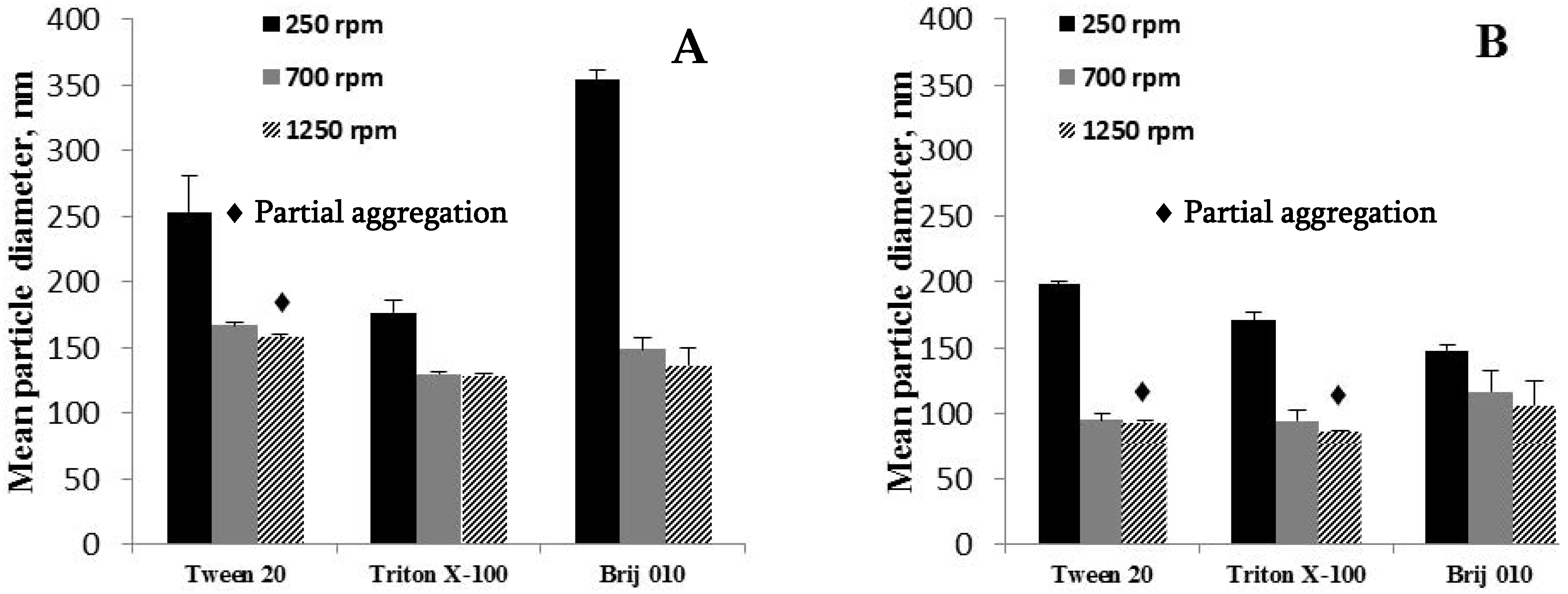
3.3.7. The Influence of the Stirring Rate of the Water Phase
| Parameters of the Process | |
| Invariable | Variable |
|
|
3.4. Modified method of the NPs fabrication
| Parameters of the Process | |
| Invariable | Variable |
|
|
3.5. ZP of the NPs
| Parameters of the Process | |
| Invariable | Variable |
|
|
3.6. Fabrication of Positively Charged NPs
| Parameters of the Process | |
| Invariable | Variable |
|
|
3.7. Stability of the NPs
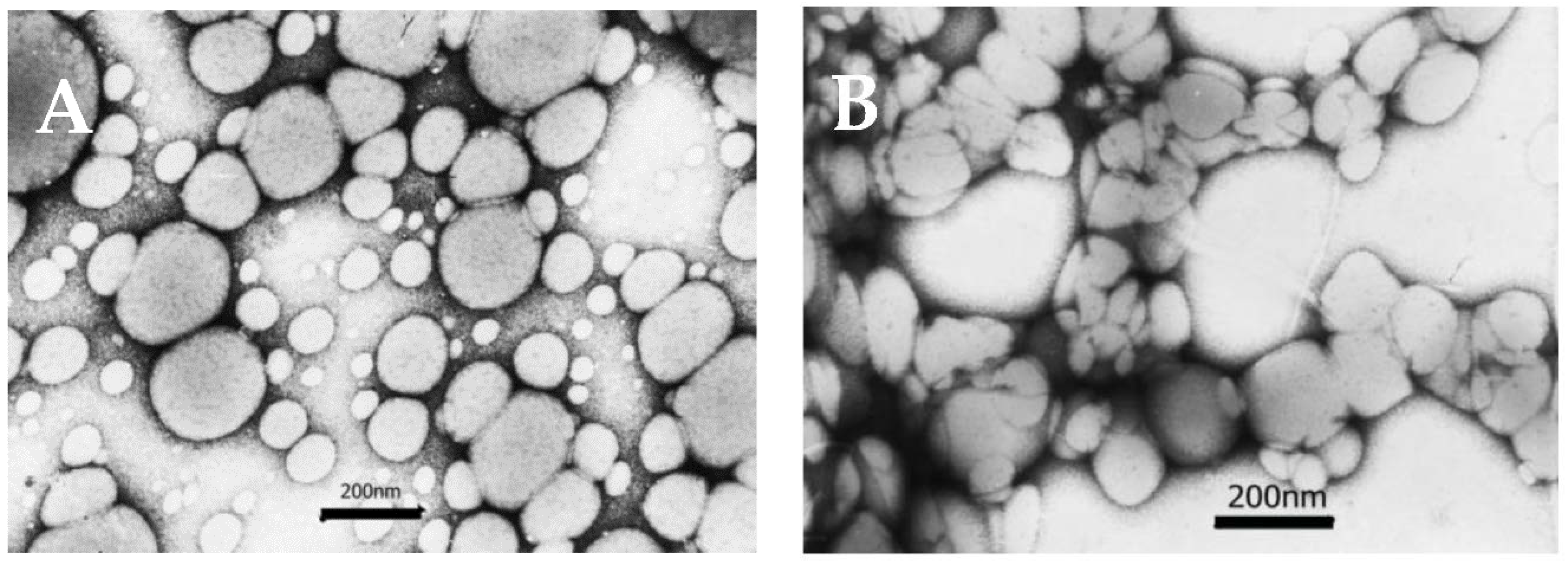
3.8. Morphology of the NPs
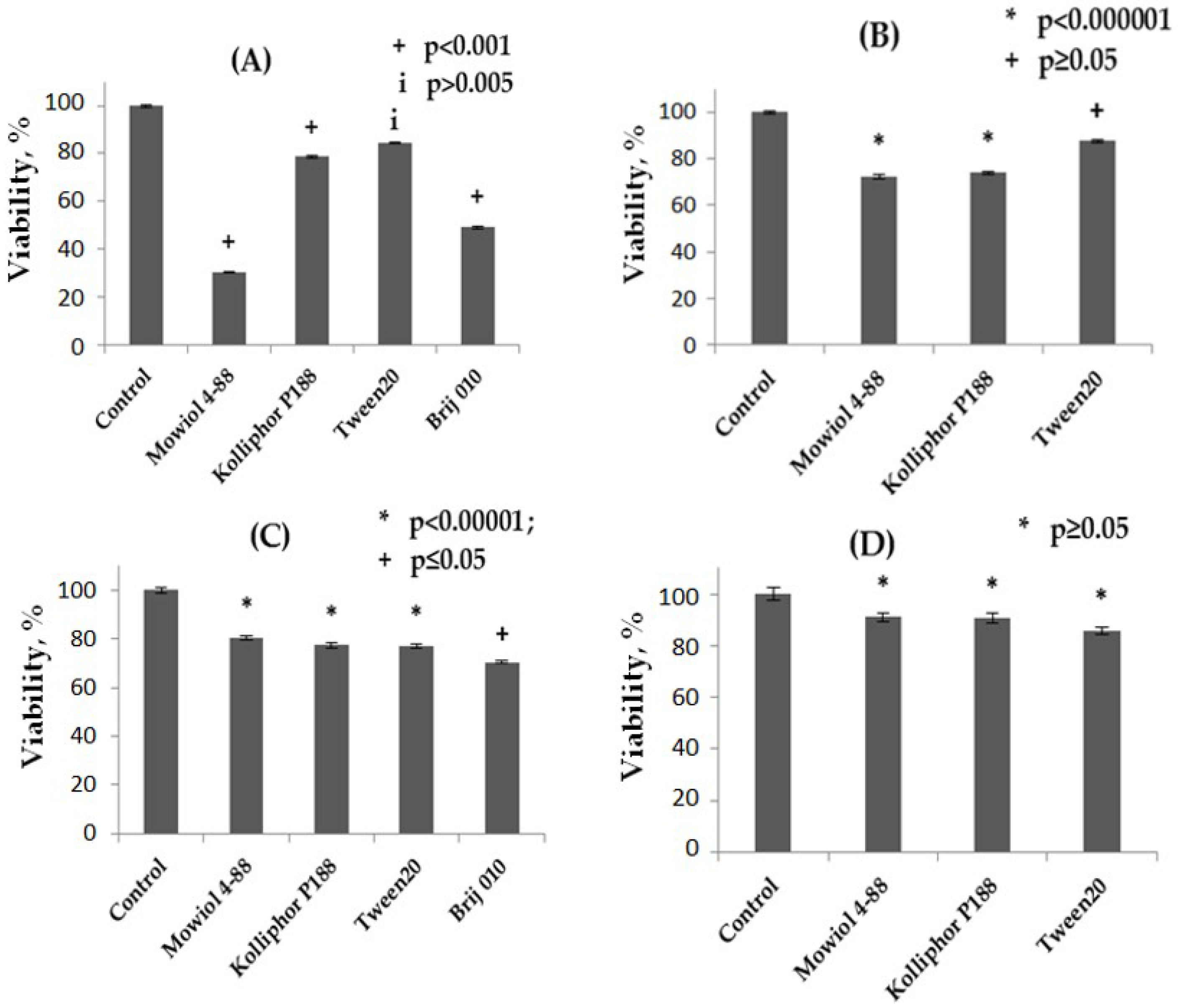
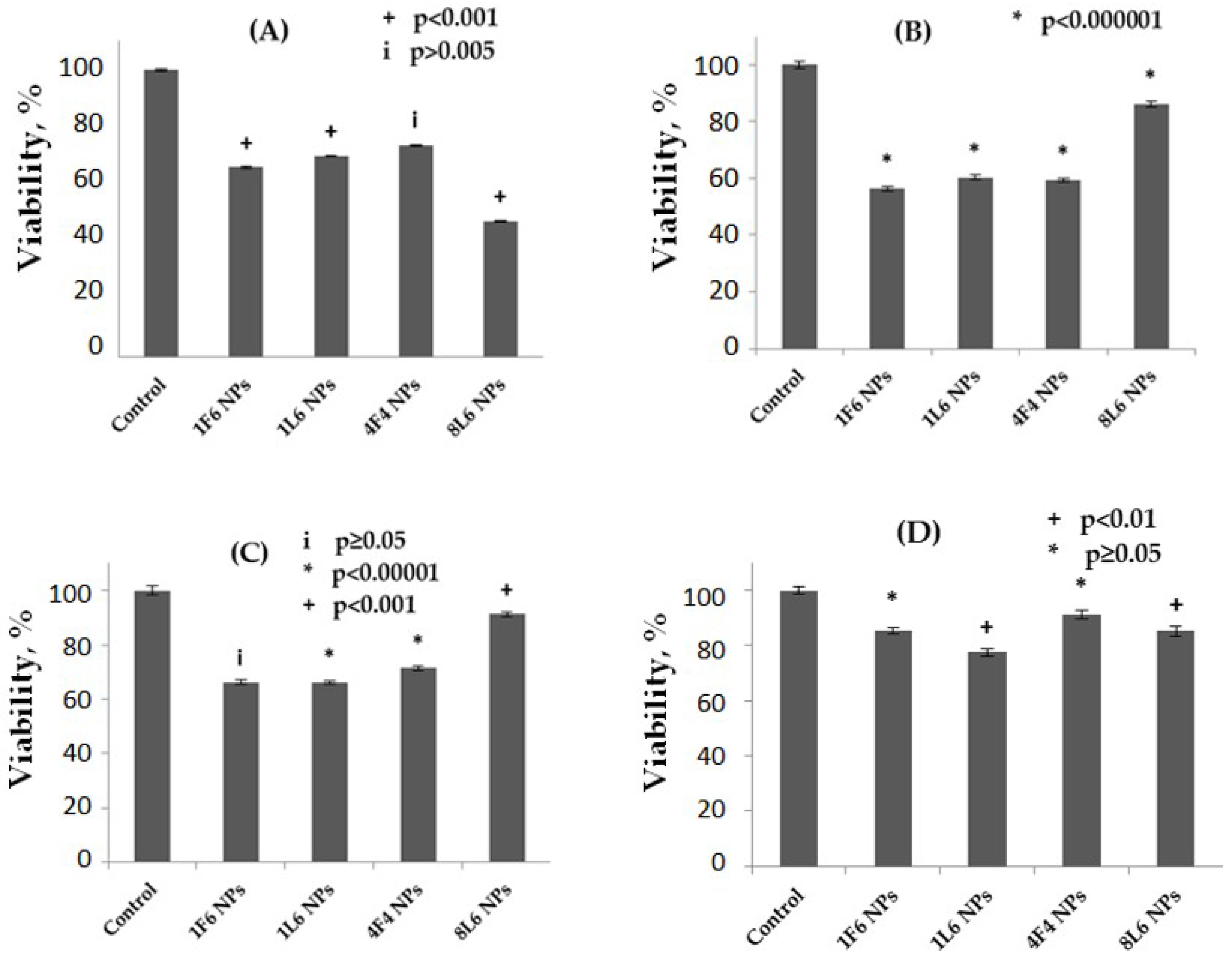
3.9. Cell Compatibility Study of the NPs
4. Discussion
4.1. Fabrication of the NPs
4.1.1. The Influence of a Polymer Concentration in the Organic Phase in the Presence of Various Surfactants in the Water Phase
4.1.2. The Influence of a Polymer’s Nature
4.1.3. The Influence of a Surfactant’s Concentration in the Water Phase
4.1.4. The Influence of an Organic Solvent
4.1.5. The Influence of O/W Ratio
4.1.6. The Influence of the Organic Phase Dropping Rate to the Water Phase
4.1.7. The Influence of the Stirring Rate of the Water Phase
4.2. Modified Method of the NPs Fabrication
4.3. ZP of the NPs
4.4. Fabrication of the Positively Charged NPs
4.5. Stability of the NPs
4.6. Morphology of the NPs
4.7. Cell Compatibility Study of the NPs
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Müller, R.H.; Jacobs, C.; Kayser, O. Nanosuspensions as particulate drug formulations in therapy: Rationale for development and what we can expect for the future. Adv. Drug Deliv. Rev. 2001, 47, 3–19. [Google Scholar] [CrossRef]
- Kaur, I.P.; Garg, A.; Singla, A.K.; Aggarwal, D. Vesicular systems in ocular drug delivery: An overview. Int. J. Pharm. 2004, 269, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, A.; Kreuter, J. Microspheres and nanoparticles used in ocular delivery systems. Adv. Drug Deliv. Rev. 1995, 16, 61–73. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Dilnawaz, F.; Krishnakumar, S. Nanotechnology in ocular drug delivery. Drug Discov. Today 2008, 13, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Bochot, A.; Fattal, E.; Boutet, V.; Deverre, J.R.; Jeanny, J.C.; Chacun, H.; Couvreur, P. Intravitreal delivery of oligonucleotides by sterically stabilized liposomes. Investig. Ophthalmol. Vis. Sci. 2002, 43, 253–259. [Google Scholar]
- Irache, J.M.; Merodio, M.; Arnedo, A.; Camapanero, M.A.; Mirshahi, M.; Espuelas, S. Albumin nanoparticles for the intravitreal delivery of anticytomegaloviral drugs. Mini Rev. Med. Chem. 2005, 5, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Jani, P.D.; Singh, N.; Jenkins, C.; Raghava, S.; Mo, Y.; Amin, S.; Kompella, U.B.; Ambati, B.K. Nanoparticles Sustain Expression of Flt Intraceptors in the Cornea and Inhibit Injury-Induced Corneal Angiogenesis. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2030–2036. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, R.; Bucolo, C.; Spedalieri, G.; Maltese, A.; Puglisi, G. Flurbiprofen-loaded acrylate polymer nanosuspensions for ophthalmic application. Biomaterials 2002, 23, 3247–3255. [Google Scholar] [CrossRef]
- Kawashima, Y.; Niwa, T.; Handa, T.; Takeuchi, H.; Iwamoto, T.; Itoh, K. Preparation of controlled-release microspheres of ibuprofen with acrylic polymers by a novel quasi-emulsion solvent diffusion method. J. Pharm. Sci. 1989, 78, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Maltese, A.; Maugeri, F.; Busà, B.; Puglisi, G.; Pignatello, R. Eudragit RL100 nanoparticle system for the ophthalmic delivery of cloricromene. J. Pharm. Pharmacol. 2004, 56, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, E.; Ozeki, H.; Kunou, N.; Ogura, Y. Effect of particle size of polymeric nanospheres on intravitreal kinetics. Ophthalm. Res. 2001, 33, 31–36. [Google Scholar] [CrossRef]
- Li, V.H.; Wood, R.W.; Kreuter, J.; Harmia, T.; Robinson, J.R. Ocular drug delivery of progesterone using nanoparticles. J. Microencapsul. 1986, 3, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Vila-Jato, J.L.; Alonso, M.J. Evaluation of cationic polymer-coated nanocapsules as ocular drug carriers. Int. J. Pharm. 1997, 153, 41–50. [Google Scholar] [CrossRef]
- De Campos, A.M.; Sánchez, A.; Gref, R.; Calvo, P.; Alonso, M.J. The effect of a PEG versus a chitosan coating on the interaction of drug colloidal carriers with the ocular mucosa. Eur. J. Pharm. Sci. 2003, 20, 73–81. [Google Scholar] [CrossRef]
- Giannavola, C.; Bucolo, C.; Maltese, A.; Paolino, D.; Vandelli, M.A.; Puglisi, G.; Lee, V.H.; Fresta, M. Influence of Preparation Conditions on Acyclovir-Loaded Poly-d,l-Lactic Acid Nanospheres and Effect of PEG Coating on Ocular Drug Bioavailability. Pharm. Res. 2003, 20, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Vidmar, V.; Pepeljnjak, S.; Jalseniak, J. The in vivo evaluation of poly(lactic acid) microcapsules of pilocarpine hydrochloride. J. Microencapsul. 1985, 2, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.K.; Gillies, E.R.; Mequanint, K. Strategies in Functional Poly(ester amide) Syntheses to Study Human Coronary Artery Smooth Muscle Cell Interactions. Biomacromolecules. 2011, 12, 2475–2487. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, M. Custom-made biomaterials. Chem. Eng. News 2001, 79, 30–35. [Google Scholar] [CrossRef]
- Arabuli, N.; Tsitlanadze, G.; Edilashvili, L.; Kharadze, D.; Goguadze, T.; Beridze, V.; Gomurashvili, Z.; Katsarava, R. Heterochain polymers based on natural α-amino acids. Synthesis and enzymatic hydrolysis of regular poly(ester amide)s based on bis(l-phenylalanine) α,ω-alkylene diesters and adipic acid. Macromol. Chem. Phys. 1994, 195, 2279–2289. [Google Scholar] [CrossRef]
- Kartvelishvili, T.; Tsitlanadze, G.; Edilashvili, L.; Japaridze, N.; Katsarava, R. Amino acid based bioanalogous polymers. Regular poly(ester urethane)s and poly(ester urea)s based on bis(phenylalanine)-α, ω-alkylene diesters. Macromol. Chem. Phys. 1997, 198, 1921–1932. [Google Scholar] [CrossRef]
- Katsarava, R.; Tugushi, D.; Gomurashvili, Z.D. Poly (ester urea) Polymers and Methods of Use. U.S. Patent 8,765,164, 1 July 2014. Available online: https://www.google.ch/patents/US8765164 (accessed on 1 July 2014). [Google Scholar]
- Katsarava, R. Active Polycondensation: From Peptide Chemistry to Amino Acid Based Biodegradable Polymers. Macromol. Symp. 2003, 199, 419–429. [Google Scholar] [CrossRef]
- Katsarava, R.; Gomurashvili, Z. Biodegradable Polymers Composed of Naturally Occurring α-Amino Acids. Available online: http://onlinelibrary.wiley.com/doi/10.1002/9783527635818.ch5/summary (accessed on 16 December 2016).
- Defife, K.; Grako, K.; Cruz-Aranda, G.; Price, S.; Chantung, R.; Macpherson, K.; Khoshabeh, R.; Gopalan, S.; Turnell, W.G. Poly(ester amide) Co-polymers Promote Blood and Tissue Compatibility. J. Biomater. Sci. 2009, 20, 1495–1511. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Meng, F.M.; Feijen, J.; Dias, A.A.; Hendriks, M.; Zhong, Z. α-Amino Acid Containing Degradable Polymers as Functional Biomaterials: Rational Design, Synthetic Pathway, and Biomedical Applications. Biomacromolecules. 2011, 12, 1937–1955. [Google Scholar] [CrossRef] [PubMed]
- Ghaffar, A.; Draaisma, G.J.J.; Mihov, G.; Dias, A.A.; Schoenmakers , P.J.; Van der Wal, S. Monitoring the in Vitro Enzyme-Mediated Degradation of Degradable Poly(ester amide) for Controlled Drug Delivery by LC-ToF-MS. Biomacromolecules. 2011, 12, 3243–3251. [Google Scholar] [CrossRef] [PubMed]
- Trollsas, M.; Maslanka, B.; Pham, N.; Lin, Q.; Hossainy, S.; Hsu, S.L.; Ngo, M.H. Polyesteramide Coatings for Drug Eluting Stents: Controlling Drug Release by Polymer Engineering. Stud. Mechanobiol. Tissue Eng. Biomater. 2011, 8, 127–143. [Google Scholar]
- Díaz, A.; Katsarava, R.; Puiggalí, J. Synthesis, properties and applications of biodegradable polymers derived from diols and dicarboxylic acids: From polyesters to poly(ester amide)s. Int. J. Mol. Sci. 2014, 15, 7064–7123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsarava, R.; Puiggali, J. Leucine Based Polymers: Synthesis and Applications. In Leucine: Biology, Consumption and Benefits; Biochemistry Research Trends; Newman, S.R., Ed.; NOVA Science Publisher: New York, NY, USA, 2015; pp. 17–76. [Google Scholar]
- Katsarava, R.; Kulikova, N.; Puiggalí, J. Amino Acid Based Biodegradable Polymers—Promising materials for the applications in regenerative medicine. J. Regener. Med. 2016, 1, 012. [Google Scholar]
- Markosishvili, K.; Tsitlanadze, G.; Katsarava, R.; Morris, J.G., Jr.; Sulakvelidze, A. Novel Sustained-Release Matrix Based on Biodegradable Poly(ester amide)s and Impregnated with Bacteriophages and an Antibiotic Shows Promise in Management of Infected Venous Stasis Ulcers and Other Poorly Healing Wounds. Int. J. Dermatol. 2002, 41, 453–458. [Google Scholar] [CrossRef]
- Jikia, D.; Chkhaidze, N.; Imedashvili, E.; Mgaloblishvili, I.; Tsitlanadze, G.; Katsarava, R.; Glenn Morris, J., Jr.; Sulakvelidze, A. The Use of a Novel Biodegradable Preparation Capable of the Sustained Release of Bacteriophages and Ciprofloxacin, in the Complex Treatment of Multidrug-Resistant Staphylococcus aureus-Infected Local Radiation Injuries Caused by Exposure to Sr90. Clin. Exp. Dermatol. 2005, 30, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.C.; Katsarava, R. Elastomeric Functional Biodegradable Copolyester Amides and Copolyester Urethanes. Available online: http://www.google.tl/patents/US7408018 (accessed on 5 August 2008).
- Lee, S.H.; Szinai, I.; Carpenter, K.; Katsarava, R.; Jokhadze, G.; Chu, C.C.; Huang, Y.; Verbeken, E.; Bramwell, O.; De Scheerder, I.; et al. In Vivo biocompatibility evaluation of stents coated with a new biodegradable elastomeric and functional polymer. Coron. Artery Dis. 2002, 13, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Gomurashvili, Z.; Zhang, H.; Da, J.; Turnell, W.G.; Jenkins, T.D.; Hughes, J.; Wu, M.; Lambert, L.; Grako, K.A.; DeFife, K.M.; et al. From drug-eluting stents to biopharmaceuticals: Poly(ester amide) a versatile new bioabsorbable biopolymer. In ACS Symposium Series 977: Polymers for Biomedical Applications; Mahapatro, A., Kulshrestha, A.S., Eds.; Oxford University Press: Oxford, UK, 2008; pp. 10–26. [Google Scholar]
- Kropp, M.; Morawa, K.-M.; Mihov, G.; Salz, A.K.; Harmening, N.; Franken, A.; Kemp, A.; Dias, A.A.; Thies, J.; Johnen, S. Biocompatibility of Poly(ester amide) (PEA) Microfibrils in Ocular Tissues. Polymers 2014, 6, 243–260. [Google Scholar] [CrossRef] [Green Version]
- Andrés-Guerrero, V.; Zongc, M.; Ramsay, E.; Rojas, B.; Sarkhel, S.; Gallego, B.; de Hoz, R.; Ramírez, A.I.; Salazar, J.J.; Triviño, A. Novel biodegradable polyesteramide microspheres for controlled drug delivery in Ophthalmology. J. Control. Release 2015, 211, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Chu, C.C. Biodegradable and Injectable Paclitaxel-Loaded Poly(ester amide)s Microspheres: Fabrication and Characterization. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 89, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lin, F.; Lin, P.; Gao, Y.; Becker, M.L. Phenylalanine-Based Poly(ester urea): Synthesis, Characterization, and in vitro Degradation. Macromolecules 2014, 47, 121–129. [Google Scholar] [CrossRef]
- Policastro, G.; Lin, F.; Esterle, A.; Harris, F.; Graham, M.; Katsarava, R.; Stakleff, K.S.; Becker, M.L. OGP Functionalized Phenylalanine-based Poly(ester urea) for Enhancing Osteoinductive Potential of human Mesenchymal Stem Cells. In Proceedings of the 249th ACS National Meeting & Exposition, Denver, CO, USA, 22–26 March 2015.
- Memanishvili, T.; Zavradashvili, N.; Kupatadze, N.; Tugushi, D.; Gverdtsiteli, M.; Torchilin, V.P.; Wandrey, C.; Baldi, L.; Manoli, S.S.; Katsarava, R. Arginine-based biodegradable ether-ester polymers of low cytotoxicity as potential gene carriers. Biomacromolecules. 2014, 15, 2839–2848. [Google Scholar] [CrossRef] [PubMed]
- Fessi, H.; Puisieux, F.; Devissaguet, J.-P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a request from the Commission related to the use of polyvinyl alcohol as a coating agent for food supplements Question number EFSA-Q-2005–017. EFSA J. 2005, 294, 1–15. [Google Scholar]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, J.C. Biological actions and medical applications of dimethyl sulfoxide. Ann. N. Y. Acad. Sci. 1983, 411, 1–402. [Google Scholar]
- Le Bourlais, C.; Acar, L.; Zia, H.; Sado, P.A.; Needham, T.; Leverge, R. Ophthalmic Drug Delivery Systems—Recent Advances. Prog. Retin. Eye Res. 1998, 17, 33–58. [Google Scholar] [CrossRef]
- Mudgil, M.; Gupta, N.; Nagpal, M.; Pawar, P. Nanotechnology: A New Approach for Ocular Drug Delivery System. Int. J. Pharm. Pharm. Sci. 2012, 4, 105–112. [Google Scholar]
- Diener, C.; Muñoz-Gonzalez, F.; Encarnación, S.; Resendis-Antonio, O. The space of enzyme regulation in HeLa cells can be inferred from its intracellular metabolome. Sci Rep. 2016, 6, 28415. [Google Scholar] [CrossRef] [PubMed]
- Drozd, E.; Krzysztoń-Russjan, J.; Marczewska, J.; Drozd, J.; Bubko, I.; Bielak, M.; Lubelska, K.; Wiktorska, K.; Chilmonczyk, Z.; Anuszewska, E.; et al. Up-regulation of glutathione-related genes, enzyme activities and transport proteins in human cervical cancer cells treated with doxorubicin. Biomed. Pharmacother. 2016, 83, 397–406. [Google Scholar] [PubMed]

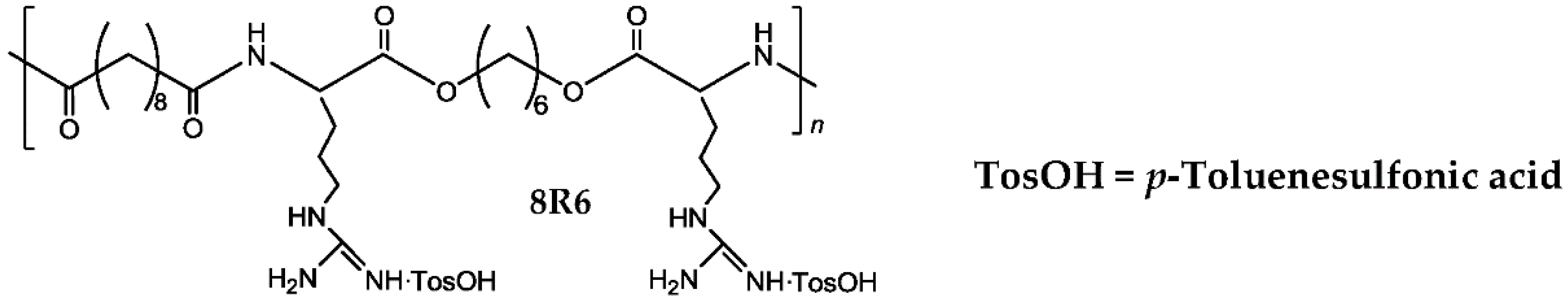
| Polymer | Class of the Polymers | Mw | Mn | Mw/Mn |
|---|---|---|---|---|
| 1F6 | PEU | 35,400 | 20,500 | 1.73 |
| 1L6 | PEU | 90,400 | 54,100 | 1.67 |
| 4F4 | PEA | 66,400 | 47,400 | 1.43 |
| 8L6 | PEA | 46,500 | 23,400 | 1.98 |
| 8R6 | PEA | 17,500 | 7,200 | 2.43 |
| Polymer | Solubility of 10 mg of Polymer in 1 mL of the Solvent * | |||
|---|---|---|---|---|
| DMI | DMSO | DMF | Acetone | |
| 1F6 | +t | +t | +t | - |
| 1L6 | + | + | + | + |
| 4F4 | + | + | + | - |
| 8L6 | + | + | + | + |
| Cpol (mg/mL) | Polymer | |
|---|---|---|
| 1F6 | 4F4 | |
| 0.6 | Full Aggregation | Full Aggregation |
| 3.0 | Full Aggregation | Full Aggregation |
| 6.0 | Full Aggregation | Full Aggregation |
| Polymer | Surfactant | ||||
|---|---|---|---|---|---|
| Tween 20 | Triton X-100 | Brij 010 | Mowiol 4-88 | Mowiol 8-88 | |
| MPD (nm) ± SD [PDI] ± SD | |||||
| 1F6 | 143 ± 6 [0.123 ± 0.019] | 148 ± 6 [0.116 ± 0.004] | 160 ± 10 [0.268 ± 0.009] | 321 ± 4 [0.053 ± 0.015] | 328 ± 2 [0.075 ± 0.019] |
| 1L6 | 132 ± 7 [0.152 ± 0.032] | 140 ± 8 [0.172 ± 0.091] | 136 ± 1 [0.145 ± 0.098] | 218 ± 6 [0.102 ± 0.056] | 260 ± 10 [0.192 ± 0.086] |
| 4F4 | 158 ± 7 [0.111 ± 0.006] | 139 ± 7 [0.121 ± 0.009] | 163 ± 10 [0.299 ± 0.013] | 173 ± 9 ♦ [0.117 ± 0.009] | 240 ± 10 ♦ [0.224 ± 0.008] |
| 8L6 | 159 ± 3 [0.131 ± 0.003] | 154 ± 9 [0.090 ± 0.003] | 166 ± 5 [0.088 ± 0.001] | 144 ± 3 [0.076 ± 0.006] | 181 ± 6 [0.095 ± 0.003] |
| Organic Solvent | Surfactant | ||||
|---|---|---|---|---|---|
| Tween 20 | Tween 80 | Kolliphor P188 | Brij 010 | Mowiol 4-88 | |
| MPD (nm) ± SD [PDI] ± SD | |||||
| DMI | 143 ± 6 [0.123 ± 0.019] | 156 ± 4 [0.218 ± 0.063] | 191 ± 6 [0.112 ± 0.018] | 160 ± 10 [0.268 ± 0.009] | 321 ± 4 [0.053 ± 0.015] |
| DMF | 338 ± 9 [0.136 ± 0.012] | 311 ± 12 [0.147 ± 0.019] | 302 ± 11 [0.214 ± 0.019] | 255 ± 12 [0.213 ± 0.011] | 398 ± 10 [0.135 ± 0.013] |
| DMSO | 122 ± 6 [0.098 ± 0.005] | 115 ± 3 [0.112 ± 0.006] | 134 ± 7 [0.215 ± 0.012] | 124 ± 7 [0.219 ± 0.030] | 249 ± 11 [0.247 ± 0.017] |
| Organic Solvent | Surfactant | ||||
|---|---|---|---|---|---|
| Tween 20 | Tween 80 | Kolliphor P188 | Brij 010 | Mowiol 4-88 | |
| MPD (nm) ± SD [PDI] ± SD | |||||
| DMI | 132 ± 7 [0.152 ± 0.032] | 168 ± 5 [0.104 ± 0.010] | 155 ± 3 [0.219 ± 0.019] | 136 ± 1 [0.145 ± 0.098] | 218 ± 6 [0.102 ± 0.056] |
| DMF | 215 ± 9 [0.139 ± 0.010] | 193 ± 7 [0.103 ± 0.030] | 194 ± 10 [0.213 ± 0.010] | 213 ± 10 [0.219 ± 0.012] | 288 ± 10 [0.135 ± 0.017] |
| DMSO | 123 ± 9 [0.223 ± 0.011] | 144 ± 8 [0.212 ± 0.013] | 101 ± 4 [0.229 ± 0.011] | 112 ± 4 [0.203 ± 0.010] | 164 ± 9 [0.219 ± 0.010] |
| Acetone | 252 ± 12 [0.216 ± 0.017] | 280 ± 14 [0.239 ± 0.019] | 278 ± 11 [0.293 ± 0.021] | 293 ± 11 [0.311 ± 0.016] | 323 ± 13 [0.245 ± 0.031] |
| Organic Solvent | Surfactant | ||||
|---|---|---|---|---|---|
| Tween 20 | Tween 80 | Kolliphor P188 | Brij 010 | Mowiol 4-88 | |
| MPD (nm) ± SD [PDI] ± SD | |||||
| DMI | 158 ± 7 [0.111 ± 0.006] | 157 ± 7 [0.091 ± 0.003] | 111 ± 1 [0.054 ± 0.012] | 163 ± 10 [0.299 ± 0.013] | 173 ± 9 ♦ [0.117 ± 0.009] |
| DMF | 138 ± 3 [0.116 ± 0.011] | 124 ± 2 [0.126 ± 0.004] | 135 ± 3 ♦ [0.132 ± 0.004] | 123 ± 2 [0.119 ± 0.032] | 204 ± 2 ♦ [0.103 ± 0.008] |
| DMSO | 93 ± 4 [0.302 ± 0.023] | 60 ± 1 [0.240 ± 0.001] | 100 ± 3 ♦ [0.260 ± 0.001] | 56 ± 2 [0.132 ± 0.078] | 98 ± 1 ♦ [0.110 ± 0.010] |
| Organic Solvent | Surfactant | ||||
|---|---|---|---|---|---|
| Tween 20 | Tween 80 | Kolliphor P188 | Brij 010 | Mowiol 4-88 | |
| MPD (nm) ± SD [PDI] ± SD | |||||
| DMI | 159 ± 3 [0.131 ± 0.003] | 162 ± 15 [0.101 ± 0.002] | 98 ± 2 [0.116 ± 0.009] | 166 ± 5 [0.088 ± 0.001] | 144 ± 3 [0.076 ± 0.006] |
| DMF | 187 ± 4 [0.180 ± 0.006] | 176 ± 10 [0.231 ± 0.013] | 186 ± 8 (P-I) 56% ♦ 575 ± 24 (P-II) 44% [0.456 ± 0.018] | 170 ± 10 [0.193 ± 0.011] | 169 ± 8 [0.131 ± 0.012] |
| DMSO | 70 ± 5 [0.115 ± 0.008] | 135 ± 8 [0.171 ± 0.011] | 85 ± 3 [0.150 ± 0.006] | 73 ± 6 [0.273 ± 0.016] | 96 ± 9 [0.214 ± 0.018] |
| Acetone | 192 ± 5 [0.105 ± 0.003] | 235 ± 10 [0.112 ± 0.008] | 172 ± 3 [0.113 ± 0.006] | 203 ± 9 [0.254 ± 0.016] | 283 ± 11 [0.183 ± 0.012] |
| O:W | Surfactant | |||
|---|---|---|---|---|
| Tween 20 | Brij 010 | Tween 20 | Brij 010 | |
| Polymer | ||||
| 1F6 | 4F4 | |||
| MPD(nm) ± SD [PDI] ± SD | ||||
| 1:2 | 449 ± 23 (P-I) 68% ♦ 2569 ± 286 (P-II) 32% [0.468 ± 0.053] | 322 ± 9 [0.258 ± 0.007] | 483 ± 19 (P-I) 73% ♦ 3534 ± 218 (P-II) 27% [0.468 ± 0.059] | 213 ± 8 [0.246 ± 0.009] |
| 1:5 | 177 ± 5 [0.118 ± 0.014] | 237 ± 6 [0.225 ± 0.009] | 166 ± 6 [0.128 ± 0.012] | 153 ± 5 [0.218 ± 0.007] |
| 1:10 | 143 ± 6 [0.123 ± 0.019] | 160 ± 10 [0.210 ± 0.005] | 158 ± 7 [0.111 ± 0.006] | 163 ± 10 [0.299 ± 0.013] |
| 1:20 | 138 ± 6 [0.102 ± 0.020] | 159 ± 9 [0.259 ± 0.012] | 165 ± 5 [0.158 ± 0.008] | 169 ± 8 [0.241 ± 0.015] |
| Method | Surfactant | ||||
|---|---|---|---|---|---|
| Tween 20 | Triton X-100 | Brij 010 | Mowiol 4-88 | Mowiol 8-88 | |
| MPD (nm) ± SD [PDI] ± SD | |||||
| NM | 143 ± 6 [0.123 ± 0.019] | 148 ± 6 [0.116 ± 0.004] | 160 ± 10 [0.268 ± 0.009] | 321 ± 4 [0.053 ± 0.015] | 328 ± 2 [0.075 ± 0.019] |
| MNM | 121 ± 4 [0.230 ± 0.024] | 113 ± 5 [0.180 ± 0.011] | 108 ± 3 [0.213 ± 0.006] | 222 ± 7 [0.092 ± 0.020] | 199 ± 4 [0.087 ± 0.014] |
| Method | Surfactant | ||||
|---|---|---|---|---|---|
| Tween 20 | Triton X-100 | Brij 010 | Mowiol 4-88 | Mowiol 8-88 | |
| MPD (nm) ± SD [PDI] ± SD | |||||
| NM | 132 ± 7 [0.152 ± 0.032] | 140 ± 8 [0.172 ± 0.091] | 136 ± 1 [0.145 ± 0.098] | 218 ± 6 [0.102 ± 0.056] | 260 ± 10 [0.192 ± 0.086] |
| MNM | 112 ± 5 [0.102 ± 0.016] | 118 ± 2 (P-I) 70% 4093 ± 684 (P-II) 20% [0.602 ± 0.016] | 111 ± 6 (P-I) 52% 2726 ± 265 (P-II) 48% [0.807 ± 0.023] | 143 ± 2 (P-I) 70% 3250 ± 182 (P-II) 30% [0.563 ± 0.005] | 212 ± 5 [0.293 ± 0.033] |
| Method | Surfactant | ||||
|---|---|---|---|---|---|
| Tween 20 | Triton X-100 | Brij 010 | Mowiol 4-88 | Mowiol 8-88 | |
| MPD (nm) ± SD [PDI] ± SD | |||||
| NM | 158 ± 7 [0.111 ± 0.006] | 139 ± 7 [0.121 ± 0.009] | 163 ± 10 [0.299 ± 0.013] | 173 ± 9 ♦ [0.117 ± 0.009] | 240 ± 10 ♦ [0.224 ± 0.008] |
| MNM | 85 ± 2 [0.125 ± 0.002] | 85 ± 6 [0.231 ± 0.014] | 79 ± 4 [0.157 ± 0.006] | 129 ± 8 ♦ [0.212 ± 0.006] | 211 ± 12 ♦ [0.214 ± 0.016] |
| Method | Surfactant | ||||
|---|---|---|---|---|---|
| Tween 20 | Triton X-100 | Brij 010 | Mowiol 4-88 | Mowiol 8-88 | |
| MPD (nm) ± SD [PDI] ± SD | |||||
| NM | 159 ± 3 [0.131 ± 0.003] | 154 ± 9 [0.090 ± 0.003] | 166 ± 5 [0.088 ± 0.001] | 144 ± 3 [0.076 ± 0.006] | 181 ± 6 [0.095 ± 0.003] |
| MNM | 111 ± 9 [0.222 ± 0.005] | 132 ± 4 [0.089 ± 0.006] | 93 ± 6 (P-I) 51% 2329 ± 225 (P-II) 49% [0.331 ± 0.082] | 143 ± 3 (P-I) 65% 3364 ± 272 (P-II) 35% [0.413 ± 0.020] | 311 ± 10 (P-I) 67% 3189 ± 195 (P-II) 33% [0.412 ± 0.052] |
| Polymer | Surfactant | ||
|---|---|---|---|
| Tween 20 | Tween 80 | Brij 010 | |
| Zeta-potential (mV) ± SD | |||
| 1F6 | −2.47 ± 2.25 | −1.58 ± 1.39 | −7.08 ± 2.55 |
| 1L6 | −2.97± 1.64 | −2.89 ± 2.78 | −5.15 ± 1.62 |
| 4F4 | −2.44 ± 1.16 | −2.49 ± 1.07 | −9.14 ± 1.51 |
| 8L6 | −12.50 ± 1.99 | −8.50 ± 2.68 | −7.70 ± 2.29 |
| Polymer | 8R6 Content (%, w/w) | Zeta-Potential (mV) ± SD |
|---|---|---|
| 8L6 | 0 | −1.73± 0.6 |
| 8L6/8R6 | 5 | +19.6 ± 3.6 |
| 10 | +23.2 ± 4.7 | |
| 20 | +25.8 ± 2.7 | |
| 30 | +27.2 ± 3.2 | |
| 50 | +28.0 ± 4.9 |
| Time | Surfactant | |||||
|---|---|---|---|---|---|---|
| Tween 20 | Tween 80 | Brij 010 | Tween 20 | Tween 80 | Brij 010 | |
| MPD (nm) ± SD | ||||||
| At r.t. (23–25 °C) | Refrigerated (4–5 °C) | |||||
| Freshly prepared | 143 ± 6 | 156 ± 4 | 160 ± 10 | 143 ± 6 | 156 ± 4 | 160 ± 10 |
| A week later | 1688 ± 628 (P-I) 55% 238 ± 75 (P -II) 45% | 1390 ± 190 (P-I) 75% 262 ± 67 (P-II) 25% | 1586 ± 42 (P-I) 88% 288 ± 84 (P-II) 12% | 138 ± 7 | 160 ± 6 | 168 ± 29 |
| A month later | 587 ± 293 (P-I) 70% 3105 ± 622 (P-II) 30% | 1482 ± 149 (P-I) 60% 5329 ± 275 (P-II) 40% | 2110 ± 592 (P-I) 89% 162 ± 7 (P-II) 11% | 153 ± 9 | 179 ± 5 | 162 ± 7 (P-I) 70% 1607 ± 32 (P-II) 30% |
| 3 months later | 2030 ± 704 (P-I) 83% 5288 ± 118 (P-II) 17% | 1353+253 (P-I) 64% 5876 ± 261 (P-II) 36% | 1965 ± 408 (P-I) 81% 270 ± 50 (P-II) 19% | 152 ± 7 (P-I) 74% 989 ± 156 (P-II) 26% | 191 ± 74 (P-I) 84% 2984 ± 198 (P-II) 16% | 208 ± 11 (P-I) 85% 2146 ± 385 (P-II) 15% |
| Time | Surfactant | |||||
|---|---|---|---|---|---|---|
| Tween 20 | Tween 80 | Brij 010 | Tween 20 | Tween 80 | Brij 010 | |
| MPD (nm) ± SD | ||||||
| At r.t. (23–25 °C) | Refrigerated (4–5 °C) | |||||
| Freshly prepared | 132 ± 7 | 168 ± 5 | 136 ± 1 | 132 ± 7 | 168 ± 5 | 136 ± 1 |
| A week later | 154 ± 5 | 166 ± 3 (P-I) 71% 4063 ± 295 (P-II) 29% | 160 ± 5 | 154 ± 1 | 169 ± 8 | 162 ± 6 |
| A month later | 156 ± 3 (P-I) 90% 4782 ± 192 (P-II) 10% | 163 ± 10 (P-I) 70% 3892 ± 428 (P-II) 30% | 155 ± 5 (P-I) 70% 4670 ± 433 (P-II) 30% | 157 ± 3 | 164 ± 6 | 167 ± 5 |
| 3 months later | 159 ± 10 (P-I) 70% 4765 ± 705 (P-II) 30% | 226 ± 35 (P-I) 88% 5000 ± 326 (P-II) 12% | 160 ± 8 (P-I) 72% 4580 ± 425 (P-II) 28% | 160 ± 5 (P-I) 75% 4959 ± 617 (P-II) 25% | 169 ± 2 (P-I) 80% 4781 ± 309 (P-II) 20% | 168 ± 1 |
| Time | Surfactant | |||||
|---|---|---|---|---|---|---|
| Tween 20 | Tween 80 | Brij 010 | Tween 20 | Tween 80 | Brij 010 | |
| MPD (nm) ± SD | ||||||
| At r.t. (23–25 °C) | Refrigerated (4–5 °C) | |||||
| Freshly prepared | 158 ± 7 | 157 ± 7 | 163 ± 10 | 158 ± 7 | 157 ± 7 | 163 ± 10 |
| A week later | 133 ± 3 (I p) 83% 1575 ± 150 (II p) 17% | 162 ± 8 | 171 ± 6 | 161 ± 4 | 155 ± 9 | 169 ± 7 |
| A month later | Full aggregation | Full aggregation | Full aggregation | 166 ± 6 | 161 ± 5 | 171 ± 8 |
| 3 months later | - | - | - | 169 ± 8 | 164 ± 8 | 173 ± 9 |
| Time | Surfactant | |||||
|---|---|---|---|---|---|---|
| Tween 20 | Tween 80 | Brij 010 | Tween 20 | Tween 80 | Brij 010 | |
| MPD (nm) ± SD | ||||||
| At r.t. (23–25 °C) | Refrigerated (4–5 °C) | |||||
| Freshly prepared | 159 ± 3 [0.131 ± 0.003] | 162 ± 15 [0.107 ± 0.015] | 166 ± 5 [0.088 ± 0.001] | 159 ± 3 [0.131 ± 0.003] | 162 ± 15 [107 ± 0.012] | 166 ± 5 [0.088 ± 0.001] |
| A week later | 179 ± 2 [0.123 ± 0.007] | 169 ± 10 [0.114 ± 0.005] | 186 ± 5 [0.102 ± 0.011] | 183 ± 8 [0.135 ± 0.005] | 167 ± 8 [0.112 ± 0.015] | 187 ± 5 [0.095 ± 0.004] |
| A month later | 186 ± 7 [0.129 ± 0.012] | 165 ± 11 [0.103 ± 0.009] | 180 ± 3 [0.094 ± 0.013] | 189 ± 2 [0.149 ± 0.008] | 188 ± 6 [0.121 ± 0.005] | 184 ± 9 [0.109 ± 0.011] |
| 3 months later | 195 ± 10 [0.145 ± 0.015] | 200 ± 15 [0.121 ± 0.004] | 181 ± 3 [0.113 ± 0.009] | 200 ± 8 [0.138 ± 0.005] | 200 ± 8 [0.115 ± 0.010] | 189 ± 2 [0.118 ± 0.015] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kantaria, T.; Kantaria, T.; Kobauri, S.; Ksovreli, M.; Kachlishvili, T.; Kulikova, N.; Tugushi, D.; Katsarava, R. Biodegradable Nanoparticles Made of Amino-Acid-Based Ester Polymers: Preparation, Characterization, and In Vitro Biocompatibility Study. Appl. Sci. 2016, 6, 444. https://doi.org/10.3390/app6120444
Kantaria T, Kantaria T, Kobauri S, Ksovreli M, Kachlishvili T, Kulikova N, Tugushi D, Katsarava R. Biodegradable Nanoparticles Made of Amino-Acid-Based Ester Polymers: Preparation, Characterization, and In Vitro Biocompatibility Study. Applied Sciences. 2016; 6(12):444. https://doi.org/10.3390/app6120444
Chicago/Turabian StyleKantaria, Temur, Tengiz Kantaria, Sophio Kobauri, Mariam Ksovreli, Tinatin Kachlishvili, Nina Kulikova, David Tugushi, and Ramaz Katsarava. 2016. "Biodegradable Nanoparticles Made of Amino-Acid-Based Ester Polymers: Preparation, Characterization, and In Vitro Biocompatibility Study" Applied Sciences 6, no. 12: 444. https://doi.org/10.3390/app6120444






