Ammonium Removal from Landfill Leachate by Means of Multiple Recycling of Struvite Residues Obtained through Acid Decomposition
Abstract
:1. Introduction
2. Description of Experiments
2.1. Materials
2.2. Struvite Production
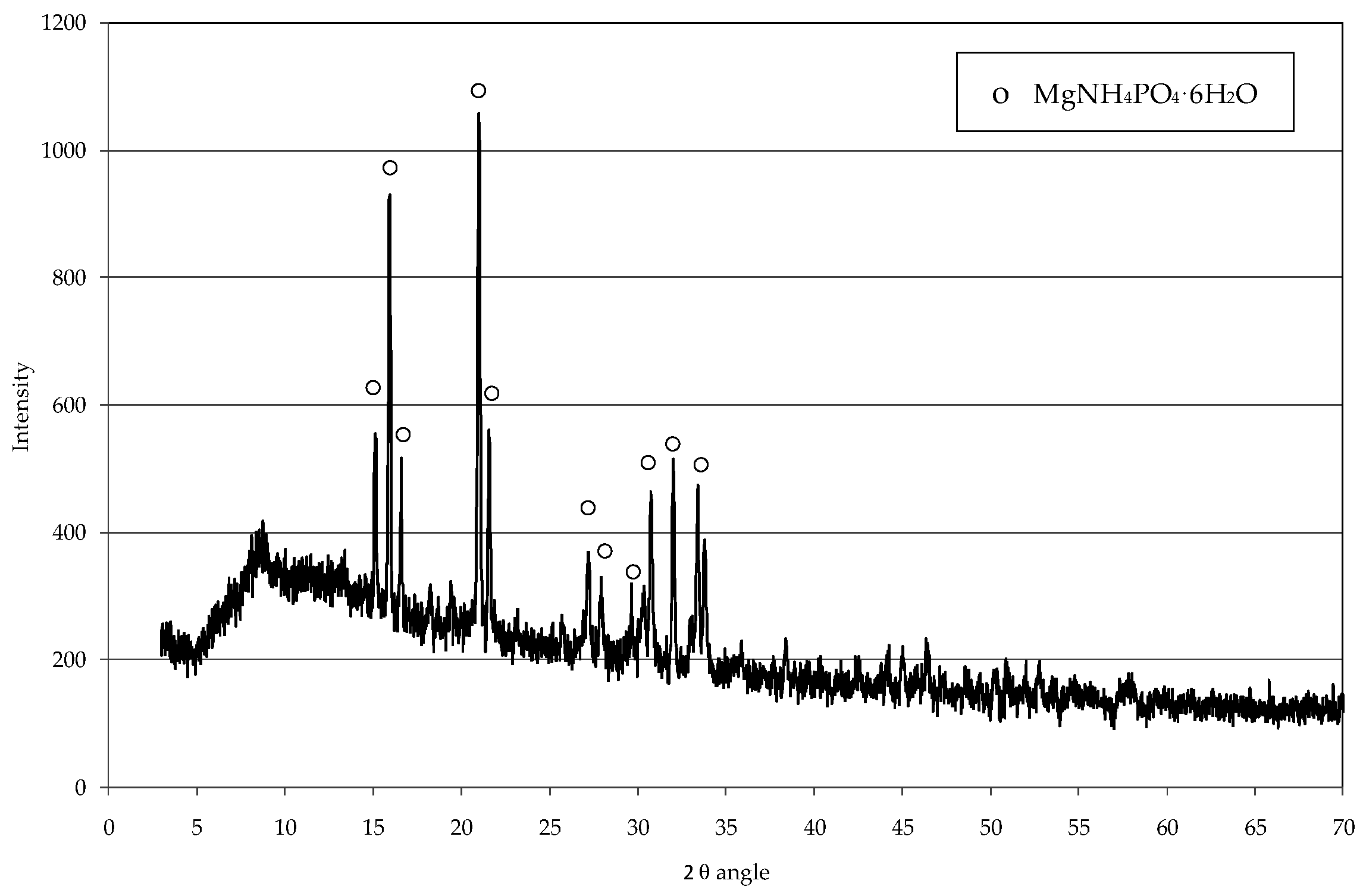
2.3. Struvite Decomposition
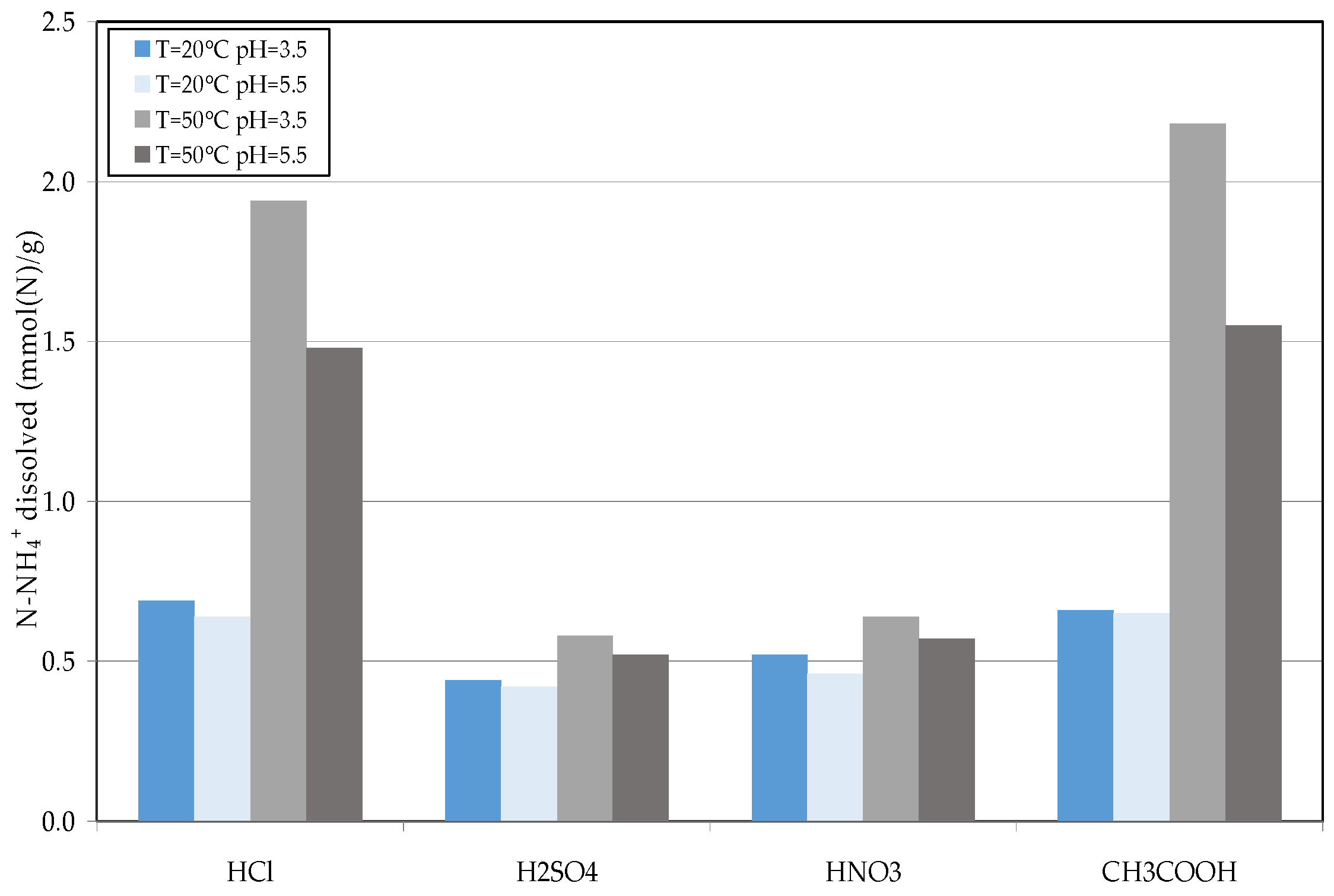
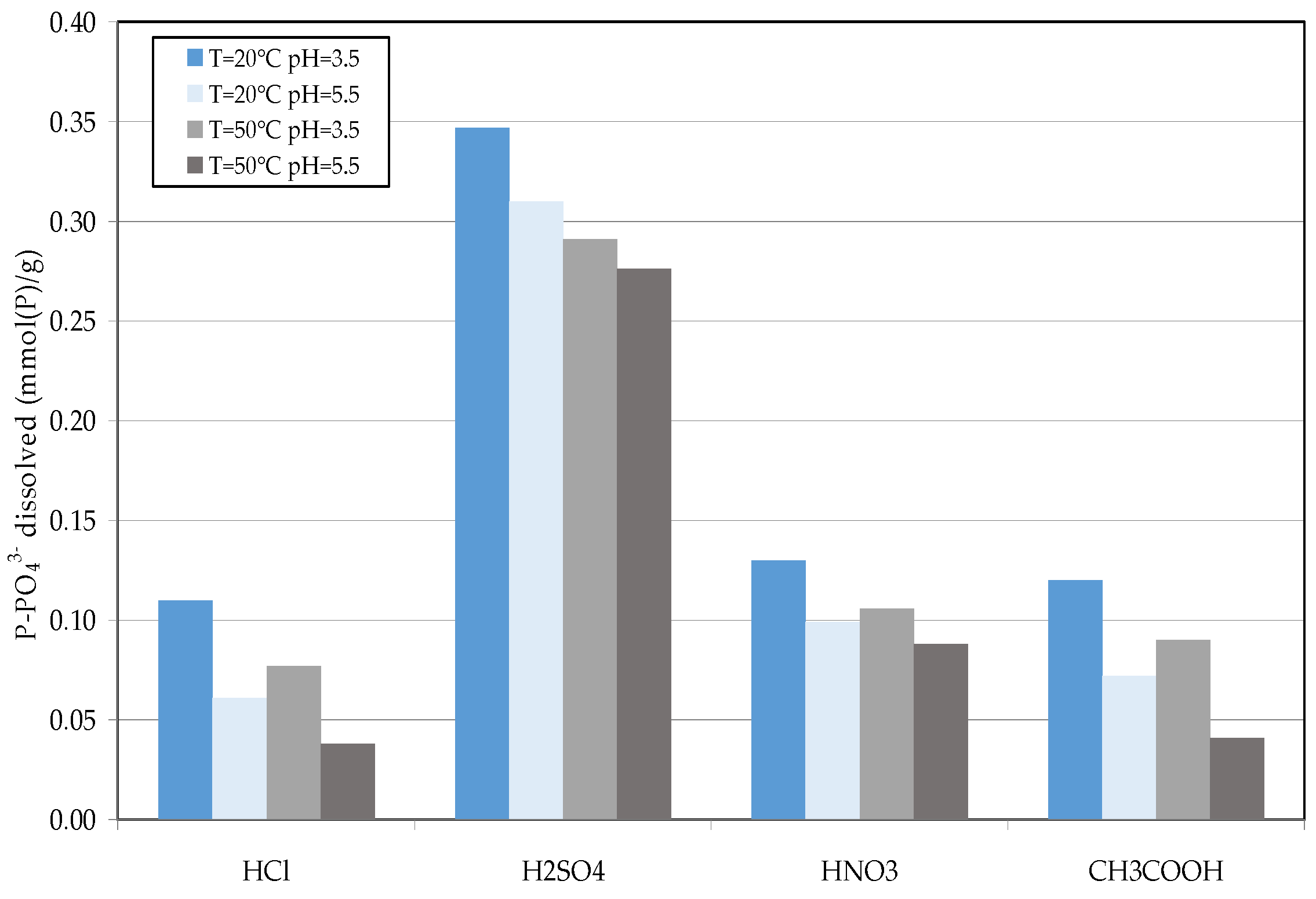
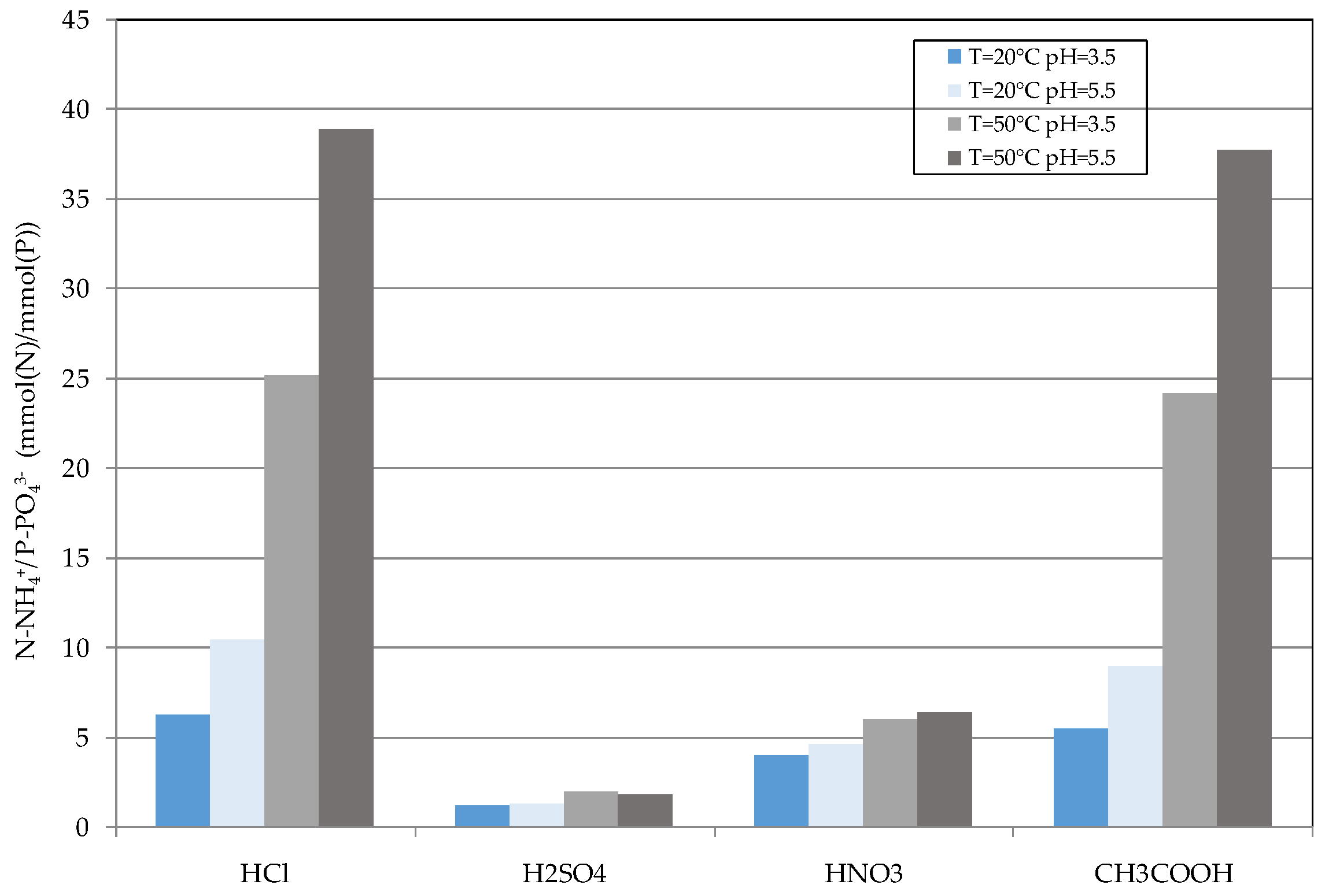
2.4. Reuse of Struvite Decomposition Residues
2.5. Analytical Methods
3. Results and Discussion
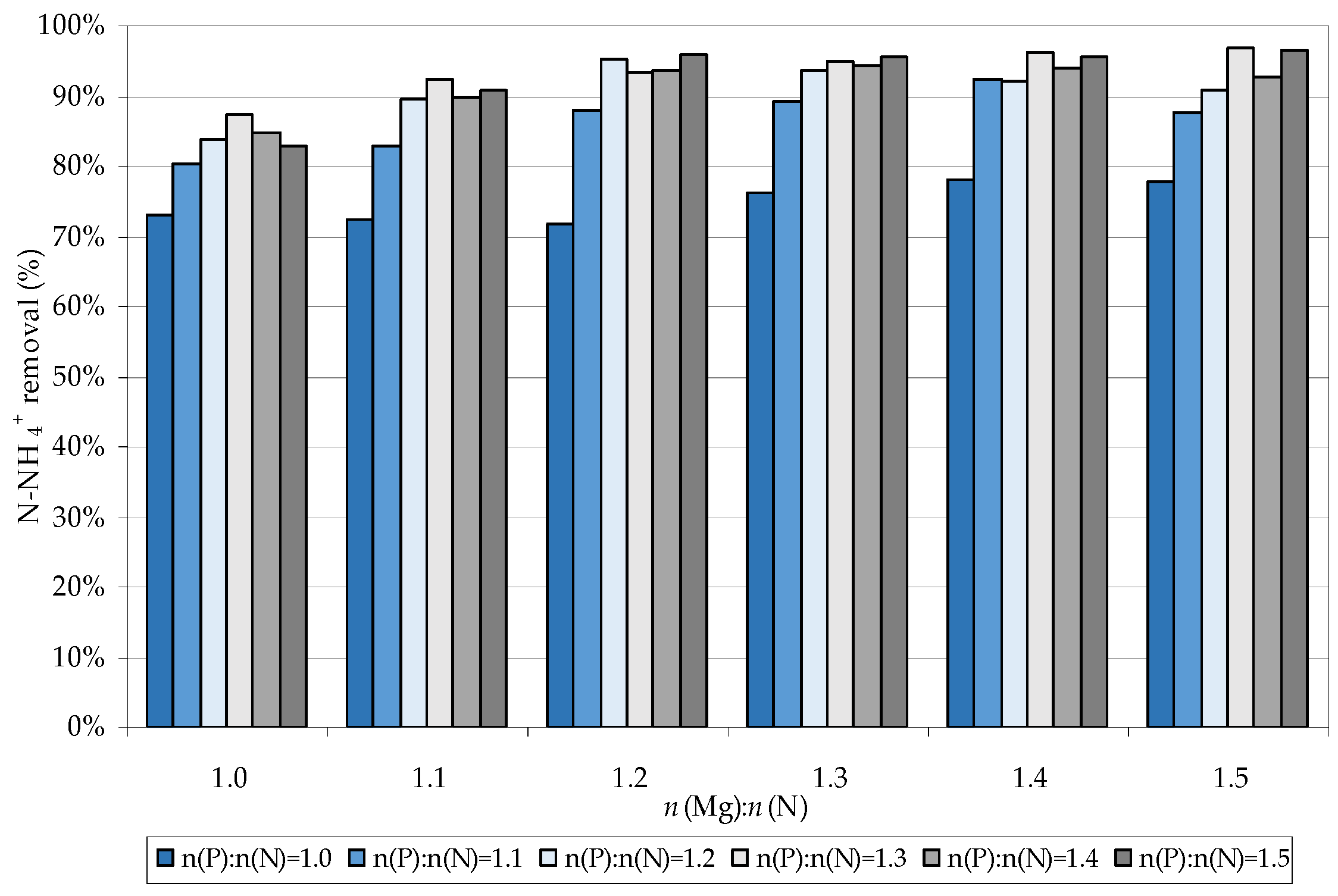
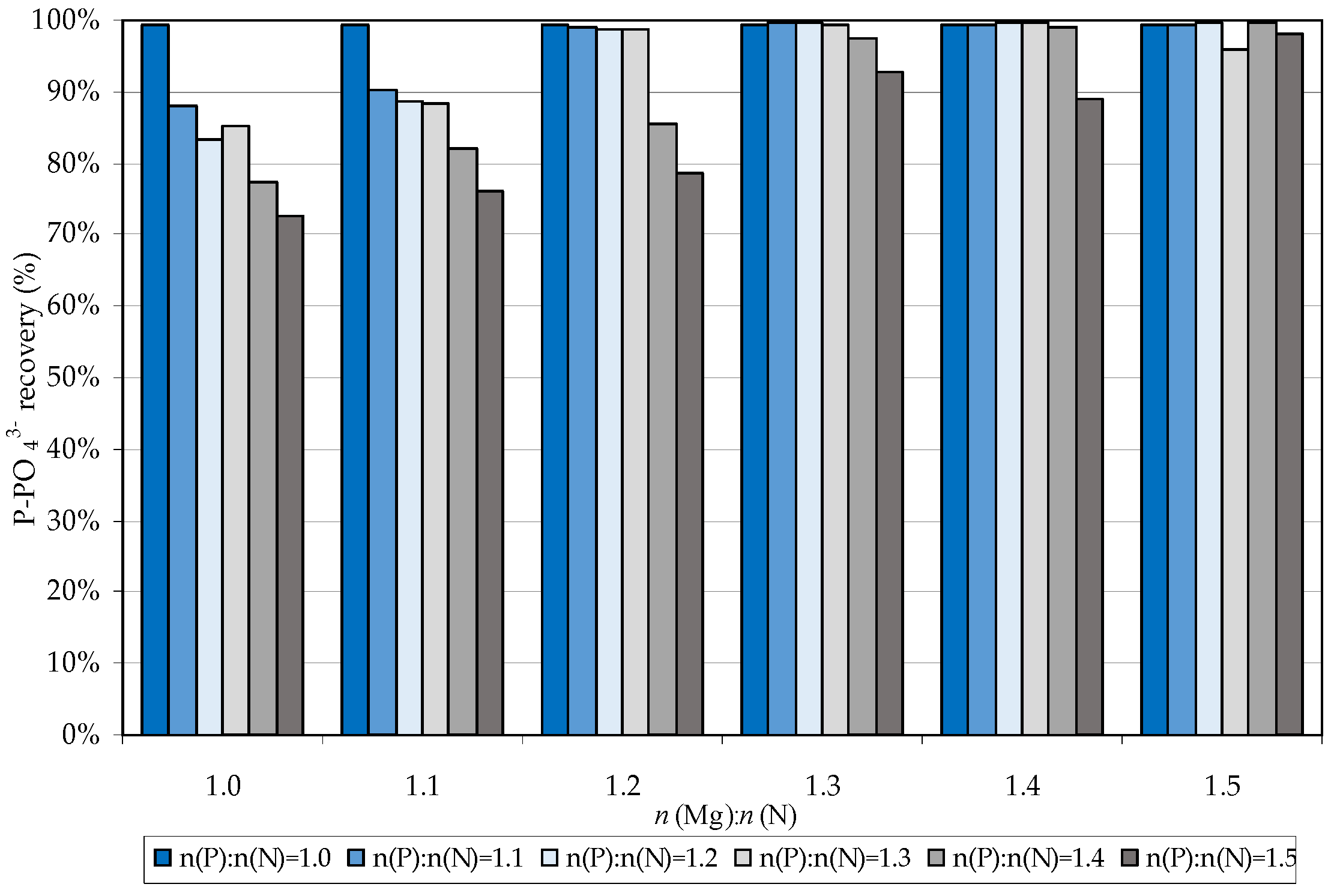
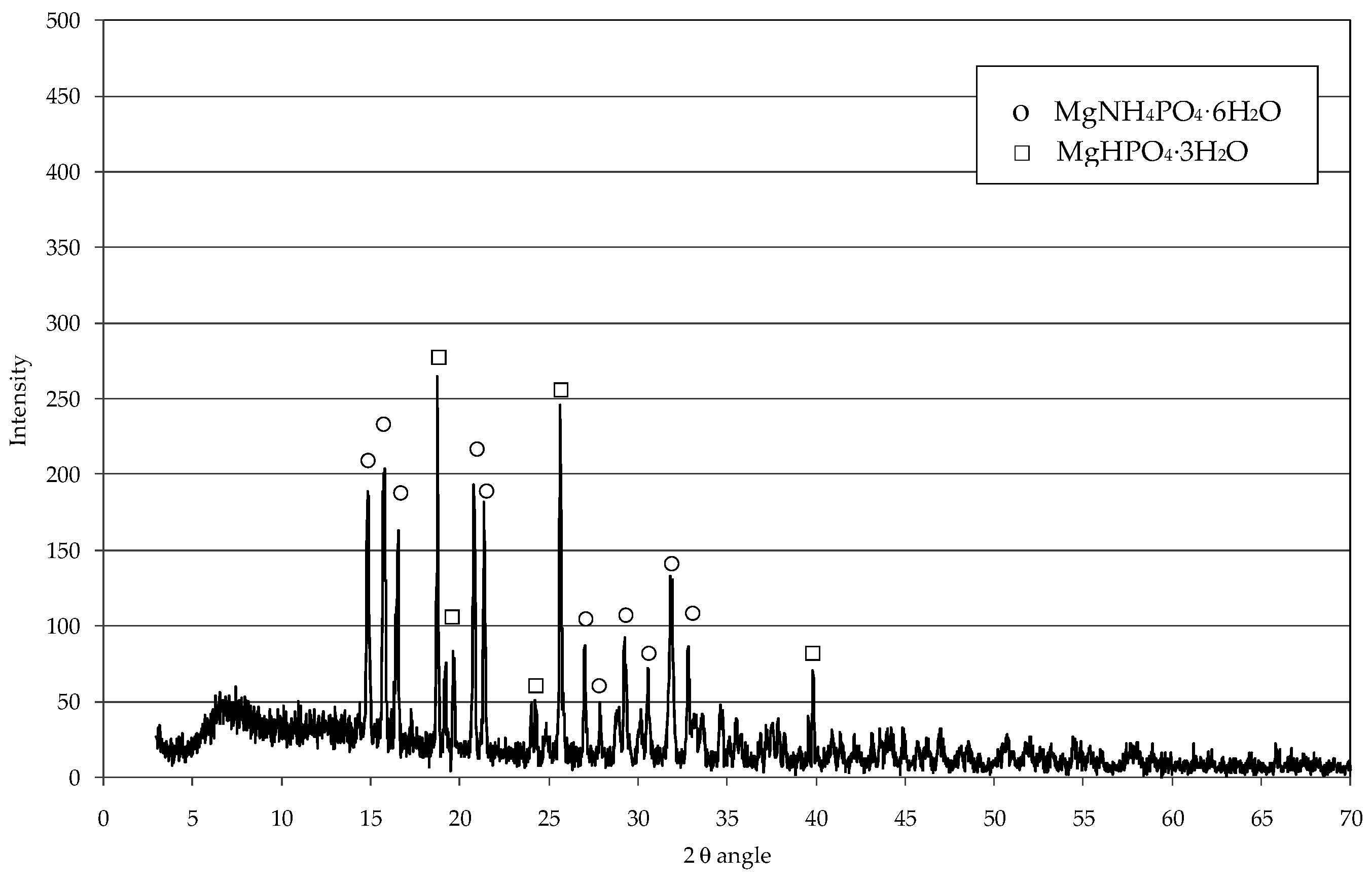
3.1. Struvite Production
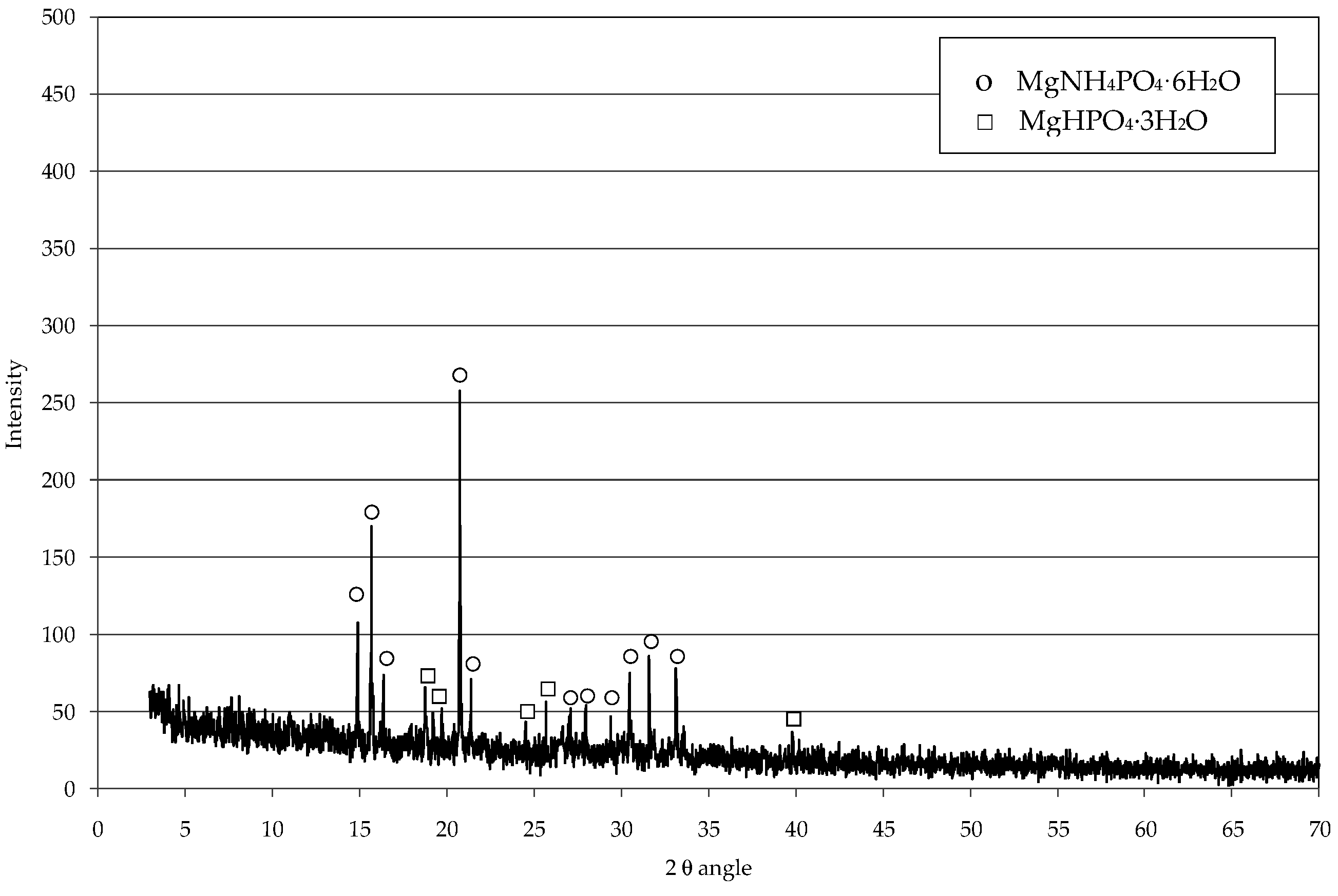
3.2. Struvite Decomposition
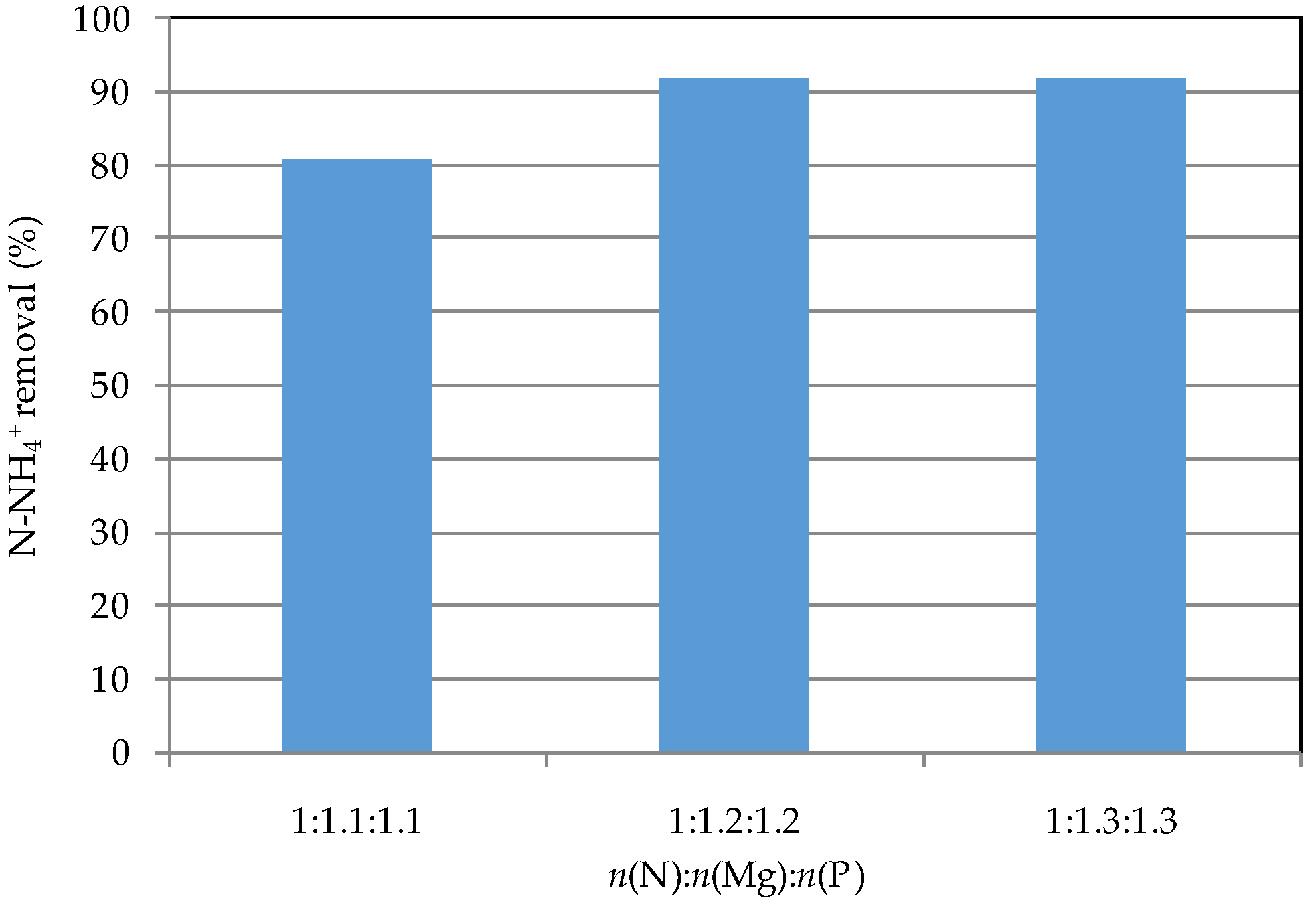
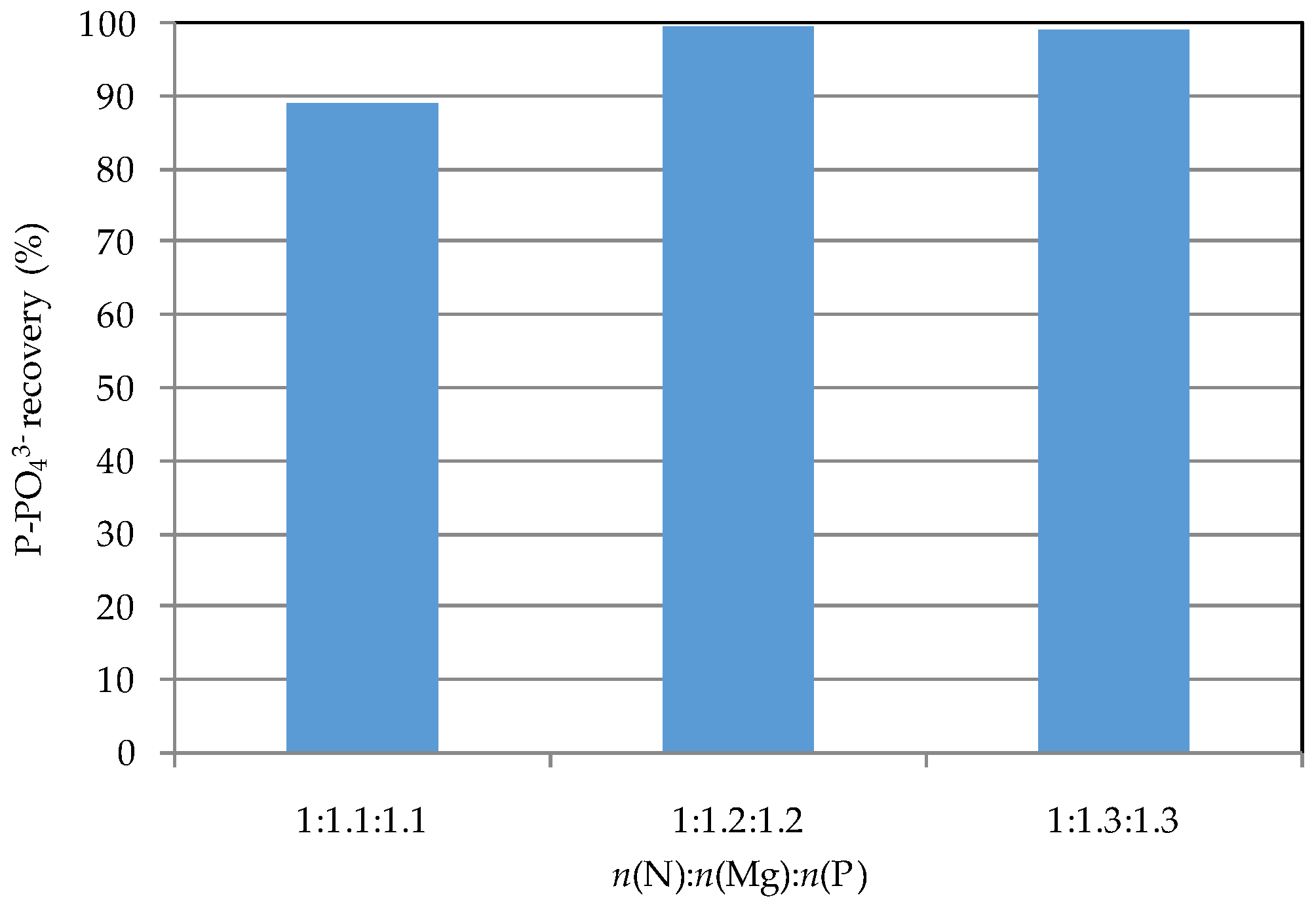
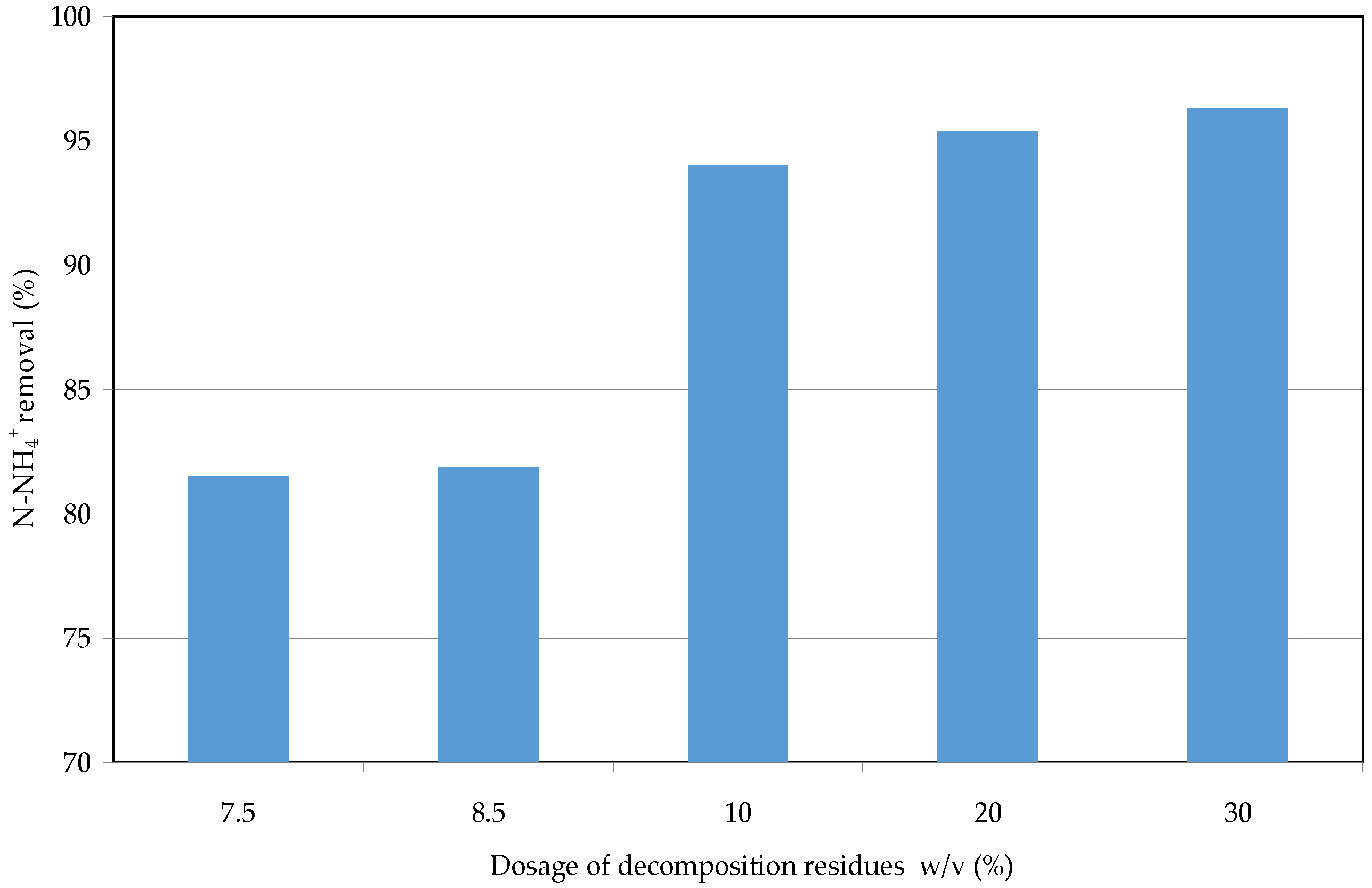
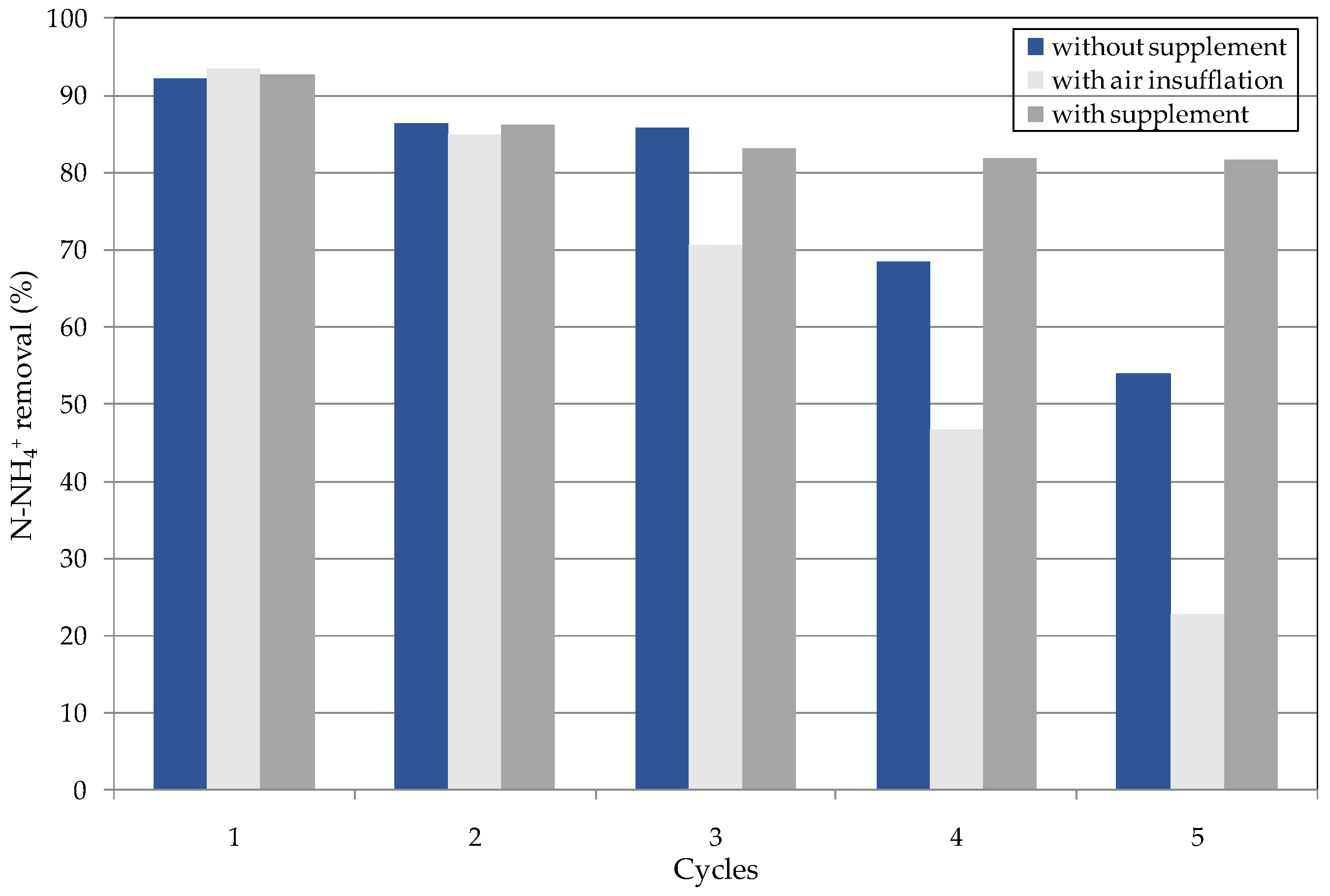
3.3. Reuse of Struvite Decomposition Residues
3.4. Economic Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Di Iaconi, C.; Pagano, M.; Ramadori, R.; Lopez, A. Nitrogen recovery from a stabilized municipal landfill leachate. Bioresour. Technol. 2010, 101, 1732–1736. [Google Scholar] [CrossRef] [PubMed]
- Kabdaşli, I.; Şafak, A.; Tünay, O. Bench-scale evaluation of treatment schemes incorporating struvite precipitation for young landfill leachate. Waste Manag. 2008, 28, 2386–2392. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Ryu, H.D.; Kim, M.S.; Kim, J.; Lee, S.I. Enhancing struvite precipitation potential for ammonia nitrogen removal in municipal landfill leachate. J. Hazard. Mater. 2007, 146, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xiao, D.; Zhang, Q.; Ding, L. Removal of ammonia from landfill leachate by struvite precipitation with the use of low-cost phosphate and magnesium sources. J. Environ. Manag. 2014, 145, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Zhao, Q.L. Recovery of ammonium-nitrogen from landfill leachate as a multi-nutrient fertilizer. Ecol. Eng. 2003, 20, 171–181. [Google Scholar] [CrossRef]
- Siciliano, A.; de Rosa, S. Experimental formulation of a kinetic model describing the nitrification process in biological aerated filters filled with plastic elements. Environ. Technol. 2015, 36, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, A.; de Rosa, S. An experimental model of COD abatement in MBBR based on biofilm growth dynamic and on substrates’ removal kinetics. Environ. Technol. 2016, 37, 2058–2071. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, T.A.; Lo, W.-H.; Chan, G.Y.S. Physico-chemical treatments for removal of recalcitrant contaminants from landfill leachate. J. Hazard. Mater. 2006, B129, 80–100. [Google Scholar] [CrossRef] [PubMed]
- Kochany, J.; Lipczynska-Kochany, E. Utilization of landfill leachate parameters for pretreatment by Fenton reaction and struvite precipitation—A comparative study. J. Hazard. Mater. 2009, 166, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; Perathoner, S.; Centi, G.; de Rosa, S.; Granato, T.; Katovic, A.; Siciliano, A.; Tagarelli, A.; Tripicchio, F. Wet hydrogen peroxide catalytic oxidation of olive oil mill wastewaters using Cu-zeolite and Cu-pillared clay catalysts. Catal. Today 2007, 124, 240–246. [Google Scholar] [CrossRef]
- De Rosa, S.; Siciliano, A. A catalytic oxidation process of olive oil mill wastewaters using hydrogen peroxide and copper. Desalt. Water Treat. 2010, 23, 187–193. [Google Scholar] [CrossRef]
- Siciliano, A.; Stillitano, M.A.; de Rosa, S. Increase of the anaerobic biodegradability of olive mill wastewaters through a pre-treatment with hydrogen peroxide in alkaline conditions. Desalt. Water. Treat. 2014, 55, 1735–1746. [Google Scholar] [CrossRef]
- Siciliano, A.; Stillitano, M.A.; de Rosa, S. Biogas production from wet olive mill wastes pretreated with hydrogen peroxide in alkaline conditions. Renew. Energy 2016, 85, 903–916. [Google Scholar] [CrossRef]
- Zhang, S.; Yao, C.; Feng, X.; Yang, M. Repeated use of MgNH4PO4·6H2O residues for ammonium removal by acid dipping. Desalination 2004, 170, 27–32. [Google Scholar] [CrossRef]
- Siciliano, A. Use of Nanoscale Zero-Valent Iron (NZVI) Particles for Chemical Denitrification under Different Operating Conditions. Metals 2015, 5, 1507–1519. [Google Scholar] [CrossRef]
- Karabegovic, L.; Uldal, M.; Werker, A.; Morgan-Sagastume, F. Phosphorus recovery potential from a waste stream with high organic and nutrient contents via struvite precipitation. Environ. Technol. 2013, 34, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Saidou, H.; Ben Moussa, S.; Ben Amor, M. Influence of airflow rate and substrate nature on heterogeneous struvite precipitation. Environ. Technol. 2009, 30, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Çelen, I.; Türker, M. Recovery of Ammonia as Struvite from Anaerobic Digester Effluents. Environ. Technol. 2001, 22, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- El Diwani, G.; El Rafie, S.H.; El Ibiari, N.N.; El-Aila, H.I. Recovery of ammonia nitrogen from industrial wastewater treatment as struvite slow releasing fertilizer. Desalination 2007, 214, 200–214. [Google Scholar] [CrossRef]
- Iqbal, M.; Bhuiyan, H.; Mavinic, D.S. Assessing struvite precipitation in a pilot-scale fluidized bed crystallizer. Environ. Technol. 2008, 29, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Korchef, A.; Saidou, H.; Ben Amor, M. Phosphate recovery through struvite precipitation by CO2 removal: Effect of magnesium, phosphate and ammonium concentrations. J. Hazard. Mater. 2011, 186, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Impact of calcium on struvite crystal size, shape and purity. J. Cryst. Growth 2005, 283, 514–522. [Google Scholar] [CrossRef] [Green Version]
- Pastor, L.; Mangin, D.; Ferrer, J.; Seco, A. Struvite formation from the supernatants of an anaerobic digestion pilot plant. Bioresour. Technol. 2010, 101, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.D.; Lim, C.S.; Kang, M.K.; Lee, S.I. Evaluation of struvite obtained from semiconductor wastewater as a fertilizer in cultivating Chinese cabbage. J. Hazard. Mater. 2012, 221–222, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Uysal, A.; Yilmazel, Y.D.; Demirer, G.N. The determination of fertilizer quality of the formed struvite from effluent of a sewage sludge anaerobic digester. J. Hazard. Mater. 2010, 181, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Uludag-Demirer, S.; Demirer, G.N.; Chen, S. Ammonia removal from anaerobically digested dairy manure by struvite precipitation. Process Biochem. 2005, 40, 3667–3674. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, S. Improving the prediction of ammonium nitrogen removal through struvite precipitation. Environ. Sci. Pollut. Res. 2012, 19, 347–360. [Google Scholar]
- Fattah, K.P.; Sabrina, N.; Mavinic, D.S.; Koch, F.A. Reducing operating costs for struvite formation with a carbon dioxide stripper. Water Sci. Technol. 2008, 58, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Chimenos, J.M.; Fernández, A.I.; Villalba, G.; Segarra, M.; Urruticoechea, A.; Artazab, B.; Espiella, F. Removal of ammonium and phosphates from wastewater resulting from the process of cochineal extraction using MgO-containing by-product. Water Res. 2003, 37, 1601–1607. [Google Scholar] [CrossRef]
- Quintana, M.; Colmenarejo, M.F.; Barrera, J.; García, G.; García, E.; Bustos, A. Use of a byproduct of magnesium oxide production to precipitate phosphorus and nitrogen as struvite from wastewater treatment liquors. J. Agric. Food Chem. 2004, 52, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Gunay, A.; Karadag, D.; Tosun, I.; Ozturk, M. Use of magnesit as a magnesium source for ammonium removal from leachate. J. Hazard. Mater. 2008, 156, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Weon, S.Y.; Lee, C.W.; Koopman, B. Removal of nitrogen and phosphate from wastewater by addition of bittern. Chemosphere 2003, 51, 265–271. [Google Scholar] [CrossRef]
- Siciliano, A.; Ruggiero, C.; de Rosa, S. A new integrated treatment for the reduction of organic and nitrogen loads in methanogenic landfill leachates. Process Saf. Environ. Prot. 2013, 91, 311–320. [Google Scholar] [CrossRef]
- Siciliano, A.; de Rosa, S. Recovery of ammonia in digestates of calf manure through a struvite precipitation process using unconventional reagents. Environ. Technol. 2014, 35, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, A. Assessment of fertilizer potential of the struvite produced from the treatment of methanogenic landfill leachate using low-cost reagents. Environ. Sci. Pollut. Res. 2016, 23, 5949–5959. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhang, Y.; Yang, M.; Du, W.; Harada, H. Repeated use of MAP decomposition residues for the removal of high ammonium concentration from landfill leachate. Chemosphere 2007, 66, 2233–2238. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xu, C.; Zhang, W. Removal of nutrients from piggery wastewater using struvite precipitation and pyrogenation technology. Bioresour. Technol. 2011, 102, 2523–2528. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Jiang, Y.; Ding, L. Recovery and removal of ammonia–nitrogen and phosphate from swine wastewater by internal recycling of struvite chlorination product. Bioresour. Technol. 2014, 172, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Kumar, S.; Kwag, J.H.; Kim, J.H.; Kim, J.D.; Ra, C.S. Recycle of electrolytically dissolved struvite as an alternative to enhance phosphate and nitrogen recovery from swine wastewater. J. Hazard. Mater. 2011, 195, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-H.; Qiu, G.-L.; Yuan, P.; Cui, X.-Y.; Peng, J.-F.; Zeng, P.; Duan, L.; Xiang, L.-C.; Qian, F. Nutrients removal and recovery from anaerobically digested swine wastewater by struvite crystallization without chemical additions. J. Hazard. Mater. 2011, 190, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ding, L.; Ren, H.; Xiong, X. Ammonium nitrogen removal from coking wastewater by chemical precipitation recycle technology. Water Res. 2009, 43, 5209–5215. [Google Scholar] [CrossRef] [PubMed]
- Türker, M.; Cęlen, I. Removal of ammonia as struvite from anaerobic digester effluents and recycling of magnesium and phosphate. Bioresour. Technol. 2007, 98, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Geng, J.; Ren, H.; Wang, Y.; Xu, K. Struvite pyrolysate recycling combined with dry pyrolysis for ammonium removal from wastewater. Bioresour. Technol. 2013, 132, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xiao, D.; Liu, J.; Hou, L.; Ding, L. Recovery and removal of nutrients from swine wastewater by using a novel integrated reactor for struvite decomposition and recycling. Sci. Rep. 2015, 5, 10183. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association, Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association; Water Environment Federation: Washington, DC, USA, 1998. [Google Scholar]
| Parameter | Value | Unit |
|---|---|---|
| pH | 8.16 | - |
| Conductivity | 28.70 | mS/cm |
| Alkalinity | 12.98 | gCaCO3/L |
| COD (chemical oxygen demand) | 9.7 | g/L |
| BOD5 (biochemical oxygen demand) | 0.56 | g/L |
| TS | 14.8 | g/L |
| VS | 5.12 | g/L |
| N-NH4+ | 2.62 | g/L |
| P-PO43− | 25.5 | mg/L |
| Mg2+ | 63.1 | mg/L |
| Ca2+ | 27.8 | mg/L |
| K+ | 11.2 | mg/L |
| n(N):n(Mg):n(P) | 1:0.014:0.004 | mol(N):mol(Mg):mol(P) |
| Cycle | Without Supplement | With Air Insufflation | With Supplement | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| Amount of wet precipitate (g) | 29.4 | 27.3 | 26.7 | 25.3 | 20.9 | 30.1 | 27.9 | 25.2 | 21.20 | 16.9 | 29.9 | 28.2 | 29.4 | 28.6 | 28.5 |
| Amount of wet decomposition residues (g) | 20.1 | 19.5 | 19.2 | 14.1 | 10.3 | 20.7 | 19.2 | 17.7 | 12.5 | 8.8 | 20.9 | 19.5 | 19.6 | 19.0 | 19.1 |
| Dissolved N-NH4+ (mmol (N)/g) | 1.51 | 1.48 | 1.45 | 1.24 | 1.07 | 1.49 | 1.46 | 1.35 | 1.08 | 0.87 | 1.52 | 1.50 | 1.48 | 1.48 | 1.45 |
| Dissolved P-PO43− (mmol (P)/g) | 0.041 | 0.038 | 0.036 | 0.035 | 0.030 | 0.04 | 0.039 | 0.038 | 0.031 | 0.029 | 0.04 | 0.038 | 0.038 | 0.041 | 0.039 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siciliano, A.; Stillitano, M.A.; Limonti, C.; Marchio, F. Ammonium Removal from Landfill Leachate by Means of Multiple Recycling of Struvite Residues Obtained through Acid Decomposition. Appl. Sci. 2016, 6, 375. https://doi.org/10.3390/app6110375
Siciliano A, Stillitano MA, Limonti C, Marchio F. Ammonium Removal from Landfill Leachate by Means of Multiple Recycling of Struvite Residues Obtained through Acid Decomposition. Applied Sciences. 2016; 6(11):375. https://doi.org/10.3390/app6110375
Chicago/Turabian StyleSiciliano, Alessio, Maria Assuntina Stillitano, Carlo Limonti, and Francesco Marchio. 2016. "Ammonium Removal from Landfill Leachate by Means of Multiple Recycling of Struvite Residues Obtained through Acid Decomposition" Applied Sciences 6, no. 11: 375. https://doi.org/10.3390/app6110375








