Angiogenic and Osteogenic Coupling Effects of Deferoxamine-Loaded Poly(lactide-co-glycolide)-Poly(ethylene glycol)-Poly(lactide-co-glycolide) Nanoparticles
Abstract
:1. Introduction
2. Results
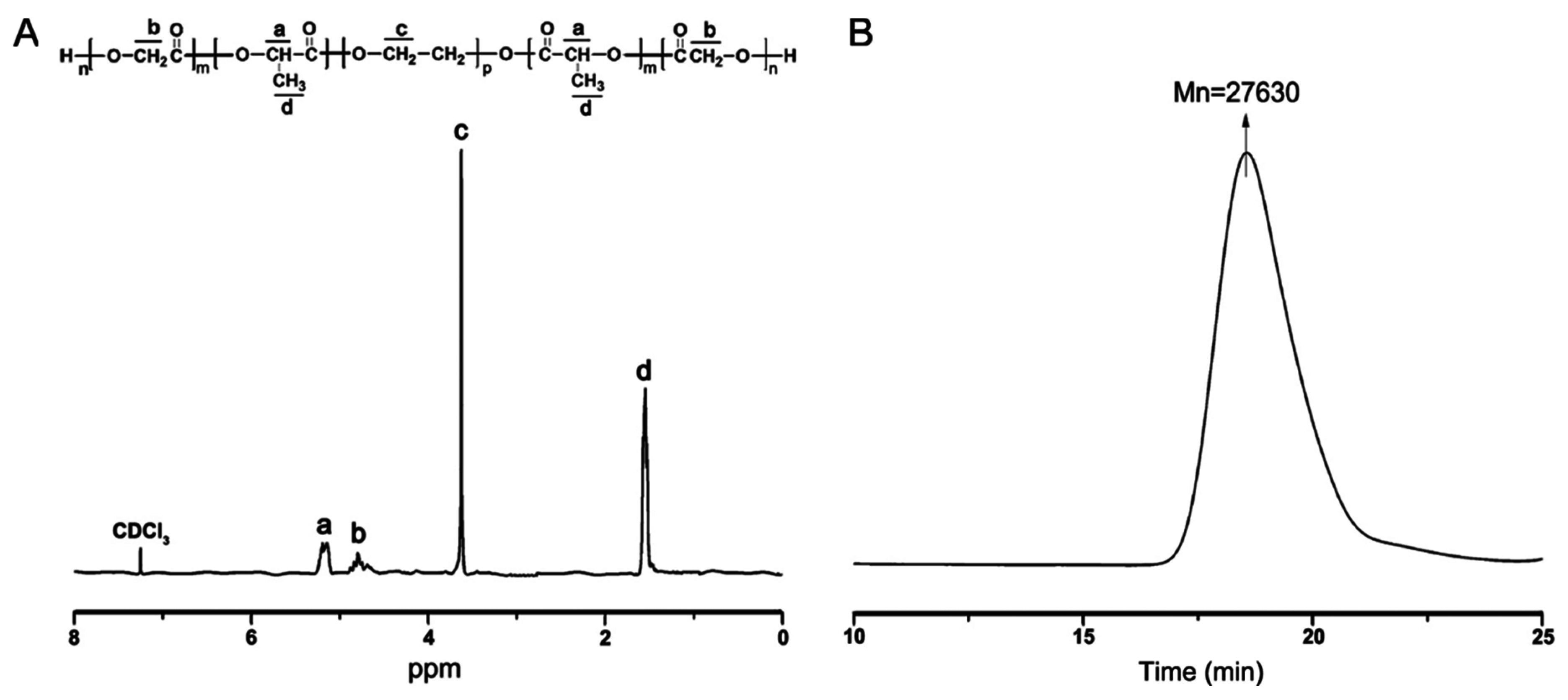
2.1. Synthesis and Characterization of Triblock Copolymer Poly(lactide-co-glycolide)-Poly(ethylene glycol)-Poly(lactide-co-glycolide) (PLGA-PEG-PLGA)
2.2. Preparation and Characterization of Deferoxamine (DFO)-Loaded PLGA-PEG-PLGA Nanoparticles (DFO-Loaded NPs)
2.3. DFO Release Profile from the PLGA-PEG-PLGA NPs
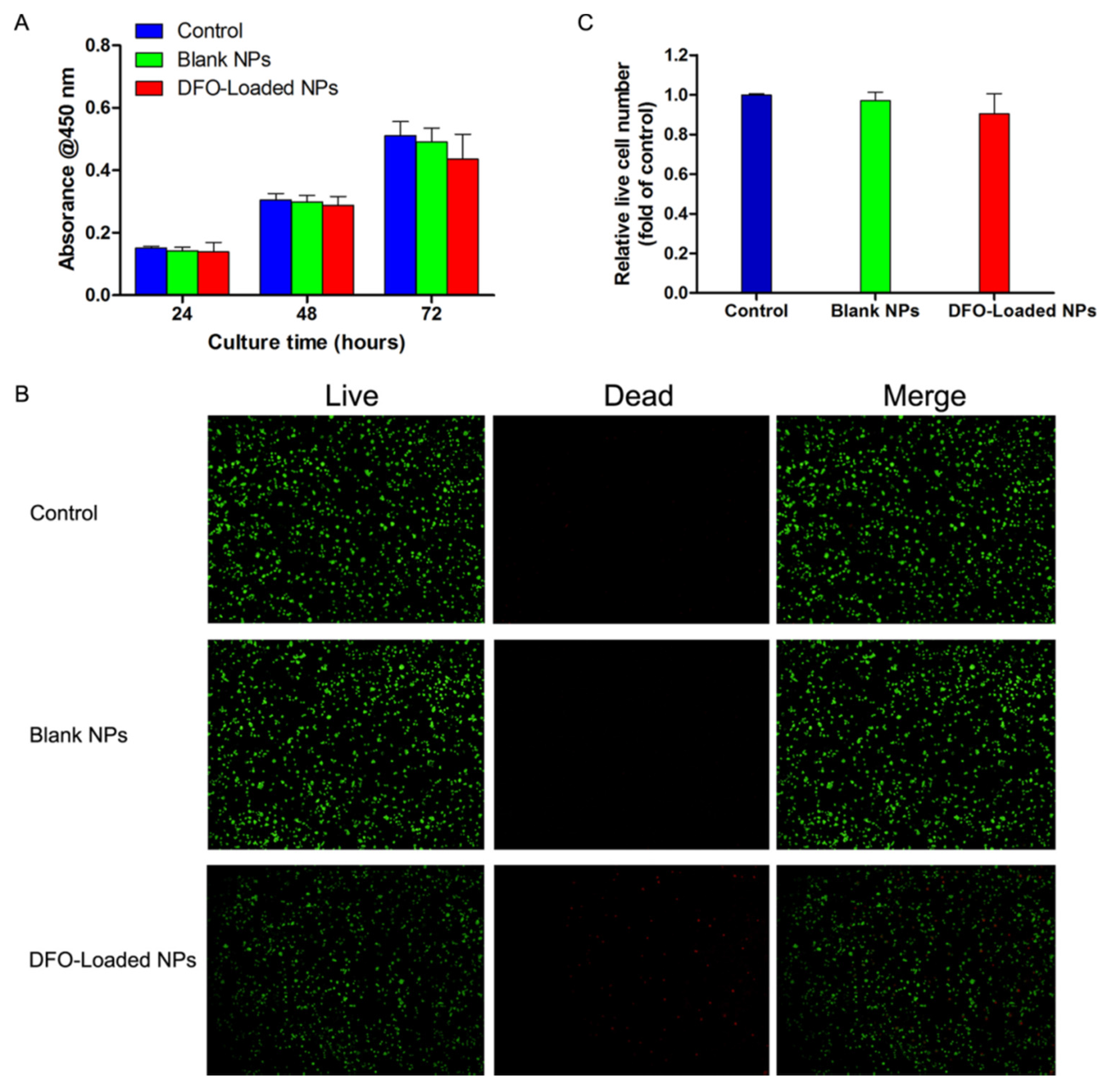
2.4. The Effect of DFO-Loaded PLGA-PEG-PLGA NPs on the Viability of Human Bone Marrow Stromal Cells (hBMSCs)
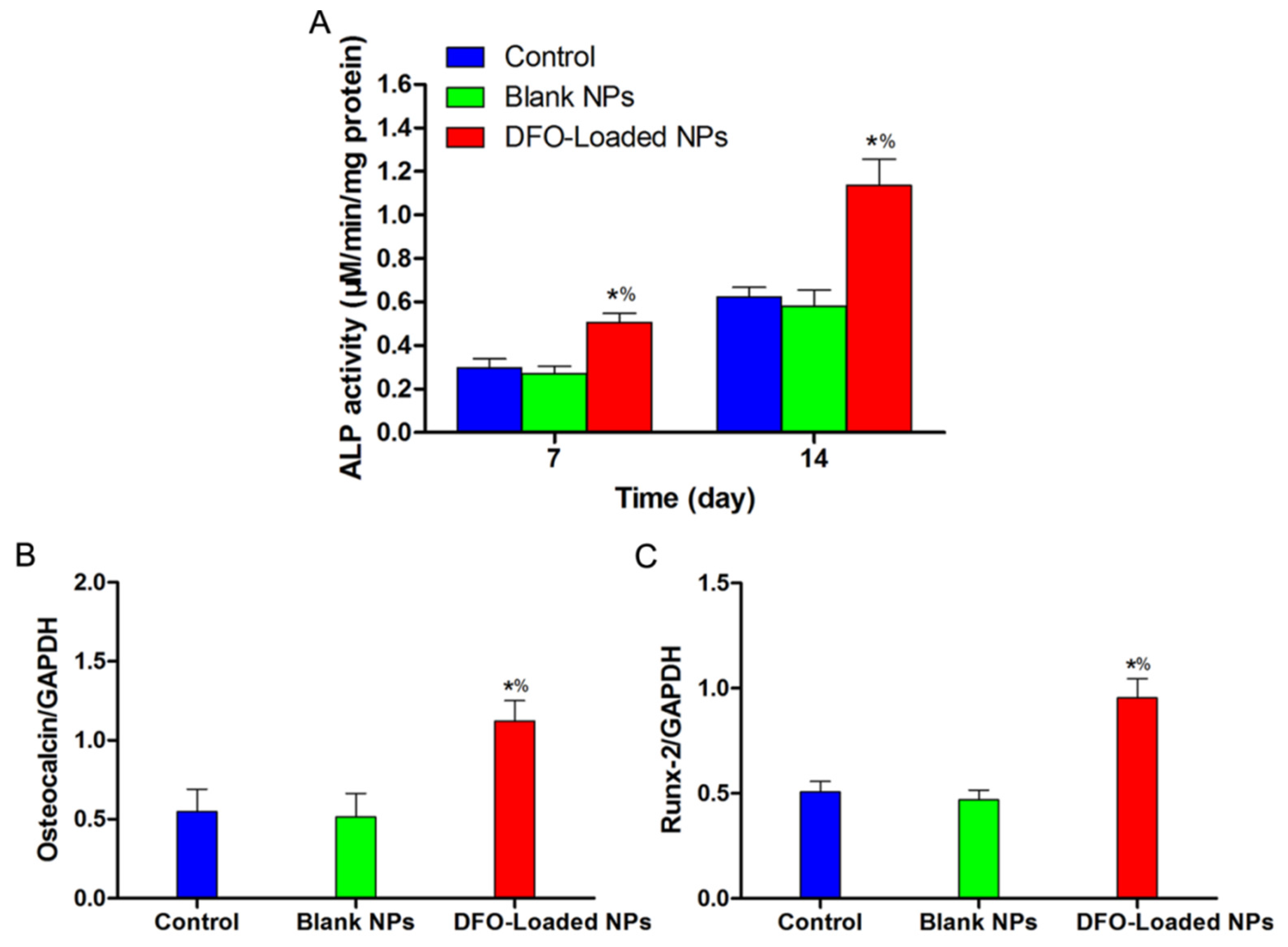
2.5. The Angiogenic and Osteogenic Effects of DFO-Loaded PLGA-PEG-PLGA NPs on hBMSCs in Vitro
2.6. The Angiogenic Effect of DFO-Loaded PLGA-PEG-PLGA NPs on HUVECs in Vitro
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis and Characterization of PLGA-PEG-PLGA Copolymers
4.3. Preparation of DFO-Loaded PLGA-PEG-PLGA NPs
4.4. Characterization of DFO-Loaded PLGA-PEG-PLGA NPs
4.5. In Vitro DFO Release Assay
4.6. Cell Culture
4.7. In Vitro Cell Viability Assessment
4.8. Enzyme-Linked Immunosorbent Assay
4.9. Western Blot Analysis
4.10. ALP Activity Evaluation
4.11. Angiogenic and Osteogenic mRNA Expressions of DFO-Loaded PLGA-PEG-PLGA NPs-Treated hBMSCs
4.12. Evalution of the Angiogenic Effect of DFO-Loaded PLGA-PEG-PLGA NPs on HUVECs
4.13. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Min, Z.; Shichang, Z.; Chen, X.; Yufang, Z.; Changqing, Z. 3D-printed dimethyloxallyl glycine delivery scaffolds to improve angiogenesis and osteogenesis. Biomater. Sci. 2015, 3, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Hankenson, K.D.; Dishowitz, M.; Gray, C.; Schenker, M. Angiogenesis in bone regeneration. Injury 2011, 42, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Zimmermann, G.; Pufe, T.; Varoga, D.; Henle, P. The systemic angiogenic response during bone healing. Arch. Orthop. Trauma Surg. 2009, 129, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Potier, E.; Ferreira, E.; Dennler, S.; Mauviel, A.; Oudina, K.; Logeart-Avramoglou, D.; Petite, H. Desferrioxamine-driven upregulation of angiogenic factor expression by human bone marrow stromal cells. J. Tissue Eng. Regen. Med. 2008, 2, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Quarto, R.; Mastrogiacomo, M.; Cancedda, R.; Kutepov, S.M.; Mukhachev, V.; Lavroukov, A.; Marcacci, M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N. Engl. J. Med. 2001, 344, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Cancedda, R.; Bianchi, G.; Derubeis, A.; Quarto, R. Cell therapy for bone disease: A review of current status. Stem Cells 2003, 21, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Petite, H.; Viateau, V.; Bensaid, W.; Meunier, A.; de Pollak, C.; Bourguignon, M.; Guillemin, G. Tissue-engineered bone regeneration. Nat. Biotechnol. 2000, 18, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guan, J.; Qi, X.; Ding, H.; Yuan, H.; Xie, Z.; Huang, Y. Dimethyloxaloylglycine Promotes the Angiogenic Activity of Mesenchymal Stem Cells Derived from iPSCs via Activation of the PI3K/Akt Pathway for Bone Regeneration. Int. J. Biol. Sci. 2016, 12, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wan, C.; Gilbert, S.R.; Clemens, T.L. Oxygen sensing and osteogenesis. Ann. N. Y. Acad. Sci. 2007, 1117, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Tabata, Y. Hypoxia-induced angiogenesis is increased by the controlled release of deferoxiamine from gelatin hydrogels. Acta Biomater. 2014, 10, 3641–3649. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, P.; Mole, D.R.; Tian, Y.M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; Maxwell, P.H. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Yang, H.; Ivan, M.; Gertler, F.; Kaelin, W.G.; Pavletich, N.P. Structure of an HIF-1α-pVHL complex: Hydroxyproline recognition in signaling. Science 2002, 296, 1886–1889. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J.; Spyrou, A.; Kolnagou, A. Iron chelation therapy in hereditary hemochromatosis and thalassemia intermedia: Regulatory and non regulatory mechanisms of increased iron absorption. Hemoglobin 2010, 34, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Kim, Y.S.; Noh, K.; Lee, Y.M.; Chang, S.W.; Kim, E.C. Deferoxamine promotes osteoblastic differentiation in human periodontal ligament cells via the nuclear factor erythroid 2-related factor-mediated antioxidant signaling pathway. J. Periodontal Res. 2014, 49, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Chen, H.; Kang, H.; Qi, J.; Zhao, P.; Jiang, M.; Guo, L.; Zhou, Q.; Qian, N.D.; Zhou, H.B.; et al. Deferoxamine released from poly (lactic-co-glycolic acid) promotes healing of osteoporotic bone defect via enhanced angiogenesis and osteogenesis. J. Biomed. Mater. Res. A 2016, 104, 2515–2527. [Google Scholar] [CrossRef] [PubMed]
- Hallaway, P.E.; Eaton, J.W.; Panter, S.S.; Hedlund, B.E. Modulation of deferoxamine toxicity and clearance by covalent attachment to biocompatible polymers. Proc. Natl. Acad. Sci. USA 1989, 86, 10108–10112. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Tajima, S.; Yoshida, S.; Yamano, N.; Kihira, Y.; Ishizawa, K.; Tamaki, T. Deferoxamine promotes angiogenesis via the activation of vascular endothelial cell function. Atherosclerosis 2011, 215, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.; Nguyen, Q.P. Effect of silica nanoparticles on clay swelling and aqueous stability of nanoparticle dispersions. J. Nanopart. Res. 2014, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhu, D.; Liu, G.; Tao, W.; Cao, W.; Zhang, L.; Zeng, X. DTX-loaded star-shaped TAPP-PLA-b-TPGS nanoparticles for cancer chemical and photodynamic combination therapy. RSC Adv. 2015, 5, 50617–50627. [Google Scholar] [CrossRef]
- Liao, S.H.; Liu, C.H.; Bastakoti, B.P.; Suzuki, N.; Chang, Y.; Yamauchi, Y.; Wu, K.C. Functionalized magnetic iron oxide/alginate core-shell nanoparticles for targeting hyperthermia. Int. J. Nanomed. 2015, 10, 3315–3328. [Google Scholar]
- Suárez-González, D.; Barnhart, K.; Saito, E.; Vanderby, R.; Hollister, S.J.; Murphy, W.L. Controlled nucleation of hydroxyapatite on alginate scaffolds for stem cell-based bone tissue engineering. J. Biomed. Mater. Res. A 2010, 95, 222–234. [Google Scholar]
- Lin, H.R.; Yeh, Y.J. Porous alginate/hydroxyapatite composite scaffolds for bone tissue engineering: Preparation, characterization, and in vitro studies. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 71, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Tomasina, J.; Lheureux, S.; Gauduchon, P.; Rault, S.; Malzert-Fréon, A. Nanocarriers for the targeted treatment of ovarian cancers. Biomaterials 2013, 34, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.H.; Liu, C.H.; Liao, S.H.; Lin, Y.Y.; Tang, H.W.; Liu, S.Y.; Wu, K.C.W. Cosynthesis of cargo-loaded hydroxyapatite/alginate core–shell nanoparticles (HAP@ Alg) as pH-responsive nanovehicles by a pre-gel method. ACS Appl. Mater. Interfaces 2012, 4, 6720–6727. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Chen, Z.; Xiao, X.; Ruan, C.; Mei, L.; Liu, Z.; Zeng, X. Surface modification of paclitaxel-loaded tri-block copolymer PLGA-b-PEG-b-PLGA nanoparticles with protamine for liver cancer therapy. J. Nanopart. Res. 2015, 17, 1–11. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, H.; Ding, J. Effects of precipitate agents on temperature-responsive sol–gel transitions of PLGA–PEG–PLGA copolymers in water. Colloid Polym. Sci. 2010, 288, 1151–1159. [Google Scholar] [CrossRef]
- Long, F. Building strong bones: Molecular regulation of the osteoblast lineage. Nat. Rev. Mol. Cell Biol. 2012, 13, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Saran, U.; Piperni, S.G.; Chatterjee, S. Role of angiogenesis in bone repair. Arch. Biochem. Biophys. 2014, 561, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Mayr-Wohlfart, U.; Waltenberger, J.; Hausser, H.; Kessler, S.; Günther, K.P.; Dehio, C.; Brenner, R.E. Vascular endothelial growth factor stimulates chemotactic migration of primary human osteoblasts. Bone 2002, 30, 472–477. [Google Scholar] [CrossRef]
- Deckers, M.M.; Karperien, M.; van der Bent, C.; Yamashita, T.; Papapoulos, S.E.; Löwik, C.W. Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology 2000, 141, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wan, C.; Deng, L.; Liu, X.; Cao, X.; Gilbert, S.R.; Haase, V.H. The hypoxia-inducible factor α pathway couples angiogenesis to osteogenesis during skeletal development. J. Clin. Investig. 2007, 117, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, O.; Amit, T.; Mandel, S.; Youdim, M.B. Novel therapeutic approach for neurodegenerative pathologies: Multitarget iron-chelating drugs regulating hypoxia-inducible factor 1 signal transduction pathway. Neurodegener. Dis. 2011, 10, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Lv, Y.; Lin, B.; Tipoe, G.L.; Youdim, M.B.; Xing, F.; Liu, Y. A Novel Antioxidant Multitarget Iron Chelator M30 Protects Hepatocytes against Ethanol-Induced Injury. Oxid. Med. Cell. Longev. 2015, 2015, 607271. [Google Scholar] [CrossRef] [PubMed]
- Golko-Perez, S.; Amit, T.; Youdim, M.B.; Weinreb, O. Beneficial Effects of Multitarget Iron Chelator on Central Nervous System and Gastrocnemius Muscle in SOD1G93A Transgenic ALS Mice. J. Mol. Neurosci. 2016, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Park, H.J.; Kim, Y.D.; Ryu, M.H.; Takata, T.; Bae, S.K.; Bae, M.K. Hinokitiol increases the angiogenic potential of dental pulp cells through ERK and p38MAPK activation and hypoxia-inducible factor-1α (HIF-1α) upregulation. Arch. Oral. Biol. 2014, 59, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.E.; Szoka, F.C.; Fréchet, J.M. Soluble polymer carriers for the treatment of cancer: The importance of molecular architecture. Acc. Chem. Res. 2009, 42, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Zhang, Y.; Zheng, Y.; Tian, G.; Song, C.; Yang, D.; Li, Z. A novel docetaxel-loaded poly (ε-caprolactone)/pluronic F68 nanoparticle overcoming multidrug resistance for breast cancer treatment. Nanoscale Res. Lett. 2009, 4, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Phromviyo, N.; Swatsitang, E.; Chompoosor, A. Effect of a surface stabilizer on the formation of polyoxalate nanoparticles and their release profiles. Vacuum 2014, 107, 208–212. [Google Scholar] [CrossRef]
- Yu, J.; Li, X.; Luo, Y.; Lu, W.; Huang, J.; Liu, S. Poly (ethylene glycol) shell-sheddable magnetic nanomicelle as the carrier of doxorubicin with enhanced cellular uptake. Colloids Surf. B Biointerfaces 2013, 107, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.F.; Chen, C.T.; Chen, S.C.; Kulkarni, A.R.; Chiu, Y.L.; Chen, M.C.; Sung, H.W. Paclitaxel-loaded poly (γ-glutamic acid)-poly (lactide) nanoparticles as a targeted drug delivery system for the treatment of liver cancer. Biomaterials 2006, 27, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Hastings, C.L.; Kelly, H.M.; Murphy, M.J.; Barry, F.P.; O’Brien, F.J.; Duffy, G.P. Development of a thermoresponsive chitosan gel combined with human mesenchymal stem cells and desferrioxamine as a multimodal pro-angiogenic therapeutic for the treatment of critical limb ischaemia. J. Control. Release 2012, 161, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Chekanov, V.S.; Zargarian, M.; Baibekov, I.; Karakozov, P.; Tchekanov, G.; Hare, J.; Akhtar, M. Deferoxamine-fibrin accelerates angiogenesis in a rabbit model of peripheral ischemia. Vasc. Med. 2003, 8, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Li, Y.; Lv, Y.; Cheng, Y.; He, F.; Zhuo, R. Biodegradable amphiphilic block-graft copolymers based on methoxy poly (ethylene glycol)-b-(polycarbonates-g-polycarbonates) for controlled release of doxorubicin. J. Mater. Sci. Mater. Med. 2014, 25, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Duffy, G.P.; Ahsan, T.; O’Brien, T.; Barry, F.; Nerem, R.M. Bone marrow–derived mesenchymal stem cells promote angiogenic processes in a time-and dose-dependent manner in vitro. Tissue Eng. Part A 2009, 15, 2459–2470. [Google Scholar] [CrossRef] [PubMed]
- Barbucci, R.; Lamponi, S.; Magnani, A.; Piras, F.M.; Rossi, A.; Weber, E. Role of the Hyal-Cu (II) complex on bovine aortic and lymphatic endothelial cells behavior on microstructured surfaces. Biomacromolecules 2005, 6, 212–219. [Google Scholar] [CrossRef] [PubMed]
- van Waarde, M.A.W.H.; van Assen, A.J.; Kampinga, H.H.; Konings, A.W.T.; Vujaskovic, Z. Quantification of Transforming Growth Factor-β in Biological Material Using Cells Transfected with a Plasminogen Activator Inhibitor-1 Promoter–Luciferase Construct. Anal. Biochem. 1997, 247, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Maire, M.; Logeart-Avramoglou, D.; Degat, M.C.; Chaubet, F. Retention of transforming growth factor β1 using functionalized dextran-based hydrogels. Biomaterials 2005, 26, 1771–1780. [Google Scholar] [CrossRef] [PubMed]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, M.; Wang, C.; Chen, D.; Shen, C.; Zhao, H.; He, Y. Angiogenic and Osteogenic Coupling Effects of Deferoxamine-Loaded Poly(lactide-co-glycolide)-Poly(ethylene glycol)-Poly(lactide-co-glycolide) Nanoparticles. Appl. Sci. 2016, 6, 290. https://doi.org/10.3390/app6100290
Qiu M, Wang C, Chen D, Shen C, Zhao H, He Y. Angiogenic and Osteogenic Coupling Effects of Deferoxamine-Loaded Poly(lactide-co-glycolide)-Poly(ethylene glycol)-Poly(lactide-co-glycolide) Nanoparticles. Applied Sciences. 2016; 6(10):290. https://doi.org/10.3390/app6100290
Chicago/Turabian StyleQiu, Manle, Chongyang Wang, Daoyun Chen, Chaoyong Shen, Huakun Zhao, and Yaohua He. 2016. "Angiogenic and Osteogenic Coupling Effects of Deferoxamine-Loaded Poly(lactide-co-glycolide)-Poly(ethylene glycol)-Poly(lactide-co-glycolide) Nanoparticles" Applied Sciences 6, no. 10: 290. https://doi.org/10.3390/app6100290





