Study on Sintering System of Calcium Barium Sulphoaluminate by XRD Quantitative Analysis
Abstract
:1. Introduction
2. Experimental Section
2.1. Sample Preparation
2.2. Test Methods
2.2.1. XRD Measurement
2.2.2. Quantitative Phase Analysis and Atomic Site Occupancy Analysis
3. Results and Discussion
3.1. Qualitative Analysis of Minerals
| Phase | COD code | Phase | COD code |
|---|---|---|---|
| CAS-Cubic | 9009938 | CA2 | 3500014 |
| CAS-Orthorhombic | 4001772 | C4A3 | 9002486 |
| BA | 1010630 | C12A7 | 4308078 |
| BŠ | 1010542 | CŠ | 9004096 |
| CA | 1528679 | C | 1011094 |
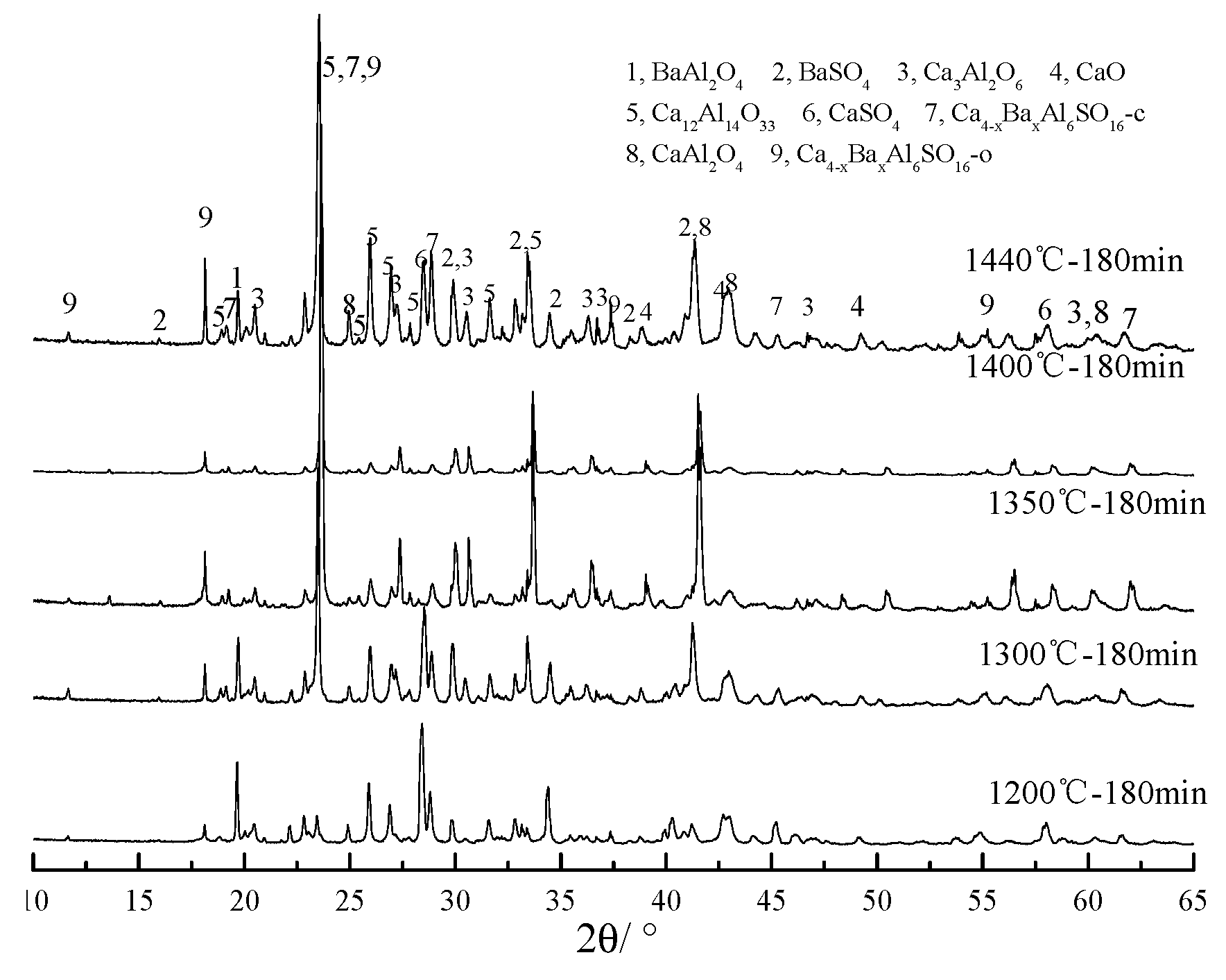
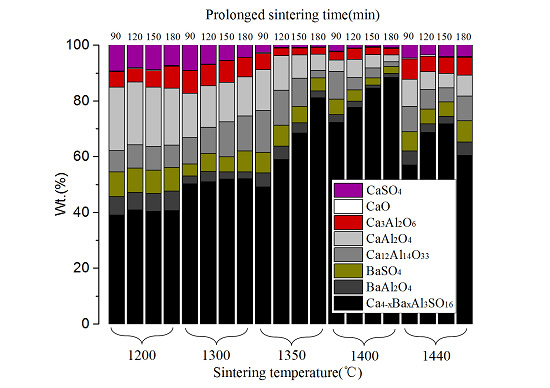
3.2. Quantitative Phase Analysis
| Phase | ICSD code | Phase | ICSD code |
|---|---|---|---|
| Ca4Al6SO16-Cubic | 9560 | CaAl4O7 | 16191 |
| Ca4Al6SO16-Orthorhombic | 80361 | Ca12Al14O33 | 261586 |
| BaAl2O4 | 246028 | CaSO4 | 183919 |
| BaO | 186427 | CaO | 261847 |
| CaAl2O4 | 180997 | - | - |
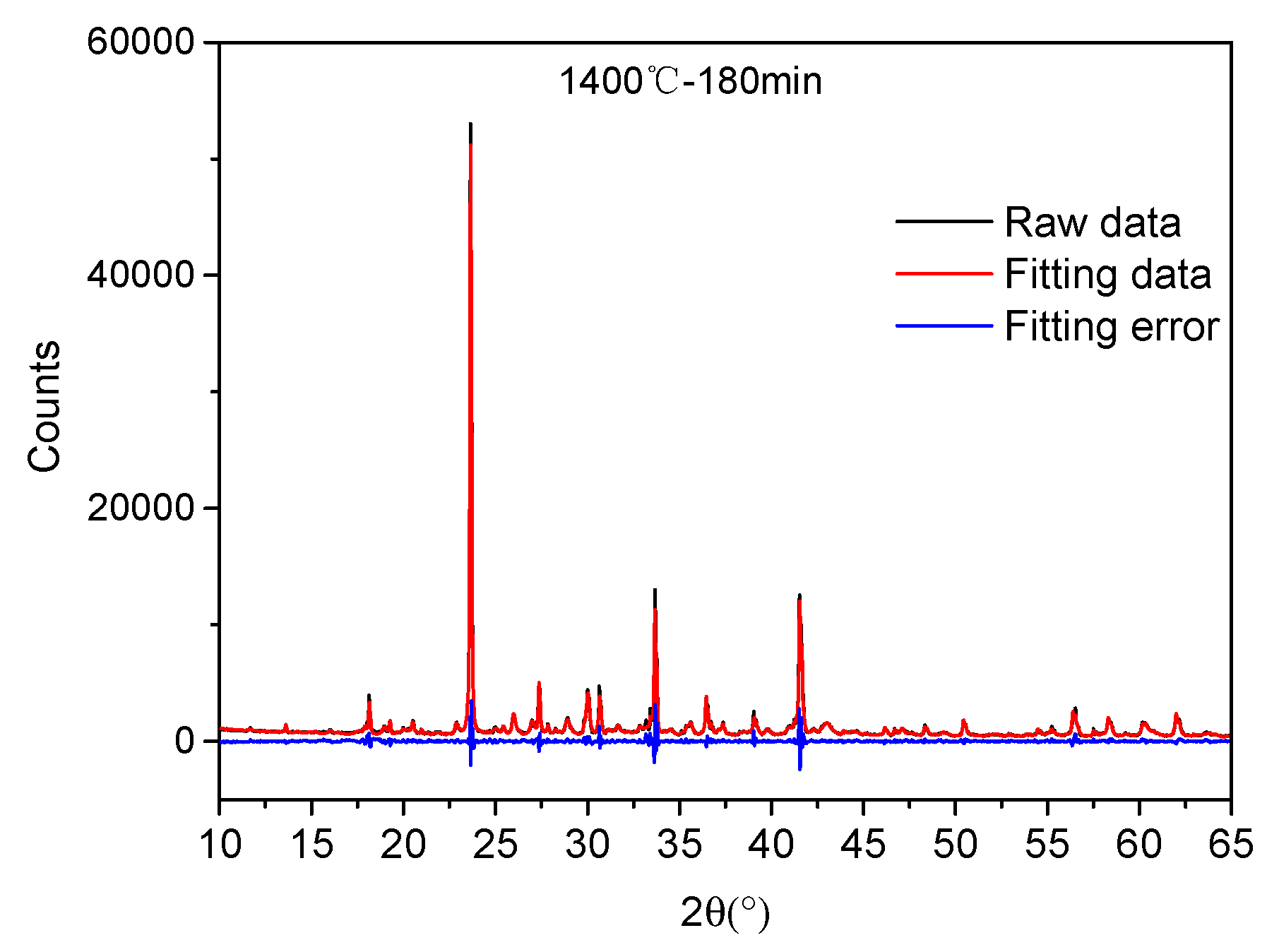
| Phase | Parameter | 1200 °C-180 min | 1300 °C-180 min | 1350 °C-180 min | 1400 °C-180 min |
|---|---|---|---|---|---|
| CBSA-O | a | 13.01032 | 13.0207508 | 13.0270247 | 13.0402404 |
| b | 12.92488 | 12.9469589 | 12.9600426 | 12.9981782 | |
| c | 9.21658 | 9.22244 | 9.22428 | 9.2330302 | |
| CBSA-C | a | 9.21896 | 9.22475 | 9.23109 | 9.23704 |
| Ba Content | Main Peak | Second Peak |
| d value | d value | |
| 0 | 3.74749 | 2.65358 |
| 0.5 | 3.75387 | 2.97368 |
| 1.0 | 3.78612 | 3.12588 |
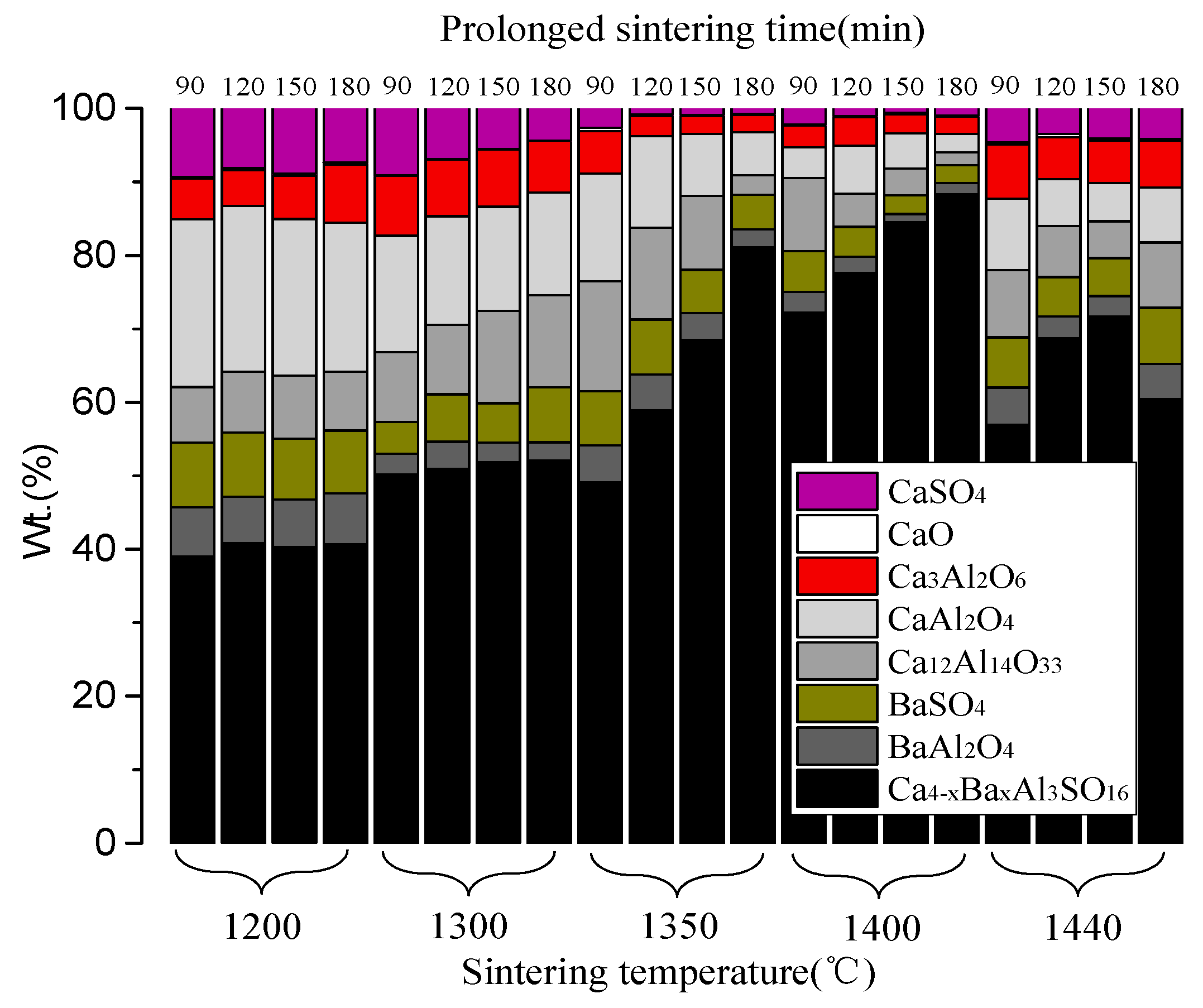
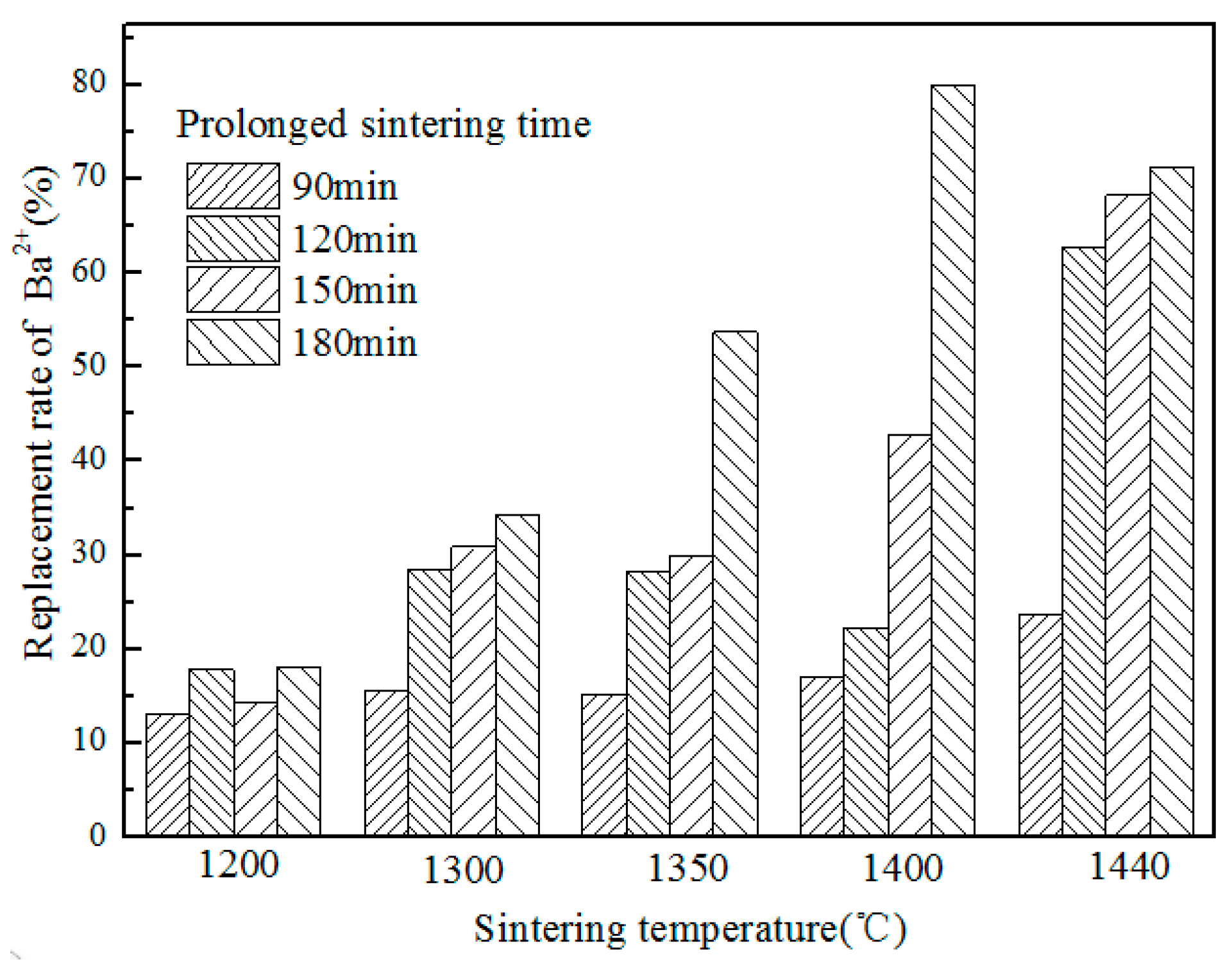
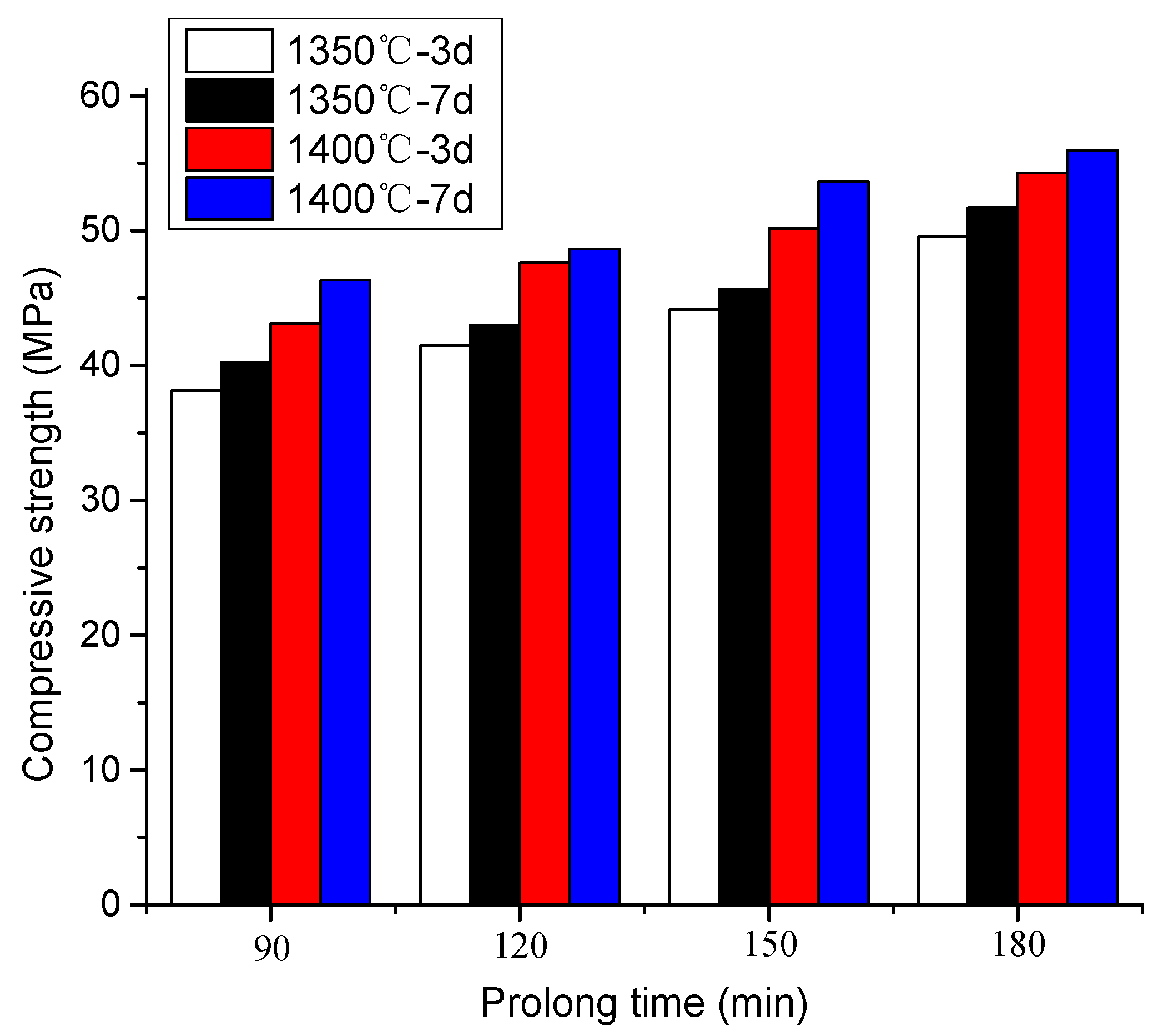
3.3. The Result of Strength
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tereanu, I.; Munten, M.; Dragnea, I. Type 3(CaO·Al2O3)·Mx(SO4)y compounds and compatibility relations in CaO-CaO·A12O3-Mx(SO4)y system. Cement 1986, 83, 39–45. [Google Scholar]
- Yan, P. Hydration of Sr and Ba-bearing sulphoaluminates in the presence of sulphates. Adv. Cem. Res. 1993, 5, 65–69. [Google Scholar] [CrossRef]
- Chang, J. Study on new type Ba-bearing cement. Mater’s Thesis, Wuhan University of Technology, Wuhan, China, 1998. [Google Scholar]
- Cheng, X.; Chang, J.; Lu, L.; Liu, F.; Teng, B. Study of Ba-bearing calcium sulphoaluminate minerals and cement. Cem. Concr. Res. 2000, 30, 77–81. [Google Scholar]
- Young, R.A. The Rietveld Method; Oxford University Press: Oxford, UK, 1993; pp. 21–22. [Google Scholar]
- Halstead, P.E.; Moore, A.E. The composition and crystallography of an anhydrous calcium aluminosulphate occurring in expanding cement. J. Appl. Chem. 1962, 12, 413–417. [Google Scholar] [CrossRef]
- Saalfeld, H.; Depmeier, W. Silicon-free compounds with sodalite structure. Krist. Tech. 1972, 7, 229–233. [Google Scholar] [CrossRef]
- Calos, N.J.; Kennard, C.H.L.; Whittaker, A.K.; Davis, R.L. Structure of calcium aluminate sulfate Ca4Al6O16 S. J. Solid State Chem. 1995, 119, 1–7. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, Y.; Shi, P.; Wang, J. The crystal structure of C4A3S. In Proceedings of 9th International Congress on the Chemistry of Cement, New Delhi, India, 23–28 November 1992; 201–208.
- Krstanović, I.; Radaković, A.; Karanović, L. X-Ray Powder Data for Ca4Al6O12SO4. Powder Diffr. 1992, 7, 47–48. [Google Scholar] [CrossRef]
- Hargis, C.W.; Moon, J.; Lothenbach, B.; Winnefeld, F.; Wenk, H.R.; Monterio, P.J. Calcium Sulfoaluminate Sodalite (Ca4Al6O12SO4) Crystal Structure Evaluation and Bulk Modulus Determination. J. Am. Ceram. Soc. 2014, 97, 892–898. [Google Scholar] [CrossRef]
- Cuesta, A.; de La Torre, A.G.; Losilla, E.R.; Peterson, V.K.; Rejmak, P.; Ayuela, A.; Aranda, M.A. Structure, atomistic simulations, and phase transition of stoichiometric yeelimite. Chem. Mater. 2013, 9, 1680–1687. [Google Scholar] [CrossRef]
- Cuesta, A.; de La Torre, A.G.; Losilla, E.R.; Santacruz, I.; Aranda, M.A. Pseudo cubic crystal structure and phase transition in doped yeelimite. Cryst. Growth Des. 2014, 10, 5158–5163. [Google Scholar] [CrossRef]
- Andac, O.; Glasser, F.P. Polymorphism of calcium sulphoaluminate (Ca4 Al6O16SO3) and its solid solutions. Adv. Cem. Res. 1994, 22, 57–60. [Google Scholar] [CrossRef]
- Álvarez-Pinazo, G.; Cuesta, A.; García-Maté, M.; Santacruz, I.; Losilla, E.R.; de La Torre, A.G.; Aranda, M.A. Rietveld quantitative phase analysis of Yeelimite-containing cements. Cem. Concr. Res. 2012, 7, 960–971. [Google Scholar] [CrossRef]
- Jansen, E.; Schäfer, W.; Will, G. R values in analysis of powder diffraction data using Rietveld refinement. J. Appl. Crystallogr. 1994, 4, 492–496. [Google Scholar] [CrossRef]
- Ma, S.; Shen, X.; Huang, Y.; Huang, Y.; Zhong, B. Preparation and formation mechanism of calcium sulphoaluminate. J. Ceram. Soc. 2008, 1, 78–81. [Google Scholar]
- Zhang, W.; Ouyang, S.; Chen, Y. Study on the compound formation in system CaO-Al2O3-BaSO4 and the formation kinetics of 3CaO3·Al2O3·BaSO4. J. Chin. Ceram. Soc. 2001, 4, 305–308. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, J.; Shang, X.; Zhao, J. Study on Sintering System of Calcium Barium Sulphoaluminate by XRD Quantitative Analysis. Appl. Sci. 2015, 5, 989-997. https://doi.org/10.3390/app5040989
Chang J, Shang X, Zhao J. Study on Sintering System of Calcium Barium Sulphoaluminate by XRD Quantitative Analysis. Applied Sciences. 2015; 5(4):989-997. https://doi.org/10.3390/app5040989
Chicago/Turabian StyleChang, Jun, Xiaopeng Shang, and Jiuye Zhao. 2015. "Study on Sintering System of Calcium Barium Sulphoaluminate by XRD Quantitative Analysis" Applied Sciences 5, no. 4: 989-997. https://doi.org/10.3390/app5040989