1. Introduction
Organic synthesis has tremendously advanced since the advent of transition metal catalysis. Over the years, not only a considerable synthetic efficiency was achieved but also concatenations of organometallic elementary steps were established that led to an unprecedented increase in structural complexity. By name, these processes are commonly referred to as metal catalyzed domino, tandem, or cascade reactions [
1]. During the past two decades, in spite of their different literal meaning all these terms have been progressively used in this context as synonyms since by design they all fall into the realm of one-pot transformations, which have been recently reviewed [
2,
3,
4]. A particular advantage of these concepts is the huge increase in structural complexity facilitated by the use of even the simplest starting materials. This is also one of the driving factors for constant research advancements in this field [
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17].
In general, transition metal catalyzed cascade reactions can be categorized into two groups, either being domino reactions that constantly repeat the same elementary step [
15,
16,
17], or they are constituted by reaction steps that consist of fundamentally different elementary transformations [
18,
19,
20,
21,
22,
23,
24,
25]. As such, transition metal catalyzed domino reactions involve organometallic intermediates which are constantly regenerated and yield complex, often polycyclic, organic products after numerous insertion steps and after a concluding elimination. Mostly in palladium catalyzed processes, unimolecular reactions that proceed intramolecularly, for instance via cyclic carbopalladation [
26,
27,
28], generate complex polycyclic structures beginning with linear polyunsaturated substrates. Moreover, metal catalyzed cascade reactions that proceed in a parallel or sequential manner [
18,
22] offer even more versatility if the catalyst bears the capability of performing different catalytic processes, especially if the adjustment of the reaction conditions from step to step with additives can trigger different catalytic steps. Conceptually however, for obvious reasons, parallel catalysis is more difficult to develop than sequential catalysis. According to Fogg’s and dos Santos’ terminology, sequential catalysis can be categorized as bi- or multicatalytic one-pot processes, assisted tandem catalysis or auto tandem catalysis, respectively [
1]. Consequently, the most demanding part in the development of such processes is the identification of an apt precatalyst which has the potential to catalyze various reactions in a one-pot fashion, thereby rapidly enabling the access to complex molecular scaffolds in diversity oriented syntheses [
29,
30,
31,
32,
33].
One of the two essential requirements to the catalytic system is the generation or retention of a functional group which is then appropriate for a subsequent reaction. Secondly, the catalyst or catalyst precursor has to be present in the reaction medium at the outset without any further addition of catalyst after the initial step. Additives to trigger the subsequent step might be added stepwise though.
To date, sequentially transition metal catalyzed processes predominantly make use of palladium [
34,
35], rhodium [
36], and ruthenium [
37] as metal sources. Recently, an overview focusing on cyclizing metal catalyzed processes in the sense of auto and assisted tandem catalysis according to Fogg’s and dos Santos’ classification in mind [
1], gave an insight into this concept [
38]. Here, we specifically focus on syntheses of heterocycles employing sequentially palladium catalyzed processes that are one-pot reactions
in sensu stricto. Special attention will be given to Pd-catalyzed alkynylation and insertion sequences, sequences based upon aminations, processes that are intercepted by cyclocondensation events, and a few specific sequentially catalyzed processes. In the context of our research, this catalytic concept of sequential catalysis turned out to be particularly useful for diversity oriented synthesis of functional chromophores [
39,
40].
2. Sequences Based upon Alkynylation and Carbopalladative Insertions
Generally speaking, the majority of Pd-catalyzed sequential, consecutive, and domino processes involve an insertion of the palladium species into a carbon-carbon bond [
41,
42,
43]. Carbopalladation of alkynes is particularly fascinating because, on the one hand, triple bonds are excellent ligands for transition metals, and on the other hand the complexes do not exit the catalytic cycle by β-hydride eliminations as known for Heck vinylations. For these complexes, possible exits are Pd-mediated nucleophilic trapping or a subsequent transmetallation, respectively. To date, the known catalytic systems for Sonogashira reactions are those with the highest capability of subsequent Pd- or Pd-Cu-cocatalyzed cyclizations. Thus, selected developments in Pd-catalyzed cascade cyclizations are discussed in the following.
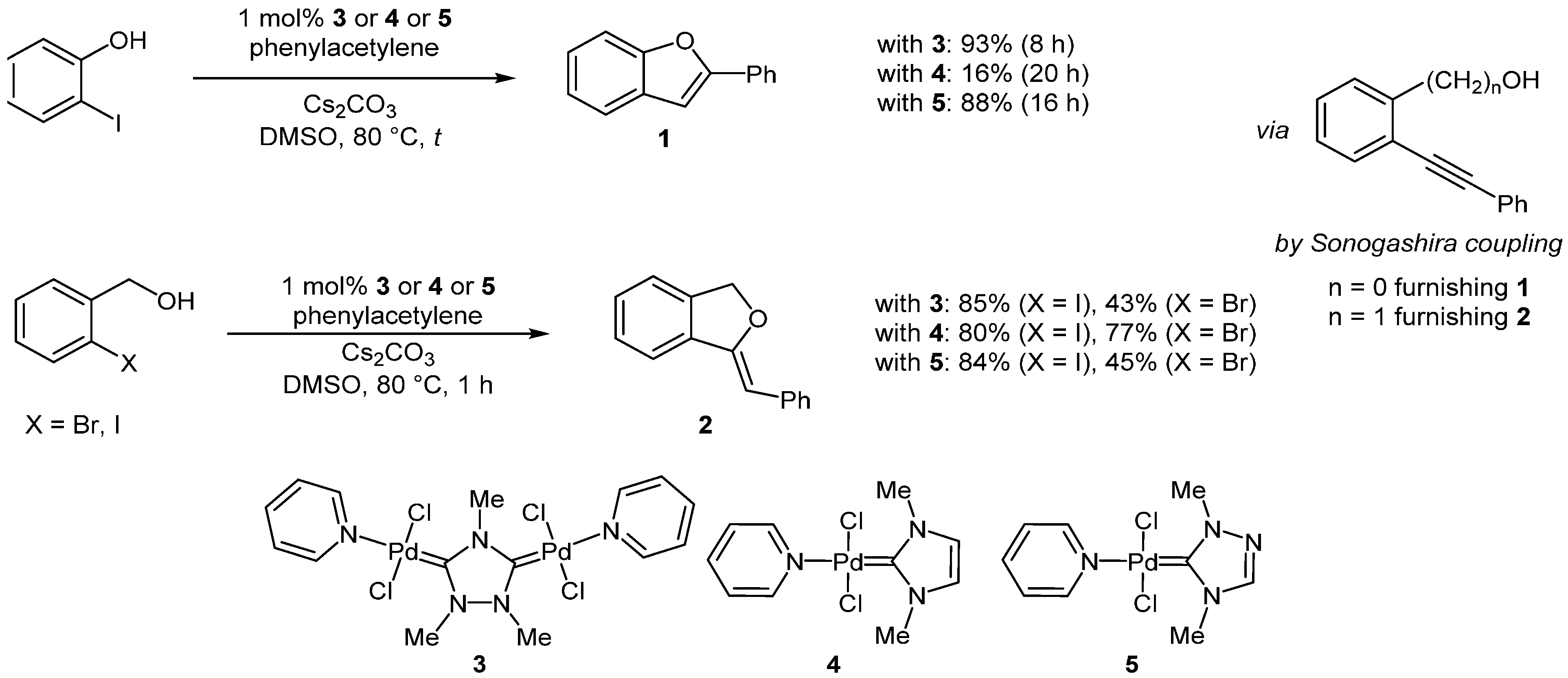
Based on
ortho-iodo phenol or an
ortho-iodo benzylalcohol as an aryl halide and phenylacetylene as an alkynyl coupling partner, a Pd-catalyzed domino sequence has been reported where cycloisomerization subsequent to the coupling step affords benzofuran
1 or benzoisofuran
2, respectively [
44]. Three different NHC-Pd-pyridine complexes
3–
5, where NHC is 1,2,4-trimethyltriazolyldiylidene (
3), 1,3-dimethylimidazolylidene (
4), and 1,4-dimethyltriazolylidene (
5), respectively, have been successfully employed (
Scheme 1).
Scheme 1.
Sequentially Pd-catalyzed alkynylation-hydroaryloxylation synthesis of benzofuran 1 and isobenzofuran 2.
Scheme 1.
Sequentially Pd-catalyzed alkynylation-hydroaryloxylation synthesis of benzofuran 1 and isobenzofuran 2.
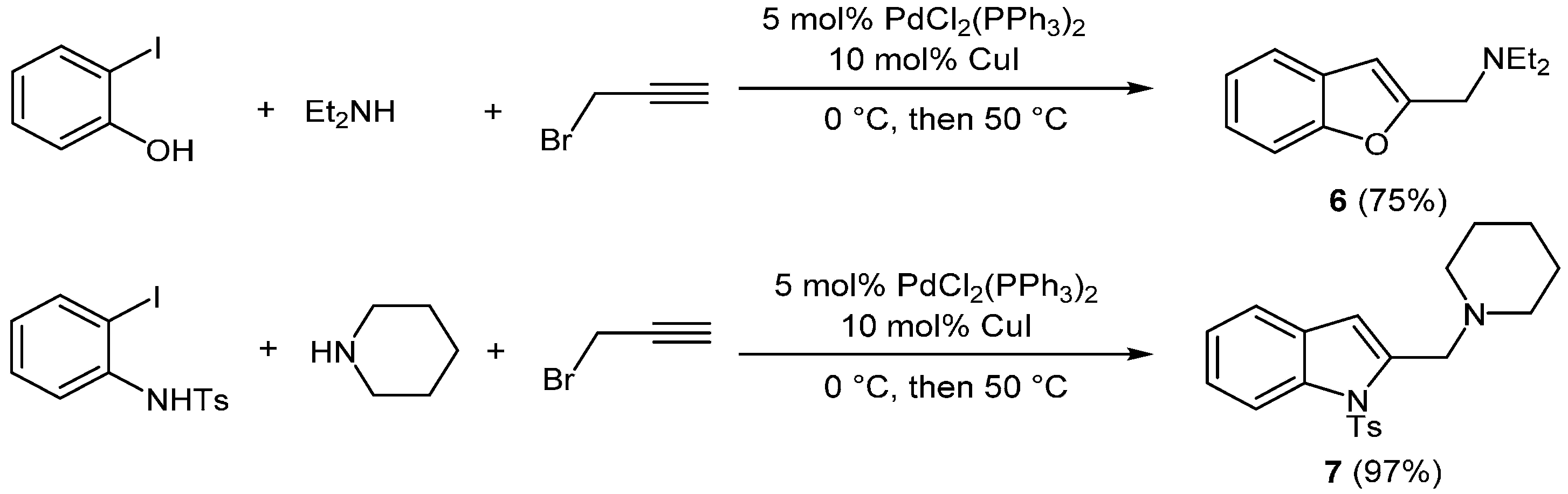
Accordingly, the group of Olivi and coworkers reported a three-component coupling-cyclization sequence affording benzofuran
6 or indole
7 involving an initial amine propargylation via classic substitution and a subsequent Sonogashira coupling-cyclization using the established Sonogashira conditions (
Scheme 2) [
45].
Scheme 2.
Consecutive amine propargylation-Sonogashira coupling-cyclization synthesis of benzofuran 6 and indole 7.
Scheme 2.
Consecutive amine propargylation-Sonogashira coupling-cyclization synthesis of benzofuran 6 and indole 7.
Another application of Sonogashira conditions in coupling and subsequent cyclization has been published by Pal and coworkers [
46]. Despite mechanistic incomprehensibilities pertaining to a formal oxidation step, the cooperation of palladium and copper as catalysts provides thiophene anellated α-pyrones
8 and
9 (
Scheme 3,
Table 1).
Scheme 3.
Sequentially Pd-catalyzed alkynylation-cyclization-alkynylation synthesis of 4-alkynylthieno[2,3-c]pyran-7-ones 8 and 7-alkynylthieno[3,2-c]pyran-4-ones 9.
Scheme 3.
Sequentially Pd-catalyzed alkynylation-cyclization-alkynylation synthesis of 4-alkynylthieno[2,3-c]pyran-7-ones 8 and 7-alkynylthieno[3,2-c]pyran-4-ones 9.
Table 1.
Selected 4-alkynylthieno[2,3-c]pyran-7-ones 8 and 7-alkynylthieno[3,2-c]pyran-4-ones 9 via sequentially Pd-catalyzed alkynylation-cyclization-alkynylation sequence.
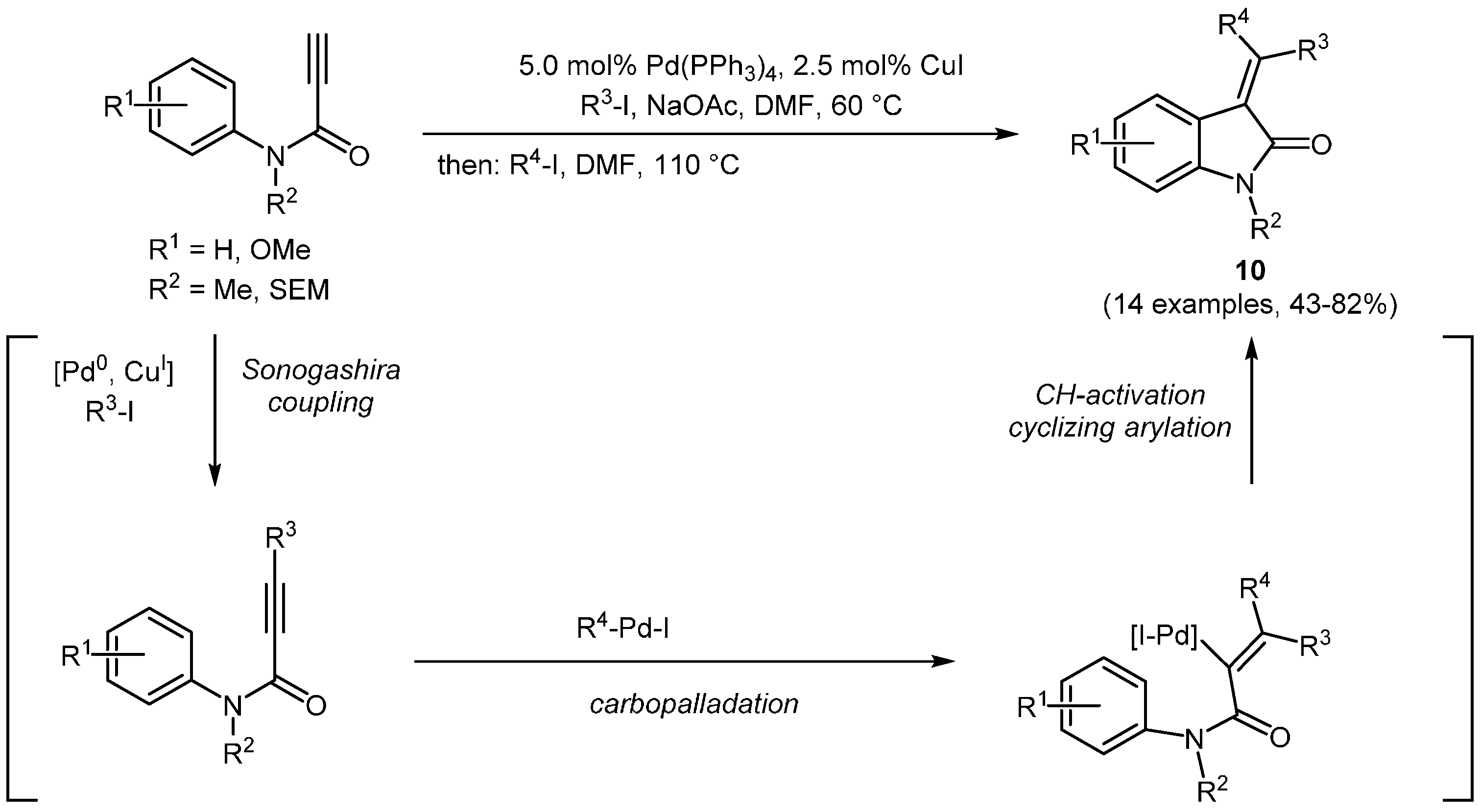
Another remarkable extension of the Sonogashira catalyst system has been published by Pinto, Neuville, and Zhu in which they employed the catalytic system in a Sonogashira alkynylation-carbopalladation-C–H activation-cyclization sequence [
47]. Their three-component synthesis afforded an access to 3-(diaryl-methylene)oxindoles
10 using the initial catalyst source for various steps (
Scheme 4,
Table 2).
Scheme 4.
Sequentially Pd-Cu-catalyzed Sonogashira-carbopalladation-CH-activation-cyclization synthesis of 3-(diarylmethylene)indolin-2-ones 10.
Scheme 4.
Sequentially Pd-Cu-catalyzed Sonogashira-carbopalladation-CH-activation-cyclization synthesis of 3-(diarylmethylene)indolin-2-ones 10.
Table 2.
Selected 3-(diarylmethylene)oxindoles 10 via sequentially Pd-Cu-catalyzed Sonogashira-carbopalladation-CH-activation-cyclization sequence.
The use of
ortho-iodo anilines provides access to a variety of substrates for consecutive domino reactions which can then be applied to the synthesis of heterocycles [
5,
6,
7,
9,
48,
49,
50,
51] and functional materials [
39]. One example is the preparation of (tetrahydroisobenzofuran) spiro-benzofuranones or spiro-indolones in moderate to excellent yields by use of the domino insertion-coupling-isomerization-intramolecular Diels-Alder sequence of alkynoyl
ortho-iodo phenolesters or alkynoyl
ortho-iodo anilides and propargyl allyl ethers [
52,
53]. The particular interest in the target compounds is based on their solution and solid state luminescence. Making use of the aforementioned concept, the intermediary enyne indolone
11 has been transformed into solid state red fluorescent push-pull indolones
12 in a consecutive one-pot three-component sequence (
Scheme 5,
Table 3) [
54].
Table 3.
Selected push-pull indolones 12.
Scheme 5.
Three-component synthesis of push-pull indolones 12.
Scheme 5.
Three-component synthesis of push-pull indolones 12.
Mechanistically, the bicatalytic system of palladium and copper catalyzes an insertion-alkynylation-amination path. Related to these results, Balalaie and coworkers published a facile entry to alkynoyl
ortho-iodo anilides using the well-established Ugi four-component reaction [
55].
Table 4.
Selected 2,4-diarylpyrano[2,3-b]indoles 13 prepared via sequentially Pd-Cu-catalyzed insertion-coupling-cycloisomerization domino synthesis.
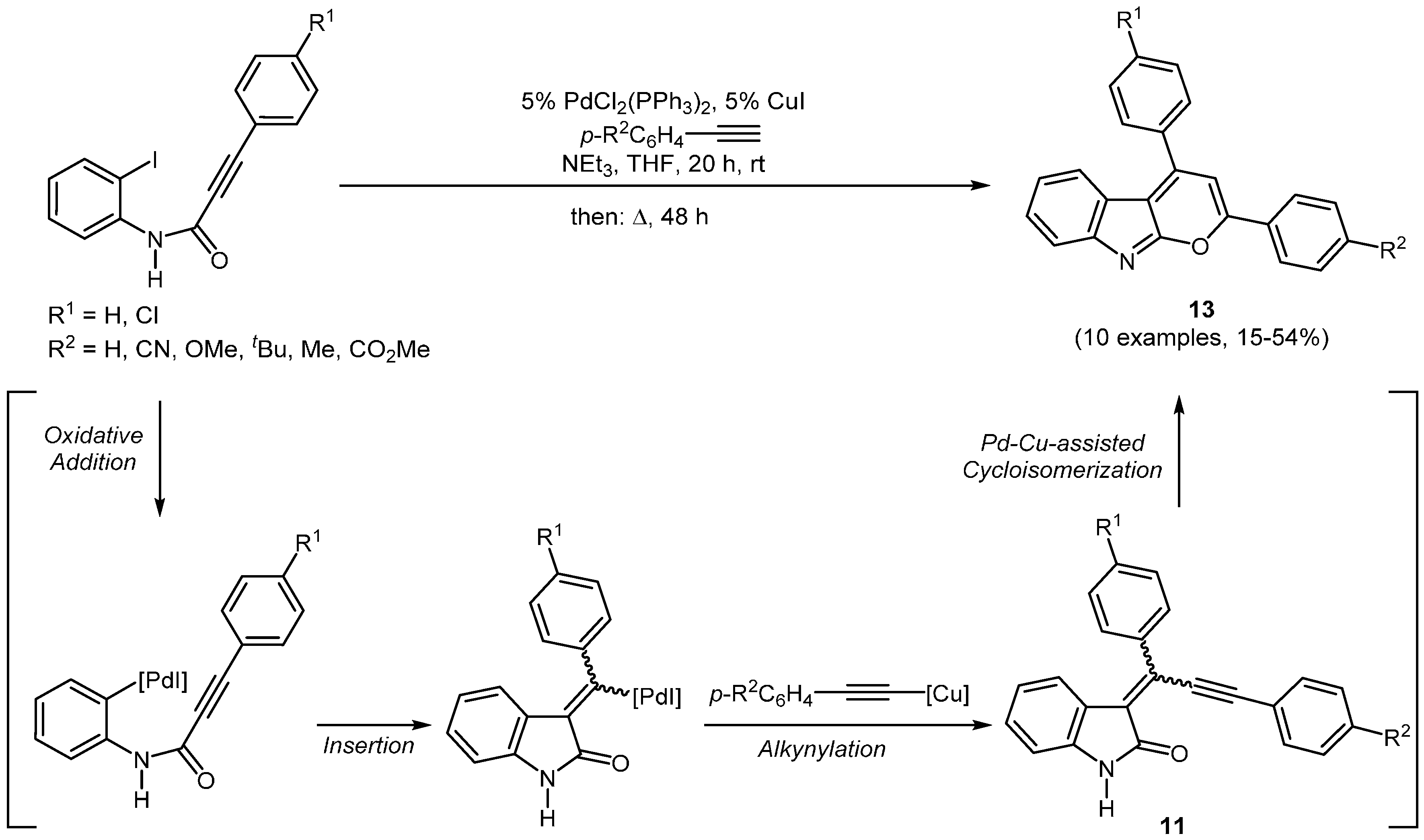
Based on previous results, Schönhaber, Frank, and Müller found, when secondary
ortho-iodo alkynoyl anilides (R
1 = H) are used, that intermediate
11 (
Scheme 5), formed by the palladium-copper co-catalyzed insertion-coupling, undergoes a transition-metal catalyzed cycloisomerization to give a new class of proto- and metallochromic luminescent 2,4-diarylpyrano[2,3-
b]indoles
13 (
Scheme 6,
Table 4) [
56].
Scheme 6.
Sequentially Pd-Cu-catalyzed insertion-coupling-cycloisomerization domino synthesis of 2,4-diarylpyrano[2,3-b]indoles 13.
Scheme 6.
Sequentially Pd-Cu-catalyzed insertion-coupling-cycloisomerization domino synthesis of 2,4-diarylpyrano[2,3-b]indoles 13.
Over the past decades, the Sonogashira alkynylation has turned out to be the most versatile catalytic synthesis of internal alkynes starting from (hetero)aryl or vinyl halides and terminal acetylenes [
42,
57,
58,
59,
60,
61,
62]. Most Sonogashira reactions are bimetallically catalyzed by palladium and copper complexes and, therefore, this unique catalyst system has also been shown to be suitable for CuAAC (Cu-catalyzed alkyne-azide cycloaddition) [
63,
64,
65,
66,
67,
68,
69], eventually, in a sequential or consecutive fashion.
Sonogashira coupling of various aryl halides with (trimethylsilyl)acetylene followed by fluoride mediated desilylation and subsequent CuAAC represents a fairly general approach to 1,4-disubstituted triazoles
14 in good to excellent yield (
Scheme 7) [
70]. Besides TBAF, CuF
2 was identified as a fluoride source that could also beneficially catalyze the CuAAC.
Scheme 7.
Consecutive three-component synthesis of 1,4-disubstituted triazoles 14 via Sonogashira alkynylation-desilylation-CuAAC sequence.
Scheme 7.
Consecutive three-component synthesis of 1,4-disubstituted triazoles 14 via Sonogashira alkynylation-desilylation-CuAAC sequence.
The ubiquity of indoles in nature combined with their established pharmaceutical role in drug development has made indole derivatives attractive targets for synthesis. Indoles substituted at
C-3 [
71,
72] and their aza analogues [
73] are known to exhibit remarkable biological activities. Particularly in the case of azaindoles, their ability to bind to the hinge region of kinases renders them promising structural motifs for kinase inhibition studies [
74,
75,
76,
77]. Thus a one-pot multicomponent strategy was developed involving the coupling of
N-Boc protected 3-iodo NH-heterocycles with (trimethylsilyl)acetylene that furnished the TMS-protected heterocyclic alkynes as intermediates, which, after in situ deprotection, gave rise to terminal alkynes that underwent subsequent CuAAC with an azide to furnish 3-triazolyl indoles or aza-indoles
15 (
Scheme 8) [
78]. Encouraged by the success of this three-component process, the strategy was further elaborated involving in situ generation of azides in a four-component one-pot sequence. Removal of the Boc group was carried out as a separate step under relatively mild conditions and some derivatives obtained by this concise strategy exhibited satisfactory kinase inhibition activity.
Scheme 8.
Consecutive three-component synthesis of N-Boc 3-triazolyl (aza)indoles and azoles 15 via Sonogashira alkynylation-desilylation-CuAAC sequence.
Scheme 8.
Consecutive three-component synthesis of N-Boc 3-triazolyl (aza)indoles and azoles 15 via Sonogashira alkynylation-desilylation-CuAAC sequence.
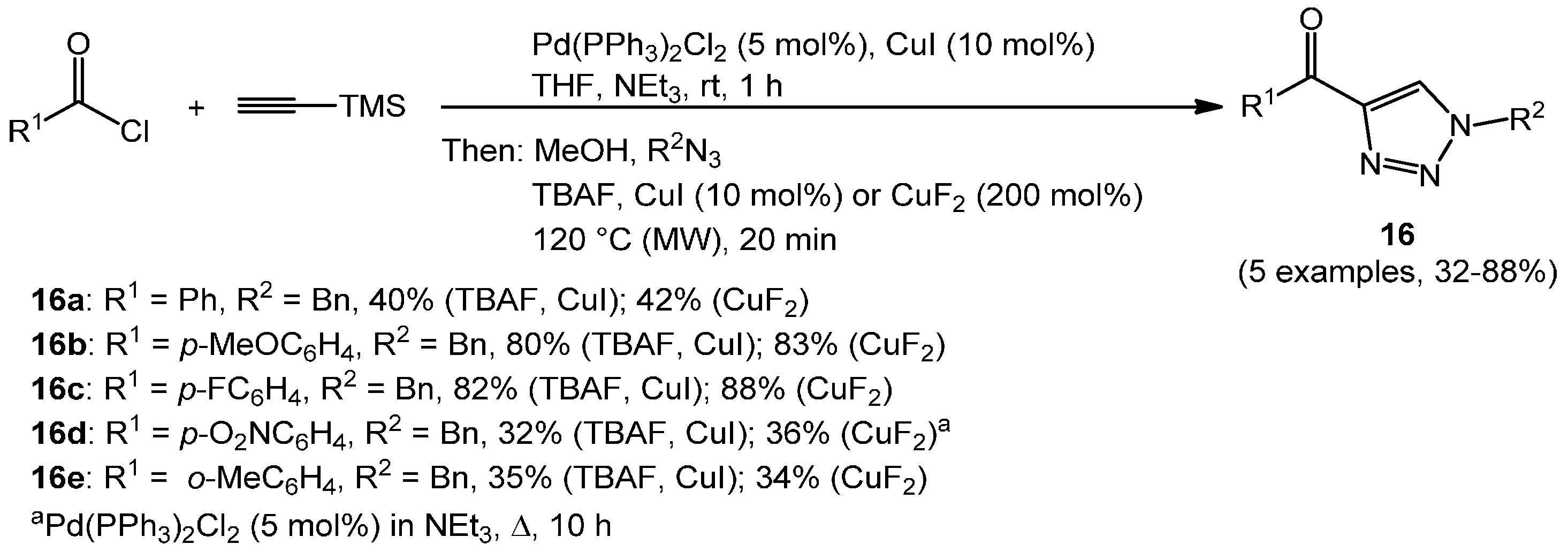
Likewise, aroyl chlorides successfully underwent Sonogashira coupling and desilylation steps to give terminally unsubstituted ynones that were subsequently transformed to 1-substituted-4-phenylacyl-1
H-1,2,3- triazoles
16 under dielectric heating (
Scheme 9) [
70].
Scheme 9.
Consecutive three-component synthesis of 1-substituted 4-acyl-1H-1,2,3-triazoles 16 via Sonogashira alkynylation-desilylation-CuAAC sequence.
Scheme 9.
Consecutive three-component synthesis of 1-substituted 4-acyl-1H-1,2,3-triazoles 16 via Sonogashira alkynylation-desilylation-CuAAC sequence.
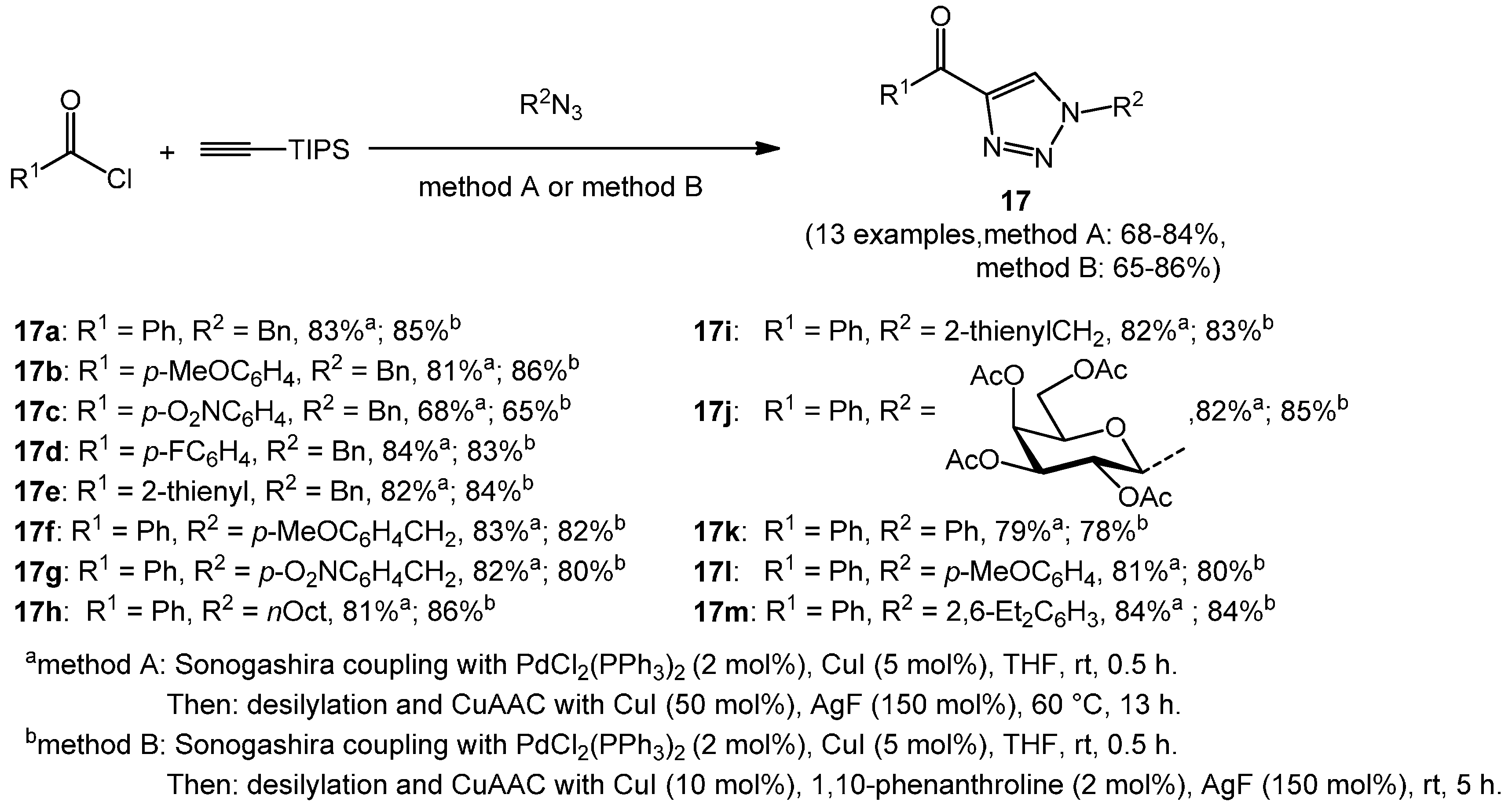
In a related study, the one-pot synthesis of 1-substituted 4-acyl-triazoles
17 was improved by (1) switching to the more robust TIPS (tris(isopropyl)silyl) protecting group; (2) reducing the amount of triethylamine in the Sonogashira step to one stoichiometrically necessary equivalent, as previously shown for TMS-alkynone formation by Karpov and Müller [
79]; and (3) employing AgF as an efficient reagent for the removal of the TIPS group [
80]. In addition, the reaction proceeds smoothly at room temperature and gives rise to good yields (
Scheme 10) [
81].
Scheme 10.
Consecutive three-component synthesis of 1-substituted 4-acyl-1H-1,2,3-triazoles 17 via Sonogashira alkynylation-desilylation-CuAAC sequence.
Scheme 10.
Consecutive three-component synthesis of 1-substituted 4-acyl-1H-1,2,3-triazoles 17 via Sonogashira alkynylation-desilylation-CuAAC sequence.
Not many methods for the synthesis of
N-unsubstituted triazoles are available [
82] and their potential biological activity encouraged the development of synthetic strategies. This led to the development of a three-component one-pot synthesis of 4,5-disubstituted-1,2,3-2
H-triazoles
18 starting from acid chlorides, terminal acetylenes, and sodium azide via ultrasonication promoted Sonogashira coupling-1,3-dipolar cycloaddition (
Scheme 11) [
83].
Scheme 11.
Consecutive three-component synthesis of 4,5-disubstituted-1,2,3-2H-triazoles 18 via Sonogashira alkynylation-cycloaddition sequence.
Scheme 11.
Consecutive three-component synthesis of 4,5-disubstituted-1,2,3-2H-triazoles 18 via Sonogashira alkynylation-cycloaddition sequence.
The developed strategy exhibited a wide range of tolerated substrates, also showing its compatibility with both electron donating and electron withdrawing substituents. Thus such 1,2,3-2H-triazoles provided an opportunity for further exploration of their inclination towards reacting in coupling reactions. The screening of various substrates showed the regioselective N-2 arylation furnishing 1,4,5-trisubstituted-1,2,3-triazoles by nucleophilic aromatic substitution with 1-chloro-4-nitrobenzene.
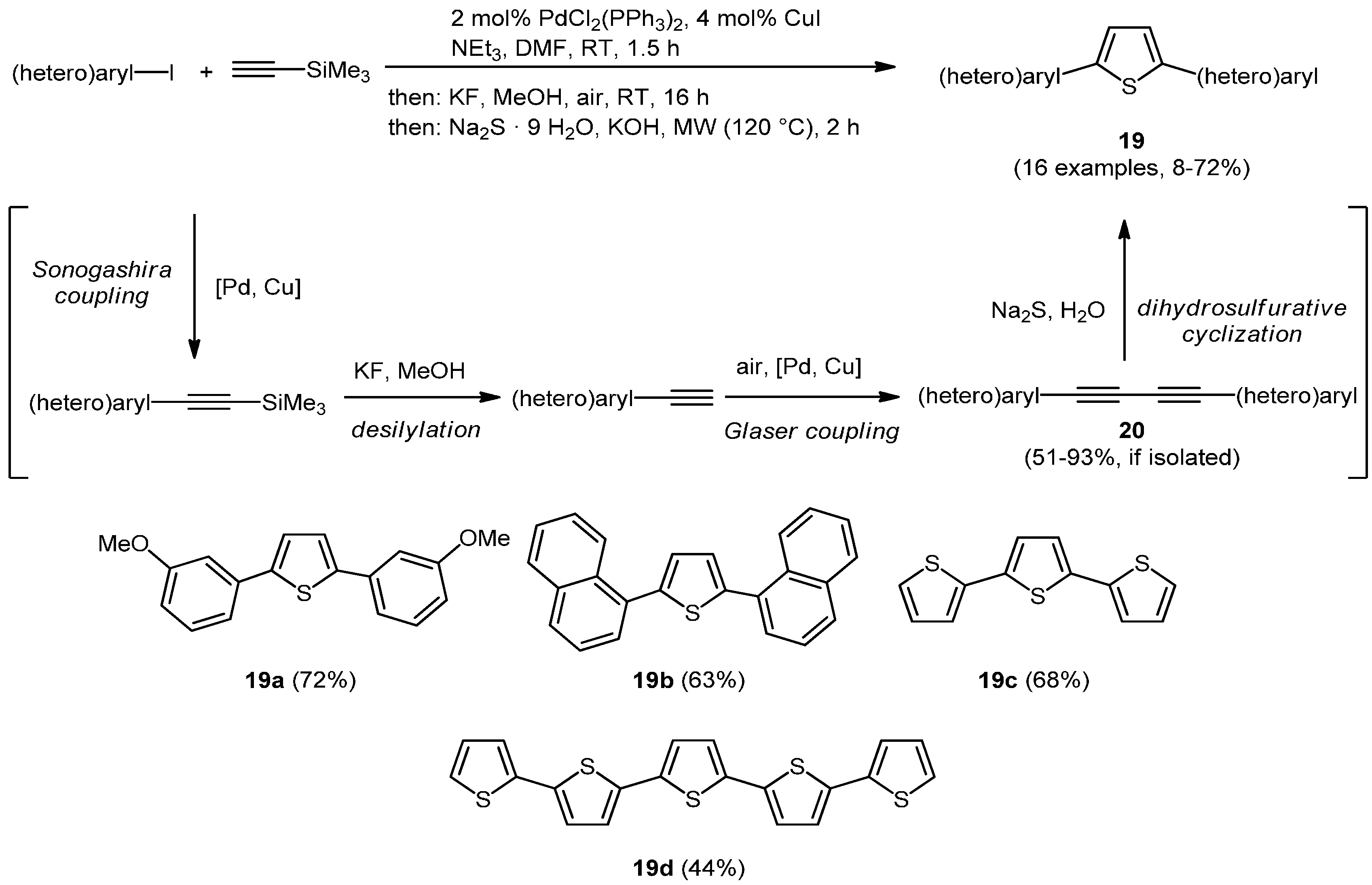
Another illustration for Pd-Cu-sequentially catalyzed processes was presented by Merkul, Urselmann, and Müller with a consecutive Sonogashira-Glaser reaction, where first a (hetero)aryliodide was coupled with (trimethylsilyl)acetylene which was subsequently desilylated by potassium fluoride. Under aerobic conditions the oxidation states of Pd and Cu are altered and an alkyne dimerization furnishes the symmetrically substituted butadiynes
20 in the sense of a pseudo four-component process [
84]. This sequence was employed as an entry to a pseudo five-component synthesis of symmetrically substituted 2,5-di(hetero)arylthiophenes
19 (
Scheme 12) [
85]. Likewise, the Müller group disclosed a rapid access to intensively blue luminescent 2,5-di(hetero)arylfurans when potassium hydroxide was used as the bifunctional nucleophile [
86]. The same concept was applied to the synthesis of 3-(hetero)arylmethyl-2,5-di(hetero)aryl-substituted thiophenes in the sense of a pseudo five-component process by employing (hetero)aryl methylthiols as masked dinucleophilic substrates [
87].
Scheme 12.
Pseudo five-component Sonogashira-Glaser cyclization synthesis of symmetrical 2,5-di(hetero)arylthiophenes 19.
Scheme 12.
Pseudo five-component Sonogashira-Glaser cyclization synthesis of symmetrical 2,5-di(hetero)arylthiophenes 19.
Another very recently reported sequence is the sequentially Pd-Cu-catalyzed Sonogashira coupling Cu-catalyzed cyclization approach towards cyclopenta[
b]naphthalene and benzo[
b]fluorene derivatives
21 starting from aryl 1-cyanoalk-5-ynyl ketones reported by the group of Shia and coworkers (
Scheme 13,
Table 5) [
88]. A tentative mechanism for the Cu-catalyzed cyclization succeeding the Sonogashira coupling starts with the oxidation of the copper(I)-species to give copper(II) and a hydroperoxide anion. The activated hydrogen atom in α-position to the cyano and the keto group is abstracted and the carbon radical undergoes a 5-
exo-
dig cyclization to produce a vinyl radical. This radical then attacks the benzene ring to give a cyclohexadienyl radical. Aromatization is reconstituted by aromatic homolytic substitution, eventually forming hydrogen peroxide.
Scheme 13.
Sequential Pd-Cu-catalyzed Sonogashira coupling-Cu-catalyzed cyclization synthesis of cyclopenta[b]naphthalene and benzo[b]fluorene derivatives 21.
Scheme 13.
Sequential Pd-Cu-catalyzed Sonogashira coupling-Cu-catalyzed cyclization synthesis of cyclopenta[b]naphthalene and benzo[b]fluorene derivatives 21.
Table 5.
Selected cyclopenta[b]naphthalene and benzo[b]fluorene derivatives 21 prepared by the sequential Pd-Cu-catalyzed Sonogashira coupling-Cu-catalyzed cyclization.
3. Sequences Based upon Amination
Initiated by Buchwald and Hartwig in the past two decades Pd-catalyzed carbon-heteroatom bond forming processes have considerably fertilized the field of heterocyclization and the catalyst sources are often identical with those for carbon-carbon bond forming processes [
89,
90,
91,
92,
93]. Several intriguing one-pot sequences have been developed on this conceptual basis furnishing specific substitution patterns on classical and novel heterocyclic core structures.
A prominent example of a two-component sequential catalysis is the intermolecular-intramolecular Buchwald-Hartwig double amination reported by Tamao and coworkers [
94]. The method uses 2,2′-dihalobiphenyls and primary amines for the synthesis of multisubstituted carbazoles
22 that possess intriguing optical properties (
Scheme 14). The methodology has then been applied to the synthesis of different compound classes, for instance to the synthesis of diazacarbazoles [
95], ladder-type π-conjugated heteroacenes [
96], dithienopyrroles [
97,
98,
99], and to the synthesis of the phenothiazine backbone of promazines [
100].
Scheme 14.
Sequentially Pd-catalyzed intermolecular intramolecular Buchwald-Hartwig double amination sequence to access multisubstituted carbazoles 22.
Scheme 14.
Sequentially Pd-catalyzed intermolecular intramolecular Buchwald-Hartwig double amination sequence to access multisubstituted carbazoles 22.
The method has also been employed in the synthesis of dithienothiazines
23 that possess interesting electronic properties involving reversible oxidation and can therefore be regarded as new donors for functional π-systems (
Scheme 15,
Table 6) [
101,
102].
Scheme 15.
Sequentially Pd-catalyzed intermolecular intramolecular Buchwald-Hartwig double amination sequence to access dithienothiazines 23.
Scheme 15.
Sequentially Pd-catalyzed intermolecular intramolecular Buchwald-Hartwig double amination sequence to access dithienothiazines 23.
Table 6.
Selected dithienothiazines 23 via Pd-catalyzed intermolecular intramolecular Buchwald-Hartwig double amination.
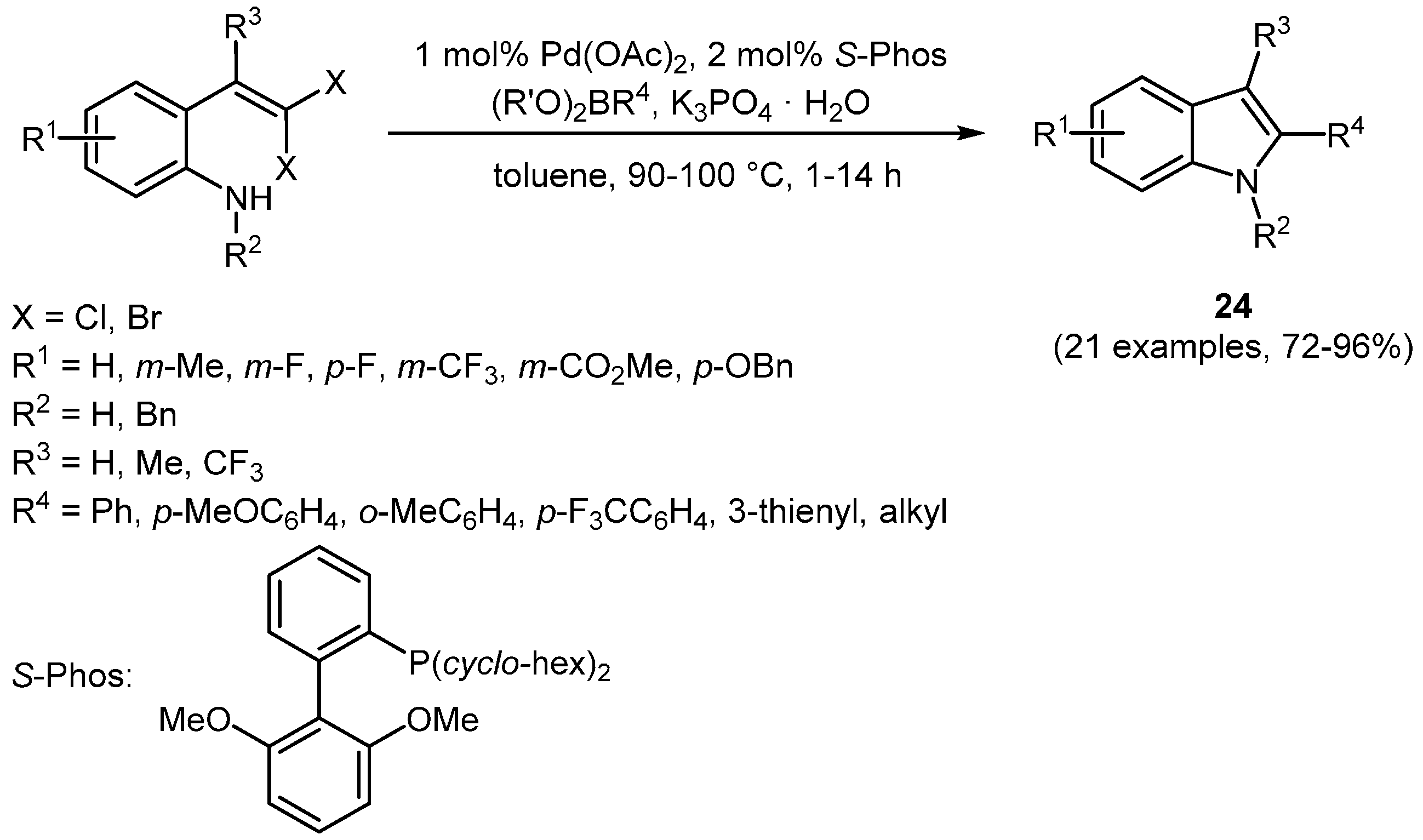
Fang and Lautens reported an assisted tandem catalysis involving an intramolecular palladium-catalyzed Buchwald-Hartwig amination and intermolecular Suzuki-Miyaura coupling [
103]. By the use of
gem-dihalovinyls as starting materials, 2-functionalized indoles
24 could be accessed using various organoboron compounds as coupling partners in the Suzuki-Miyaura-coupling (
Scheme 16,
Table 7).
The developed method has been further elaborated by the same group to provide access to substituted azaindoles and thienopyrroles, respectively [
104,
105,
106]. If the
gem-dihalovinyl group was placed
ortho to the amino group in amino-pyridines or anilines, the catalytic system performed a Buchwald-Hartwig amination followed by subsequent Suzuki- [
104,
105] or Heck-coupling [
106], respectively. In a related fashion, the Lautens group could employ the same concept for a thioanellation furnishing 2-substituted benzothiophenes by sequentially Pd-catalyzed CS– and CC–bond formation [
107]. Furthermore, the developed Pd-catalyzed Buchwald-Hartwig amination Suzuki-coupling methodology has been applied to the synthesis of kinase insert domain-containing receptor (KDR) kinase inhibitors, which pose as promising compounds in anti-tumor therapy [
105].
Scheme 16.
Sequentially Pd-catalyzed Buchwald-Hartwig amination Suzuki-Miyaura coupling sequence for the synthesis of 2-functionalized indoles 24.
Scheme 16.
Sequentially Pd-catalyzed Buchwald-Hartwig amination Suzuki-Miyaura coupling sequence for the synthesis of 2-functionalized indoles 24.
Table 7.
Selected 2-functionalized indoles 24 via sequentially Pd-catalyzed Buchwald-Hartwig amination Suzuki-Miyaura-coupling sequence.
Table 7.
Selected 2-functionalized indoles 24 via sequentially Pd-catalyzed Buchwald-Hartwig amination Suzuki-Miyaura-coupling sequence.
| Entry | gem-Dihalovinyl | Boronate | 2-Functionalized Indoles 24 (Yield) |
|---|
| 1 | R1 = H, R2 = H, R3 = H, X = Br | R4 = Ph | ![Applsci 05 01803 i037]() |
| 2 | R1 = H, R2 = H, R3 = H, X = Cl | R4 = Ph | ![Applsci 05 01803 i038]() |
| 3 | R1 = H, R2 = H, R3 = CF3, X = Br | R4 = Ph | ![Applsci 05 01803 i039]() |
| 4 | R1 = H, R2 = H, R3 = H, X = Br | R4 = p-MeOC6H4 | ![Applsci 05 01803 i040]() |
| 5 | R1 = H, R2 = H, R3 = H, X = Br | R4 = o-MeC6H4 | ![Applsci 05 01803 i041]() |
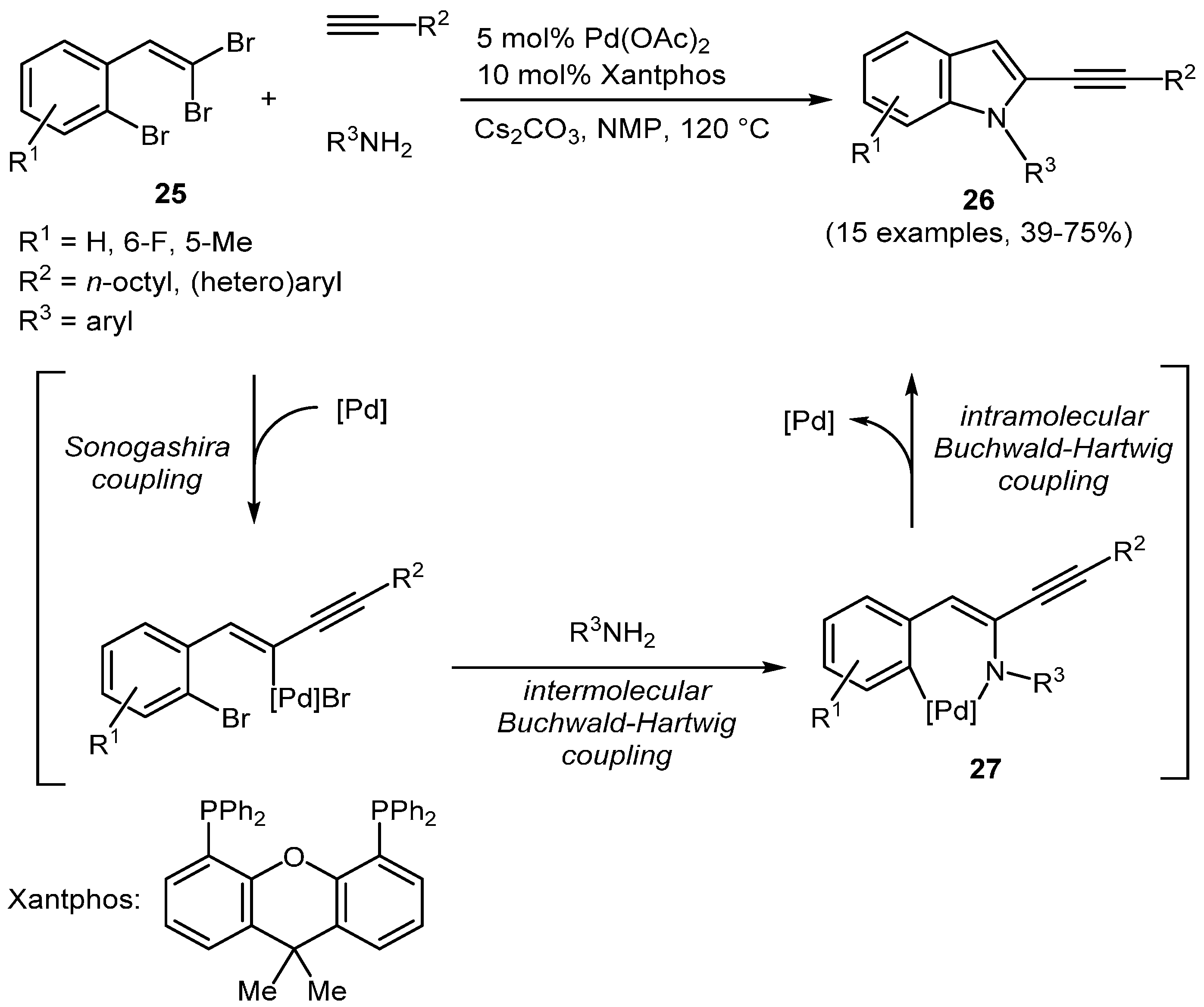
β,β′-Dibromo-
ortho-bromo styrenes
25 are fascinating substrates for multiple cross-coupling reactions where all carbon-bromine bonds display minute differences in their propensity to undergo oxidative addition. Liang
et al. have reported a three-component synthesis of α-alkynyl indoles
26 in moderate to good yields (
Scheme 17,
Table 8) [
108]. The sequence commences with an intermolecular site-selective Sonogashira coupling at the
E-configured bromo styrene position followed by an intermolecular Buchwald-Hartwig amination furnishing the α-alkynyl enaminyl Pd-species
27 which concludes the process with an intramolecular catalytic amination, thereby establishing the indole annulation.
Scheme 17.
Three-component synthesis of α-alkynyl indoles 26 via Sonogashira-inter- and intra-molecular Buchwald-Hartwig sequence.
Scheme 17.
Three-component synthesis of α-alkynyl indoles 26 via Sonogashira-inter- and intra-molecular Buchwald-Hartwig sequence.
Table 8.
Selected α-alkynyl indoles 26 via Sonogashira-inter- and intramolecular Buchwald-Hartwig sequence.
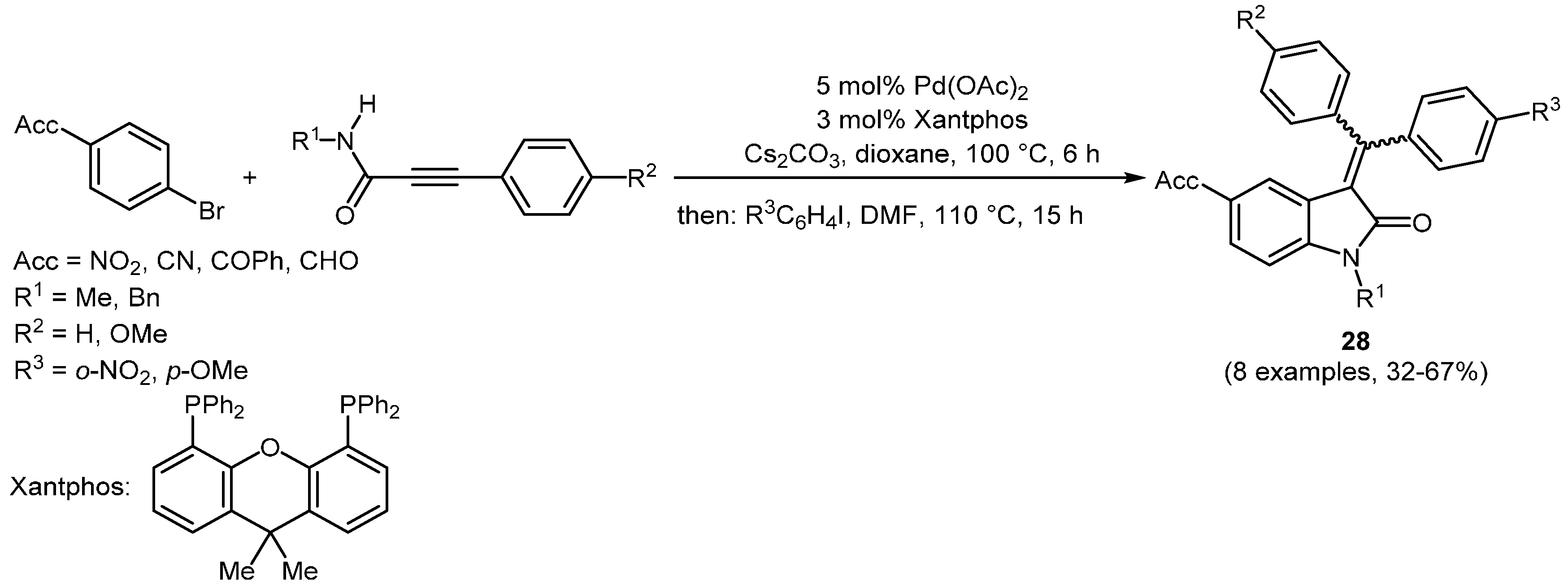
A Pd-catalyzed three-component
N-arylation-carbopalladation-CH-activation-cyclization sequence to afford the indolone scaffold has been reported by Zhu’s group [
109]. Using Xantphos as the initial ligand no further addition of the same is needed after the
N-arylation of the secondary propiolamide. Entering an intermolecular carbopalladation-CH-activation-arylation, the addition of a second aryliodide completes the formation of 3-(diarylmethylene)indolin-2-ones
28 (
Scheme 18,
Table 9).
Scheme 18.
Sequentially Pd-catalyzed N-arylation-carbopalladation-CH-activation-cyclization synthesis of 3-(diarylmethylene)indolin-2-ones 28.
Scheme 18.
Sequentially Pd-catalyzed N-arylation-carbopalladation-CH-activation-cyclization synthesis of 3-(diarylmethylene)indolin-2-ones 28.
Table 9.
Selected 3-(diarylmethylene)indolin-2-ones 28 via sequentially Pd-catalyzed N-arylation-carbopalladation-CH-activation-cyclization.
4. Sequences Intercepted by Cyclizations
Reactive members of the class of three-carbon building blocks are alkynones [
110] and 1,3-diaryl propenones [
111], so-called chalcones, which can be rapidly transformed into five-, six-, and even seven-membered heterocycles in a Michael-addition cyclocondensation sequence.
A catalytic access to ynones [
79,
112] and enones [
113,
114,
115,
116] by Sonogashira-coupling therefore provides an entry to the preparation of a variety of heterocycles. Based on this motivation, the concept has been studied in great detail and elaborated into well-established protocols by the group of Müller in the last decade. Since major advancements of this one-pot methodology have been already reviewed [
117,
118,
119,
120] only recent conceptual achievements with respect to sequentially Pd-catalyzed processes [
34] will be highlighted here.
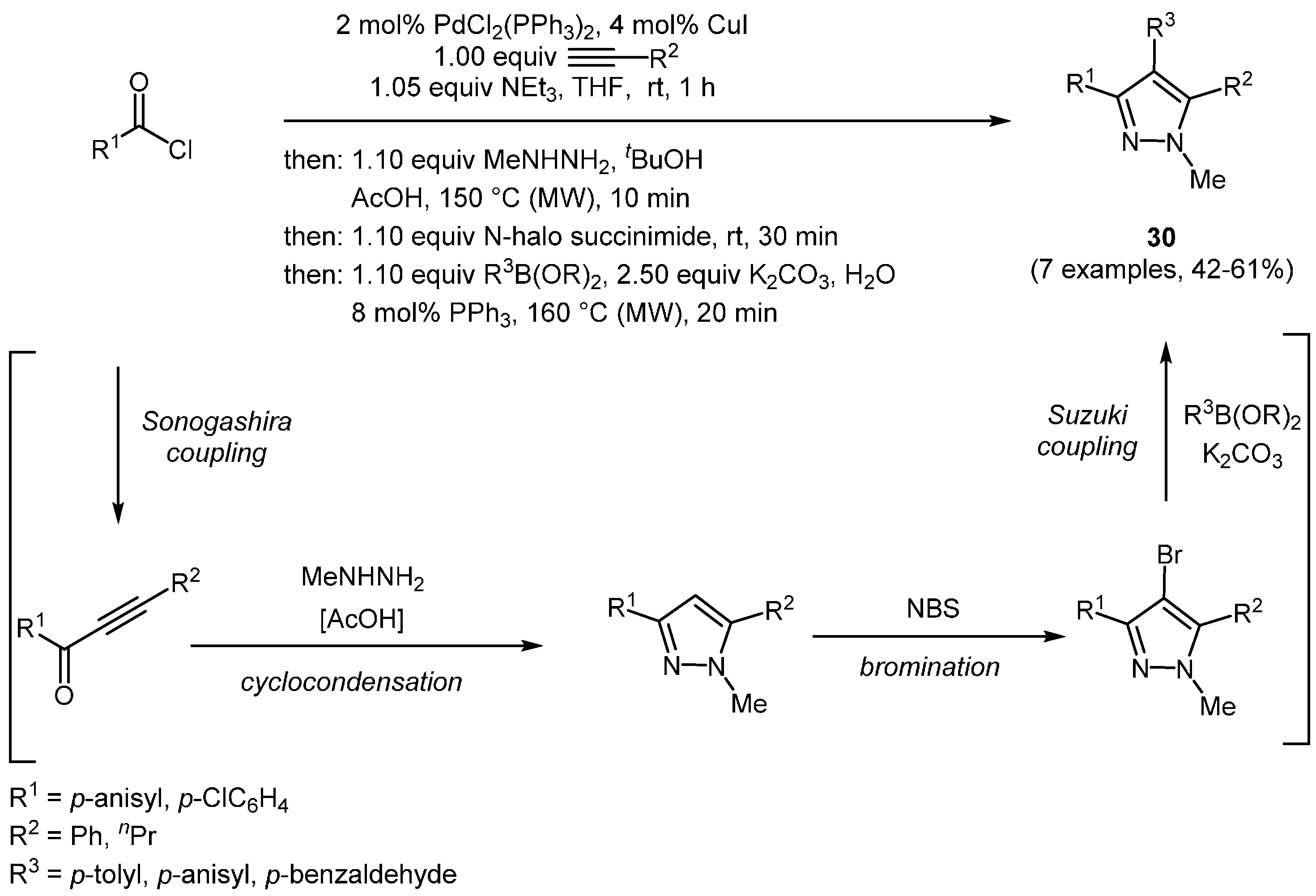
One of these achievements is a consecutive three-component synthesis of pyrazoles using (hetero)aroyl chlorides, terminal alkynes, and hydrazines to regioselectively access substituted heterocyclic structures [
121,
122]. As an example the combination with an electrophilic halogenation led to a one-pot four-component sequence of 4-halo pyrazoles
29 (
Scheme 19) [
123].
Scheme 19.
Four-component synthesis of 4-halo pyrazoles 29.
Scheme 19.
Four-component synthesis of 4-halo pyrazoles 29.
Further elaboration of the previously reported concept afforded the transformation of the in situ generated 4-halo pyrazole
29 in the presence of catalytic metal complexes in a Suzuki-coupling [
123]. In the sense of an assisted tandem catalysis, the addition of catalytic amounts of triphenylphosphane to the reaction mixture for the retention of the correct oxidation state of the Pd source gave rise to the formation of 1,3,4,5-tetrasubstituted pyrazoles
30 in a four-step sequence comprising Sonogashira-coupling, addition-cyclocondensation, halogenation, and Suzuki-coupling in a one-pot fashion (
Scheme 20,
Table 10). All title compounds feature intense blue fluorescence and high quantum yields.
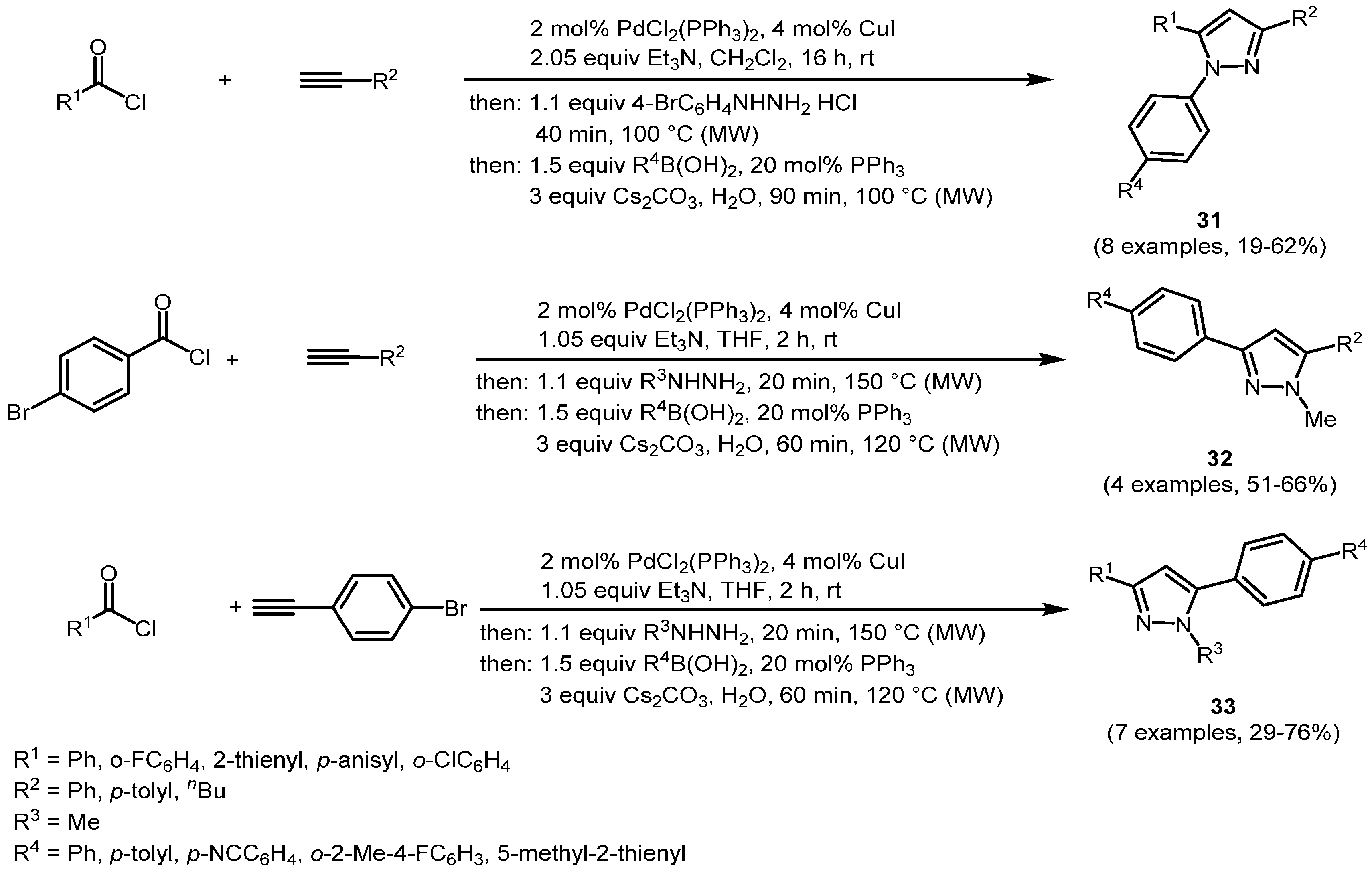
The concatenation of Sonogashira and Suzuki coupling in a one-pot process, intercepted by cyclocondensation with hydrazines, was successfully applied to the efficient microwave-assisted consecutive four-component synthesis of 1-, 3-, and 5-biarylsubstituted pyrazoles
31,
32, and
33 (
Scheme 21,
Table 11) [
124]. This robust protocol allows the introduction of bromo aryl substituents on all three substrates as points of diversification. In every case the one-pot sequence terminates with a Suzuki arylation by adding (hetero)aryl boronates or boronic acids to the reaction mixture. This diversity-oriented approach enables tailoring, fine-tuning, and optimization of absorption and emission properties. By physical organic studies, including molecular modelling, high fluorescence quantum yields up to 97% (compound
33a) were achieved.
Scheme 20.
Sequentially Pd-Cu-catalyzed Sonogashira alkynylation-cyclocondensation-bromination-Suzuki arylation synthesis of 1,3,4,5-tetrasubstituted pyrazoles 30.
Scheme 20.
Sequentially Pd-Cu-catalyzed Sonogashira alkynylation-cyclocondensation-bromination-Suzuki arylation synthesis of 1,3,4,5-tetrasubstituted pyrazoles 30.
Table 10.
Selected 1,3,4,5-tetrasubstituted pyrazoles 30 via sequentially Pd-Cu-catalyzed Sonogashira alkynylation-cyclocondensation-halogenation-Suzuki arylation synthesis.
Scheme 21.
Sequentially Pd-Cu-catalyzed Sonogashira alkynylation-cyclocondensation-Suzuki arylation synthesis of 1-, 3-, and 5-biarylsubstituted pyrazoles 31, 32, and 33.
Scheme 21.
Sequentially Pd-Cu-catalyzed Sonogashira alkynylation-cyclocondensation-Suzuki arylation synthesis of 1-, 3-, and 5-biarylsubstituted pyrazoles 31, 32, and 33.
Table 11.
Selected 1-, 3-, and 5-biarylsubstituted pyrazoles 31, 32, and 33 via sequentially Pd-Cu-catalyzed Sonogashira alkynylation-cyclocondensation-Suzuki arylation synthesis.
Since the reaction conditions used for the alkynylation of aroyl chlorides can easily be conditioned to be Brønsted acidic, Müller and coworkers could show that using THP-protected propargyl alcohols as the alkyne species and sodium chloride or iodide leads to an effective coupling-acetal cleavage-hydrogen halide Michael addition-cyclocondensation sequence giving the compound class of 3-halofurans [
125,
126]. Of equal interest is the fact that the conditions are compatible with a subsequent Suzuki-coupling by mere addition of the desired boronic acids and Na
2CO
3 in excess to the reaction mixture. The intermediary iodo furan then affords the corresponding trisubstituted furan [
125].
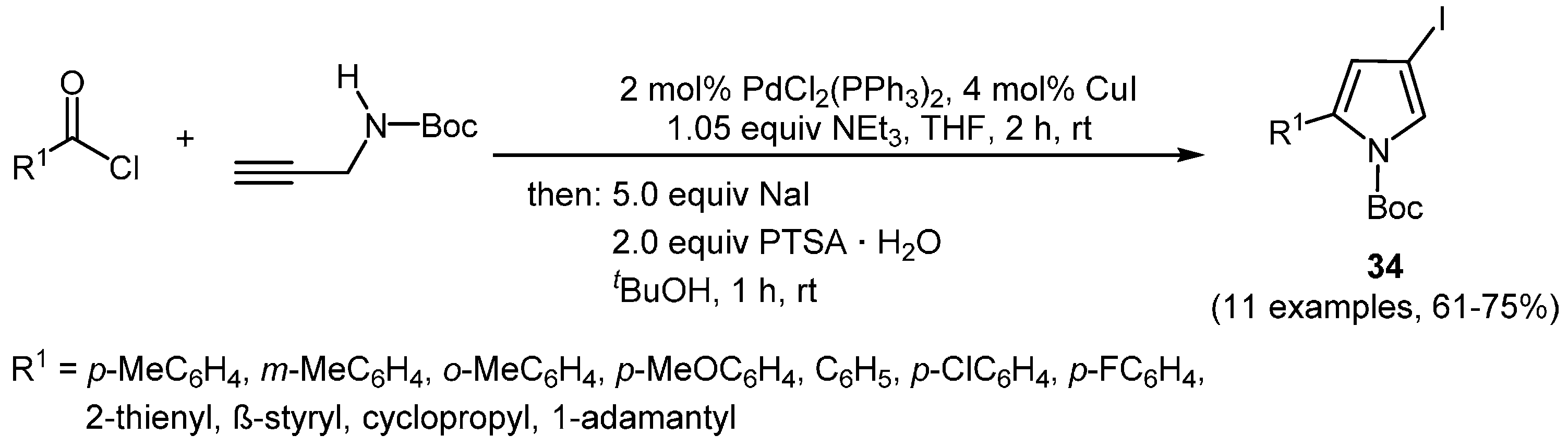
Applying the previously mentioned concept to the synthesis of the corresponding 4-iodo pyrroles however requires the choice of an apt protecting group for the nitrogen. As such, the Boc group is a convenient choice due to its tolerance towards several reaction conditions and the inherent easy removability of a carbamate. As an example, (hetero)aroyl chlorides and
N-Boc-protected propargyl amine have been coupled to give rise to an intermediary alkynone which is then rapidly transformed by addition-cyclocondensation with sodium iodide and PTSA into
N-Boc-4-iodo-2-substituted pyrroles
34 (
Scheme 22) [
127].
Scheme 22.
Three-component synthesis of N-Boc-4-iodo-2-substituted pyrroles 34.
Scheme 22.
Three-component synthesis of N-Boc-4-iodo-2-substituted pyrroles 34.
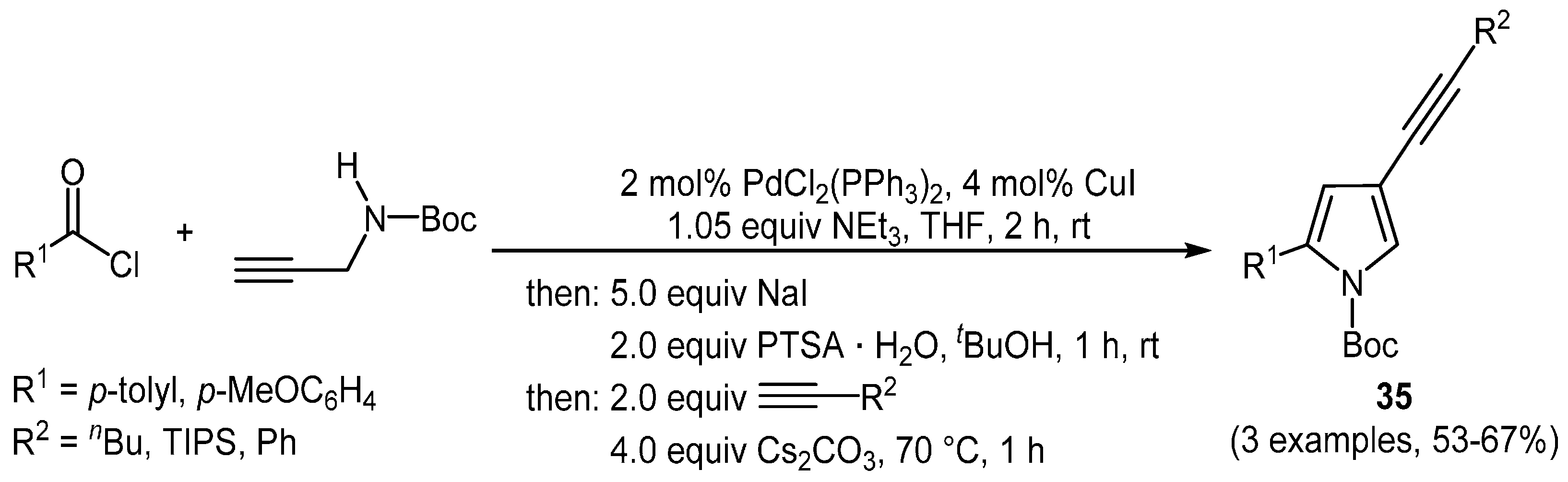
Due to the introduced iodide into the pyrrole core, the
N-Boc-protected 4-iodo pyrroles
34 can now be easily coupled with various alkyne species in the sense of a subsequent Sonogashira-coupling, based on the assumption that the catalyst system is still intact. Addition of a terminal alkyne to the reaction mixture therefore allowed the transformation of
34 into
N-Boc-2-aryl-4-alkynyl pyrroles
35 in the sense of a one-pot four-step coupling-addition-cyclocondensation-coupling sequence that retains the nitrogen protecting group (
Scheme 23) [
127].
Scheme 23.
Sequentially Pd-Cu-catalyzed three-component synthesis of N-Boc-2-aryl-4-alkynyl pyrroles 35.
Scheme 23.
Sequentially Pd-Cu-catalyzed three-component synthesis of N-Boc-2-aryl-4-alkynyl pyrroles 35.
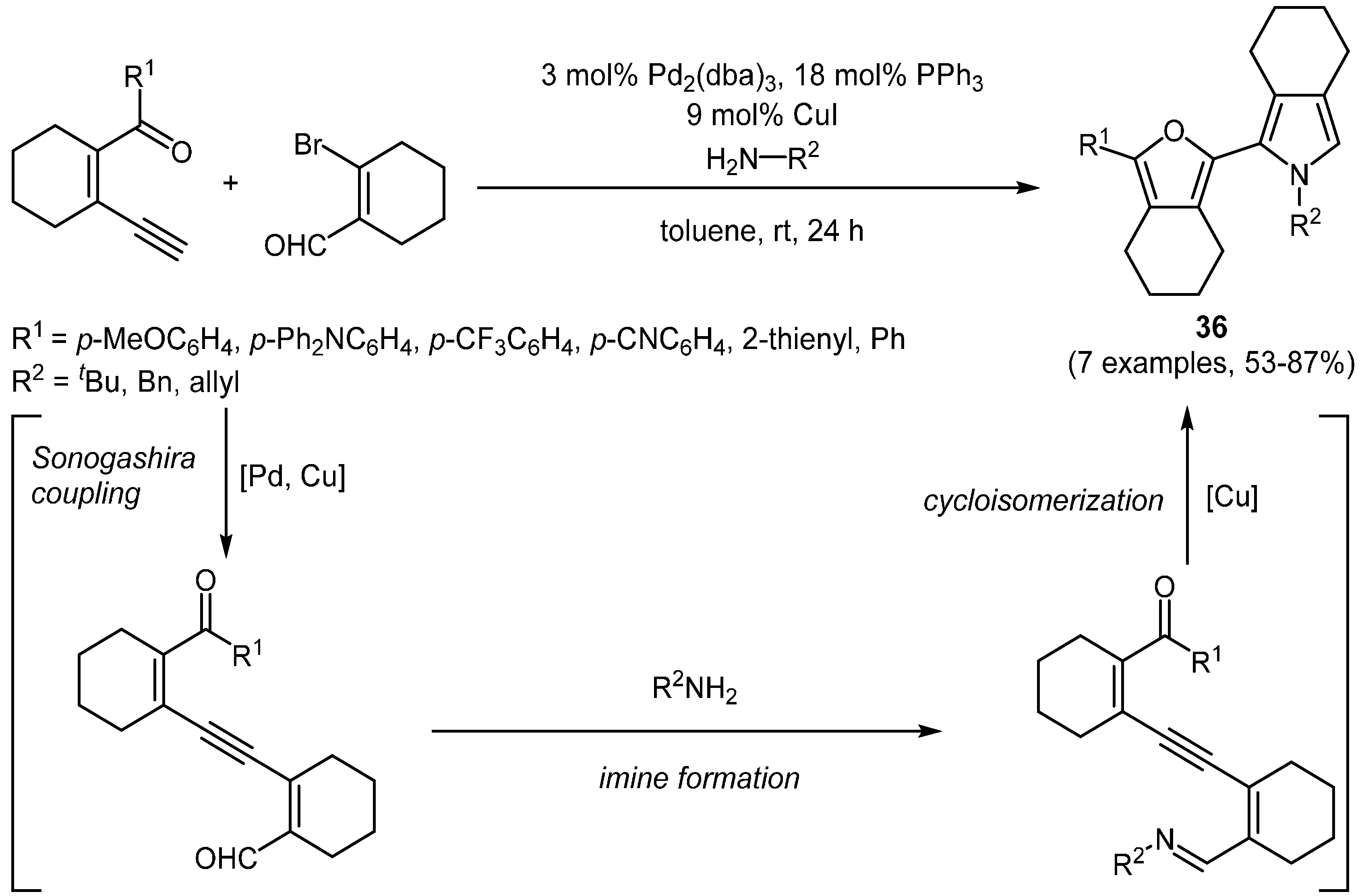
Another recently disclosed sequence is the sequentially Pd-Cu-catalyzed three-component Sonogashira-coupling imine formation double cycloisomerization reaction yielding hetero α,α′-dimers of heterocycles
36, reported by Murata and coworkers (
Scheme 24,
Table 12) [
128]. The sequence starts with the alkynylation of 2-bromo cyclohexen-1-carbaldehyde with a conformationally predetermined enyne carbonyl compound followed by imine formation and eventual double cycloisomerization to the final product.
Scheme 24.
Sequentially Pd-Cu-catalyzed three-component synthesis of hetero α,α′-dimers of heterocycles 36.
Scheme 24.
Sequentially Pd-Cu-catalyzed three-component synthesis of hetero α,α′-dimers of heterocycles 36.
Table 12.
Selected hetero α,α′-dimers of heterocycles 36 via sequentially Pd-Cu-catalyzed three-component synthesis.
The very same protocol could also be exemplified in the preparation of 2-(5′-furanyl)furan and -pyrrole
37 and the monodisperse heterooligomer
38 (
Scheme 25).
Scheme 25.
Sequentially Pd-Cu-catalyzed synthesis of 2-(5′-furanyl)furan and -pyrrole 37 and the monodisperse heterooligomer 38.
Scheme 25.
Sequentially Pd-Cu-catalyzed synthesis of 2-(5′-furanyl)furan and -pyrrole 37 and the monodisperse heterooligomer 38.
5. Miscellaneous Sequentially Pd-Catalyzed Cyclizations
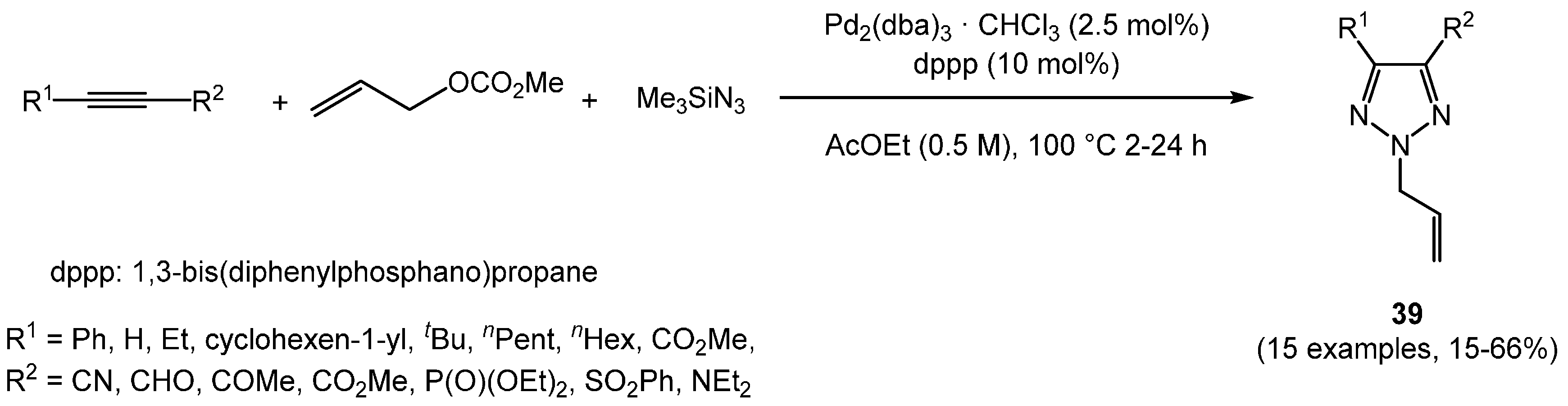
Before the advent of CuAAC for regioselective triazole synthesis [
63], a regiospecific synthesis of 2-allyl-1,2,3-triazoles
39 by palladium-catalyzed cyclization via azidation of allyl methyl carbonate with TMSN
3 at elevated temperature was reported by Yamamoto (
Scheme 26) [
129].
This palladium-catalyzed three-component reaction of activated alkynes (substituted with electron withdrawing group(s)), allyl carbonate, and trimethylsilyl azide also represented a regiospecific formation of 2-allyl-1,2,3-triazoles.
While various Pd catalyst species were not able to transform nonactivated alkynes solely, copper co-catalysis finally led to a significant extension of the methodology, tolerating nonactivated alkynes (
Scheme 26) [
130]. Two sets of reaction conditions were optimized for regioselective one-pot syntheses of 1-allyl 1,2,3-triazoles
40 and 2-allyl 1,2,3-triazoles
41 employing nonactivated alkynes as substrates (
Scheme 27). The optimization studies disclosed the regioselective role exerted by phosphane ligands.
Scheme 26.
Pd-catalyzed three-component reaction of alkynes, allyl carbonate, and trimethylsilyl azide to regiospecifically give 2-allyl-1,2,3-triazole 39.
Scheme 26.
Pd-catalyzed three-component reaction of alkynes, allyl carbonate, and trimethylsilyl azide to regiospecifically give 2-allyl-1,2,3-triazole 39.
Scheme 27.
Regioselective Pd-Cu-catalyzed three-component syntheses of 1-allyl 1,2,3-triazoles 40 and 2-allyl 1,2,3-triazoles 41.
Scheme 27.
Regioselective Pd-Cu-catalyzed three-component syntheses of 1-allyl 1,2,3-triazoles 40 and 2-allyl 1,2,3-triazoles 41.
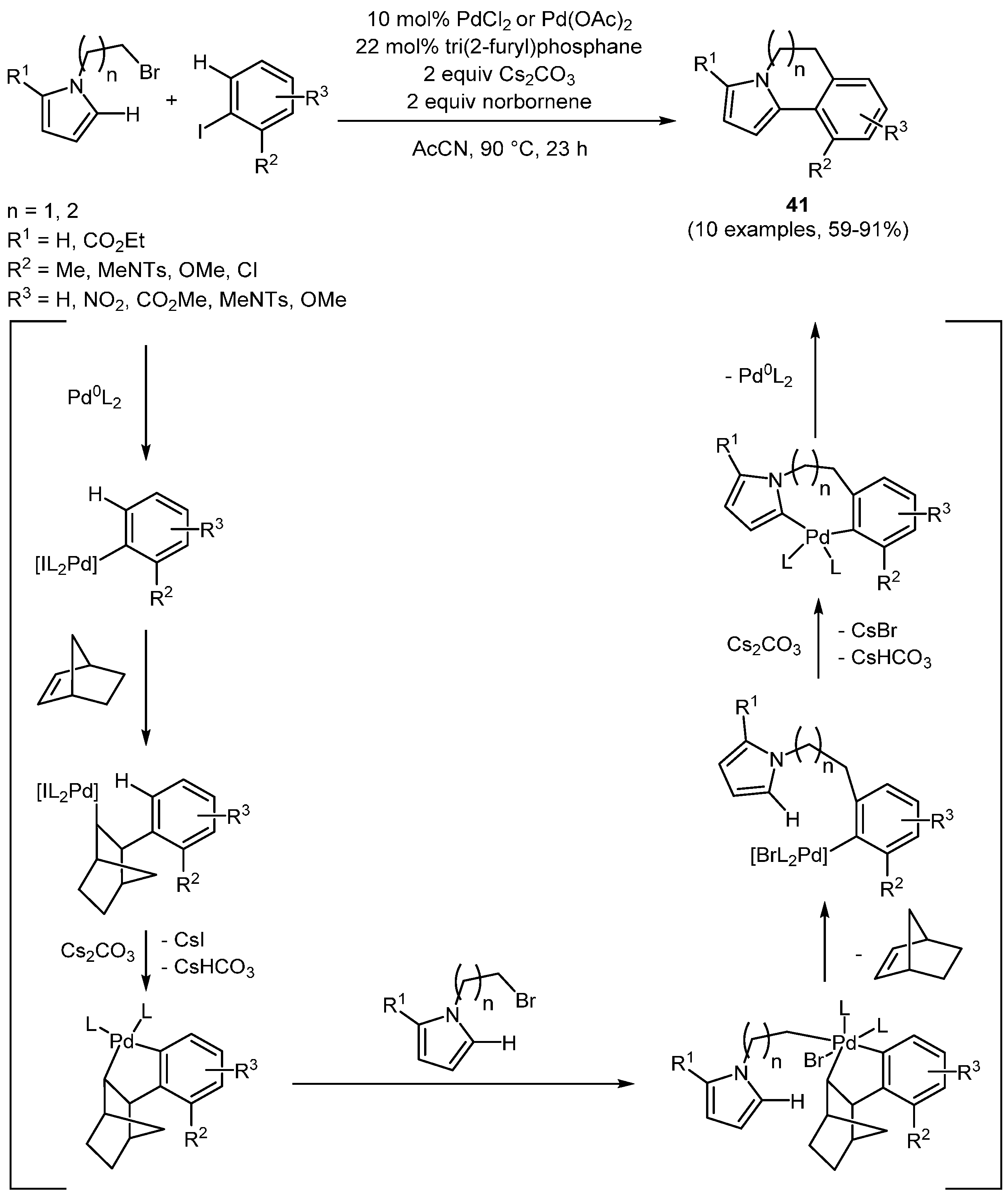
The Lautens group has further developed Catellani’s norbornene-mediated Pd-catalyzed
ortho-alkylation of iodo arenes under Heck conditions [
131] and published an efficient methodology accessing functionalized polycyclic heterocycles by employing a norbornene-mediated, palladium-catalyzed tandem alkylation direct arylation sequence in a one-pot fashion [
132,
133,
134,
135]. After oxidative addition of the palladium(0) species to the employed iodoarene a carbopalladation of norbornene precedes the sp
2 CH activation that enables the alkylation. Reductive elimination of the postulated intermediary palladium(IV) complex followed by dismissal of norbornene gives rise to a second CH activation that results in the heteroannulated pyrroles
42 (
Scheme 28,
Table 13). Following this methodology, six- and seven-membered annulated pyrroles and pyrazoles [
132], polycyclic thiophenes and furans [
133], annulated 2-
H-indazoles and 1,2,3- and 1,2,4-triazoles [
134], and, even more remarkable, sterically hindered bi- and tricyclic benzonitriles [
135] have been prepared.
Scheme 28.
Sequentially Pd-catalyzed, norbornene mediated tandem alkylation direct arylation sequence in a one-pot fashion affording heteroannulated pyrroles 42.
Scheme 28.
Sequentially Pd-catalyzed, norbornene mediated tandem alkylation direct arylation sequence in a one-pot fashion affording heteroannulated pyrroles 42.
Table 13.
Selected heteroannulated pyrroles 42 via sequentially Pd-catalyzed, norbornene mediated tandem alkylation direct arylation sequence.