Microwave-Assisted Conversion of Levulinic Acid to γ-Valerolactone Using Low-Loaded Supported Iron Oxide Nanoparticles on Porous Silicates
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials Synthesis
2.1.1. Synthesis of Al- and Zr-SBA-15
2.1.2. Continuous Flow Synthesis of Supported Iron Oxide Nanoparticles on Al- and Zr-SBA-15

2.1.3. Mechanochemical Synthesis of Pd/Al-SBA-15
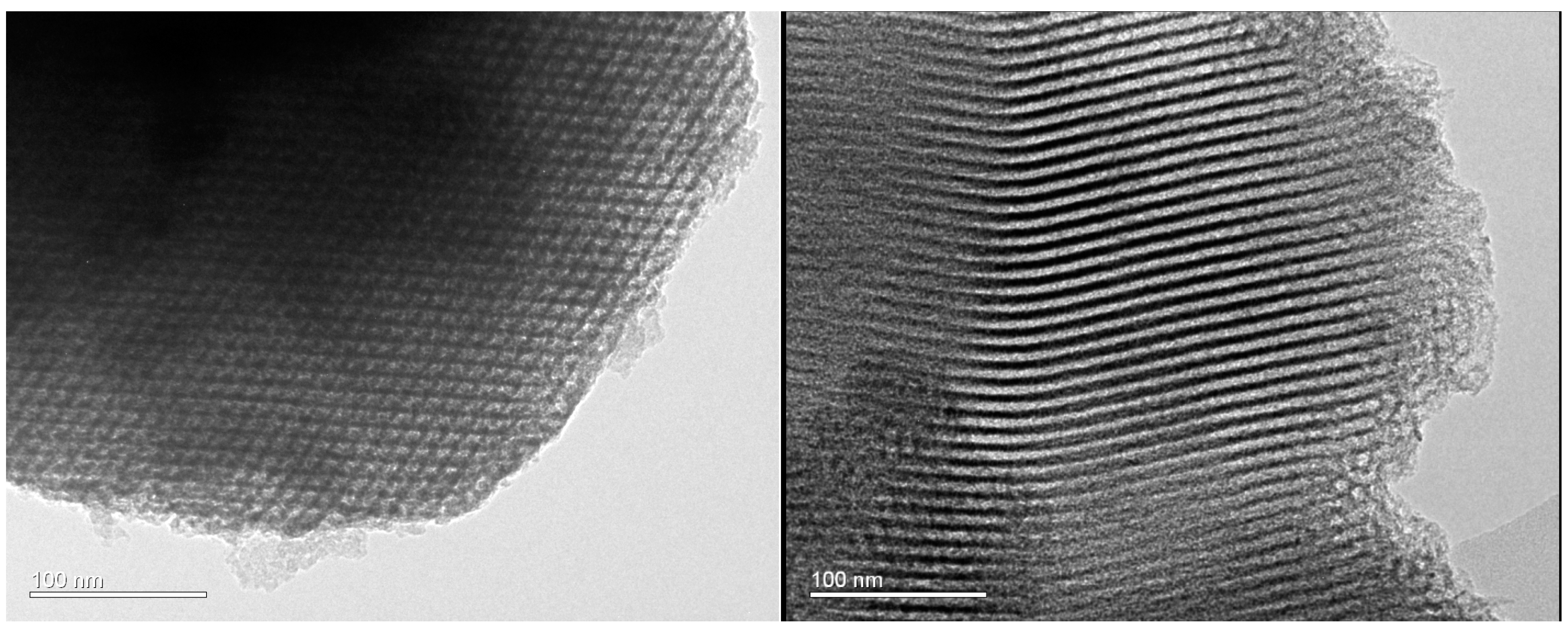
2.2. Materials Characterization
2.3. Catalytic Experiments
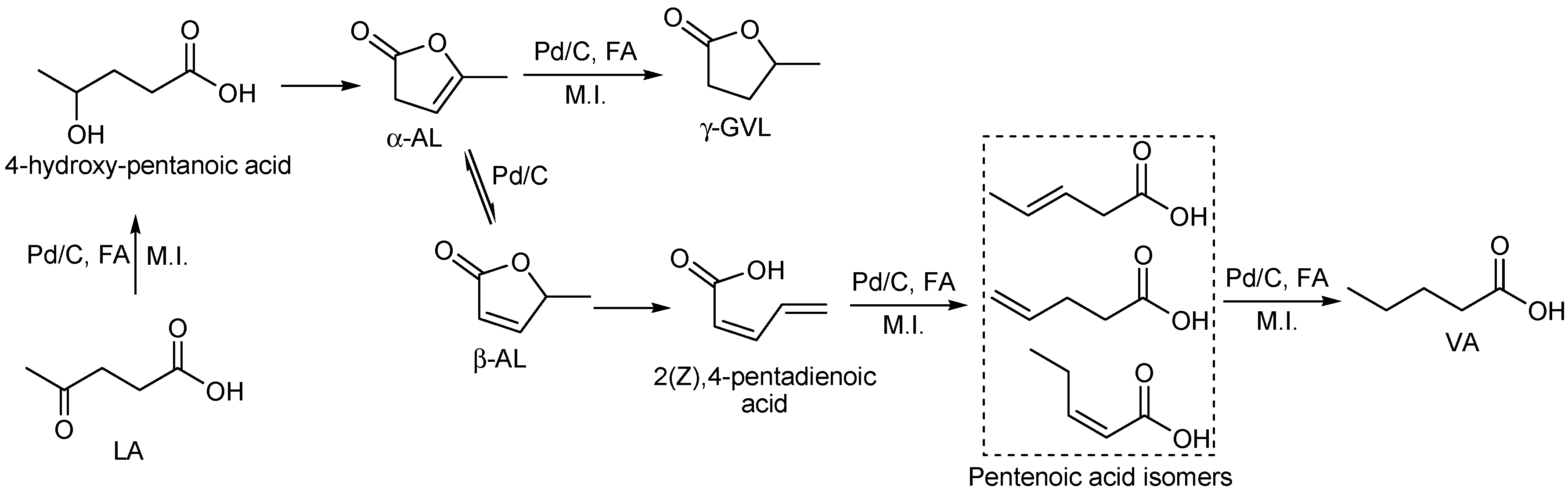
Microwave-assisted Conversion of Levulinic Acid to γ-valerolactone
3. Results and Discussion
| Material | Surface area a (m2g−1) | Pore size (nm) | Pore volume b (mLg−1) | Elemental composition c (%) | ||
|---|---|---|---|---|---|---|
| Fe d | Al | Zr | ||||
| Al-SBA-15 | 720 | 8.5 | 0.80 | - | 2.0 | - |
| Zr-SBA-15 | 765 | 7.4 | 0.74 | - | - | 4.0 |
| FeAlCF | 659 | 8.2 | 0.77 | 0.85 (0.6) | 1.1 | - |
| FeZrCF | 687 | 7.0 | 0.66 | 0.53 (0.7) | - | 3.9 |
| PdAlCF | 633 | 7.9 | 0.70 | - | 1.2 | - |
| PdAlSBA-BM | 507 | 8.5 | 0.92 | - | 1.1 | - |
| 5%Pd/C | 1220 | <2 | 1.50 | - | - | - |
| Entry | Catalyst | Mass catalyst (g) | Conv. (mol%) | TOF (h−1) | Selectivity (mol%) | |||
|---|---|---|---|---|---|---|---|---|
 |  |  |  | |||||
| 1 | Blank | - | <5 | - | - | - | - | - |
| 2 | Al-SBA-15 | 0.1 | <5 | - | - | - | - | - |
| 3 | Zr-SBA-15 | 0.1 | <5 | - | - | - | - | - |
| 4 | FeZrCF | 0.05 | 30 | 125 | 22 | 78 | - | - |
| 5 | FeAlCF | 0.05 | 20 | 52 | 36 | 64 | - | - |
| 6 | PdAlCF | 0.05 | <10 | - | - | 96 | - | 4 |
| 7 | PdAlSBA-BM | 0.05 | <10 | - | - | 94 | - | 6 |
| 8 | 5%Pd/C | 0.05 | 22 | 19 | - | 66 | 27 | 7 |
| 9 | 5%Pd/C b | 0.1 | 33 | 14 | - | 88 | 6 | 6 |
| 10 | 5%Pd/C | 0.1 | 36 | 14 | - | 86 | 7 | 7 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bereczky, A.; Lukács, K.; Farkas, M.; Dóbé, S. Effect of γ-valerolactone blending on engine performance, combustion characteristics and exhaust emissions in a diesel engine. Nat. Resour. 2014, 5, 177–191. [Google Scholar]
- Bond, J.Q.; Alonso, D.M.; Wang, D.; West, R.M.; Dumesic, J.A. Integrated catalytic conversion of γ-valerolactone to liquid alkenes for transportation fuels. Science 2010, 327, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.M.; Bond, J.Q.; Serrano-Ruiz, J.C.; Dumesic, J.A. Production of liquid hydrocarbon transportation fuels by oligomerization of biomass-derived C9 alkenes. Green Chem. 2010, 12, 992–999. [Google Scholar] [CrossRef]
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. γ-Valerolactone—A sustainable liquid for energy and carbon-based chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Wettstein, S.G.; Alonso, D.M.; Chong, Y.; Dumesic, J.A. Production of levulinic acid and gamma-valerolactone (GVL) from cellulose using GVL as a solvent in biphasic systems. Energy Environ. Sci. 2012, 5, 8199–8203. [Google Scholar] [CrossRef]
- Qi, L.; Mui, Y.F.; Lo, S.W.; Lui, M.Y.; Akien, G.R.; Horváth, I.T. Catalytic conversion of fructose, glucose, and sucrose to 5-(hydroxymethyl) furfural and levulinic and formic acids in γ-valerolactone as a green solvent. ACS Catal. 2014, 4, 1470–1477. [Google Scholar] [CrossRef]
- Luterbacher, J.S.; Rand, J.M.; Alonso, D.M.; Han, J.; Youngquist, J.T.; Maravelias, C.T.; Pfleger, B.F.; Dumesic, J.A. Nonenzymatic sugar production from biomass using biomass-derived γ-valerolactone. Science 2014, 343, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Chalid, M.; Broekhius, A.A.; Heeres, H.J. Experimental and kinetic modeling studies on the biphasic hydrogenation of levulinic acid to γ-valerolactone using a homogeneous water-soluble Ru-(TPPTS) catalyst. J. Mol. Catal. A 2011, 341, 14–21. [Google Scholar] [CrossRef]
- Tukacs, J.M.; Kiraly, D.; Stradi, A.; Novodarszki, G.; Eke, Z.; Dibo, G.; Kegl, T.; Mika, L.T. Efficient catalytic hydrogenation of levulinic acid: A key step in biomass conversion. Green Chem. 2012, 14, 2057–2065. [Google Scholar] [CrossRef]
- Fábos, V.; Mika, L.T.; Horváth, I.T. Selective conversion of levulinic and formic acids to γ-valerolactone with the shvo catalyst. Organometallics 2014, 33, 181–187. [Google Scholar] [CrossRef]
- Li, W.; Xie, J.H.; Lin, H.; Zhou, Q.L. Highly efficient hydrogenation of biomass-derived levulinic acid to γ-valerolactone catalyzed by iridium pincer complexes. Green Chem. 2012, 14, 2388–2390. [Google Scholar] [CrossRef]
- Deng, J.; Wang, Y.; Pan, T.; Xu, Q.; Guo, Q.X.; Fu, Y. Conversion of carbohydrate biomass to γ-valerolactone by using water-soluble and reusable iridium complexes in acidic aqueous media. ChemSusChem 2013, 6, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Lin, L.; Liu, S. Synthesis of γ-valerolactone by hydrogenation of biomass-derived levulinic acid over Ru/C catalyst. Energy Fuels 2009, 23, 3853–3858. [Google Scholar] [CrossRef]
- Galletti, A.M.R.; Antonetti, C.; Luise, V.D.; Martinelli, M. A sustainable process for the production of γ-valerolactone by hydrogenation of biomass-derived levulinic acid. Green Chem. 2012, 14, 688–694. [Google Scholar] [CrossRef]
- Selva, M.; Gottardo, M.; Perosa, A. Upgrade of biomass-derived levulinic acid via Ru/C-catalyzed hydrogenation to γ-valerolactone in aqueous–organic–ionic liquids multiphase systems. ACS Sustain. Chem. Eng. 2013, 1, 180–189. [Google Scholar] [CrossRef] [Green Version]
- Yana, K.; Lafleura, T.; Wua, G.; Liaob, J.; Cenga, C.; Xie, X. Highly selective production of value-added γ-valerolactone frombiomass-derived levulinic acid using the robust Pd nanoparticles. Appl. Catal. A 2013, 468, 52–58. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Hasan, M.A. Pd/C catalyzed conversion of levulinic acid to γ-valerolactone using alcohol as a hydrogen donor under microwave conditions. Catal. Commun. 2015, 60, 5–7. [Google Scholar] [CrossRef]
- Ruppert, A.M.; Grams, J.; Jedrzejczyk, J.; Matras-Michalska, J.; Keller, N.; Ostojska, K.; Sautet, P. Titania-supported catalysts for levulinic acid hydrogenation: influence of support and its impact on γ-valerolactone yield. ChemSusChem 2015. [Google Scholar] [CrossRef]
- Son, P.A.; Nishimura, S.; Ebitani, K. Production of γ-valerolactone from biomass-derived compounds using formic acid as a hydrogen source over supported metal catalysts in water solvent. RSC Adv. 2014, 4, 10525–10530. [Google Scholar] [CrossRef]
- Bourne, R.A.; Stevens, J.G.; Ke, J.; Poliakoff, M. Maximising opportunities in supercritical chemistry: The continuous conversion of levulinic acid to γ-valerolactone in CO2. Chem. Commun. 2007, 44, 4632–4634. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.M.; Wettstein, S.G.; Bond, J.Q.; Root, T.W.; Dumesic, J.A. Production of biofuels from cellulose and corn stover using alkylphenol solvents. ChemSusChem 2011, 4, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, G.; Zhang, X.; Li, F. Facile fabrication of composition-tuned Ru-Ni bimetallics in ordered mesoporous carbon for levulinic acid hydrogenation. ACS Catal. 2014, 4, 1419–1425. [Google Scholar] [CrossRef]
- Lange, J.P.; Price, R.; Ayoub, P.M.; Louis, J.; Petrus, L.; Clarke, L.; Gosslink, H. Valeric biofuels: A platform of cellulosic transportation fuels. Angew. Chem. Int. Ed. 2010, 49, 4479–4483. [Google Scholar] [CrossRef] [PubMed]
- Braden, D.J.; Henao, C.A.; Heltzel, J.; Maravelias, C.C.; Dumesic, J.A. Production of liquid hydrocarbon fuels by catalytic conversion of biomass-derived levulinic acid. Green Chem. 2011, 13, 1755–1765. [Google Scholar] [CrossRef]
- Hengne, A.M.; Rode, C.V. Cu-ZrO2 nanocomposite catalyst for selective hydrogenation of levulinic acid and its ester to γ-valerolactone. Green Chem. 2012, 14, 1064–1072. [Google Scholar] [CrossRef]
- Shimizu, K.; Kanno, S.; Kon, K. Hydrogenation of levulinic acid to γ-valerolactone by Ni and MoOx co-loaded carbon catalysts. Green Chem. 2014, 16, 3899–3903. [Google Scholar] [CrossRef]
- Mai, E.F.; Machado, M.A.; Davies, T.E.; Lopez-Sanchez, J.A.; Teixeira da Silva, V. Molybdenum carbide nanoparticles within carbon nanotubes as superior catalysts for γ-valerolactone production via levulinic acid hydrogenation. Green Chem. 2014, 16, 4092–4097. [Google Scholar] [CrossRef]
- Song, J.; Wu, L.; Zhou, B.; Zhou, H.; Fan, H.; Yang, Y.; Meng, Q.; Han, B. A new porous Zr-containing catalyst with a phenate group: An efficient catalyst for the catalytic transfer hydrogenation of ethyl levulinate to γ-valerolactone. Green Chem. 2015. [Google Scholar] [CrossRef]
- Wang, J.; Jaenicke, S.; Chuah, G.K. Zirconium-Beta zeolite as a robust catalyst for the transformation of levulinic acid to γ-valerolactone via Meerwein-Ponndorf-Verley reduction. RSC Adv. 2014, 4, 13481–13489. [Google Scholar] [CrossRef]
- Bermudez, J.M.; Menéndez, J.A.; Romero, A.A.; Serrano, E.; Garcia-Martinez, J.; Luque, R. Continuous flow nanocatalysis: Reaction pathways in the conversion of levulinic acid to valuable chemicals. Green Chem. 2013, 15, 2786–2792. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Li, J.; Lai, D.; Fu, Y.; Guo, Q. Catalytic conversion of biomass-derived carbohydrates into γ-valerolactone without using an external H2 supply. Angew. Chem. Int. Ed. 2009, 48, 6529–6532. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zhao, Y.; Li, J.; Fu, Y.; Liao, B.; Guo, Q.X. Conversion of levulinic acid and formic acid into γ-valerolactone over heterogeneous catalysts. ChemSusChem 2010, 3, 1172–1175. [Google Scholar] [CrossRef] [PubMed]
- Yepez, A.; Lam, F.L.Y.; Romero, A.A.; Kappe, C.O.; Luque, R. Continuous flow preparation of iron oxide nanoparticles supported on porous silicates. ChemCatChem 2015, 7, 276–282. [Google Scholar] [CrossRef]
- Toledano, A.; Serrano, L.; Pineda, A.; Balu, A.M.; Luque, R.; Labidi, J. Fractionation of organosolv lignin from olive tree clippings and its valorization to simple phenolic compounds. ChemSusChem 2013, 6, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Toledano, A.; Serrano, L.; Labidi, J.; Balu, A.M.; Pineda, A.; Luque, R. Heterogeneously catalysed mild hydrogenolytic depolymerisation of lignin under microwave irradiation with hydrogen-donating solvents. ChemCatChem 2013, 5, 977–985. [Google Scholar] [CrossRef]
- Al-Shaal, M.G.; Hausoul, P.J.C.; Palkovits, R. Efficient, solvent-free hydrogenation of α-angelica lactone catalysed by Ru/C at atmospheric pressure and room temperature. Chem. Commun. 2014, 50, 10206–10209. [Google Scholar] [CrossRef] [PubMed]
- Palkovits, R. Pentenoic acid pathways for cellulosic biofuels. Angew. Chem. Int. Ed. 2010, 49, 4336–4338. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yepez, A.; De, S.; Climent, M.S.; Romero, A.A.; Luque, R. Microwave-Assisted Conversion of Levulinic Acid to γ-Valerolactone Using Low-Loaded Supported Iron Oxide Nanoparticles on Porous Silicates. Appl. Sci. 2015, 5, 532-543. https://doi.org/10.3390/app5030532
Yepez A, De S, Climent MS, Romero AA, Luque R. Microwave-Assisted Conversion of Levulinic Acid to γ-Valerolactone Using Low-Loaded Supported Iron Oxide Nanoparticles on Porous Silicates. Applied Sciences. 2015; 5(3):532-543. https://doi.org/10.3390/app5030532
Chicago/Turabian StyleYepez, Alfonso, Sudipta De, Maria Salud Climent, Antonio A. Romero, and Rafael Luque. 2015. "Microwave-Assisted Conversion of Levulinic Acid to γ-Valerolactone Using Low-Loaded Supported Iron Oxide Nanoparticles on Porous Silicates" Applied Sciences 5, no. 3: 532-543. https://doi.org/10.3390/app5030532






